Abstract
Objectives
To report the current elastography methods used to quantify back muscles’ biomechanical characteristics in patients with musculoskeletal disorders (MSKd) and inform on their reliability, validity, and responsiveness.
Methods
MEDLINE, Embase, CINAHL, Cochrane library and grey literature were consulted. Predefined criteria allowed for study selection and data extraction. The quality of evidence was rated using the COSMIN tool. Data were meta-analyzed in terms of pooled intraclass correlation coefficient (pICC) for reliability and pooled standardized mean difference (pSMD) for validity and responsiveness. Heterogeneity was assessed.
Results
Seventy-nine studies were included in the meta-analysis (total number of participants N = 3178). Three elastography methods were identified: strain imaging (SI; number of cohorts M = 26), shear wave imaging (SWI; M = 50), and vibration sonoelastography (VSE; M = 3). Strain imaging and SWI studies reported good reliability measurement properties (pICC > 0.70) and a medium pSMD (0.58 for SI and 0.60 for SWI; p ≤ 0.020) in discriminating MSKd from controls’ condition (validity). Strain imaging studies reported a medium pSMD (0.64; p = 0.005) in detecting within-group changes over time, whereas SWI pSMD was very high (1.24; p = 0.005). Only SWI reported significant but small pSMD (0.30; p = 0.003) in detecting between-group changes over time. The small number of VSE studies could not be meta-analyzed. Heterogeneity was high (I-squared > 90%; p < 0.001).
Conclusions
Elastography presents good reliability results and a medium pSMD in discriminating MSKd from control conditions. Responsiveness data suggest detectable changes within groups over time using SI and SWI, calling for long-term longitudinal studies. Assessing changes between groups over time using elastography still needs to be proven. Highly significant heterogeneity limits meta-analytic results.
Critical relevance statement
While still in its early-stage exploration phase, musculoskeletal ultrasound elastography may reliably quantify back muscles’ biomechanics in asymptomatic individuals, moderately discriminate back musculoskeletal disorders and detect biomechanical changes over time in these conditions, calling for long-term longitudinal studies.
Key Points
-
Ultrasound elastography is reviewed for back pain and related musculoskeletal disorder assessments.
-
Growing literature supports good reproducibility, some validity and responsiveness.
-
Back muscle elastography considers assumptions calling for standardized protocols.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Musculoskeletal disorders (MSKd) are the most common cause of severe long-term pain and disability worldwide, responsible for 166 million (21.3%) years of life lived with disability in the general population [1]. In adults with MSKd, the most common pain is back pain, with neck and thoracic pain affecting up to 15% of older people, and low back pain (LBP) concerning up to 45% of the working-age population representing a major economic issue [1,2,3,4]. The interaction of forces and movement with the anatomy of back muscles, defined as biomechanics [5], is of prime importance for the comprehension of back pain [5,6,7,8]. Biomechanical models suggest that the activation of back muscles may depend on their inherent viscoelastic properties, which, placed under increased demand, may expose them to disorders and injuries [9,10,11,12]. Biomechanical properties such as viscoelasticity are influenced by the contraction or relaxation of the musculature [13], which makes them difficult to assess by conventional clinical imaging technologies such as computed tomography or magnetic resonance imaging. To answer this, the previous decades witnessed substantial research in the field of ultrasound imaging [14] with the aim of evaluating muscle and related soft tissue morphology and function [15]. In this regard, using ultrasound elastography (a group of techniques for objectively assessing tissue strain or stiffness [16]), researchers quantified muscle movements and deformations in various pathologies and anatomical structures [17,18,19], including back muscles [16, 20,21,22].
However, to date, there is no consensus on which elastography method or parameter is useful to assess back muscle biomechanical characteristics. Moreover, the role of elastography in measuring biomechanical characteristics of back muscles depends on the extent to which measurements are consistent and free from error, namely reliability. In addition, this also implies that accurate conclusions can be drawn about measurements to make predictions or diagnosis on biomechanical outcomes, discriminating among patients with and without the disorder or treatment; that is validity. Moreover, elastography must demonstrate the ability to detect changes over time, namely responsiveness [23, 24]. To allow clinicians to make decisions and research with accurate data and substantial indicators of back muscle biomechanical behavior in patients with MSKd, it is therefore necessary to report on the reliability, validity, and responsiveness of elastography.
Hence, the questions of this systematic review were:
-
What are the current elastography methods and parameters used to quantify back muscles’ biomechanical properties in patients with MSKd and/or asymptomatic individuals?
-
Are these measurements reproducible, valid, and responsive?
Methods
Search strategy
This systematic review was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [25] and the Methodological Expectations of Cochrane Intervention Reviews (MECIR) [26] recommendations. The protocol was registered in the PROSPERO database (#CRD42020186482).
The following databases were searched for relevant studies on April 22, 2020: MEDLINE (via Ovid, 1946 to April 21, 2020; via PubMed, on April 15, 2020); Embase (via Ovid, 1974 to April 21, 2020); Cochrane Database of Systematic Reviews (via Ovid, 2005 to April 17, 2020), Cochrane Central Register of Controlled Trials (via Ovid) and, CINAHL Complete from inception. Search strategies designed by a librarian (B.N.) used text words and relevant indexing to identify studies about ultrasound elastography and back muscles. The MEDLINE strategy (Supplementary Table S1) was peer-reviewed by a second librarian and then applied to all databases, with modifications to search terms as necessary. No language limits were applied. Case reports and animal studies were excluded. A grey literature search was conducted on February 10, 2021, to complement missing articles using the tool “Grey Matters: a practical tool for searching health-related grey literature” developed by the Canadian Agency for Drugs and Technologies in Health (CADTH) [27]. Further studies were identified by screening references of selected studies. The MEDLINE strategy was rerun on February 16, 2023 (2213 new citations were found).
Study selection
Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) was used for data management. One reviewer (M.D.) screened titles and abstracts for eligibility according to criteria. Two independent reviewers (M.D. and K.D.) screened full texts for final inclusion. Inclusion criteria were: (1) a peer-reviewed full article, (2) focusing on back muscles or fascia as listed in the Terminologia Anatomica [28, 29], (3) in vivo measurements conducted on humans, (4) using ultrasound elastography, (5) recording biomechanical outcomes, (6) reporting some form of reliability, validity or responsiveness. Studies were considered assessing reliability if inter-rater, intra-rater or test-retest intraclass correlation coefficient (ICC) single measure (ICC Model 1, 2 or 3 form 1) were reported [24]. Studies reporting standard error of the measurement (SEM) or minimum detectable change (MDC) reliability scores were also included [30]. Studies were considered reporting validity if they were dealing with hypothesis testing for construct validity. “Hypothesis testing for construct validity” was defined as an ongoing process of learning more about ultrasound techniques used to quantify back muscles’ biomechanical properties, making new predictions, and testing them [23, 24, 31,32,33]. It could take the form of known-groups validation or convergent and discriminant validation. “Known-groups validation” was reported in terms of significant difference between measurements of extreme groups known to be different (i.e., MSKd versus controls). “Convergent or discriminant validation” reported how closely ultrasound scores are correlated to other variables linked to the disease (i.e., pain, worsening sensation, disease duration, disability, clinical diagnosis) or measures of the same construct to which it should be related (i.e., stiffness as measured with a muscle hardness meter). Finally, studies provided evidence of “responsiveness” if they measured changes over time (“within-group responsiveness”) or differences between groups after treatment (“between-groups responsiveness”) [23, 24, 31,32,33]. Exclusion criteria were: (1) no back muscle; (2) studies conducted only on cadavers, animals, tendons, or ligaments; (3) no elastography measurements; (4) no quantitative measurements; (5) no biomechanical outcome; (6) no evidence of reliability, validity, or responsiveness.
Data extraction
Data were extracted from included studies by one reviewer (M.D.) and double-checked for accuracy by a second reviewer (K.D.). The following study characteristics were compiled: (1) authors’ details and demographics; (2) evidence of reliability, validity, and responsiveness; (3) elastography method, ultrasound probe, and biomechanical parameters; (4) anatomical structure, participant’s position, muscle/fascia state; and (5) clinical diagnosis.
Based on the World Federation for Ultrasound in Medicine and Biology (WFUMB) elastography classification [34], studies were sorted by (1) elastography method (i.e., strain imaging (SI), shear wave imaging (SWI), and vibration sonoelastography (VSE) [35]), (2) muscle excitation method (i.e., manual compression, body movement, tissue ultrasound palpation system (TUPS), acoustic radiation force, and controlled external vibration), (3) implemented elastography technique (i.e., strain elastography, shear strain elastography, point-shear wave speed (p-SWS), shear wave speed (SWS) imaging, vibration amplitude sonoelastography, and vibration phase gradient sonoelastography), and (4) elastography outcome (i.e., displacement, strain, shear strain, strain ratio, strain rate, SWS, shear modulus, shear elastic modulus, Young’s modulus, stiffness index, elastic modulus coefficient, and mechanical heterogeneity index).
Data pooling and analysis
A multistage grouping of outcomes facilitated data pooling. First, we segregated data into reliability, validity, or responsiveness categories (some studies could serve multiple classifications). Second, using a random-effect model, ICCs (for reliability) and biomechanical outcomes (for validation and responsiveness) of studies assessing multiple muscles or conditions were pooled to have only one ICC (pooled ICC, pICC) or pooled standardized mean difference (pSMD; the standardized mean difference being defined as the ratio of the difference in means with the pooled standard deviation [36, 37]) and their respective 95% confidence interval (CI) by study. Pooled ICCs were based on estimates derived from Fisher transformation z = 0.5 ln ((1 + ICC) / (1 − ICC)), which has an approximate variance of Var(z) = 1 / (N − 3), where N is the study sample size (number of participants). Third, these pICC and pSMD by study were meta-analyzed to compute reliability, validity, and responsiveness pooled results. Note that reliability data were returned to their original metric for ease of interpretation of results [38]. As the meta-analysis was a priori set to assess only the ICCs and standardized mean difference, the number of studies included in the meta-analysis was maximized by estimating ICCs of articles reporting only the MDC or the SEM, following [30, 39]:
with SD the standard deviation and SEM being:
Heterogeneity was assessed using Tau-squared, Q and I-squared statistics estimating the between-studies variance, the existence of true heterogeneity, and the percentage of the variability in effect estimates that it is due to heterogeneity, respectively [40]. I-squared percentages of 25%, 50%, and 75% were considered to report, respectively, low, medium, and high heterogeneity [40]. Heterogeneity statistics were calculated if there were sufficient studies included in the category of interest to perform a meta-regression analysis (rule of thumb of at least ten studies per variable). To look for potential variables predicting the variance in pSMD across studies, the meta-regression analysis was performed with a random-effects model using the restricted maximum likelihood estimation and applying the Knapp Hartung adjustment. The SPSS software (version 28.0.1.0) was used for statistical analysis. The significance level was set at p < 0.05.
Quality of evidence grading
As done before [39, 41], the quality of evidence of included studies was graded as high, moderate, low, and very low using the consensus-based standards for selecting health status measurement instruments (COSMIN) three-step methodology, initially dedicated to patients’ reported outcomes [31,32,33]. First, one reviewer (M.D.) used the COSMIN subscales for reliability, validity, and responsiveness to rate every single study as very good, adequate, doubtful, or inadequate (Supplementary Table S2). Twenty-six studies were rated by a second reviewer (K.D.) to validate the outcomes of the first reviewer (Cohen’s Kappa = 0.72). Disagreements were resolved by consensus or intercession of a third reviewer (G.C.). Second, pICCs and pSMD were rated against the criteria for good measurement properties as sufficient (pICC ≥ 0.70) or insufficient (pICC < 0.70) (for reliability) [31,32,33] and as very small (pSMD = 0.01), small (pSMD = 0.20), medium (pSMD = 0.50), large (pSMD = 0.80), very large (pSMD = 1.20), and huge (pSMD = 2.00) standardized mean difference (for validation and responsiveness) [36, 37, 42]. Rating was not determinate for unpooled ICC or standardized mean difference (categories including only one study). Third, a modified Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to assign a final quality-evidence score that was downgraded when there was a risk of bias, imprecision (e.g., small study sample sizes) or inconsistency (appreciated by visual inspection of forest-plots (e.g., Fig. 1 and Supplementary Fig. S1)). Indirectness and publication bias were not considered in this modified GRADE approach, as the first was solved by exclusion criteria, and the second was discarded by the presence of natural heterogeneity of the populations included in the subgroups.
Results
Search results
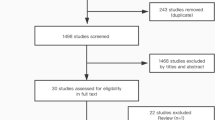
Figure 2 presents the flow chart of the meta-analysis selection process. The initial database search identified 8086 records. A search in the grey literature yielded 211 additional articles. Seven papers were added from other sources. After removing duplicates, titles/abstracts, and full-text screening, we considered 124 studies reporting some form of reliability, validity, and responsiveness. From these studies, 79 presented sufficient consistency in data reporting to allow for data pooling and were included in the meta-analysis [43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121].
Characteristics of studies included in the meta-analysis
Study design and demographics
Publications extended from 2008 to 2023. Fifty-seven studies [43,44,45, 48, 50,51,52,53,54,55, 57, 59, 61, 64, 65, 67, 68, 71,72,73, 75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97, 99,100,101,102, 104, 105, 111,112,113,114,115,116, 118, 121] were cross-sectional, nine were longitudinal, eight were randomized controlled trials (RCT), and five were non-RCTs. The total number of participants N was 3178, with an average of 40.2 participants per study (minimum = 6 [55], maximum = 278 [64]). The age was 36.9 (mean) ± 11.9 (SD). Women represented 27% of the population. See Supplementary Table S3 for a breakdown of study characteristics.
Diagnosis of included subjects
Thirty-one studies recruited asymptomatic participants, whereas patients with LBP (M = 19), neck or shoulder pain (M = 6), whiplash-associated disorders (M = 5), myofascial pain (M = 8), and rotator cuff tendinopathy (M = 3) were investigated. Among low back pain patients, studied groups presented unilateral lumbar disk herniation [43, 51], nonspecific lumbopelvic pain [50], and asymmetric lumbar somatic dysfunction [59, 60]. One cohort was also composed of medical workers [84] and another of middle-aged and elderly women [85]. Twelve studies did not provide any further clarification as to the type of LBP [49, 52, 72, 74, 79, 87, 95, 103, 104, 110, 111, 120]. Among neck and shoulder pain patients, studied groups presented rounded shoulders [63], migraine with and without ictal neck pain [65], neck and shoulder complaints [66], chronic neck pain [77, 105], and frozen shoulder in the freezing or frozen phase [112]. Two studies recruited subjects with cervical disc diseases. Each of the following conditions—knee flexion contracture, osteoporosis, fibromyalgia, and participants aged over 60 years—has been the subject of only one study [56, 64, 68, 117].
Patient position, muscle state and anatomical structure assessed
Most of the measurements were taken in seated (M = 36) and prone (M = 37) positions. In five studies, participants were upright. One study reported unclear position information [111]. Protocols were designed mostly with muscles at rest (M = 61) versus contracted (M = 16), stretched (M = 1) and passively mobilized (M = 3). In two studies, the muscle state was unclear [55, 111]. Thirty-nine studies evaluated intrinsic back muscles compared to 34 dedicated to extrinsic ones. Five studies assessed both categories. One study reported unclear information [83].
Applied elastography technology
Twenty-five papers reported the use of SI, 50 the use of SWI, and 3 the use of VSE (see Table 1 and Fig. 3 for definitions and classification, respectively). One manuscript used both SI and SWI [99] and was classified in the SI category for the statistical analysis. Among studies using SI, 12 applied manual compression, whereas 14 used body movement as the muscle excitation method. Amongst manual compression studies, four used a muscle excitation method with known applied stress (conversely to other manual compression studies for which the applied stress was unknown). Chan et al [52] and Ma et al [83] used a TUPS with an in-series force sensor included in the ultrasound probe to infer the Young modulus from the known applied stress. Tamartash et al [103, 104] built a custom-made system with a force gauge attached to the ultrasound transducer with the aim of inferring an elastic modulus coefficient from the stress/strain ratio. Amongst body movement excitation method studies, Wong et al [115] asked participants to perform a contraction of the latissimus dorsi while a load cell apparatus recorded the force output. Displacement and stiffness were inferred from the force and muscle-fascia junction displacement recordings. An acoustic radiation force is used in SWI, whereas a controlled external vibration excites the tissue in VSE. The acoustic radiation force produced by the focused beam of the ultrasound probe locally vibrates the tissue to produce propagating shear waves in SWI [34]. In VSE, controlled external vibrations are generated by an adapted external hand-held vibrating massager. We identified six elastography techniques: strain (number of cohorts or studies M = 22) or shear strain-based elastography (M = 3), SWS imaging (M = 50), p-SWS measurements (M = 1), vibration amplitude sonoelastography (M = 2), and vibration phase gradient sonoelastography (M = 1). For SI, strain ratio was the most reported outcome (M = 8). For SWI, the shear elastic modulus was to most reported outcome (M = 28). Essentially, with four exceptions [43, 48, 52, 54], all ultrasound probes were linear arrays. All were placed longitudinally to the muscle fibers’ direction. The most common frequencies used were within 2–10 MHz (M = 19). Fifty-five authors used a clinical scanner versus 24 that used a scanner equipped with a non-commercial postprocessing research software.
Applied elastography technologies classification inspired by Shiina et al [34]. Each column shows methods and measured physical quantities for elastography. Each row shows methods for inducing displacement. Each cell shows a type of elastography
Evidence of reliability, validity, and responsiveness
The final 79 papers included in the meta-analysis contain 52 reliability coefficients (reported in 35 studies), 35 known-groups validation outcomes (participants with MSKd versus controls), 21 within-group responsiveness outcomes, and 8 between-groups responsiveness outcomes, as a single study may contribute to more than one category (Supplementary Table S4 presents pooled results by study). Due to inconsistent data reporting, we could not meta-analyze known-groups validation outcomes from groups varying in experimental settings (trunk posture, muscular strength) or muscles assessed. The same applies to convergent and discriminant validation outcomes that were too inconsistent to be meta-analyzed.
Pooled ICCs for reliability studies
Table 2 presents pICCs obtained for the different elastography methods. For SI, data from two test-retest, seven intra-rater and two inter-rater reliability studies accounting for moderate, high and low quality of evidence, respectively, suggested sufficient criteria for good measurement properties (test-retest pICC (95% CI) = 0.93 (0.42–0.99), M = 2, N = 87; intra-rater pICC (95% CI) = 0.85 (0.70–0.92), M = 7, N = 226; inter-rater pICC (95% CI) = 0.79 (0.67–0.87), M = 2, N = 36). The SI ICCs were gathered only from data of asymptomatic participants if one subtracted a study reporting unclear information [79]. For SWI, data from 8 test-retest, 20 intra-rater and 10 inter-rater reliability studies accounting for high quality of evidence suggested sufficient criteria for good measurement properties (test-retest pICC (95% CI) = 0.87 (0.73–0.94), M = 8, N = 149; intra-rater pICC (95% CI) = 0.87 (0.82–0.92), M = 20, N = 522; inter-rater pICC (95% CI) = 0.88 (0.82–0.92), M = 10, N = 204). Although SWI ICCs were mostly gathered from data of asymptomatic participants, noted however three exceptions where ICCs were gathered from participants with a unilateral disk herniation [43], a whiplash-associated disorder [46], and myofascial pain [99]. Noted also two studies providing unclear information on this aspect [44, 60]. For VSE, the rating was not determinate as data were coming from only one test-retest study [106] and one intra and inter-rater reliability study [118].
Pooled standardized mean differences
Results on pSMD are presented in Table 3 and discussed below.
Pooled standardized mean differences for known-groups validation studies
Data from twelve studies accounting together for high quality of evidence suggested a medium and significant (p = 0.020) pSMD for SI in discriminating between patients with MSKd and controls (pSMD = 0.58 (95% CI = 0.11–1.05), M = 12, N = 569) [50, 52, 57, 66, 67, 78, 79, 90, 91, 92, 97, 104]. Data from 22 studies accounting for high quality of evidence suggested a medium and significant (p < 0.001) pSMD for SWI in discriminating between patients with MSKd and controls (pSMD = 0.60 (95% CI = 0.37–0.83), M = 22, N = 1578) [44, 45, 51, 54, 56, 58,59,60, 64, 65, 68, 72, 77, 80, 82, 84, 85, 87, 95, 105, 108, 112]. For VSE, pSMD rating was not determinate as there was only one single study in this category [102].
Pooled standardized mean differences for within-group responsiveness studies
Data from nine studies accounting together for high quality of evidence suggested a medium and significant (p = 0.005) pSMD for SI in detecting changes in biomechanical properties of muscles over time (pSMD = 0.64 (95% CI = 0.25–1.02), M = 9, N = 302) [61, 62, 92, 99, 103, 110, 111, 115, 120]. Data from twelve studies accounting together for high quality of evidence suggested a very large and significant (p = 0.005) pSMD for SWI in detecting changes in biomechanical properties of muscles over time (pSMD = 1.24 (95% CI = 0.46–2.02), M = 12, N = 390) [47, 49, 56, 59, 63, 70, 76, 98, 107, 109, 117, 119].
Pooled standardized mean differences for between-groups responsiveness studies
Data from three studies accounting together for low quality of evidence suggested a very small and non-significant (p = 0.278) pSMD for SI in detecting changes in biomechanical properties of muscles between groups after treatment (pSMD = 0.19 (95% CI = −0.36 to 0.73), M = 3, N = 163) [61, 99, 114]. Data from five studies accounting together for high quality of evidence suggested a small and significant (p = 0.003) pSMD for SWI in detecting changes in biomechanical properties of muscles between groups after treatment (pSMD = 0.30 (95% CI = 0.17–0.43), M = 5, N = 363) [45, 49, 63, 74, 107].
Heterogeneity and meta-regression
Heterogeneity statistics were performed on SI and SWI studies reporting known-groups validation and within-group responsiveness outcomes. The Q-statistic value was significant for each category (p < 0.001). Tau-squared and I-squared values ranged between 0.182–1.219 and 90–99.3%, respectively (Table 4). There was a significant and large amount of heterogeneity within the selected dataset. To explain this variance in pSMD, we tested several predictors using univariate meta-regression (Supplementary Table S5). The analysis indicated that the excitation method (body movement versus manual compression) significantly explained 76% of the variance among pSMD for SI studies discriminating between patients with MSKd and controls (p < 0.001) (Table 5 and Fig. 1). No other significant predictor was found in any category, nor for SWI.
Discussion
This systematic review included 79 studies reporting the use of strain imaging (SI), shear wave imaging (SWI) and vibration sonoelastography (VSE) to assess the biomechanical properties of back muscles in MSKd. Whereas the small number of VSE studies could not be meta-analyzed, SI and SWI studies demonstrated good reliability results, moderate validity to discriminate between patients with MSKd and controls, and moderate to very high within-group responsiveness, for SI and SWI, respectively. Strain imaging and SWI between-groups responsiveness is more questionable, partly due to the lack of sufficient studies available.
Reliability considers the sum of measurement errors and patient variability, that is, the interaction between the tool used and the population of interest [24]. Given its context-dependency, reliability is essential to be evaluated before any testing process. In this meta-analysis, we summarized the reliability characteristics of elastography to estimate back muscle biomechanics in participants with MSKd or, where necessary, asymptomatic participants. However, notice that the reliability coefficients of studies included in our review were all but three [43, 46, 99] calculated on young (31.1 ± 12.5 years) asymptomatic participants. Therefore, it is questionable whether the reliability characteristics demonstrated here apply to the MSKd population.
Both test-retest, intra and inter-rater reliability of SI, SWI and VSE studies presented sufficient criteria for good measurement properties (pICC > 0.70). Strain imaging studies demonstrated slightly higher values than SWI studies for test-retest reliability (pICC (95% CI) = 0.93 (0.42–0.99) for SI versus 0.87 (0.73–0.94) for SWI). Both SI and SWI indicated good inter and intra-rater reliability with pICC (95% CI) ranging from 0.79 (0.67–0.87) to 0.88 (0.82–0.92). Note, however, that most SI evidence comes from moderate (test-retest) to low-quality (inter-rater) evidence contrary to high-quality SWI evidence. The discrepancy in the quality of evidence between SI and SWI studies may be due to insufficient details on the SI data collection or analysis procedure to permit replication. By contrast, intra-rater reliability procedures in SI studies were more documented, resulting in a higher quality of evidence index.
Forty-three of the 52 reliability coefficients contained in the meta-analysis (83%) were acquired at rest. Interestingly, four studies also reported the reliability of SI during active movements in trapezius, neck muscles and lumbar multifidus [52, 57, 78, 89], allowing investigation of soft-tissues torque-dependent biomechanical changes in MSKd physiopathology [122]. Altogether, moderate to excellent reliability was reported (minimum pICC (95% CI) = 0.61 (0.45–0.90); maximum pICC (95% CI) = 0.99 (0.98–0.99)). Five SWI studies also reported moderate to excellent reliability coefficients (minimum pICC (95% CI) = 0.73 (0.59–0.81); maximum pICC (95% CI) = 0.99 (0.98–1.00)) during muscle contraction on the trapezius [75, 81, 98, 121] and lumbar multifidus [73]. While one can expect fewer measurement errors in SWI acquisitions (performed in isometric conditions) compared to SI (performed during concentric movements, except for TUPS), it is instructive to note that both SWI and SI reliability values were in the same range.
Strain imaging and SWI significantly (p ≤ 0.020) discriminate between participants with MSKd and controls. Both presented high-quality evidence and a medium pSMD. In the particular case of two normal distributions with the same variance, a pSMD of 0.01 (very small), 0.2 (small), 0.5 (medium), 0.8 (large), 1.2 (very large), 2.0 (huge) means that 0%, 14.7%, 33%, 47.4%, 62.2% and 81.1%, respectively, of the area covered by the two distributions is not overlapping [37].
Therefore, elastography allows discriminating between MSKd and controls in 33% of cases, the remaining 67% being subject to false positives or negatives. Increasing the discriminatory power could be done by enhancing the sample size or reducing variability. Knowing that the prevalence of MSKd in the general population is 21.3% [1], to discriminate among people with MSKd and controls with a power equal to 0.80 and α = 0.05, 254 participants should be recruited to meet these specifications [123]. The average known-groups validation study sample size in this review was 63 ± 55, which suggests insufficient numbers of participants to demonstrate discrimination among populations. On the other hand, heterogeneity statistics confirmed variability among studies. While the meta-regression has shown no evidence of variable influencing the variance among SWI studies, forest plot visual inspection (Fig. 1b) suggests a reduction in the magnitude of the confidence intervals as studies become more recent, while variance among studies does not seem to be improved. The reason for this could be a natural heterogeneity between studied groups (limiting meta-analytic results) combined with a possible improvement in protocols over time. Strain imaging meta-regression results pointed out a 76% contribution of the excitation method (body movement versus manual compression) to predict the variance among studies (Table 5). If SI studies are dichotomized according to their excitation method (Fig. 1a), the pSMD of studies using body movement as the excitation method was very small (0.09) and non-significant (p = 0.51). On the contrary, the pSMD of manual compression studies was very large (1.32) and significant (p < 0.001). Strain imaging using manual compression, cardiovascular pulsation or respiratory motion has successfully been used to characterize breast and prostate cancers [124], carotid plaques [125], or diaphragmatic breathing [126], respectively. However, using body movement to characterize large muscle structures may be a source of variability as it depends on the individual anatomy and force-sharing strategies, movement control, and muscle complex biomechanical behavior. Or the reason may lie elsewhere, as SI exhibits good reliability (pICC > 0.79). The hypothesis used to classify data by groups to calculate pSMD also deserves reflection. We dichotomized studies’ results as “data of muscles assumed stiffer/presenting less displacement” versus “data of muscles assumed less stiff/presenting more displacement” according to the hypothesis made by the authors or, if necessary, according to the state of the art. However, many authors did not disclose assumptions. Moreover, the original hypothesis underpinning that MSKd patients present stiffer soft tissues with less displacement due to pathophysiological processes (e.g., fibrosis, fatty infiltration, inflammation, or adhesions) [127], could perhaps be a specific case from the relatively wide range of assumptions applicable to all cases. For example, Dones et al [57] and Peolsson et al [91] reported conclusions against the state of the art, resulting in negative pooled ES disadvantaging the overall measure of pSMD in SI.
Strain imaging as well as SWI significantly (p < 0.005) detected changes within groups over time with high-quality evidence, reporting medium (0.64) and very large (1.24) pSMD, respectively. Therefore, SI can detect changes over time within subjects in 33% of cases versus 62.2% for SWI, the remaining 67% and 37.8%, respectively, being subject to false positives or negatives. As data were heterogeneous (Table 4), we performed a meta-regression to consider the delay between treatment and measurements influencing variability. Although the analysis was inconclusive (Supplementary Table S5), it is tempting to point out the relatively limited time between the treatment and ultrasound measurements, showing little regard for the chronicity of MSKd to identify changes in biomechanical outcomes. Indeed, aside from Sakaki et al [98] assessing changes in trapezius characteristics 1 year after arthroscopic rotator cuff surgery, measurements were made within 2 weeks [56], 72 h [99], 48 h [47], 24 h [119] or immediately after one-session treatment [49, 59, 61, 62, 76, 92, 107, 109, 111, 115]. Although some authors also measured biomechanical parameters at baseline and after 2 months [110], 4 weeks [63, 117], 3 weeks [120], and 2 weeks [103] treatments, long-term longitudinal studies are lacking and required. The same is true for between-groups responsiveness studies where only SWI presented, even if small (0.30) but significant (p = 0.003) pSMD with high-quality evidence (changes detected between groups over time in 14.7% of cases, the remaining 85.3% being subject to false positives or negatives). Aljinovic et al [45] found no difference in SWI values between recovered and non-recovered participants with whiplash injuries at 6 months of follow-up. They suggested a possible increase in adherence to physical medicine interventions post-trauma to distort the short-term biomechanical data collected. Koppenhaver et al [74] found significant differences in the erector spinae shear elasticity modulus (but failed for the multifidus) between groups 1 week after receiving dry needling (DN) or sham DN providing arguments that elasticity parameters should be studied in long-lasting studies conducted in the same context as clinical care, combined with other treatments. Moreover, as biomechanical characteristics may vary according to the muscle typology, designating the more sensitive structure to detect changes over time throughout the disease may be required [122].
Limitations
Sixteen studies were excluded from the meta-analysis because an inaccurate ICC model or form was reported. This reduced the number of available evidence and weakened reported results. More, 18 reliability studies included in the meta-analysis mentioned no model or form details and were assumed to report a one-way mixed-effects ICC model for single measurements. Quality of evidence ratings were double-checked for only 1/3 of the papers by another reviewer. Due to limited resources, the screening of titles and abstract was performed by only one reviewer (however, two reviewers did the data extraction). The small number of responsiveness data reduced the chance of finding potential predictors for within-group responsiveness variability and did not allow to perform meta-regression for between-groups responsiveness. The body mass index was reported in only half of the studies, distorting predictor analysis for this variable. Finally, the highly significant heterogeneity between primary studies may limit meta-analytic results.
Conclusions
This meta-analysis reported three elastography methods used to quantify back muscles’ biomechanical properties: SI, SWI and VSE. Despite good reliability results, muscular elastography is still in an early-stage exploration phase, partly able to discriminate between patients with MSKd and controls in cross-sectional studies. The type of methods used to excite the tissue may be a variability factor. Strain imaging and SWI seem to detect changes within groups over time, but this needs to be confirmed by long-term longitudinal studies. Assessing changes between groups over time using elastography still needs to be proven.
Data availability
Data analyzed are available upon request by contacting the corresponding author.
Abbreviations
- CI:
-
Confidence interval
- COSMIN:
-
Consensus-based standards for the selection of health status measurement instruments
- DN:
-
Dry needling
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- ICC:
-
Intraclass correlation coefficient
- LBP:
-
Low back pain
- M:
-
Number of cohorts or studies
- MDC:
-
Minimum detectable change
- MSKd:
-
Musculoskeletal disorders
- N:
-
Number of participants
- pICC:
-
Pooled ICC
- pSMD:
-
Pooled standardized mean difference
- p-SWS:
-
Point-shear wave speed measurements
- RCT:
-
Randomized controlled trials
- SD:
-
Standard deviation
- SEM:
-
Standard error of the measurement
- SI:
-
Strain imaging
- SWI:
-
Shear wave imaging
- SWS:
-
Shear wave speed
- TUPS:
-
Tissue ultrasound palpation system
- VSE:
-
Vibration sonoelastography
References
Vos T, Flaxman AD, Naghavi M et al (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2163–2196
Woolf AD, Erwin J, March L (2012) The need to address the burden of musculoskeletal conditions. Best Pract Res Clin Rheumatol 26:183–224
Wu A, March L, Zheng X et al (2020) Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the Global Burden of Disease Study 2017. Ann Transl Med 8:299
Ferreira ML, de Luca K (2017) Spinal pain and its impact on older people. Best Pract Res Clin Rheumatol 31:192–202
Panjabi MM, White AA 3rd (1980) Basic biomechanics of the spine. Neurosurgery 7:76–93
Kamibayashi LK, Richmond FJR (1998) Morphometry of human neck muscles. Spine (Phila Pa 1976) 23:1314–1323
Vasavada AN, Li S, Delp SL (1998) Influence of muscle morphometry and moment arms on the moment-generating capacity of human neck muscles. Spine (Phila Pa 1976) 23:412–422
Roussouly P, Pinheiro-Franco JL (2011) Biomechanical analysis of the spino-pelvic organization and adaptation in pathology. Eur Spine J 20:609–618
Freddolini M, Strike S, Lee RY (2014) Stiffness properties of the trunk in people with low back pain. Hum Mov Sci 36:70–79
Howarth SJ, Kingston DC, Brown SH, Graham RB (2013) Viscoelastic creep induced by repetitive spine flexion and its relationship to dynamic spine stability. J Electromyogr Kinesiol 23:794–800
Olson MW, Li L, Solomonow M (2009) Interaction of viscoelastic tissue compliance with lumbar muscles during passive cyclic flexion-extension. J Electromyogr Kinesiol 19:30–38
Stokes IA, Gardner-Morse M (2003) Spinal stiffness increases with axial load: another stabilizing consequence of muscle action. J Electromyogr Kinesiol 13:397–402
Hill AV (1950) The series elastic component of muscle. Proc R Soc Lond B Biol Sci 137:273–280
Whittaker JL, Teyhen DS, Elliott JM et al (2007) Rehabilitative ultrasound imaging: understanding the technology and its applications. J Orthop Sports Phys Ther 37:434–449
Teyhen DS (2011) Rehabilitative ultrasound imaging for assessment and treatment of musculoskeletal conditions. Man Ther 16:44–45
Brandenburg JE, Eby SF, Song P et al (2014) Ultrasound elastography: the new frontier in direct measurement of muscle stiffness. Arch Phys Med Rehabil 95:2207–2219
Blank J, Blomquist M, Arant L, Cone S, Roth J (2022) Characterizing musculoskeletal tissue mechanics based on shear wave propagation: a systematic review of current methods and reported measurements. Ann Biomed Eng 50:751–768
Creze M, Nordez A, Soubeyrand M, Rocher L, Maître X, Bellin MF (2018) Shear wave sonoelastography of skeletal muscle: basic principles, biomechanical concepts, clinical applications, and future perspectives. Skeletal Radiol 47:457–471
Van Hooren B, Teratsias P, Hodson-Tole EF (2020) Ultrasound imaging to assess skeletal muscle architecture during movements: a systematic review of methods, reliability, and challenges. J Appl Physiol (1985) 128:978–999
Cheung WK, Cheung JPY, Lee WN (2020) Role of ultrasound in low back pain: a review. Ultrasound Med Biol 46:1344–1358
Heidari P, Farahbakhsh F, Rostami M, Noormohammadpour P, Kordi R (2015) The role of ultrasound in diagnosis of the causes of low back pain: a review of the literature. Asian J Sports Med 6:e23803
Opara M, Kozinc Ž (2023) Which muscles exhibit increased stiffness in people with chronic neck pain? A systematic review with meta-analysis. Front Sports Act Living 5:1172514
Streiner DL, Norman GR, Cairney J (2016) Health measurement scales: a practical guide to their development and use, 5th edn. Oxford University Press, Oxford
Portney LG, Watkins MP (2009) Foundations of clinical research: applications to practice, 3rd edn. Pearson/Prentice Hall, Upper Saddle River
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 88:105906
Higgins JPTLT, Thomas J, Flemyng E, Churchill R (2023) Methodological Expectations of Cochrane Intervention Reviews. Cochrane, London
CADTH (2022) Grey matters: a tool for searching health-related grey literature. Available via https://greymatters.cadth.ca. Accessed 01 Apr 2020
Gilroy AM, MacPherson BR, Ross LM, Schuenke M, Schulte E, Schumacher U (2012) Atlas of anatomy. Thieme Medical Publishers, New York
Hromádka R (2007) Anatonomina. Available via http://terminologia-anatomica.org/en. Accessed 13 Apr 2022
Weir JP (2005) Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res 19:231–240
Mokkink LB, de Vet HCW, Prinsen CAC et al (2018) COSMIN Risk of Bias checklist for systematic reviews of patient-reported outcome measures. Qual Life Res 27:1171–1179
Prinsen CAC, Mokkink LB, Bouter LM et al (2018) COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res 27:1147–1157
Terwee CB, Prinsen CAC, Chiarotto A et al (2018) COSMIN methodology for evaluating the content validity of patient-reported outcome measures: a Delphi study. Qual Life Res 27:1159–1170
Shiina T, Nightingale KR, Palmeri ML et al (2015) WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol 41:1126–1147
Gao L, Parker KJ, Lerner RM, Levinson SF (1996) Imaging of the elastic properties of tissue: a review. Ultrasound Med Biol 22:959–977
Andrade C (2020) Mean difference, standardized mean difference (SMD), and their use in meta-analysis: as simple as it gets. J Clin Psychiatry 81:20f13681
Cohen J (1987) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum Associates Publishers, New York
Sánchez-Meca J, López-López JA, López-Pina JA (2013) Some recommended statistical analytic practices when reliability generalization studies are conducted. Br J Math Stat Psychol 66:402–425
Martínez-Romero MT, Ayala F, De Ste Croix M et al (2020) A meta-analysis of the reliability of four field-based trunk extension endurance tests. Int J Environ Res Public Health 17:3088
Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J (2006) Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 11:193–206
Mijnarends DM, Meijers JM, Halfens RJ et al (2013) Validity and reliability of tools to measure muscle mass, strength, and physical performance in community-dwelling older people: a systematic review. J Am Med Dir Assoc 14:170–178
Sawilowsky SS (2009) New effect size rules of thumb. J Mod Appl Stat Methods 8:597–599
Alis D, Durmaz ESM, Alis C et al (2019) Shear wave elastography of the lumbar multifidus muscle in patients with unilateral lumbar disk herniation. J Ultras Med 38:1695–1703
Aljinovic J, Barisic I, Poljicanin A et al (2020) Can measuring passive neck muscle stiffness in whiplash injury patients help detect false whiplash claims? Wien Klin Wochenschr 132:506–514
Aljinovic J, Barun B, Benzon B, Poljicanin A, Vlak T (2022) Lack of objective measurement in the initial screening and follow-up of patients who report whiplash injury-Is elastography of the trapezius muscle an answer? J Clin Med 11:3851
Barun B, Barisic I, Krnic A, Benzon B, Vlak T, Aljinovic J (2021) Neck disability index is better in classification of recovery after whiplash Injury in comparison with ultrasound shear wave elastography of trapezius muscle. Diagnostics 11:2077
Bethers AH, Swanson DC, Sponbeck JK et al (2021) Positional release therapy and therapeutic massage reduce muscle trigger and tender points. J Bodyw Mov Ther 28:264–270
Blain M, Bedretdinova D, Bellin MF et al (2019) Influence of thoracolumbar fascia stretching on lumbar back muscle stiffness: a supersonic shear wave elastography approach. Clin Anat 32:73–80
Buran Cirak Y, Yurdaisik I, Elbasi ND, Tutuneken YE, Koce K, Cinar B (2021) Effect of sustained natural apophyseal glides on stiffness of lumbar stabilizer muscles in patients with nonspecific low back pain: randomized controlled trial. J Manip Physiol Ther 44:445–454
Calvo-Lobo C, Diez-Vega I, Martinez-Pascual B et al (2017) Tensiomyography, sonoelastography, and mechanosensitivity differences between active, latent, and control low back myofascial trigger points. Medicine (Baltimore) 96:e6287
Can ST, Yilmaz BK, Ozdemir S (2022) Multifidus muscle stiffness in single-level unilateral lumbar disc herniation: comparison of two shear-wave elastography methods. Haseki Tip Bulteni 60:45–51
Chan ST, Fung PK, Ng NY et al (2012) Dynamic changes of elasticity, cross-sectional area, and fat infiltration of multifidus at different postures in men with chronic low back pain. Spine J 12:381–388
Chen B, Cui S, Xu M, Zhang Z, Liu C (2021) Effects of isometric plantar-flexion on the lower limb muscle and lumbar tissue stiffness. Front Bioeng Biotechnol 9:810250
Creze M, Nyangoh Timoh K, Gagey O, Rocher L, Bellin MF, Soubeyrand M (2017) Feasibility assessment of shear wave elastography to lumbar back muscles: a radioanatomic study. Clin Anat 30:774–780
Dieterich AV, Andrade RJ, Le Sant G et al (2017) Shear wave elastography reveals different degrees of passive and active stiffness of the neck extensor muscles. Eur J Appl Physiol 117:171–178
Ding Y, Liu B, Qiao H et al (2020) Can knee flexion contracture affect cervical alignment and neck tension? A prospective self-controlled pilot study. Spine J 20:251–260
Dones 3rd VC, Tangcuangco LPD, Regino JM (2021) The reliability in determining the deep fascia displacement of the upper trapezius during cervical movement: a pilot study. Bodyw Mov Ther 27:239–246
Ertekin E, Kasar ZS, Turkdogan FT (2021) Is early diagnosis of myofascial pain syndrome possible with the detection of latent trigger points by shear wave elastography? Pol J Radiol 86:e425–e431
Gao J, Caldwell J, McLin K, Zhang M, Park D (2020) Ultrasound shear wave elastography to assess osteopathic manipulative treatment on the iliocostalis lumborum muscle: a feasibility study. J Med Ultrasound 39:157–164
Gao J, Caldwell J, Wells M, Park D (2020) Ultrasound shear wave elastography to assess tissue mechanical properties in somatic dysfunction: a feasibility study. J Am Osteopath Assoc 120:677–684
Griefahn A, Knicker A, von Piekartz H (2021) Efficacy of foam rolling with additional vibration stimulation on the mobility of the thoracolumbar fascia. An observational study. Bodyw Mov Ther 26:84–93
Griefahn A, Oehlmann J, Zalpour C, von Piekartz H (2017) Do exercises with the Foam Roller have a short-term impact on the thoracolumbar fascia?—A randomized controlled trial. Bodyw Mov Ther 21:186–193
Gunaydin OE, Ertekin E, Gunaydin G (2022) Four weeks of exercise regimen for sedentary workers with rounded shoulder posture: a randomized controlled study. Sao Paulo Med J 141:e2022257
Heizelmann A, Tasdemir S, Schmidberger J, Grater T, Kratzer W, Gruner B (2017) Measurements of the trapezius and erector spinae muscles using virtual touch imaging quantification ultrasound-elastography: a cross section study. BMC Musculoskelet Disord 18:1–8
Hvedstrup J, Kolding LT, Ashina M, Schytz HW (2020) Increased neck muscle stiffness in migraine patients with ictal neck pain: a shear wave elastography study. Cephalalgia 40:565–574
Ishikawa H, Muraki T, Morise S et al (2017) Changes in stiffness of the dorsal scapular muscles before and after computer work: a comparison between individuals with and without neck and shoulder complaints. Eur J Appl Physiol 117:179–187
Ishikawa H, Muraki T, Morise S, Yamamoto N, Itoi E, Izumi SI (2021) Differences in scapular motion and parascapular muscle activities among patients with symptomatic and asymptomatic rotator cuff tears, and healthy individuals. JSES Int 5:238–246
Karayol KC, Karayol SS (2021) A comparison of visual analog scale and shear-wave ultrasound elastography data in fibromyalgia patients and the normal population. J Phys Ther Sci 33:40–44
Kelly JP, Koppenhaver SL, Michener LA, Proulx L, Bisagni F, Cleland JA (2018) Characterization of tissue stiffness of the infraspinatus, erector spinae, and gastrocnemius muscle using ultrasound shear wave elastography and superficial mechanical deformation. J Electromyogr Kinesiol 38:73–80
Kisilewicz A, Madeleine P, Ignasiak Z, Ciszek B, Kawczynski A, Larsen RG (2020) Eccentric exercise reduces upper trapezius muscle stiffness assessed by shear wave elastography and myotonometry. Front Bioeng Biotechnol 8:928
Kitamura G, Tateuchi H, Ichihashi N (2019) Greater lumbar extension during dolphin kick and psoas major tightness in swimmers with low back pain. J Sport Rehabil 29:716–722
Koppenhaver S, Gaffney E, Oates A et al (2020) Lumbar muscle stiffness is different in individuals with low back pain than asymptomatic controls and is associated with pain and disability, but not common physical examination findings. Musculoskelet Sci Pract 45:102078
Koppenhaver S, Kniss J, Lilley D et al (2018) Reliability of ultrasound shear-wave elastography in assessing low back musculature elasticity in asymptomatic individuals. J Electromyogr Kinesiol 39:49–57
Koppenhaver SL, Weaver AM, Randall TL et al (2022) Effect of dry needling on lumbar muscle stiffness in patients with low back pain: a double blind, randomized controlled trial using shear wave elastography. J Man Manip Ther 30:154–164
Kozinc Z, Sarabon N (2020) Shear-wave elastography for assessment of trapezius muscle stiffness: reliability and association with low-level muscle activity. PLoS One 15:e0234359
Kumamoto T, Seko T, Matsuda R, Miura S (2021) Repeated standing back extension exercise: influence on muscle shear modulus change after lumbodorsal muscle fatigue. Work 68:1229–1237
Kuo WH, Jian DW, Wang TG, Wang YC (2013) Neck muscle stiffness quantified by sonoelastography is correlated with body mass index and chronic neck pain symptoms. Ultrasound Med Biol 39:1356–1361
Landen Ludvigsson M, Peterson G, Jull G, Trygg J, Peolsson A (2016) Mechanical properties of the trapezius during scapular elevation in people with chronic whiplash associated disorders—a case-control ultrasound speckle tracking analysis. Man Ther 21:177–182
Langevin HM, Fox JR, Koptiuch C et al (2011) Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet Disord 12:203
Leong HT, Hug F, Fu SN (2016) Increased upper trapezius muscle stiffness in overhead athletes with rotator cuff tendinopathy. PLoS One 11:e0155187
Leong HT, Ng GY, Leung VY, Fu SN (2013) Quantitative estimation of muscle shear elastic modulus of the upper trapezius with supersonic shear imaging during arm positioning. PLoS One 8:e67199
Liang XN, Guo RJ, Li S (2021) New application of multimodal ultrasound imaging for identification of myofascial trigger points in the trapezius muscle. Ann Palliat Med 10:9784–9791
Ma CZ, Ren LJ, Cheng CL, Zheng YP (2020) Mapping of back muscle stiffness along spine during standing and lying in young adults: a pilot study on spinal stiffness quantification with ultrasound imaging. Sensors (Basel) 20:7317
Masaki M, Aoyama T, Murakami T et al (2017) Association of low back pain with muscle stiffness and muscle mass of the lumbar back muscles, and sagittal spinal alignment in young and middle-aged medical workers. Clin Biomech 49:128–133
Masaki M, Ikezoe T, Yanase K et al (2019) Association of pain history and current pain with sagittal spinal alignment and muscle stiffness and muscle mass of the back muscles in middle-aged and elderly women. Clin Spine Surg 32:E346–E352
Moreau B, Vergari C, Gad H, Sandoz B, Skalli W, Laporte S (2016) Non-invasive assessment of human multifidus muscle stiffness using ultrasound shear wave elastography: a feasibility study. Proc Inst Mech 230:809–814
Murillo C, Falla D, Rushton A, Sanderson A, Heneghan NR (2019) Shear wave elastography investigation of multifidus stiffness in individuals with low back pain. J Electromyogr Kinesiol 47:19–24
Nagai T, Schilaty ND, Krause DA, Crowley EM, Hewett TE (2020) Sex differences in ultrasound-based muscle size and mechanical properties of the cervical-flexor and -extensor muscles. J Athl Train 55:282–288
Peolsson A, Brodin LA, Peolsson M (2010) A tissue velocity ultrasound imaging investigation of the dorsal neck muscles during resisted isometric extension. Man Ther 15:567–573
Peolsson A, Peolsson M, Jull G, Lofstedt T, Trygg J, O’Leary S (2015) Preliminary evaluation of dorsal muscle activity during resisted cervical extension in patients with longstanding pain and disability following anterior cervical decompression and fusion surgery. Physiotherapy 101:69–74
Peolsson AL, Peolsson MN, Jull GA, O’Leary SP (2013) Cervical muscle activity during loaded arm lifts in patients 10 years postsurgery for cervical disc disease. J Manip Physiol Ther 36:292–299
Peolsson M, Larsson B, Brodin LA, Gerdle B (2008) A pilot study using tissue velocity ultrasound imaging (TVI) to assess muscle activity pattern in patients with chronic trapezius myalgia. BMC Musculoskelet Disord 9:1–15
Peterson G, Leary SO, Nilsson D et al (2019) Ultrasound imaging of dorsal neck muscles with speckle tracking analyses—the relationship between muscle deformation and force. Sci Rep 9:13688
Pimentel-Santos F, Rodrigues Manica S, Masi AT et al (2021) Lumbar myofascial physical properties in healthy adults: myotonometry vs. shear wave elastography measurements. Acta Reumatol Port 46:110–119
Pinto SM, Cheung JPY, Samartzis D et al (2022) Are morphometric and biomechanical characteristics of lumbar multifidus related to pain intensity or disability in people with chronic low back pain after considering psychological factors or insomnia? Front Psychiatry 13:809891
Rafsanjani Deh Qazi H, Mohseni Bandpei MA, Rahmani N (2022) Sonoelastography of multifidus, piriformis, quadratus lumborum, and gluteus medius muscles in patients with unilateral discogenic lumbar pain and healthy subjects: a reliability study. Arch Bone Jt Surg 10:621–626
Rahnama L, Peterson G, Kazemnejad A, Trygg J, Peolsson A (2018) Alterations in the mechanical response of deep dorsal neck muscles in individuals experiencing whiplash-associated disorders compared to healthy controls: an ultrasound study. Am J Phys Med Rehabil 97:75–82
Sakaki Y, Taniguchi K, Sato F et al (2022) Time-course changes in active stiffness of the supraspinatus muscle after arthroscopic rotator cuff repair. J Med Ultrason 49:77–84
Sánchez-Infante J, Bravo-Sánchez A, Jiménez F, Abián-Vicén J (2021) Effects of dry needling on muscle stiffness in latent myofascial trigger points: a randomized controlled trial. J Pain 22:817–825
Sawada T, Okawara H, Nakashima D et al (2020) Reliability of trapezius muscle hardness measurement: a comparison between portable muscle hardness meter and ultrasound strain elastography. Sensors (Basel) 20:7200
Shimoyama D, Shitara H, Hamano N et al (2021) Reliability of shoulder muscle stiffness measurement using strain ultrasound elastography and an acoustic coupler. J Med Ultrason 48:91–96
Takla MK, Razek NM, Kattabei O, El-Lythy MA (2016) A comparison between different modes of real-time sonoelastography in visualizing myofascial trigger points in low back muscles. J Man Manip Ther 24:253–263
Tamartash H, Bahrpeyma F, Mokhtari Dizaji M (2022) Comparative effect of lumbar myofascial release with electrotherapy on the elastic modulus of lumbar fascia and pain in patients with non-specific low back pain. Bodyw Mov Ther 29:174–179
Tamartash H, Bahrpeyma F, Mokhtari Dizaji M (2023) Ultrasound evidence of altered lumbar fascia in patients with low back pain. Clin Anat 36:36–41
Tas S, Korkusuz F, Erden Z (2018) Neck muscle stiffness in participants with and without chronic neck pain: a shear-wave elastography study. J Manip Physiol Ther 41:580–588
Turo D, Otto P, Hossain M et al (2015) Novel use of ultrasound elastography to quantify muscle tissue changes after dry needling of myofascial trigger points in patients with chronic myofascial pain. J Med Ultrasound 34:2149–2161
Valera-Calero JA, Sanchez-Jorge S, Buffet-Garcia J, Varol U, Fernandez-de-Las-Penas C, Alvarez-Gonzalez J (2022) Changes in stiffness at active myofascial trigger points of the upper trapezius after dry needling in patients with chronic neck pain: a randomized controlled trial. Med Acupunct 41:121–129
Valera-Calero JA, Sanchez-Jorge S, Buffet-Garcia J, Varol U, Gallego-Sendarrubias GM, Alvarez-Gonzalez J (2021) Is shear-wave elastography a clinical severity indicator of myofascial pain syndrome? An observational study. J Clin Med 10:2895
Vatovec R, Kozinc Z, Voglar M (2022) The effects of isometric fatigue on trunk muscle stiffness: implications for shear-wave elastography measurements. Sensors (Basel) 22:9476
Vining R, Onifer SM, Twist E, Ziegler A-M, Corber L, Long CR (2022) Thoracolumbar fascia mobility and chronic low back pain: phase 2 of a pilot and feasibility study including multimodal chiropractic care. Chiropr Man Therap 30:1–12
Wachi M, Jiroumaru T, Satonaka A et al (2022) Effects of capacitive and resistive electric transfer therapy on pain and lumbar muscle stiffness and activity in patients with chronic low back pain. J Phys Ther Sci 34:400–403
Wada T, Itoigawa Y, Yoshida K, Kawasaki T, Maruyama Y, Kaneko K (2020) Increased stiffness of rotator cuff tendons in frozen shoulder on shear wave elastography. J Med Ultrasound 39:89–97
Wang M, Liu J, Meng L et al (2022) Mapping of lumbar multifidus stiffness quantification in ankylosing spondylitis with shear-wave elastography. BMC Musculoskelet Disord 23:1–10
Weber P, Klingler W, Schleip R, Weber N, Joisten C (2022) The influence of a single instrument-assisted manual therapy (IAMT) for the lower back on the structural and functional properties of the dorsal myofascial chain in female soccer players: a randomised, placebo-controlled trial. J Clin Med 11:7110
Wong KK, Chai HM, Chen YJ, Wang CL, Shau YW, Wang SF (2017) Mechanical deformation of posterior thoracolumbar fascia after myofascial release in healthy men: a study of dynamic ultrasound imaging. Musculoskelet Sci Pract 27:124–130
Xie Y, Thomas L, Hug F, Johnston V, Coombes BK (2019) Quantifying cervical and axioscapular muscle stiffness using shear wave elastography. J Electromyogr Kinesiol 48:94–102
Xu D, Xu H, Liu J et al (2018) Effect of thunder-fire moxibustion on pain, quality of life, and tension of multifidus in patients with primary osteoporosis: a randomized controlled trial. Med Sci Monit 24:2937–2945
Yamamoto A, Yamakoshi Y, Ohsawa T et al (2018) Shear wave velocity measurement of upper trapezius muscle by color Doppler shear wave imaging. Med Ultrasound 45:129–136
Yamaura K, Mifune Y, Inui A et al (2021) Sequential changes in posterior shoulder muscle elasticity after throwing as assessed via ultrasound shear wave elastography. Orthop J Sports Med 9:23259671211021362
Yurdakul OV, Beydogan E, Yilmaz Yalcinkaya E (2019) Effects of physical therapy agents on pain, disability, quality of life, and lumbar paravertebral muscle stiffness via elastography in patients with chronic low back pain. Turk J Phys Med Rehabil 65:30–39
Zhang J, Yu J, Liu C, Tang C, Zhang Z (2019) Modulation in elastic properties of upper trapezius with varying neck angle. Appl Bionics Biomech 2019:6048562
Hug F, Tucker K, Gennisson JL, Tanter M, Nordez A (2015) Elastography for muscle biomechanics: toward the estimation of individual muscle force. Exerc Sport Sci Rev 43:125–133
Charan J, Biswas T (2013) How to calculate sample size for different study designs in medical research? Indian J Psychol Med 35:121–126
Daniaux M, Auer T, De Zordo T et al (2016) Strain elastography of breast and prostata cancer: similarities and differences. Rofo 188:253–258
Roy Cardinal MH, Durand M, Chartrand-Lefebvre C, Soulez G, Tremblay C, Cloutier G (2022) Associative prediction of carotid artery plaques based on ultrasound strain imaging and cardiovascular risk factors in people living with HIV and age-matched control subjects of the CHACS cohort. J Acquir Immune Defic Syndr 91:91–100
Chuang HC, Hsu HY, Chiu WH, Tien DC, Wu RH, Hsu CH (2015) Verification and compensation of respiratory motion using an ultrasound imaging system. Med Phys 42:1193–1199
Langevin HM, Stevens-Tuttle D, Fox JR et al (2009) Ultrasound evidence of altered lumbar connective tissue structure in human subjects with chronic low back pain. BMC Musculoskelet Disord 10:1–9
Funding
M.D. received a doctoral research fellowship from the Fonds de recherche du Québec–Nature et technologies (FRQNT), grant #302254.
Author information
Authors and Affiliations
Contributions
M.D. screened available literature (titles and abstracts) for eligibility under the direct supervision of G.C. M.D. and K.D. performed full text screening for final inclusion, helped by G.C. for the resolution of disagreements. M.D. performed the meta-analysis under the supervision of M.C. M.D. wrote the first version of the manuscript, which was reviewed and corrected by G.C. The subsequent versions of the manuscript were reviewed and improved by G.C., N.B., N.G., M.K., and M.C. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
David, M., Devantéry, K., Nauche, B. et al. Ultrasound elastography of back muscle biomechanical properties: a systematic review and meta-analysis of current methods. Insights Imaging 15, 206 (2024). https://doi.org/10.1186/s13244-024-01785-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-024-01785-7