Abstract
Background
Studies have suggested that blood circulating phytosterols, plant-derived sterols analogous to cholesterol, were associated with blood lipid levels and the risk of Alzheimer’s disease (AD) and Parkinson’s disease (PD). This Mendelian randomization (MR) study is performed to determine the causal effect of circulating phytosterols on AD and PD and evaluate the mediation effect of blood lipids.
Methods
Leveraging genome-wide association studies summary-level data for phytosterols, blood lipids, AD, and PD, univariable and multivariable MR (MVMR) analyses were conducted. Four types of phytosterols (brassicasterol, campesterol, sitosterol, and stigmasterol), three blood lipids parameters (high-density lipoprotein cholesterol [HDL-C], non-HDL-C, and triglyceride), two datasets for AD and PD were used. Inverse-variance weighted method was applied as the primary analysis, and false discovery rate method was used for adjustment of multiple comparisons.
Results
Using the largest AD dataset, genetically proxied higher levels of stigmasterol (OR = 0.593, 95%CI = 0.431–0.817, P = 0.004) and sitosterol (OR = 0.864, 95%CI = 0.791–0.943, P = 0.004) significantly correlated with a lower risk of AD. No significant associations were observed between all four types of phytosterols levels and PD. MVMR estimates showed that the above causal associations were missing after integrating the blood lipids as exposures. Sensitivity analyses confirmed the robustness of these associations, with no evidence of pleiotropy and heterogeneity.
Conclusion
The study supports a potential beneficial role of blood stigmasterol and sitosterol in reducing the risk of AD, but not PD, which is dependent on modulating blood lipids. These insights highlight circulating stigmasterol and sitosterol as possible biomarkers and therapeutic targets for AD.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) and Parkinson’s disease (PD), the two most common neurodegenerative diseases, place a substantial burden to global public health systems [1,2,3]. Both AD and PD are characterized by the accumulation of misfolding peptides/proteins and progressive neuronal impairment in the brain, including beta-amyloid and p-tau for AD and alpha-synuclein for PD respectively [4]. Despite extensive efforts, the mechanisms driving the development of AD and PD remain not fully understood, and there is currently lack of effective treatment options to delay the progression of the disease [5]. Cholesterol is indispensable for maintaining neuronal membrane integrity, which is involved in synaptic plasticity and neuronal polarity [6, 7]. A large body of research indicates that dysregulation of cholesterol metabolism has been implicated in the pathogenesis of AD and PD [8, 9], which might serve as a potential therapeutic target for both conditions.
Phytosterols, plant-derived sterols, have been recognized for their role in regulating cholesterol homeostasis, immune response, and brain health [10, 11]. Due to similar structure to cholesterol, it is suggested that phytosterols might compete with dietary and biliary cholesterol absorption in the intestine to reduce blood cholesterol levels [12]. Additionally, previous studies showed that phytosterols also exert anti-inflammatory and antioxidant function [13], which were involved in the pathological changes in AD and PD. Increasing studies have suggested that circulating levels of phytosterols were associated the risk of AD and PD [14, 15]. However, since observational studies are often confounded by factors such as diet, lifestyle, lipids levels and medication use, the associations between phytosterols and AD and PD risk remain inconsistent [13, 16].
Mendelian randomization (MR) offers a method to minimize confounding inherent to observational studies by using genetic variants as instrumental variables (IVs) to infer causal relationships [17, 18]. We employ both univariable and multivariable MR approaches to investigate whether circulating phytosterols are causally linked to the risk of AD and PD, and to what extent this relationship is mediated by blood lipids profile.
Materials and methods
Study design and data
Univariable MR (UVMR) design was used to explore the causal impact of circulating phytosterols on the risk of AD and PD, while multivariable MR (MVMR) was applied to evaluate the mediating effect of blood lipids in the relationship between phytosterols and AD and PD (Fig. 1). This study was conducted utilizing publicly available genome-wide association studies (GWAS) summary-level data on phytosterols, blood lipids, AD, and PD of European decent (Table 1).
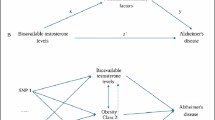
Flowchart for this study. The red cross sign means genetic variables not associated with confounding factors and outcomes. Assumption 1 indicates that SNPs are strongly associated with circulating phytosterols levels; Assumption 2 indicates that SNPs are not associated with confounding factors; Assumption 3 indicates that SNPs affect the risk of outcomes via circulating phytosterols levels directly. AD, Alzheimer’s disease; PD, Parkinson’s disease; PD AAO, Parkinson’s disease age at onset; SNP, single nucleotide polymorphism; LD, linkage disequilibrium; IPDGC, International Parkinson Disease Genomics Consortium; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; UVMR, univariable Mendelian randomization; MVMR, multivariable Mendelian randomization; UKB, UK Biobank; IGAP, International Genomics of Alzheimer’s Project; N, number
For phytosterols, four subtypes of phytosterols, including total brassicasterol (N = 8,790), campesterol (N = 9,758), sitosterol (N = 9,755), and stigmasterol (N = 8,663), were used in this study [19]. The serum concentrations of the above phytosterols were measured using the liquid chromatography tandem mass spectrometry [19]. For AD, one dataset was from the International Genomics of Alzheimer’s Project (IGAP) Consortium with 63,926 participants available [20], and another dataset were obtained from combined summary-level data on both IGAP and UK BioBank (UKB, N = 472,868) [21]. For PD, one dataset for PD (N = 482,730) [22] and another for PD age at onset (PD AAO, N = 28,568) [23] were from the International Parkinson Disease Genomics Consortium (IPDGC). For blood lipids as potential mediators, summary-level data on triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and non-HDL cholesterol (non-HDL-C, N = up to 1,320,016) were obtained from the latest large-scale GWAS meta-analysis by the Global Lipids Genetics Consortium (GLGC) [24]. The lipid levels were measured more than 8 h after fasting and were corrected for age, sex, and medication use. To avoid the impact from sample overlapping, only summary statistics without UKB samples were used for blood lipids [24]. The detailed information for each included study were well described in original studies (Table 1). Given that this study is based on anonymized summary-level data publicly available, no separated ethical approval and individual consent were required. The MR analysis was conducted adheres to the STROBE-MR guideline for MR study [25].
Selection of instrumental variables
Genetic variants, namely single nucleotide polymorphisms in GWAS summary-level data, identified as IVs for circulating levels of each phytosterol should meet three principal assumptions [18]. First, IVs should pass the genome-wide significance threshold (P < 5E-08) and had no linkage disequilibrium (LD: r2 < 0.001 within 10 M bases). Second, IVs should not be associated with confounders related to both phytosterols levels and AD or PD. Third, IVs should affect the outcome indirectly through the exposure. In addition, the F-statistic value was calculated for each IV to avoid potential bias introduced by weaken IVs with F-statistic less than 10. Finally, a proxy SNP with r2 > 0.8 (LD) was used as a replacement for those IVs missing in corresponding outcome. A detailed list of IVs for each phytosterol was presented in Additional file 1: Table S1.
Statistical analysis
The primary MR analysis was conducted using the inverse-variance weighted (IVW) method, which are confirmed in sensitivity analysis using MR-Egger, weighted median, weighted mode, simple median, and maximum likelihood approaches. To ensure the validity of MR estimates, the MR-Egger intercept test was used to detect directional pleiotropy [26] and the MR pleiotropy residual sum and outlier (MR-PRESSO) test was applied to identify and correct for outliers that could bias the results [27]. To validate the robustness of our MR estimates, the heterogeneity was assessed using the Cochran’s Q statistic test. The MVMR analysis was performed to evaluate the mediating effect of blood lipids in the relationship between phytosterols and AD and PD by treating both phytosterols and blood lipids as exposures simultaneously. All analyses were performed using the TwoSampleMR (0.5.6) package in R statistical software [28]. P-values were adjusted for multiple testing using the false discovery rate (FDR) method for each outcome and an adjusted P-value less than 0.05 was considered significant.
Results
In summary, there were four, three, six, and four valid IVs for total brassicasterol, campesterol, sitosterol, and stigmasterol respectively. The F-statistics value for all IVs were above the threshold of 10, indicating a low risk of weak instrument bias.
UVMR analysis
Using the IVW approach, genetically determined higher circulating stigmasterol levels were significantly associated with a reduced risk of AD (IGAP: odds ratio [OR] = 0.452, 95%CI = 0.278–0.733, P = 0.004; IGAP + UKB: OR = 0.593, 95%CI = 0.431–0.817, P = 0.004). Additionally, a significant inverse relationship was found between sitosterol levels and AD using the IGAP + UKB dataset (OR = 0.864, 95%CI = 0.791–0.943, P = 0.004), but not replicated in the IGAP dataset. However, there were no significant causal effects of circulating levels of campesterol and brassicasterol on the risk of AD (Fig. 2A). Moreover, no significant relationships were observed between all four phytosterols subtypes and the risk of PD or PD age one set (Fig. 2B). Estimates from other MR approaches showed similar trend to the primary analysis (Additional file 1: Table S2), as displayed in the scatter plots (Fig. 3). This finding suggests a potential protective effect of phytosterols against AD. The MR-Egger regression did not indicate the presence of directional pleiotropy (Table 2). No obvious outlier(s) was found in the MR-PRESSO test (Table 2) and the leave-one-out plots showed good stability of the estimates (Additional file 1: Figure S1).
Effects of circulating phytosterols levels on the risk of Alzheimer’s disease and Parkinson’s disease. Using two different summary-level data on AD, genetically predicted circulating levels of stigmasterol and sitosterol were associated with a decreased risk of AD (A). There was no effect of phytosterols levels on the risk of PD and age at onset of PD (B). Alzheimer’s disease; PD, Parkinson’s disease; PD OAA, PD age at onset; UKB, UK Biobank; SNP, single nucleotide polymorphism; OR, odds ratio; IGAP, International Genomics of Alzheimer’s Project; IVs, instrumental variables; N, number
Scatter plots for the causal effect of circulating stigmasterol and sitosterol levels and Alzheimer’s disease. A and B showed the SNPs’ effect on circulating stigmasterol levels and AD using summary-level data from IGAP and IGAP + UKB, respectively. C and D showed the SNPs’ effect on circulating sitosterol levels and AD using summary-level data from IGAP and IGAP + UKB, respectively. The slope indicated the causal estimates for each method. AD, Alzheimer’s disease; MR, Mendelian randomization; SNP, single nucleotide polymorphism; UKB, UK Biobank; IGAP, International Genomics of Alzheimer’s Project
MVMR analysis
Since UVMR showed that circulating levels of stigmasterol and sitosterol were only significantly associated with AD, but not PD, we performed a MVMR analysis to evaluate the potential role of blood lipids in the relationship between these two phytosterols and AD. By using either stigmasterol or sitosterol and blood lipids as exposures, the estimates showed that the impact of stigmasterol and sitosterol on AD was loss (P > 0.05) (Fig. 4). Among the HDL-C, non-HDL-C, and triglyceride, only non-HDL-C showed a significantly inverse association with AD using the IGAP + UKB dataset (Stigmasterol: OR = 0.856, 95%CI = 0.769–0.954, P = 0.038; Sitosterol: OR = 0.844, 95%CI = 0.760–0.936, P = 0.011). These data suggested that the protective effect of stigmasterol and sitosterol on AD may be potentially mediated by their impact on blood lipid profiles, especially on non-HDL-C levels.
Effects of circulating stigmasterol and sitosterol levels on the risk of Alzheimer’s disease in multivariable Mendelian randomization. The causal effect of circulating stigmasterol (A) and sitosterol (B) on AD disappeared after adjustment of blood lipids in MVMR analysis. AD, Alzheimer’s disease; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; OR, odds ratio; MVMR, multivariable Mendelian randomization; UKB, UK Biobank; IGAP, International Genomics of Alzheimer’s Project
Discussion
The present study provides evidence supporting an inverse relationship between circulating levels of stigmasterol and sitosterol and the risk of AD, but not PD. In addition, MVMR analysis reveals that blood lipids, especially for non-HDL-C, may serve as a potential mediator in the relationship between phytosterols and AD risk. Our findings underscore the importance of considering the phytosterols (stigmasterol and sitosterol) in AD risk assessment and intervention strategies.
There are four common subtypes of phytosterols used in this MR study, but the results showed that only stigmasterol and sitosterol levels were significantly associated with a decreased risk of AD. It is worth noting that the OR estimates for campesterol and brassicasterol on AD was also smaller than one, despite lacking statistical significance. These results suggested that the protective effect of phytosterols on AD might be a class effect rather than an action limited to individual compounds. However, as both campesterol and brassicasterol are derived from sitosterol after removing the 24th carbon atom [29], it cannot be ruled out that the slight differences in their chemical structures might lead to different functions in AD. Thus, further studies remain deserved to assess whether there is specificity of the protective effect across different phytosterols subtypes.
Numerous studies have suggested a relationship between lipids metabolism disorder and the risk of neurodegenerative diseases [30,31,32], but the role of phytosterols in AD and PD was far from conclusive [13, 16, 33]. As phytosterols could reduce intestinal cholesterol absorption and blood cholesterol levels, it generally believed that phytosterols may exert a protective effect against AD and PD [11, 34]. Additionally, previous animal studies have demonstrated that phytosterols exerted a neuroprotective role via inhibiting inflammatory response and oxidative stress, which were tightly implicated in the pathogenesis of both AD and PD [13]. Our MR study revealed that blood phytosterols levels were only significantly associated with a decreased risk of AD, but not PD. The potential reason contributing to the inconsistent impact of phytosterols on AD and PD may stem from differing sensitivities to blood LDL-C status [35]. For example, it is well established that apolipoprotein E4 (APOE4), a crucial lipoprotein crucial for lipid homeostasis, plays a more significant role in AD compared to PD [36,37,38], suggesting that AD may be particularly vulnerable to disturbances in lipid metabolism.
Our MVMR analysis showed that the protective effect of phytosterols on AD were dependent on blood lipids. A recent cohort study with more than 86,000 older adults showed that individuals with higher triglyceride levels had a reduced risk of cognitive decline and dementia compared to those with lower triglyceride levels [39]. In addition, another study on more than 80 triglyceride species indicated that serum polyunsaturated fatty acid-containing triglycerides were decreased in patients with mild cognitive impairment or AD, and were significantly linked to hippocampal volume and entorhinal cortical thickness [40]. These data suggested that triglyceride might mediate the effect of phytosterols on AD, but no significant relationship between TG levels and AD was observed in our MVMR analysis. Moreover, previous studies have showed that higher levels of non–HDL-C were related with a reduced risk of AD [41]. Our MVMR results support the concept that non-HDL-C levels was causally associated with a decreased risk of AD, even after adjustment of HDL-C and TG. However, additional studies showed that individuals aged 60–79 years with low and high non-HDL-C levels (120 mg/dl < or > 210 mg/dl) had a higher risk of AD than those with intermediate levels (160 mg/dl) [42], suggesting a nonlinear relationship (U shape) of non-HDL-C levels on AD [43]. Further research is needed to elucidate the underlying biological mechanisms by which non-HDL mediates the protective effect of phytosterols on AD.
Strength and limitation
Our study used two different AD GWAS datasets to assess the relationship between phytosterols and AD and obtained consistent results. In addition, the MR estimates had the same trend across different MR methods in sensitivity analyses, suggesting good stability. Moreover, no significant pleiotropy and outlier(s) was identified by MR-Egger intercept and MR-PRESSO test respectively, supporting the validity of the causal inference. However, there are several limitations to be addressed. First, less than 10 IVs were available for each phytosterols subtypes, which might weaken the estimates between phytosterols levels and AD or PD. Second, although several approaches were used to validate the MR assumptions, we cannot fully exclude all possible violations of these assumptions, such as undetected pleiotropy. Third, since the present MR study was based on participants of European ancestry, whether this association exists in other population still need to be further elucidated. Fourth, due to only summary-level data available, we could not rule out whether there was a non-linear association between phytosterols levels and AD or PD. Fifth, the inverse relationship between sitosterol levels and AD was only observed in the IGAP + UKB dataset, but not in the IGAP dataset, which might be due to the smaller sample size in IGAP (N = 63,926) compared to IGAP + UKB (N = 472,868). Further studies with a larger sample size are warranted to confirm these findings.
Conclusion
The present MR study reveals that phytosterols play a protective role against AD, but not PD. In addition, MVMR analysis indicates that the beneficial role of phytosterols on AD is mainly mediated by blood lipids, especially non-HDL-C, suggesting that dietary interventions to increase phytosterol intake could be potential strategies for AD prevention. Future studies like randomized controlled trials are warrant to confirm the causality and effectiveness of phytosterol-rich diets or supplements in reducing AD risk and translating these findings into practical dietary guidelines.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- AD:
-
Alzheimer’s disease
- APOE4:
-
Apolipoprotein E4
- CI:
-
Confidence interval
- FDR:
-
False discovery rate
- GLGC:
-
Global Lipids Genetics Consortium
- GWAS:
-
Genome-wide association studies
- HDL-C:
-
High-density lipoprotein cholesterol
- IGAP:
-
International Genomics of Alzheimer’s Project
- IPDGC:
-
International Parkinson Disease Genomics Consortium
- IVs:
-
Instrumental variables
- IVW:
-
Inverse-variance weighted
- LD:
-
Linkage disequilibrium
- MR:
-
Mendelian randomization
- MR-PRESSO:
-
MR-Pleiotropy RESidual Sum and Outlier
- MVMR:
-
Multivariable Mendelian randomization
- OR:
-
Odds ratio
- PD:
-
Parkinson’s disease
- SNPs:
-
Single nucleotide polymorphisms
- TG:
-
Triglyceride
- UKB:
-
UK BioBank
- UVMR:
-
Univariable Mendelian randomization
References
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–46.
Cui L, Hou NN, Wu HM, Zuo X, Lian YZ, Zhang CN, Wang ZF, Zhang X, Zhu JH. Prevalence of Alzheimer’s Disease and Parkinson’s Disease in China: an updated Systematical Analysis. Front Aging Neurosci. 2020;12:603854.
Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397(10291):2284–303.
Kulenkampff K, Wolf Perez AM, Sormanni P, Habchi J, Vendruscolo M. Quantifying misfolded protein oligomers as drug targets and biomarkers in Alzheimer and Parkinson diseases. Nat Rev Chem. 2021;5(4):277–94.
Chopade P, Chopade N, Zhao Z, Mitragotri S, Liao R, Chandran Suja V. Alzheimer’s and Parkinson’s disease therapies in the clinic. Bioeng Transl Med. 2023;8(1):e10367.
Jose M, Sivanand A, Channakeshava C. Membrane cholesterol is a critical determinant for hippocampal neuronal polarity. Front Mol Neurosci. 2021;14:746211.
Koudinov AR, Koudinova NV. Essential role for cholesterol in synaptic plasticity and neuronal degeneration. Faseb j. 2001;15(10):1858–60.
Dai L, Zou L, Meng L, Qiang G, Yan M, Zhang Z. Cholesterol metabolism in neurodegenerative diseases: Molecular mechanisms and therapeutic targets. Mol Neurobiol. 2021;58(5):2183–201.
Roca-Agujetas V, de Dios C, Abadin X, Colell A. Upregulation of brain cholesterol levels inhibits mitophagy in Alzheimer disease. Autophagy. 2021;17(6):1555–7.
Dierckx T, Bogie JFJ, Hendriks JJA. The impact of phytosterols on the healthy and diseased brain. Curr Med Chem. 2019;26(37):6750–65.
Jie F, Yang X, Wu L, Wang M, Lu B. Linking phytosterols and oxyphytosterols from food to brain health: origins, effects, and underlying mechanisms. Crit Rev Food Sci Nutr. 2022;62(13):3613–30.
Cedó L, Farràs M, Lee-Rueckert M, Escolà-Gil JC. Molecular insights into the mechanisms underlying the cholesterol- lowering effects of Phytosterols. Curr Med Chem. 2019;26(37):6704–23.
Dash R, Mitra S, Ali MC, Oktaviani DF, Hannan MA, Choi SM, Moon IS. Phytosterols: Targeting Neuroinflammation in Neurodegeneration. Curr Pharm Des. 2021;27(3):383–401.
Vanmierlo T, Popp J, Kölsch H, Friedrichs S, Jessen F, Stoffel-Wagner B, Bertsch T, Hartmann T, Maier W, von Bergmann K, et al. The plant sterol brassicasterol as additional CSF biomarker in Alzheimer’s disease. Acta Psychiatr Scand. 2011;124(3):184–92.
Doria M, Maugest L, Moreau T, Lizard G, Vejux A. Contribution of cholesterol and oxysterols to the pathophysiology of Parkinson’s disease. Free Radic Biol Med. 2016;101:393–400.
Vanmierlo T, Bogie JF, Mailleux J, Vanmol J, Lütjohann D, Mulder M, Hendriks JJ. Plant sterols: friend or foe in CNS disorders? Prog Lipid Res. 2015;58:26–39.
Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. 2017;318(19):1925–6.
Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafò MR, Palmer T, Schooling CM, Wallace C, Zhao Q, et al. Mendelian randomization. Nat Rev Methods Primers. 2022;2:6.
Scholz M, Horn K, Pott J, Gross A, Kleber ME, Delgado GE, Mishra PP, Kirsten H, Gieger C, Müller-Nurasyid M, et al. Genome-wide meta-analysis of phytosterols reveals five novel loci and a detrimental effect on coronary atherosclerosis. Nat Commun. 2022;13(1):143.
Kunkle BW, Grenier-Boley B, Sims R, Bis JC, Damotte V, Naj AC, Boland A, Vronskaya M, van der Lee SJ, Amlie-Wolf A, et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet. 2019;51(3):414–30.
Schwartzentruber J, Cooper S, Liu JZ, Barrio-Hernandez I, Bello E, Kumasaka N, Young AMH, Franklin RJM, Johnson T, Estrada K, et al. Genome-wide meta-analysis, fine-mapping and integrative prioritization implicate new Alzheimer’s disease risk genes. Nat Genet. 2021;53(3):392–402.
Nalls MA, Blauwendraat C, Vallerga CL, Heilbron K, Bandres-Ciga S, Chang D, Tan M, Kia DA, Noyce AJ, Xue A, et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 2019;18(12):1091–102.
Blauwendraat C, Heilbron K, Vallerga CL, Bandres-Ciga S, von Coelln R, Pihlstrøm L, Simón-Sánchez J, Schulte C, Sharma M, Krohn L, et al. Parkinson’s disease age at onset genome-wide association study: defining heritability, genetic loci, and α-synuclein mechanisms. Mov Disord. 2019;34(6):866–75.
Graham SE, Clarke SL, Wu KH, Kanoni S, Zajac GJM, Ramdas S, Surakka I, Ntalla I, Vedantam S, Winkler TW, et al. Author correction: the power of genetic diversity in genome-wide association studies of lipids. Nature. 2023;618(7965):E19–e20.
Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, VanderWeele TJ, Higgins JPT, Timpson NJ, Dimou N, et al. Strengthening the reporting of Observational studies in Epidemiology using mendelian randomization: the STROBE-MR Statement. JAMA. 2021;326(16):1614–21.
Burgess S, Thompson SG. Interpreting findings from mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–89.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408.
Moreau RA, Nyström L, Whitaker BD, Winkler-Moser JK, Baer DJ, Gebauer SK, Hicks KB. Phytosterols and their derivatives: structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog Lipid Res. 2018;70:35–61.
Yin F. Lipid metabolism and Alzheimer’s disease: clinical evidence, mechanistic link and therapeutic promise. Febs j. 2023;290(6):1420–53.
Jo DS, Park NY, Cho DH. Peroxisome quality control and dysregulated lipid metabolism in neurodegenerative diseases. Exp Mol Med. 2020;52(9):1486–95.
Galper J, Dean NJ, Pickford R, Lewis SJG, Halliday GM, Kim WS, Dzamko N. Lipid pathway dysfunction is prevalent in patients with Parkinson’s disease. Brain. 2022;145(10):3472–87.
Sharma N, Tan MA, An SSA. Phytosterols: potential metabolic modulators in neurodegenerative diseases. Int J Mol Sci. 2021;22(22):12255.
Babazadeh A, Vahed FM, Liu Q, Siddiqui SA, Kharazmi MS, Jafari SM. Natural bioactive molecules as Neuromedicines for the Treatment/Prevention of Neurodegenerative Diseases. ACS Omega. 2023;8(4):3667–83.
Benn M, Nordestgaard BG, Frikke-Schmidt R, Tybjærg-Hansen A. Low LDL cholesterol, PCSK9 and HMGCR genetic variation, and risk of Alzheimer’s disease and Parkinson’s disease: mendelian randomisation study. BMJ. 2017;357:j1648.
Emrani S, Arain HA, DeMarshall C, Nuriel T. APOE4 is associated with cognitive and pathological heterogeneity in patients with Alzheimer’s disease: a systematic review. Alzheimers Res Ther. 2020;12(1):141.
Liu CC, Zhao J, Fu Y, Inoue Y, Ren Y, Chen Y, Doss SV, Shue F, Jeevaratnam S, Bastea L, et al. Peripheral apoE4 enhances Alzheimer’s pathology and impairs cognition by compromising cerebrovascular function. Nat Neurosci. 2022;25(8):1020–33.
Yin Z, Rosenzweig N, Kleemann KL, Zhang X, Brandão W, Margeta MA, Schroeder C, Sivanathan KN, Silveira S, Gauthier C, et al. APOE4 impairs the microglial response in Alzheimer’s disease by inducing TGFβ-mediated checkpoints. Nat Immunol. 2023;24(11):1839–53.
Zhou Z, Ryan J, Tonkin AM, Zoungas S, Lacaze P, Wolfe R, Orchard SG, Murray AM, McNeil JJ, Yu C, et al. Association between Triglycerides and Risk of Dementia in Community-Dwelling older adults: a prospective cohort study. Neurology. 2023;101(22):e2288–99.
Bernath MM, Bhattacharyya S, Nho K, Barupal DK, Fiehn O, Baillie R, Risacher SL, Arnold M, Jacobson T, Trojanowski JQ, et al. Serum triglycerides in Alzheimer disease: relation to neuroimaging and CSF biomarkers. Neurology. 2020;94(20):e2088–98.
Reitz C, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch Neurol. 2010;67(12):1491–7.
Marcum ZA, Walker R, Bobb JF, Sin MK, Gray SL, Bowen JD, McCormick W, McCurry SM, Crane PK, Larson EB. Serum cholesterol and Incident Alzheimer’s Disease: findings from the adult changes in Thought Study. J Am Geriatr Soc. 2018;66(12):2344–52.
Zhang Q, Zhang M, Chen Y, Cao Y, Dong G. Nonlinear relationship of Non-high-density Lipoprotein Cholesterol and cognitive function in American elders: a cross-sectional NHANES Study (2011–2014). J Alzheimers Dis. 2022;86(1):125–34.
Acknowledgements
The authors thank all the participants, investigators, and consortia for their contributing studies with data publicly available.
Funding
This work was supported by the Shaanxi Provincial Traditional Chinese Medicine Administration Double Chain Integration Middle Youth Research and Innovation Team Project (2022-SLRH-LJ-007). The funding agency had no role in the design, implementation, or interpretation of this study.
Author information
Authors and Affiliations
Contributions
X.G., N.P., and R.L. conceived and designed the project. X.G., J.Y., and R.W. collected and analyzed the data. X.G. and J.Y. drafted the manuscript. N.P. and R.L. revised the manuscript. All authors have reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed based on publicly available data and no separate ethical approval was required.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guo, X., Yu, J., Wang, R. et al. Deciphering the effect of phytosterols on Alzheimer’s disease and Parkinson’s disease: the mediating role of lipid profiles. Alz Res Therapy 16, 53 (2024). https://doi.org/10.1186/s13195-024-01424-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-024-01424-9