Abstract
Objective
The purpose of this study is a new update on the resistance profile, Macrolide–Lincosamide–Streptogramin B resistance mechanisms and biofilm formation in the Staphylococcus aureus isolated from health care workers (HCWs) nasal carriage at a children’s teaching hospital in Babol (Northern Iran).
Results
A total of 143 non-repetitive nasal swab samples were collected from volunteers, where 53.8% (n; 77/143) were HCWs, 33.6% (n; 48/143) medical students, and 12.6% (n; 18/143) resident students. The prevalence of nasal carriers of S. aureus was 22.4% (n; 32/143), among them, 40.6% (n; 13/32) were identified as methicillin-resistant Staphylococcus aureus (MRSA( carriers. Antimicrobial susceptibility testing showed that erythromycin (68.8%, n; 22/32) and ciprofloxacin (15.6%, n; 5/32) had the highest and lowest resistance rate, respectively. The frequency of resistance genes in the strains was as follows; ermC (n; 17/32, 53.1%), ermA (n; 11/32, 34.4%), ermB (n; 6/32, 18.7%), ereA (n; 3/32, 9.4%). Moreover, 50.0% (n; 16/32), 28.1% (n; 9/32) and 21.8% (n; 7/32) of isolates were strongly, weakly and moderately biofilm producer, respectively. Macrolides-lincosamides-streptogramins B (MLSB) antibiotic resistance among S. aureus isolates from HCWs nasal carriage have found significant prevalence rates throughout the globe. It is crucial to remember that the development of biofilms and MLS B antibiotic resistance are both dynamic processes.
Similar content being viewed by others
Introduction
Staphylococcus aureus, a catalase- and coagulase Gram-positive cocci is a prominent pathogenic microorganism that can lead to a multiple infections from minor skin and soft tissue infections (SSTIs) to severe and potentially fatal diseases [1]. The nostril is the most common carriage site for S. aureus and anterior nasal carriers are at high risk of developing S. aureus infections. Human colonization with S. aureus occurs in the first days of life [2]. Nasal carriers are divided into transient and permanent. Antibiotic resistance and biofilm formation are important factors in maintaining the carrier state [3].
Macrolide, lincosamide and streptogramin B (MLSB) are effective as a limited and alternative treatment regimen in Staphylococcal infections, especially in SSTIs. Multiple mechanisms have been identified that confer resistance to MLSB antibiotics. These mechanisms include the presence of an active efflux pump encoded by the msr gene, drug inactivation by the lun gene, and the presence of the erm cluster, which induces changes in the ribosomal binding site via methylation and/or point mutation [4,5,6,7]. The msrA gene has been found to be present in S. aureus and is responsible for the ATP-dependent transport of erythromycin and streptogramin B out of the cell [6]. Also, msr plasmid genes encoding macrolide efflux pump have been described in these bacteria [6].
Biofilm, an extracellular polysaccharide matrix that surrounds bacteria, is one of the important survival and resistance factors in the carriage. The presence of polysaccharide intercellular adhesive (PIA) encoded and regulated by the intercellular adhesion operon (ica ADCB) is essential in biofilm formation [8]. The operon consists of three components: a N-acetylglucosamine transferase (icaA and icaB), a predicted exporter (icaC), and a deacetylase (icaD).
Understanding the mechanisms of antimicrobial resistance and biofilm formation in S. aureus nasal carriers can offer valuable insights for enhancing infection control measures and improving clinical treatment strategies in the future [9]. Therefore, the purpose of this study is to provide a new update on the resistance profile, MLSB resistance mechanisms, and biofilm formation in S. aureus isolated from health care workers (HCWs) nasal carriage at a children’s teaching hospital in Babol, Northern Iran.
Main text
Materials and methods
Study design, sampling and laboratory identification
The cross-sectional study was performed with the committee ethical number of IR. MUBABOL.HRI.REC.1400.159 from the one-year period of time (2022) at the Amirkola children’s teaching hospital (Babol, north of Iran). Exclusion criteria was HCWs who received antibiotics for the previous two weeks or those suffering signs and symptoms of upper respiratory tract infections.
Collection and processing of nasal swabs
A single nasal sample was obtained from each participant, by gently inserting a swab into their nostril and rotating it three times. The swabs were then transported to the laboratory under sterile conditions. Following this, the samples underwent culturing on Mannitol agar that had been supplemented with 7.5% sodium chloride (Merck Co., Germany), and were then incubated for a period of 24 h at a temperature of 37˚C. Standard microbiological and biochemical methods were employed to identify all resulting colonies. PCR of nuc gene (encoding thermonuclease) was used to confirm S. aureus strains [4, 10].
Antimicrobial Susceptibility Testing (AST)
In the current study, the Kirby-Bauer method was utilized in adherence to the guidelines set forth by the Clinical and Laboratory Standards Institute (CLSI document M100, 28th ed), for conducting antibiotic susceptibility testing (AST). For this, Mueller-Hinton agar plates (Merck, Darmstadt, Germany) were used, and the disk agar diffusion technique was employed to test the following antibiotics: ampicillin (AMP; 20 µg), erythromycin (ERY; 15 µg), gentamicin (GM; 10 µg), clindamycin (CD; 2 µg), ciprofloxacin (CIP; 5 µg), mupirocin (MUP; 5 µg), trimethoprim-sulfamethoxazole (SXT; 5 µg), tetracycline (TET; 30 µg), and cefoxitin (FOX; 30 µg) (Padtan-Teb, Iran). The methicillin-resistant Staphylococcus aureus (MRSA) isolates were screened based on resistance to cefoxitin (30 µg) discs (MAST, UK) by the disc diffusion method according to the CLSI guidelines S. aureus ATCC 25,923 was used as a quality control.
Determination of inducible resistant phenotypes
To identify resistant phenotypes, a double disk test was conducted by placing ERY and CD disks 20 mm apart as previously described [11].
Crystal violet biofilm formation assay
Biofilm production ability was assessed using 96-well flat bottom microtiter plate procedure as previously described.
Molecular detection of resistance determinants
Bacterial cells were lysed as follows: five pure colonies liquefied in a 25 µl of 0.25% sodium dodecyl sulfate (SDS)–0.05 N NaOH solutions and heated for 15 min. After adding 200 µL of ddH2O to the microtube, 5 µL of the diluted mixture was used in the PCR method. Successful DNA isolation was verified via agarose gel electrophoresis. Multiplex-PCR assay was performed by DNA amplification device (Eppendorf, Germany) to detect the icaA, icaB. icaD, ermA, ermC ,ereA, msrA, msrB using the specific primers (Table 1) [4, 12].
PCRs were conducted in an Eppendorf Co. (Germany) master cycler gradient, with a final reaction volume of 25 µl composed of 2.5 µl of template DNA, 13.5 µl of Taq DNA Polymerase Master Mix RED (Ampliqon, Stenhuggervej, Odense M, Denmark), 1.0 µl of each primer, and 7.0 µl of ddH2O water.
Statistical analysis
Statistical analysis in this study was carried out with SPSS software version 22.0 (IBM, Armonk, NY, USA), and the chi-square test was utilized to compare the data related to biofilm formation and resistance genes. A significance level of less than 0.05 was considered statistically significant.
Results
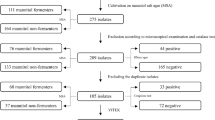
A total of 143 non-repetitive nasal swab samples were collected from volunteers, where 53.8% (n; 77/143) were HCWs, 33.6% (n; 48/143) medical students, and 12.6% (n; 18/143) resident students.
Antimicrobial Susceptibility Testing (AST)
In general, the prevalence of nasal carriers of S. aureus was 22.4% (n; 32/143), among them, 40.6% (n; 13/32) were identified as MRSA carriers (Table 2). AST showed that the highest and lowest resistance rate were related to ERY (68.8%, n; 22/32) and CIP (15.6%, n; 5/32), respectively. All FOX-resistant strains carried the mecA gene and were considered as MRSA.
Results of biofilm formation
According to our results, 50.0% (n; 16/32), 28.1% (n; 9/32) and 21.8% (n; 7/32) of isolates were strongly, weakly and moderately biofilm producer, respectively. The MRSA isolates exhibited significantly higher biofilm production compared to Meticillin-Sensitive Staphylococcus aureus (MSSA).
Results of resistance determinants
As you can see in Table 3, the diversity of resistance genes in the strains was as follows; ermC (n; 17/32, 53.1%), ermA (n; 11/32, 34.4%), ermB (n; 6/32, 18.7%), ereA (n; 3/32, 9.4%). The genes of ereB, msrA, and msrB were not found in any isolate. Also, ereA gene was present only in MRSA strains. On the other hand, the prevalence of biofilm coding genes were as follows; icaD (n; 26/32, 81.3%), icaA (n; 22/32, 68.7%), icaC (n; 19/32, 59.4%) and icaB (n; 14/32, 43.7%). All MRSA strains carried icaA gene.
The data indicated that 34.4% (n = 11/32) of the isolates demonstrated resistance to both CD and ERY. Specifically, 9.4% (n = 3/32) of the strains displayed a resistant phenotype to cMLSB (i.e., resistant to both ERY and CD), 18.8% (n = 6/32) showed inducible resistance iMLSB (i.e., resistant to ERY but susceptible to CD), and 6.3% (n = 2/32) of the isolates had the MS phenotype (i.e., susceptible to ERY and resistant to CD).
Discussion
In fact, about 20–30% of humans can carry this organism continuously and asymptomatically. Therefore, nasal carriers can increase the risk of infection transmission, which leads to the serious infections, especially in hospitalized patients and immunocompromised, which is linked to increased risk of death and prolonged hospital stays [13,14,15]. In the present study, the prevalence of S. aureus nasal carriers was 22.4% (n; 32/143), of which 40.6% (n; 13/32) were MRSA. Sedaghat et al., (2017) showed that the MRSA rate in 272 collected nasal swabs was 13% [16]. Danelli et al.. (2020) found that 42.9% of 324 nasal samples were determined to be S. aureus, with 28.8% of those being MRSA [17]. Fard-Mousavi et al., (2015) showed that of 813 subjects screened, 10.2% (n; 83), 10.6% (n; 86) and 79.2% (n; 644) were persistent, transient and non-carriers, respectively [18]. These differences can be the result of the study population, the place of sampling (specialized hospital compared to general hospitals) and people’s awareness of personal medical-hygiene. The rate of colonization was significantly higher in people who did not use antibiotics at least in the last 3 months (0.03 and 0.05 for MSSA and MRSA, respectively). Significantly, colonization was more in people who were in contact with the patient, which suggests an increase in the incidence of iatrogenic disease. The highest colonization rates of MSSA and MRSA were respectively in nurses (42.1% and 38.5%) and then medical students (21.1% and 23.1%). In a contrast study at the Brazil, Danelli et al.. (2020) demonstrated that males and students had a significantly higher prevalence of S. aureus carriage (OR = 2.898); However, no factors were found to be correlated with the carriage of MRSA [17].
A high prevalence (40.0%) of MRSA from nasal-carrier HCWs was reported from Ghana in 2020 [19]. From a reported of Moghadam et al., (2015) out of 270 nasal swabs collected from HCWS, 14.4% of S. aureus were detected [20]. Remarkably, there was a notable discrepancy observed in terms of MRSA carriage in relation to gender (P = 0.041) and occupation (P = 0.034). Pourramezan et al., (2019) showed that the incidence of S. aureus and MRSA in the nasal cavities of HCWs were 39.8% (n; 53/133) and 22.5% (n; 30/ 133), respectively [21].
However, the observed differences in S. aureus and MRSA carriage rate in the country and other parts of the world can be attributed to variations in sample size, identification methods and local infection control polices [22, 23].
On the other hand, several probable factors contribute to the high prevalence of MRSA among HCWs. These include inadequate cleaning and disinfection protocols, high patient-to-staff ratios that may result in lapses in hygiene practices, and the frequent interaction of HCWs with patients who are positive for MRSA as opposed to those who are negative for MRSA, particularly in intensive care units or during medical procedures [24, 25].
As a highlight achievement, the resistance to MUP -a topical anti-staphylococcal ointment used for eradication of nasal carriage was 50.0%. Tabandeh et al., (2022) and Moghadam et al., (2015) showed that 14.4% (n; 14/97) and 29.4% (n; 5/17) isolates were resistance to MUP [20, 26]. On the other hand, in the study of Sedaghat et al., (2018) and Askarian et al., (2009) no isolates were resistant to MUP [16, 27]. This inconsistency in the results can be a related to the rational prescription of antibiotics, geographical distance, place and year of the study. The presence of cMLSB, iMLSB, and MS phenotypes were detected in 9.4%, 18.8%, and 6.3% of the isolates, respectively, as revealed by the D-test. Also, 53.1%, 34.4%, 18.7% and 9.4% of isolates were harbored ermC, ermA, ermB and ereA, respectively. Danelli et al.. (2020) reported that the majority of ERY-resistant isolates (82.8%, 77/93) exhibited the iMLSB phenotype, while a small proportion (3.2%, 3/93) displayed cMLSB, and the remaining 14.0% (13/93) fell in the MS category [17].
Consistent with Tabandeh et al.. (2022), the current study found that the cMLSB, iMLSB, and MS phenotypes were present in 61.1%, 22.2%, and 14.8% of isolates, respectively [26].
The prevalence of inducible-resistance genes in the MRSA isolates (n = 97) was ermA (21.6%), ermB (16.5%), ermC (44.3%), and ereA (9.3%). However, studies by Solgi et al. [28]. , Khodabandeh et al. [4]. , , Gupta et al. [29]. , , Adhikari et al. [30]. , , Ruiz-Ripa et al. [31]. , , and Deotale et al. [32]. , , reported conflicting results regarding the prevalence of these genes.
In this study, inducible-resistant strains were significantly higher in MRSA as well as biofilm producing strains. In total, 50.0% (n; 16/32), 28.1% (n; 9/32) and 21.8% (n; 7/32) of isolates have strong, weak and moderate biofilm, respectively. In this regards the prevalence of biofilm genes was as follows; icaD (n; 26/32, 81.3%), icaA (n; 22/32, 68.7%), icaC (n; 19/32, 59.4%) and icaB (n; 14/32, 43.7%). All MRSA strains carried icaA gene.
Consistent with the findings of Tabandeh et al.. (2022), the 97 MRSA isolates in our study exhibited the following prevalence of ica genes: icaA (84.5%), icaB (70.1%), icaC (74.2%), and icaD (81.4%) [26]. In a similar study conducted by Sedaghat et al., (2018) the prevalence of icaA gene was 74.0% (n; 39/53) and 72.0% (n; 18/25) and icaD was 81.0% (n; 43/53) and 64.0% (n; 16/64) in MRSA and MSSA, respectively [33].
Contrary to our study, Omidi et al., (2020) showed that 76.0% (n; 111/146) and 87.5% (n; 21/24) of S. aureus and MRSA strains were able to strong biofilm production, respectively. 75% (n = 18/24) of MRSA isolates tested were found to possess the icaA gene, whereas no icaD gene was detected [34]. This difference can be due to the presence of genes other than ica that play a role in biofilm formation. Biofilm formation by S. aureus has been suggested to be primarily driven by the PIA pathway, encoded by the ica operon. However, there is evidence of an ica-independent pathway, linked to the expression of Bap [35]. In addition, it has been observed that methicillin resistance is linked to the inhibition of PIA and biofilm formation dependent on surface proteins.
Conclusion
Our findings indicate a considerable prevalence of MRSA colonization among HCWs, highlighting a persistent and significant healthcare challenge within our region. Conversely, the management of antibiotic prescriptions to mitigate selective pressures is essential for addressing the emergence of multidrug-resistant (MDR) isolates, including vancomycin-intermediate Staphylococcus aureus (VISA) and vancomycin-resistant Staphylococcus aureus (VRSA) strains. To enhance the management of S. aureus infections, techniques for avoiding biofilm development and dissolving existing biofilms should be investigated.
Limitations
This study is subject to certain limitations: the primary limitation pertains to the incomplete availability of comprehensive patient history background information. Furthermore, it is imperative to acknowledge that the isolation of MRSA strains was confined to different hospitals, necessitating a cautious approach to the interpretation of the results.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- SSTIs:
-
skin and soft tissue infections
- HCWs:
-
healthcare workers
- MRSA:
-
methicillin- resistant Staphylococcus aureus
- ERY:
-
erythromycin
- CD:
-
Clindamycin
- iMLSB:
-
inducible MLSB phenotype
- cMLSB:
-
constitutive resistance
- MLSB Bap:
-
biofilm-associated proteins
- PIA:
-
polysaccharide intercellular adhesive
References
Taylor TA, Unakal CG. Staphylococcus aureus. StatPearls [Internet]. edn.: StatPearls Publishing; 2022.
Sollid JUE, Furberg AS, Hanssen AM, Johannessen M. Staphylococcus aureus: determinants of human carriage. Infect Genet Evol. 2014;21:531–41.
Jaradat ZW, Ababneh QO, Sha’aban ST, Alkofahi AA, Assaleh D, Al Shara A. Methicillin Resistant Staphylococcus aureus and public fomites: a review. Pathog Glob Health. 2020;114:426–50.
Khodabandeh M, Mohammadi M, Abdolsalehi MR, et al. Analysis of resistance to Macrolide-Lincosamide-Streptogramin B among Meca-positive Staphylococcus Aureus isolates. Osong Public Health Res Perspect. 2019;10:25–31.
Uzun B, Güngör S, Pektaş B, et al. [Macrolide-lincosamide-streptogramin B (MLSB) resistance phenotypes in clinical Staphylococcus isolates and investigation of telithromycin activity]. Mikrobiyol Bul. 2014;48:469–76.
Seifi N, Kahani N, Askari E, Mahdipour S, Naderi NM. Inducible clindamycin resistance in Staphylococcus aureus isolates recovered from Mashhad, Iran. Iran J Microbiol. 2012;4:82–6.
Prabhu K, Rao S, Rao V. Inducible Clindamycin Resistance in Staphylococcus aureus isolated from clinical samples. J Lab Physicians. 2011;3:25–7.
Limoli DH, Jones CJ, Wozniak DJ. Bacterial extracellular polysaccharides in Biofilm formation and function. Microbiol Spectr. 2015;3.
Hasannejad-Bibalan M, Mojtahedi A, Biglari H, Halaji M, Sedigh Ebrahim-Saraie H. Antibacterial activity of Tedizolid, a Novel Oxazolidinone against Methicillin-resistant Staphylococcus aureus: a systematic review and Meta-analysis. Microb drug Resist (Larchmont NY). 2019;25:1330–7.
Karimzadeh R, Ghassab RK. Identification of nuc nuclease and sea enterotoxin genes in Staphylococcus aureus isolates from nasal mucosa of burn hospital staff: a cross-sectional study. New Microbes new Infections. 2022;47:100992–100992.
STEPANOVIĆ S, VUKOVIĆ D. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS. 2007;115:891–9.
Deighton MA, Capstick J, Domalewski E, van Nguyen T. Methods for studying biofilms produced by Staphylococcus epidermidis. Methods Enzymol. 2001;336:177–95.
Sakr A, Brégeon F, Mège JL, Rolain JM, Blin O. Staphylococcus aureus Nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections. Front Microbiol. 2018;9:2419.
Sakr A, Brégeon F, Mège J-L, Rolain J-M, Blin O. Staphylococcus aureus Nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections. Front Microbiol. 2018;9.
Emaneini M, Jabalameli F, Rahdar H, Leeuwen WBv, Beigverdi R. Nasal carriage rate of methicillin resistant Staphylococcus aureus among Iranian healthcare workers: a systematic review and meta-analysis. Rev Soc Bras Med Trop. 2017;50:590–7.
Sedaghat H, Esfahani BN, Halaji M, et al. Genetic diversity of Staphylococcus aureus strains from a teaching hospital in Isfahan, Iran: the emergence of MRSA ST639- SCCmec III and ST343- SCCmec III. Iran J Microbiol. 2018;10:82–9.
Danelli T, Duarte FC, de Oliveira TA et al. Nasal Carriage by Staphylococcus aureus among Healthcare Workers and Students Attending a University Hospital in Southern Brazil: Prevalence, Phenotypic, and Molecular Characteristics. Interdisciplinary perspectives on infectious diseases. 2020;2020: 3808036–3808036.
Fard-Mousavi N, Mosayebi G, Amouzandeh-Nobaveh A, Japouni-Nejad A, Ghaznavi-Rad E. The dynamic of Staphylococcus aureus Nasal Carriage in Central Iran. Jundishapur J Microbiol. 2015;8:e20760–20760.
Walana W, Bobzah BP, Kuugbee ED, et al. Staphylococcus aureus nasal carriage among healthcare workers, inpatients and caretakers in the Tamale Teaching Hospital, Ghana. Sci Afr. 2020;8:e00325.
Ohadian Moghadam S, Pourmand MR, Davoodabadi A. The detection of Mupirocin Resistance and Nasal Carriage of Methicillin Resistant Staphylococcus aureus among Healthcare Workers at University Hospitals of Tehran, Iran. Iran J Public Health. 2015;44:361–8.
Pourramezan N, Ohadian Moghadam S, Pourmand MR. Methicillin-resistant Staphylococcus aureus tracking spread among health-care workers and hospitalized patients in critical wards at a university hospital, Tehran, Iran. New Microbes New Infect. 2019;27:29–35.
Baroja I, Guerra S, Coral-Almeida M, et al. Methicillin-Resistant Staphylococcus aureus Nasal Colonization among Health Care Workers of a Tertiary Hospital in Ecuador and Associated Risk factors. Infect drug Resist. 2021;14:3433–40.
Karimi M, Esfahani BN, Halaji M, et al. Molecular characteristics and antibiotic resistance pattern of Staphylococcus aureus nasal carriage in tertiary care hospitals of Isfahan, Iran. Le Infezioni in Medicina. 2017;25:234–40.
Lena P, Ishak A, Karageorgos SA, Tsioutis C. Presence of Methicillin-Resistant Staphylococcus aureus (MRSA) on Healthcare workers’ attire: a systematic review. Trop Med Infect Disease. 2021;6:42.
Moshtagheian S, Halaji M, Sedaghat H, et al. Molecular characteristics of methicillin-resistant Staphylococcus aureus nasal carriage from hospitalized patients and medical staff in Isfahan, Iran. Annali Di Igiene: Med Preventiva e di Comunita. 2018;30:237–44.
Tabandeh M, Kaboosi H, Taghizadeh Armaki M, Pournajaf A, Peyravii Ghadikolaii F. New update on molecular diversity of clinical Staphylococcus aureus isolates in Iran: antimicrobial resistance, adhesion and virulence factors, biofilm formation and SCCmec typing. Mol Biol Rep. 2022;49:3099–111.
Askarian M, Zeinalzadeh A, Japoni A, Alborzi A, Memish ZA. Prevalence of nasal carriage of methicillin-resistant Staphylococcus aureus and its antibiotic susceptibility pattern in healthcare workers at Namazi Hospital, Shiraz, Iran. Int J Infect Dis. 2009;13:e241–7.
Solgi S, Razavi S, Nateghian A, et al. Resistance-related determinants in clinically relevant Staphylococcus aureus isolated from teaching therapeutic centers, Tehran, Iran. Reviews Med Microbiol. 2019;30:142–7.
Gupta V, Datta P, Rani H, Chander J. Inducible clindamycin resistance in Staphylococcus aureus: a study from North India. J Postgrad Med. 2009;55:176–9.
Adhikari RP, Shrestha S, Barakoti A, Amatya R. Inducible clindamycin and methicillin resistant Staphylococcus aureus in a tertiary care hospital, Kathmandu, Nepal. BMC Infect Dis. 2017;17:483.
Ruiz-Ripa L, Alcalá L, Simón C, et al. Diversity of Staphylococcus aureus clones in wild mammals in Aragon, Spain, with detection of MRSA ST130-mecC in wild rabbits. J Appl Microbiol. 2019;127:284–91.
Deotale V, Mendiratta DK, Raut U, Narang P. Inducible clindamycin resistance in Staphylococcus aureus isolated from clinical samples. Ind J Med Microbiol. 2010;28:124–6.
Hossein S, Bahram Nasr E, Mehrdad H et al. Genetic diversity of Staphylococcus aureus strains from a teaching hospital in Isfahan, Iran: the emergence of MRSA ST639- SCCmec III and ST343- SCCmec III. Iran J Microbiol. 2018;10.
Omidi M, Firoozeh F, Saffari M, Sedaghat H, Zibaei M, Khaledi A. Ability of biofilm production and molecular analysis of spa and ica genes among clinical isolates of methicillin-resistant Staphylococcus aureus. BMC Res Notes. 2020;13:19.
Haghi Ghahremanloi Olia A, Ghahremani M, Ahmadi A, Sharifi Y. Comparison of biofilm production and virulence gene distribution among community- and hospital-acquired Staphylococcus aureus isolates from northwestern Iran. Infect Genet Evol. 2020;81:104262.
Acknowledgements
We would like to thank Babol University of Medical Sciences for funding this study.
Funding
This study was financially supported by the Babol University of Medical Sciences (grant number: 724133815).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: AP, MM, MDF and MH, Performed the experiments: MDF, PH and MS, performed statistical and spatial analyses and interpreted all the results. MH, MDF, SY, contributed to the writing of the manuscript and revised the final version manuscript: AP, MM, MDF, SY and MH. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of Babol University of Medical Sciences; Babol, Iran with code number IR.MUBABOL.HRI.REC.1400.159. However, consent to participate was waived by Research Ethics Committee of Babol University of Medical Sciences, due to bacteria isolated from clinical samples in the clinical microbiology laboratory routinely.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Firouzjaei, M.D., Halaji, M., Yaghoubi, S. et al. Inducible clindamycin-resistant and biofilm formation in the Staphylococcus aureus isolated from healthcare worker’s anterior nasal carriage. BMC Res Notes 17, 252 (2024). https://doi.org/10.1186/s13104-024-06926-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-024-06926-1