Abstract
Nasally colonized staphylococci carry antibiotic resistance genes and may lead to serious opportunistic infections. We are investigating nasal carriage of Staphylococcus aureus and Staphylococci other than S. aureus (SOSA) among young volunteers in Egypt to determine their risk potential. Nasal swabs collected over 1 week in June 2019 from 196 volunteers were cultured for staphylococcus isolation. The participants were interviewed to assess sex, age, general health, hospitalization and personal hygiene habits. Identification was carried out using biochemical tests and VITEK 2 automated system. Disc diffusion and minimum inhibitory concentration tests were performed to determine antibiotic susceptibility. Screening for macrolide resistance genes (ermA, ermB, ermC, ermT and msrA) was performed using polymerase chain reaction. Thirty four S. aureus and 69 SOSA were obtained. Multi-drug resistance (MDR) was detected among most staphylococcal species, ranging from 30.77% among S. hominis to 50% among S. epidermidis. Phenotypic resistance to all tested antibiotics, except for linezolid, was observed. Susceptibility to rifampicin, vancomycin and teicoplanin was highest. ermB showed the highest prevalence among all species (79.41% and 94.2% among S. aureus and SOSA, respectively), and constitutive macrolide-lincosamide-streptogramin B (MLSB) resistance was equally observed in S. aureus and SOSA (11.11% and 16.22%, respectively), whereas inducible MLSB resistance was more often found in S. aureus (77.78% and 43.24%, respectively). The species or resistance level of the carried isolates were not significantly associated with previous hospitalization or underlying diseases. Although over all colonization and carriage of resistance genes are within normal ranges, the increased carriage of MDR S. aureus is alarming. Also, the fact that many macrolide resitance genes were detected should be a warning sign, particularly in case of MLSB inducible phenotype. More in depth analysis using whole genome sequencing would give a better insight into the MDR staphylococci in the community in Egypt.
Similar content being viewed by others
Introduction
Staphylococci are common infectious agents, with Staphylococcus aureus being the prominent species whose pathogenic potential can range from mild superficial skin infections to severe pneumonia and life-threatening systemic diseases such as septicemia1. Staphylococcal species other than S. aureus (SOSA), formerly grouped as coagulase-negative staphylococci, play a role as human pathogens as well, particularly in the context of nosocomial and foreign-body-associated infections, where they pose a serious threat, especially to immunocompromised patients and premature infants2. A common theme in staphylococcal infections, both in the community and in health care settings, is the pronounced capacity of staphylococci to readily acquire a multitude of antibiotic resistance (ABR) determinants, often leading to the emergence of multi-drug resistant (MDR) strains. A prime example for ABR staphylococci are methicillin resistant S. aureus (MRSA) which evolved through acquisition of mec genes, mediating resistance to all beta-lactam antibiotics3. MRSA, which often exhibit additional ABRs to other antibiotic classes, are prevalent worldwide and represent a significant public health issue, which caused more than 100,000 deaths in 2019 alone4. However, apart from their importance as pathogens, staphylococci are primarily commensals that have their natural habitat on human skin and mucous membranes (such as the anterior nares), where they make up a significant part of the healthy microbiota. Staphylococcus epidermidis, Staphylococcus hominis and a number of other SOSA species are typical human skin commensals2,5,6. In addition, 30% of the healthy human population is permanently colonized by S. aureus without showing clinical symptoms7. Of note, the majority of both S. aureus and SOSA infections are caused by strains that were originally present on the patient's skin, suggesting that colonization is a prerequisite and source for (later) infection8,9,10. Also, commensal microbiota are increasingly recognized as reservoirs in which ABR genes are exchanged and in which MDR isolates can evolve and spread11. Therefore, studying commensal staphylococci and their ABR patterns is currently in the focus to both assess the infection risk by MDR staphylococci and to gain insight into the ABR genes currently circulating in staphylococcal populations in various habitats. Most work in the field, also on the African continent, has been done on staphylococci originating from patients and staff in health care facilities12,13. Thus, a most recent meta-analysis from Egypt revealed that the MRSA prevalence is high (i.e. 63%) among infection-associated clinical S. aureus isolates from humans14. However, we currently lack comparable data on staphylococci in the community. With this study, we aim to fill this knowledge gap by investigating the species composition and ABR situation in colonizing staphylococci isolated from young volunteers in the urban region of Alexandria, Egypt. We specifically focus on colonization rates by S. aureus and SOSA in this group of individuals as well as on resistance rates to macrolides which are among the most commonly prescribed antibiotics in Egypt15,16. Finally, we ask the question if personal hygiene habits have an influence on staphylococcal skin colonization and ABR rates.
Materials and methods
Study population
This is a cross-sectional study where nasal swabs were collected from both anterior nares of young pharmacy students, at Alexandria University over a week in June 2019. At the time of swab collection, the students filled a survey to assess their personal hygiene habits, diseases they are suffering from, recurrent infections as skin infections, and the times of hospitalization in the previous year.
The collected swabs were inoculated into nutrient broth (Himedia, India) and incubated overnight at 37 °C. The cultures were then plated onto nutrient agar (Oxoid Ltd; Basingostok; Hampshire, England) for colony isolation. Staphylococci were identified based on conventional methods, including microscopic examination, growth onto mannitol-salt agar (Oxoid Ltd; Basingostok; Hampshire, England) and deoxyribonuclease (DNase) agar (Lab M Ltd; Heywood, Lancashire, United Kingdom), catalase test using 3% hydrogen peroxide (H2O2) reagent—10 volume—(LUNA cosmetics company, Egypt) and tube coagulase test using coagulase rabbit plasma (Himedia, India). Identification to the species level was performed by VITEK 2 automated system (bioMerieux, France) for Gram-positive identification test (GPI).
Inclusion and exclusion criteria
Pharmacy students present on campus at the time of the survey were approached, and the study was explained. All students who agreed to join the study and signed an informed consent were recruited. Refusal to join the study and/or to sign the informed consent form was the only exclusion criterion applied.
Antimicrobial susceptibility testing
Antimicrobial susceptibility tests were performed using disk diffusion on Mueller–Hinton agar (Oxoid Ltd; Basingostok; Hampshire, England), according to the Clinical and Laboratory Standards Institute (CLSI 2020)17, (CLSI 2007 for vancomycin)18 and European Committee on Antimicrobial Susceptibility Testing (EUCAST 2023)19 guidelines. The following antibiotic disks (Oxoid, UK, and Himedia, India) were used: azithromycin (AZM, 15 µg), cefoxitin (CX, 30 µg), chloramphenicol (C, 30 µg), clindamycin (CD, 2 µg), co-trimoxazole (COT, 25 µg), erythromycin (E, 15 µg), fusidic acid (FC, 10 µg), gentamicin (GEN, 10 µg), linezolid (LZ, 30 µg), moxifloxacin (MO, 5 µg), mupirocin (MUP, 200 µg), rifampicin (RIF, 5 µg), teicoplanin (TEI, 30 µg), tetracycline (TE, 30 µg) and vancomycin (VA, 30 µg).
The minimum inhibitory concentrations of azithromycin (as Zithromax®, Pfizer), cefoxitin (as Primafoxin®, ZAD), chloramphenicol (as IsoptoFenicol®, Alcon), doxycycline (as Vibramycin®, Pfizer), fusidic acid (a gift from Orchidia pharmaceutical industries), gentamicin (as Epigent®, EIPICO), linezolid (as Voxazoldin®, Rofabiogen), and vancomycin (as Vancomycine®, Mylan S.A.S) were determined using agar dilution method following the guidelines of CLSI 202017 and EUCAST 202319. MIC50 and MIC90 were calculated according to Schwarz et al.20. Except for fusidic acid, all antibiotics were purchased on the Egyptian market. Multi-drug resistant isolates were defined as isolates showing resistance towards at least one antimicrobial agent from three or more categories21,22.
D-test
In order to investigate the inducibility of clindamycin resistance, erythromycin-resistant isolates were subjected to ‘D test’. In this test, erythromycin (15 μg) and clindamycin (2 μg) discs were placed at a distance of 15 mm apart (edge to edge) on a Mueller Hinton agar plate previously inoculated with 0.5 McFarland bacterial suspension. Following overnight incubation at 37 °C, flattening of the inhibition zone (formation of D shape) around the clindamycin disc in the area between the two antibiotic discs was analysed, indicating inducible clindamycin resistance23,24.
Characterization of macrolide resistance genes
For genomic DNA extraction, a few colonies were suspended in 100 µl of sterile distilled water, boiled for 30 min at 95 °C and immediately cooled at − 20 °C for 30 min prior to centrifugation (microcentrifuge, Hettich, Germany) at 14,000 rpm for 10 min. The supernatant containing genomic DNA was transferred to a new Eppendorf tube and was used for subsequent polymerase chain reaction (PCR) amplification of the target genes using PCR thermal cycler, (Perkin Elmer, USA). The sequences of the primers used to detect ermC, ermA, ermT, ermB and msrA together with annealing temperatures are listed in (Supplementary file 1). The PCR conditions were as follows: an initial denaturation at 95 °C for 1 min followed by 35 cycles of denaturation at 95 °C for 15 s, annealing for 15 s, and extension at 72 °C for 10 s, then a final extension step at 72 °C for 10 min.
Statistical analysis
The collected data were analyzed using GraphPad Prism (version 9.5.1) (Dotmatics, Boston, Massachusetts, USA) or Statistical Package for Social Science (SPSS v25.0) (IBM, USA). Fisher's exact test was performed on significance level of 0.05.
Ethical approval and consent to participate
The study was approved by The Research Ethics Committee at the Faculty of Pharmacy, Alexandria University prior to study commencement and the Ethics Committee of Alexandria University, Faculty of Medicine under IRB number: 00012098 and the Federal Wide Assurance FWA number: 00018699 (Date: 9/4/2023) (http://www.hhs.gov/ohrp/assurances/index.html). All potential study participants were provided with a participant information sheet prior to taking part in the study, and informed consent was obtained from all participants for the collection of swabs and use of their data before beginning the study. All experiments were conducted according to the relevant guidelines and regulations.
Results
Sample collection and species identification
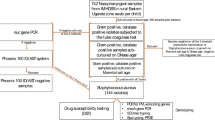
Nasal swabs were collected from 196 young pharmacy students aged 19–23 years. Processing of the swabs according to the scheme shown in Fig. 1 yielded a total of 103 staphylococcal isolates which were obtained from 86 participants (Fig. 1, Supplementary file 2). The assignment of the isolates as staphylococci was based on microscopic examination, catalase production and growth on mannitol salt agar (Supplementary files 3 and 4). S. aureus isolates were identified by coagulase tests, fermentation of mannitol as well as by growth on DNase agar. Final species determination was performed using the VITEK 2 system, identifying 34 (33.01%) S. aureus and 69 (66.99%) Staphylococci other than S. aureus (SOSA) (Fig. 1, Supplementary files 3 and 4). The SOSA isolates belonged to six species: S. haemolyticus (n = 33, 32.04%), S. hominis subspecies hominis (n = 13, 12.62%), S. epidermidis (n = 12, 11.65%), S. warneri (n = 9, 8.74%), S. capitis and S. lentus (n = 1, 0.97%, each) (Fig. 2, Table 1).
Participant characteristics and staphylococcal colonization
Of the 196 participants originally enrolled in the study, staphylococci were recovered from 86 individuals. The participants’ female to male ratio was 42:33, with 11 participants not providing information. According to self-disclosure (Supplementary file 2), 20/86 participants (23.26%) suffered from recurrent skin infections, and 11/86 (12.79%) had been hospitalized in the previous year. 4/86 (4.65%) participants reported suffering from allergic reactions, including asthma, and 11/86 (12.79%) participants experienced recurrent infections, mostly of the respiratory tract (Supplementary file 2). Further, the subjects were asked about their personal hygiene habits in order to assess a putative correlation with colonization by S. aureus and SOSA species. The participants washed their nose between 1 to 7 times (an average of 4.3 times) per day and showered between 1 to 8 times (an average of 4.5 times) weekly.
A total of 30/86 subjects were colonized by S. aureus, with 12/30 (40%) of the isolates being MRSA, resulting in a total S. aureus colonization rate of 34.89% and an MRSA carriage rate of 14%. Two of the 11 individuals (18.18%) who reported a hospital stay in the previous year carried an S. aureus from which one isolate was an MRSA (Supplementary files 2 and 5). Hospitalization did not significantly influence S. aureus colonization (p = 1). The majority of S. aureus nasal carriers (24/30, 80%) were exclusively colonized by S. aureus, while six individuals additionally harbored SOSA. Among the 12 MRSA carriers, two were found to be co-colonized by SOSA. Exclusive colonization with SOSA was found in 56/86 (65.12%) of the participants, with the majority (50/56; 89.29%) being colonized with only one species or strain, while 6/56 (10.71%) of the subjects carried more than one SOSA species or different strains of the same species (Supplementary file 2). Of the 20/86 (23.26%) individuals with recurrent skin infections, 7/20 (35%) were colonized by S. aureus, with 4/7 (57.14%) isolates being MRSA (Supplementary file 5). However, there was no statistically significant correlation between skin infection and S. aureus colonization (p > 0.5; ns). Also, analysis of the data with respect to other reported previous illnesses did not reveal significant correlations with S. aureus and/or MRSA carriage. Likewise, presence of a distinct staphylococcal species was neither correlated with gender (p > 0.5; ns) nor the frequencies of nasal washings or frequencies of weekly showers (p > 0.5; ns) (Table1).
Antimicrobial susceptibility testing and multidrug-resistance
Resistance towards the tested antibiotics was widespread among all isolates (Fig. 3, Supplementary files 5 and 6). S. aureus isolates (n = 34) showed high resistance rates to fusidic acid (52.94%), tetracycline (38.24%) as well as to erythromycin and azithromycin (26.47%, each). Based on cefoxitin resistance screening17, 35.29% (12/34) of the S. aureus isolates were identified as MRSA. Among SOSA, S. epidermidis isolates (n = 12) displayed highest resistance levels to cefoxitin (50%), clindamycin (25%), erythromycin (66.67%) and azithromycin (58.33%) (Fig. 3). Moxifloxacin resistance was only observed among S. haemolyticus isolates (n = 33) (9.09%). Moderate to high rates of resistance to azithromycin, erythromycin, cefoxitin and tetracycline were recorded across all isolates, ranging between 26.47–58.3%, 26.47–66.7%, 23.08–50%, 7.69–50%, respectively. Also, resistance to fusidic acid was common in the sample (52.91–93.94%). All isolates were susceptible to linezolid and resistance to rifampicin and vancomycin was detected only in a single S. aureus isolate each, whereas resistance to teicoplanin was detected in one S. haemolyticus isolate (Fig. 3, and Supplementary files 5 and 6).
(A) Antibiotic resistance patterns of individual staphylococci. Heatmap represents resistance (R) in black, intermediate (I) in gray and susceptible (S) results in white, determined using disc diffusion assay. GEN—gentamicin, CX—cefoxitin, MO—moxifloxacin, VA—vancomycin, TEI—teicoplanin, CD—clindamycin, LZ—linezolid, AZM—azithromycin, E—erythromycin, C—chloramphenicol, COT—co-trimoxazole, TE—tetracycline, FC—fusidic acid, MUP—mupirocin, RIF—rifampicin. (B) Distribution of resistance profiles to the tested antibiotics. Numbers in graph show percentage of resistant isolates. Total number of isolates n = 103. (C) Distribution of resistance levels among individual species. Percentage of multi-drug resistant (MDR; red), resistant (R; black) and susceptible (S; white) isolates per species. Percentages given for MDR, R and S.
Apart from S. capitis (n = 1), multi-drug resistance (MDR) was detected among all staphylococcal species, ranging from 30.77% among S. hominis to 50% among S. epidermidis. The only S. lentus isolate present in the study also displayed MDR (Fig. 3).
The susceptibility results were confirmed by MIC determination (Supplementary files 7 and 8). Azithromycin had the widest MIC range (1– > 1024 µg/ml for S. aureus and 0.5– > 1,024 µg/ml for SOSA isolates), while linezolid had the narrowest MIC range (0.5–1 µg/ml for S. aureus and SOSA), followed by vancomycin (0.25–2 µg/ml for S. aureus and 0.5–2 µg/ml for SOSA) (Table 2). Apart from fusidic acid where the highest MIC values recorded for SOSA were eightfold higher than S. aureus (1024 vs. 128 µg/ml), comparable highest and lowest MIC values in the range were detected for SOSA and S. aureus. MIC50 and MIC90 were determined using our dataset. At least four-fold elevated MIC50 values were observed for azithromycin against S. haemolyticus and S. epidermidis (32 µg/ml) and doxycycline against S. epidermidis (4 µg/ml). Elevated MIC90 values were observed for fusidic acid against S. haemolyticus (1024 µg/ml) and gentamicin against S. haemolyticus, S. epidermidis (MIC90 8 µg/ml) and S. aureus (MIC90 16 µg/ml) (Table 2). Of note, the MIC90 for doxycycline against S. aureus is four- to five-fold reduced (MIC90 0.25 µg/ml) compared to the other species.
Individual colonization by MDR staphylococci
40/86 (46.51%) of the volunteers were found to be colonized by at least one MDR isolate, with 14/86 (16.28%) individuals carrying MDR S. aureus and 26/86 (30.2%) harboring MDR SOSA isolates. Four individuals carried more than one MDR isolate, two of them carried concomitantly MDR S. aureus and MDR SOSA, while the other two participants were colonized by different MDR-SOSA. No significant correlation existed between previous hospitalization and MDR carriage (p = 0.33; ns).
Characterization of macrolide resistance
Regardless of phenotypic resistance exhibition, all isolates were tested for macrolide resistance gene carriage. Thus, presence of ermA, ermB, ermC, ermT and msrA genes was determined using PCR (Supplementary files 9 and 10). ermB showed the highest prevalence among all species (79.41% and 94.2% among S. aureus and SOSA, respectively), followed by ermC (38.24% and 86.96% among S. aurues and SOSA, respectively) (Table 3). Of note, prevalence of ermB and ermC was lower in S. aureus than in SOSA. ermA was detected in only one S. epidermidis isolate. On species level and excluding S. lentus, S. epidermidis had the highest rates of azithromycin resistance (58.33%) and the highest prevalence of resistance genes among all species, ermB (100%), ermC (91.67%), msrA (75%), and ermA and ermT (8.33%, each) (Table 3). Presence of ermC, ermB and msrA was significantly associated with S. aureus (p < 0.05). This holds also true for ermC, msrA and S. haemolyticus (p < 0.05). In S. aureus, azithromycin resistance was significantly associated with the presence of msrA (p < 0.05). S. aureus showed the highest percentage of D-test positive isolates (77.78%), followed by S. haemolyticus (57.89%). Constitutive MLSB resistance was equally observed in S. aureus and SOSA (11.11% and 16.22%, respectively), whereas inducible MLSB resistance was more often found in S. aureus (77.78% and 43.24%, respectively) (Table 4).
Discussion
Health care-associated (HA) infections are a public health issue worldwide, including Egypt25,26 Data from Mansoura New General Hospital in 2017 showed that 3.7% of hospitalized patients experienced a HA infection during the study period25. In addition, community-acquired (CA) infections contribute to the disease burden as well. In this context, some bacteria circulating in the population, such as CA-MRSA, are particularly feared when they enter hospitals due to their high virulence potential27,28. A study from Egypt in 2020 reports that the leading cause of heart failure exacerbation in hospitalized patients with former heart failure were due to infections by CA-bacteria, with an in-hospital mortality rate of up to 7.7%29. One explanation why bacteria from the community are difficult to treat might be antibiotic resistance which in turn is often associated with the abuse and overuse of antibiotics outside of hospitals.
In this study, we investigated nasal carriage of staphylococci in young volunteers, in order to gain insight into the staphylococcal species circulating in individuals in an urban setting in Egypt, and their ABR profiles. From a total of 196 volunteers, we obtained 103 staphylococcal isolates from 86 people. The detected total S. aureus colonization rate of 34.88% (30/86) is not unusual and corresponds very well with previously reported numbers in similar cohorts30. However, an MRSA carriage rate of 14% (12/86) is remarkable and suggests that MRSA are widespread and circulate in the community in the Alexandria urban region31. Here, multi-locus sequence typing (MLST) upon whole genome sequencing (WGS) of the strains would be very useful in the future to identify the exact MRSA clonal lineages spreading in the region. With respect to SOSA, we found (as expected) a broad range of typical species with S. haemolyticus, S. hominis and S. epidermidis being the most common ones (Fig. 2). Methicillin resistance among SOSA was strikingly frequent, with 39.13% (27/69) of the isolates displaying cefoxitin resistance (as a proxy for methicillin resistance). In general, SOSA displayed higher antimicrobial resistance rates than S. aureus (Fig. 3A, C) which is in good agreement with previous studies32,33. The percentage of multidrug resistant (MDR) isolates among SOSA species was 43.48% (Fig. 3C) which is twice the rate reported in a recent German study in volunteers in the community34. The numbers detected in our study, rather resemble ABR rates reported previously for health care workers in various countries across the globe35. MDR rates for S. aureus were 41.18%, slightly lower than reported for non-hospitalized individuals in Nigeria (52.2%)36, but nearly three times the rate reported for health care workers in a global study35, suggesting again a high antibiotic selective pressure in the Alexandria region.
Regarding individual antibiotics, we found high rates of fusidic acid resistance in both S. aureus and SOSA (52.94% and 81.16%, respectively, which is in agreement with a previous study also reporting high levels of fusidic acid resistance in S. aureus (71.9%) and coagulase negative staphylococci (52.6%)37. The current prevalence of fusidic acid resistance is higher than the rate presented by our group (32.1%) in 2017 among a collection of staphylococci from various hospitals in Alexandria38. The high susceptibility levels detected here with vancomycin mirror the rates detected by our group among a collection of S. aureus and S. haemolyticus clinical isolates and selected mutants39. Macrolides are among the most commonly used antibiotics in outpatients in Egypt and worldwide15,16, and accordingly, we found high resistance rates for azithromycin (44.66%) and erythromycin (45.63%) in all staphylococcal species (Fig. 3C), with data matching well with previous studies40. We analyzed the prevalence of macrolide resistance genes present in the isolates by PCR and found them in most staphylococcal species with prevalence ranging between 8.3% and 100%, which agrees with the phenotypic data. ermB showed the highest prevalence (Table3), differently to what was reported before41,42, which could be explained on the basis of regional differences43. In Africa, ermB is more prevalent than in other regions, fitting with our observation. On the other hand, ermA was only detected in one S. epidermidis isotate, which agrees with the data from Africa too, where it was the least detected erm gene43. While phenotypic reistance data fit what was previouly reported, the prevalence of resistance genes in the current study is lower41. Additionally, D-test was perferomed to investigate prevalence of consitutive and inducible MLSB resistance. Inducible phenotype was previously reported in staphylcococci at low ranges up to 30% which is less than we detected (S. aureus: 77.78% and SOSA: 43.24%)23,40,44,45. Constitutive MLSB resistance was equally obersved in S. aureus and SOSA (11.11% and 16.22%, respectively). That is in concordance with published data, where consitivitve MLSB resistance was reported up to 11%23,44. The high rates of inducible MLSB resistance are very alarming, as this harbous a huge risk for treatment failure in case of an infection where treatment is attempted with clindamycin in the absence of D-test data.
Finally, all isolates showed complete susceptibility to linezolid which is in good agreement with previous studies40,46.
Analysis of the data set with regard to such criteria as previous diseases or hospitalization of the participants did not reveal any statistically robust correlation to colonization with specific species or (resistant) strains. Previous studies suggested that personal hygiene, more precisely nose ablution, may influence nasal microbiota47,48. However, we could not demonstrate such an association in our study (Table 1).
Conclusions
Taken together our data show that in the Alexandria region (multidrug) resistant staphylococci are no longer restricted to hospital environments but are now also widespread in the community. It is tempting to speculate that the high MRSA and MDR detection rates observed are associated with high antibiotic selection pressure in the region. The alarming rates of inducible MLSB resistance phenotypes among commensal isolates call for making D-test data available to infectious disease physicians alongside susceptibility data. Whether the detected high resistance rates are also coupled with high virulence rates is an important research question for future studies. A limitation of the study is that it only focused on staphylococcal carriage among pharmacy students and didn’t include any healthcare workers. In general, our study is among the very few studies on record of staphylococcal nasal colonization (both S. aureus and SOSA) among Egyptians. Future plans include conducting whole genome sequencing of the isolates for further elucidation of their resistance and virulence genotypes as well as a detailed description of the isolate epidemiology.
Data availability
The datasets generated and analysed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- S. aureus :
-
Staphylococcus aureus
- SOSA:
-
Staphylococci other than S. aureus
- erm :
-
Erythromycin ribosomal methylase
- msrA :
-
Macrolide streptogramin resistance A
- MDR:
-
Multi-drug resistance
- S. hominis :
-
Staphylococcus hominis
- S. epidermidis :
-
Staphylococcus epidermidis
- S. haemolyticus :
-
Staphylococcus haemolyticus
- S. warneri :
-
Staphylococcus warneri
- S. lentus :
-
Staphylococcus lentus
- S. capitis :
-
Staphylococcus capitis
- MLSB :
-
Macrolide-lincosamide-streptogramin B
- ABR:
-
Antibiotic resistance
- MRSA:
-
Methicillin resistant S. aureus
- DNase:
-
Deoxyribonuclease
- GPI:
-
Gram-positive identification test
- CLSI:
-
Clinical and Laboratory Standards Institute
- EUCAST:
-
European Committee on Antimicrobial Susceptibility Testing
- PCR:
-
Polymerase chain reaction
- SPSS:
-
Statistical Package for Social Science
- MSA:
-
Mannitol salt agar
- MIC:
-
Minimum inhibitory concentration
- HA:
-
Health care-associated
- CA:
-
Community-acquired
- MLST:
-
Multi-locus sequence typing
- WGS:
-
Whole genome sequencing
- CX:
-
Cefoxitin
- C:
-
Chloramphenicol
- CD:
-
Clindamycin
- VA:
-
Vancomycin
- TE:
-
Tetracycline
- MUP:
-
Mupirocin
- TEI:
-
Teicoplanin
- LZ:
-
Linezolid
- MO:
-
Moxifloxacin
- RIF:
-
Rifampicin
- COT:
-
Co-trimoxazole
- GEN:
-
Gentamicin
- FC:
-
Fusidic acid
- E:
-
Erythromycin
- AZM:
-
Azithromycin
References
Tong, S. Y., Davis, J. S., Eichenberger, E., Holland, T. L. & Fowler, V. G. Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 28(3), 603–661 (2015).
Heilmann, C., Ziebuhr, W. & Becker, K. Are coagulase-negative staphylococci virulent?. Clin. Microbiol. Infect. 25(9), 1071–1080 (2019).
Lee, A. S. et al. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers. 4(1), 1–23 (2018).
Murray, C. J. et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 399(10325), 629–655 (2022).
Becker, K., Heilmann, C. & Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 27(4), 870–926 (2014).
Krismer, B., Weidenmaier, C., Zipperer, A. & Peschel, A. The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota. Nat. Rev. Microbiol. 15(11), 675–687 (2017).
Laux, C., Peschel, A. & Krismer, B. Staphylococcus aureus colonization of the human nose and interaction with other microbiome members. Microbiol. Spectr. 7(2), 34 (2019).
Von Eiff, C., Becker, K., Machka, K., Stammer, H. & Peters, G. Nasal carriage as a source of Staphylococcus aureus bacteremia. New Engl. J. Med. 344(1), 11–16 (2001).
von Eiff, C., Peters, G. & Heilmann, C. Pathogenesis of infections due to coagulase negative staphylococci. Lancet Infect. Dis. 2(11), 677–685 (2002).
Cheung, G. Y., Bae, J. S. & Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 12(1), 547–569 (2021).
Brinkac, L., Voorhies, A., Gomez, A. & Nelson, K. E. The threat of antimicrobial resistance on the human microbiome. Microbial Ecol. 74(4), 1001–1008 (2017).
Asante, J. et al. Review of clinically and epidemiologically relevant coagulase-negative staphylococci in Africa. Microbial Drug Resist. 26(8), 951–970 (2020).
Abdulgader, S. M., Shittu, A. O., Nicol, M. P. & Kaba, M. Molecular epidemiology of Methicillin-resistant Staphylococcus aureus in Africa: A systematic review. Front. Microbiol. 6, 348 (2015).
Azzam, A. et al. Epidemiology of clinically isolated methicillin-resistant Staphylococcus aureus (MRSA) and its susceptibility to linezolid and vancomycin in Egypt: A systematic review with meta-analysis. BMC Infect. Dis. 23(1), 1–15 (2023).
Dooling, K. L. et al. Understanding antibiotic use in Minya District, Egypt: Physician and pharmacist prescribing and the factors influencing their practices. Antibiotics 3(2), 233–243 (2014).
Sabry, N. A., Farid, S. F. & Dawoud, D. M. Antibiotic dispensing in Egyptian community pharmacies: An observational study. Res. Soc. and Admin. Pharm. 10(1), 168–184 (2014).
CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed. CLSI Supplement M100 (Clinical and Laboratory Standards Institute, Wayne, PA, 2020).
Performance standards for antimicrobial susceptibility testing; seventeenth information supplement. CLSI document M100-S17 (M2-A7 and M7-A7) VW, PA: Clinical and Laboratory Standards institute.).
The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 13.0 hweo.
Schwarz, S. et al. Assessing the antimicrobial susceptibility of bacteria obtained from animals. J. Antimicrob. Chemother. 65(4), 601–604 (2010).
Magiorakos, A.-P. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18(3), 268–281 (2012).
Rafailidis, P. I. & Kofteridis, D. Proposed amendments regarding the definitions of multidrug-resistant and extensively drug-resistant bacteria. Expert Rev. Anti Infect. Ther. 20(2), 139–146 (2022).
Deotale, V., Mendiratta, D., Raut, U. & Narang, P. Inducible clindamycin resistance in Staphylococcus aureus isolated from clinical samples. Indian J. Med. Microbiol. 28(2), 124–126 (2010).
Raut, S., Bajracharya, K., Adhikari, J., Pant, S. S. & Adhikari, B. Prevalence of methicillin resistant Staphylococcus aureus in Lumbini medical college and teaching hospital, Palpa, Western Nepal. BMC Res. Notes 10(1), 1–7 (2017).
Hassan, R., El-Gilany, A.-H., Abdelaal, A. M., El-Mashad, N. & Azim, D. A. An overview of healthcare-associated infections in a tertiary care hospital in Egypt. Infect. Prevent. Pract. 2(3), 100059 (2020).
Raoofi, S. et al. Global prevalence of nosocomial infection: A systematic review and meta-analysis. PLOS ONE 18(1), e0274248 (2023).
DeLeo, F. R., Otto, M., Kreiswirth, B. N. & Chambers, H. F. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375(9725), 1557–1568 (2010).
De Oliveira, D. M. et al. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. https://doi.org/10.1128/cmr.00181-19 (2020).
Hassanin, A., Hassanein, M., Bendary, A. & Maksoud, M. A. Demographics, clinical characteristics, and outcomes among hospitalized heart failure patients across different regions of Egypt. Egypt. Heart J. 72(1), 49 (2020).
Sakr, A., Brégeon, F., Mège, J. L., Rolain, J. M. & Blin, O. Staphylococcus aureus Nasal colonization: An update on mechanisms, epidemiology, risk factors, and subsequent infections. Front Microbiol. 9, 2419 (2018).
Abdel-Maksoud, M. et al. Methicillin-resistant Staphylococcus aureus recovered from healthcare-and community-associated infections in Egypt. Int. J. Bacteriol. 2016, 5751785 (2016).
Becker, K. et al. Does nasal cocolonization by methicillin-resistant coagulase-negative staphylococci and methicillin-susceptible Staphylococcus aureus strains occur frequently enough to represent a risk of false-positive methicillin-resistant S. aureus determinations by molecular methods?. J. Clin. Microbiol. 44(1), 229–231 (2006).
Lebeaux, D. et al. Evolution of nasal carriage of methicillin-resistant coagulase-negative staphylococci in a remote population. Antimicrob. Agents Chemother. 56(1), 315–323 (2012).
Marincola, G. et al. Antimicrobial resistance profiles of coagulase-negative staphylococci in community-based healthy individuals in Germany. Front. Public Health 9, 796 (2021).
Morgenstern, M. et al. Antibiotic resistance of commensal Staphylococcus aureus and coagulase-negative staphylococci in an international cohort of surgeons: A prospective point-prevalence study. PloS One 11(2), e0148437 (2016).
Onanuga, A. & Temedie, T. Nasal carriage of multi-drug resistant Staphylococcus aureus in healthy inhabitants of Amassoma in Niger delta region of Nigeria. Afr. Health Sci. 11(2), 176–181 (2011).
Budri, P. E. et al. Observational cross-sectional study of nasal staphylococcal species of medical students of diverse geographical origin, prior to healthcare exposure: Prevalence of SCCmec fusc fusB and the arginine catabolite mobile element (ACME) in the absence of selective antibiotic pressure. BMJ Open 8(4), e020391 (2018).
Abouelfetouh, A., Kassem, M., Naguib, M. & El-Nakeeb, M. Investigation and treatment of fusidic acid resistance among methicillin-resistant staphylococcal isolates from Egypt. Microb. Drug Resist. 23(1), 8–17 (2017).
Maarouf, L., Omar, H., El-Nakeeb, M. & Abouelfetouh, A. Prevalence and mechanisms of linezolid resistance among staphylococcal clinical isolates from Egypt. Eur. J. Clin. Microbiol. Infect. Dis. 40, 815–823 (2021).
de Benito, S. et al. Prevalence of Staphylococcus spp. nasal colonization among doctors of podiatric medicine and associated risk factors in Spain. Antimicrob. Resist. Infect. Control 7(1), 1–7 (2018).
Gatermann, S., Koschinski, T. & Friedrich, S. Distribution and expression of macrolide resistance genes in coagulase-negative staphylococci. Clin. Microbiol. Infect. 13(8), 777–781 (2007).
Leclercq, R. Mechanisms of resistance to macrolides and lincosamides: Nature of the resistance elements and their clinical implications. Clin. Infect. Dis. 34(4), 482–492 (2002).
Miklasińska-Majdanik, M. Mechanisms of resistance to macrolide antibiotics among Staphylococcus aureus. Antibiotics 10(11), 1406 (2021).
Hamilton-Miller, J. & Shah, S. Patterns of phenotypic resistance to the macrolide–lincosamide–ketolide–streptogramin group of antibiotics in staphylococci. J. Antimicrob. Chemother. 46(6), 941–949 (2000).
Mahmoud, A. M. et al. Inducible clindamycin resistance and nasal carriage rates of Staphylococcus aureus among healthcare workers and community members. Afr. Health Sci. 15(3), 861–867 (2015).
Rongpharpi, S. R., Hazarika, N. K. & Kalita, H. The prevalence of nasal carriage of Staphylococcus aureus among healthcare workers at a tertiary care hospital in Assam with special reference to MRSA. J. Clin. Diagn. Res. JCDR 7(2), 257 (2013).
Fawzi, M. M. & Ibraheem, H. A. Nasal Staphylococcus aureus carriage in ablution performers. Egypt J. Med. Microbiol. 27, 57–63 (2018).
Zaini, R., Ismail, K., Rezk, H. & Dahlawi, H. Pilot study to detect the presence of MRSA among healthcare workers who practice ablution. J. Transm. Dis. Immun. 1, 1 (2017).
Acknowledgements
The authors would like to acknowledge clinical pharmacy students, Alexandria University (class 2022) for their role in swab collection and survey administration.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This study was supported by the German Research Council (DFG) through grant ZI665/3-1 and Faculty of Pharmacy, Alexandria University Research grant (Academic Thesis Research Fund (ATRF) cycle 4).
Author information
Authors and Affiliations
Contributions
A.H: design and performance of experiments, data analysis and manuscript writing. M.A.: design of experiment and revision of manuscript. T.M.: data analysis, figure design and manuscript writing. W.Z. secured funding and manuscript revision. E.A.: conceptualization of the study and revision of the manuscript. AA: Conceptualization of the study, secured funding, data analysis, manuscript writing and revision. All authors have read and agreed on the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamdy, A., Marciniak, T., Alseqely, M. et al. Phenotypic and genotypic characterization of commensal staphylococci isolated from young volunteers in Alexandria, Egypt. Sci Rep 14, 14850 (2024). https://doi.org/10.1038/s41598-024-60924-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60924-8
- Springer Nature Limited