Abstract
Background
In vitro data suggested reduced neutralizing capacity of sotrovimab, a monoclonal antibody, against Omicron BA.2 subvariant. However, limited in vivo data exist regarding clinical effectiveness of sotrovimab for coronavirus disease 2019 (COVID-19) due to Omicron BA.2.
Methods
A multicentre, retrospective cohort study was conducted at three Canadian academic tertiary centres. Electronic medical records were reviewed for patients ≥ 18 years with mild COVID-19 (sequencing-confirmed Omicron BA.1 or BA.2) treated with sotrovimab between February 1 to April 1, 2022. Thirty-day co-primary outcomes included hospitalization due to moderate or severe COVID-19; all-cause intensive care unit (ICU) admission, and all-cause mortality. Risk differences (BA.2 minus BA.1 group) for co-primary outcomes were adjusted with propensity score matching (e.g., age, sex, vaccination, immunocompromised status).
Results
Eighty-five patients were included (15 BA.2, 70 BA.1) with similar baseline characteristics between groups. Adjusted risk differences were non-statistically significant between groups for 30-day hospitalization (− 14.3%; 95% confidence interval (CI): − 32.6 to 4.0%), ICU admission (− 7.1%; 95%CI: − 20.6 to 6.3%), and mortality (− 7.1%; 95%CI: − 20.6 to 6.3%).
Conclusions
No differences were demonstrated in hospitalization, ICU admission, or mortality rates within 30 days between sotrovimab-treated patients with BA.1 versus BA.2 infection. More real-world data may be helpful to properly assess sotrovimab’s effectiveness against infections due to specific emerging COVID-19 variants.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19) treatment have rapidly changed over time [1]. In particular, many monoclonal antibody treatments for COVID-19 have come and go [2], quickly withdrawn from recommendations as new strains showed reduced neutralizing activity based on in vitro studies [3].
As an example, sotrovimab, a monoclonal antibody, was once a favoured therapeutic option for COVID-19 given its therapeutic advantages versus other approved drug options, requiring only a single intravenous dose with fewer drug-drug interactions [4]. An in vitro study demonstrated reduced neutralizing capacity of sotrovimab against Omicron BA.2, where the FNRT50 value (titer of sotrovimab required for 50% reduction in number of infectious foci) was approximately 50-fold higher for BA.2 compared to the SARS-CoV-2 ancestral strain [3]. By March 2022, BA.2 represented about 25% of confirmed COVID-19 cases in Ontario, Canada [5]. In response, both United States and Canadian (Ontario) guidelines revoked recommendations on therapeutic use of sotrovimab for COVID-19 by early April 2022 [6, 7]. However, the question remains whether in vitro data translated to decreased efficacy in vivo of sotrovimab against BA.2.
As BA.2 emerged before large-scale discontinuation of sotrovimab in clinical practice, a few observational studies had been able to evaluate BA.2-infected patients who received sotrovimab as treatment [8,9,10]. These studies demonstrated no significant differences in hospitalizations in BA.1 versus BA.2-infected patients treated with sotrovimab, although the analyses were either unadjusted for baseline risks or adjusted for age and immunization status only. In Canada, mild COVID-19 patients who met criteria to receive sotrovimab were considered high risk either from immunocompromising conditions or having risk factors for progression to severe disease, per local (e.g., Ontario, British Columbia) guidelines (Additional file 1: Text S1) [4]. Adjusting for potential bias from age, vaccination status, as well as immunocompromised status and risk factors for severe COVID-19 may provide a better estimate of effectiveness of sotrovimab between variants in infected individuals.
To our knowledge, there is no existing data that used a propensity-matched analysis to compare the effectiveness of sotrovimab in patients with mild COVID-19 who were infected with Omicron BA.2 versus those infected with Omicron BA.1. We conducted a retrospective cohort study in patients with mild COVID-19 due to Omicron collecting local Canadian patient data. Our primary objective was to compare the effectiveness of sotrovimab in confirmed BA.2 cases versus BA.1 cases in terms of hospitalization, intensive care unit (ICU) admission and mortality risk.
Methods
Study design and participants
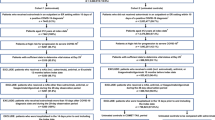
We conducted a multicentre retrospective cohort study in three Canadian academic tertiary care centres. Patients aged ≥ 18 years who received single-dose intravenous sotrovimab (500 mg) as treatment for mild COVID-19 were included. Based on the treatment algorithms from local guidelines [4], patients would have only received sotrovimab as COVID-19 directed therapy, and thus would not have received any other therapeutics (e.g., remdesivir, nirmatrelvir/ritonavir). The study period February 1 to April 1, 2022 was chosen to capture then-predominant BA.1 and BA.2 cases who received sotrovimab as treatment prior to its discontinuation within Canada. Mild COVID-19 was defined as those not requiring additional supplementary oxygen from their clinical baseline as per local and international guidelines [4, 11]. Initial diagnosis was established by rapid antigen test or real-time reverse transcriptase-polymerase chain reaction (PCR) from upper or lower respiratory tract specimens. Omicron BA.1 or BA.2 confirmation via whole genome sequencing (WGS) or targeted single-nucleotide polymorphism (SNP) PCR was required for inclusion.
Data collection
Patient data were collected retrospectively from medical records for baseline demographic characteristics, prior COVID-19 infection and vaccination status, symptoms before receiving sotrovimab, immunocompromised status, and comorbidities at high risk for complication or severe COVID-19, as derived from local guidelines (Additional file 1: Text S1) [4]. Omicron subvariant WGS or targeted SNP PCR results were provided by the microbiology laboratory of each respective study centre.
Co-primary outcomes within 30 days of sotrovimab administration included (1) hospitalization due to moderate or severe COVID-19; (2) all-cause admission to ICU, and; (3) all-cause mortality. Investigators were blinded to subvariant status during the data entry process.
Statistical analysis
For descriptive analysis, continuous (mean and standard deviation) and categorical variables (counts and percentages) were used. BA.1 and BA.2 groups were compared using Student’s t-test for continuous variables and Fisher’s exact test for categorical variables. For co-primary outcomes, risk difference of BA.2 minus BA.1 group was calculated with estimated two-sided 95% confidence intervals (CI) based on the method of Agresti and Caffo [12].
To address potential bias, propensity score for BA.2 subvariant was estimated by logistic regression of the following prognostic factors determined a priori: age, sex, vaccination status, immunocompromised status, and number of risk factors for progression to severe disease. BA.2 and BA.1 cases were matched using nearest neighbour matching with a specified caliper width of 0.3 times the standard deviation of the logit of propensity scores. Standardized mean difference was used to assess for balance of prognostic factors. Risk difference and two-sided 95% CI were estimated for each outcome; CI estimates were calculated for matched patients using the method of Agresti and Min [13, 14].
All tests were two-sided, with significance defined as P < 0.05. All analyses were done using statistical software R (version 4.1.2), with statistical packages DescTools and MatchIt for risk difference CI and propensity score matching, respectively [15, 16].
Results
Eighty-five patients with COVID-19 (70 BA.1, 15 BA.2) were included with similar baseline characteristics (Table 1). None of the patients have received any other COVID-19 therapeutics or adjunctive therapies prior to or at the time of receiving sotrovimab. Co-primary outcomes within 30 days of sotrovimab administration are presented in Table 2 and Additional file 1: Fig. S1. Following matching by propensity scores (14 BA.1 and 14 BA.2 patients), the maximum standardized difference was 0.0839, suggesting good balance of baseline characteristics (Additional file 1: Table S1). The adjusted risk difference at 30 days for BA.2 group minus BA.1 group was (Table 3 and Additional file 1: Fig. S2): − 14.3% (95% CI: − 32.6 to 4.0%) for hospitalization; − 7.1% (95% CI: − 20.6 to 6.3%) for ICU admission; and − 7.1% (95% CI: − 20.6 to 6.3%) for death.
Discussion
Though sotrovimab has clinical trial data demonstrating efficacy in reducing risk of hospitalization or death in at-risk COVID-19 outpatients [17], there were concerns of real-world effectiveness since the Omicron surge in late 2021, and its subvariants [1].
Our propensity-matched study showed no statistically significant differences in 30-day hospitalization, ICU admission and mortality rates between sotrovimab-treated patients with BA.2 versus BA.1 infection. All the estimates favoured better outcomes in the BA.2 group. As an example, in the propensity-matched group, the absolute risk for hospitalization in the BA.2 group was 14.3% less than the BA.1 group. However, there is uncertainty in this estimate as the 95% confidence interval is wide and ranges from 32.6% less to 4% more risk.
Of note, our findings are corroborated by two other studies. A French multicentre prospective cohort study (ANRS 0003S CoCoPrev) of high-risk mild-to-moderate COVID-19 patients showed low rates of disease-related hospitalization at day 28 following sotrovimab administration in 1/42 BA.2 (2.4%, 95% CI: 0–13%) and 3/125 BA.1-infected patients (2.4%, 95% CI: 1–7%), and no deaths [9]. However, the study results were not adjusted for baseline risks.
An English retrospective cohort study of COVID-19 outpatients treated with sotrovimab reported that 133 of 3,230 (4.1%) BA.1 cases and 140 of 3566 (3.9%) BA.2 cases were hospitalized with an adjusted hazard ratio of 1.02 (95% CI: 0.70–1.47) [8]. Although this study adjusted for age group and vaccination status to account for confounders, additional risk factors such as immunocompromised status were not accounted for.
When compared with other approved therapies for mild COVID-19, another study from the same French cohort (ANRS 0003S CoCoPrev) demonstrated similar COVID-19-related hospitalization rates 28 days following treatment administration between sotrovimab-treated (4/193) and nirmatrelvir/ritonavir-treated (0/55) cohorts (p = 0.24) [10]. The N gene Ct value slopes of both BA.1 and BA.2-infected patients were steeper amongst the nirmatrelvir/ritonavir-treated cohort, although the median time to negative PCR conversion amongst BA.2-infected patients did not significantly differ between the sotrovimab- and nirmatrelvir/ritonavir-treated groups [10]. This may be due to lack of statistical power from the relatively small number of nirmatrelvir/ritonavir-treated patients (in particular, BA.1-infected patients).
Our study’s strengths include its multicentre and propensity-matched study design where real-world comparative data on sotrovimab effectiveness against BA.2 versus BA.1 infections can be optimally estimated by risk differences with minimal baseline differences and biases.
Limitations
Limitations included the small sample size—resulting in large confidence intervals for the calculated risk differences that cannot exclude small but clinically important differences—and the applicability of these findings for current Omicron subvariants. This was especially true for our BA.2 group (i.e., 15 patients), as our small sample size may fail to detect a signal of true differences between the two groups.
Conclusion
In infectious diseases, in vitro assays have traditionally offered early insight into potential effectiveness of therapies such as monoclonal antibodies against emerging COVID-19 variants. The evidence for similar effectiveness of sotrovimab against BA.1 and BA.2 presents as an example where in vivo real-world clinical efficacy may potentially deviate from those initially suggested by in vitro data. More real-world data may be helpful to properly assess sotrovimab’s effectiveness against infections due to specific emerging COVID-19 variants, and whether they corroborate with laboratory data to translate to significant clinical differences for patient relevant outcomes.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- COVID-19:
-
Coronavirus disease 2019
- ICU:
-
Intensive care unit
- PCR:
-
Polymerase chain reaction
- SNP:
-
Single-nucleotide polymorphism
- WGS:
-
Whole genome sequencing
References
Siemieniuk RA, Bartoszko JJ, Zeraatkar D, Kum E, Qasim A, Martinez JPD, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. https://doi.org/10.1136/bmj.m2980.
Hernandez AV, Piscoya A, Pasupuleti V, Phan MT, Julakanti S, Khen P, et al. Beneficial and harmful effects of monoclonal antibodies for the treatment and prophylaxis of COVID-19: systematic review and meta-analysis. Am J Med. 2022;135(11):1349-61.e18. https://doi.org/10.1016/j.amjmed.2022.06.019.
Takashita E, Kinoshita N, Yamayoshi S, Sakai-Tagawa Y, Fujisaki S, Ito M, et al. Efficacy of antiviral agents against the SARS-CoV-2 Omicron subvariant BA.2. N Engl J Med. 2022;386(15):1475–7. https://doi.org/10.1056/nejmc2201933.
Ontario COVID-19 Drugs and Biologics Clinical Practice Guidelines Working Group. Clinical practice guideline summary: recommended drugs and biologics in adult patients with COVID-19 (version 10.0). Ontario COVID-19 Science Advisory Table. 2022. https://doi.org/10.47326/ocsat.cpg.2022.10.0. Accessed 7 June 2023.
Ontario Agency for Health Protection and Promotion (Public Health Ontario). COVID-19 Omicron variant sub-lineage BA.2: evidence and risk assessment (up to date as of March 22, 2022). Queen's Printer for Ontario. 2022. https://www.publichealthontario.ca/-/media/Documents/nCoV/voc/2022/03/omicron-variant-ba2-risk-assessment-mar-22.ashx?la=fr. Accessed 27 May 2023.
U.S. Food and Drug Administration. FDA updates Sotrovimab emergency use authorization. https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-sotrovimab-emergency-use-authorization. Updated Apr 5, 2022. Accessed 12 Apr 2023.
Ontario COVID-19 Drugs and Biologics Clinical Practice Guidelines Working Group. Clinical practice guideline summary: recommended drugs and biologics in adult patients with COVID-19 (version 11.0). Ontario COVID-19 Science Advisory Table. 2022. https://doi.org/10.47326/ocsat.cpg.2022.11.0. Accessed 7 June 2023.
Harman K, Nash SG, Webster HH, Groves N, Hardstaff J, Bridgen J, et al. Comparison of the risk of hospitalisation among BA.1 and BA.2 COVID-19 cases treated with sotrovimab in the community in England. Influenza Other Respir Viruses. 2023;17(5):e13150. https://doi.org/10.1111/irv.13150.
Martin-Blondel G, Marcelin A-G, Soulié C, Kaisaridi S, Lusivika-Nzinga C, Dorival C, et al. Sotrovimab to prevent severe COVID-19 in high-risk patients infected with Omicron BA.2. J Infect. 2022;85(4):e104–8. https://doi.org/10.1016/j.jinf.2022.06.033.
Martin-Blondel G, Marcelin AG, Soulié C, Kaisaridi S, Lusivika-Nzinga C, Zafilaza K, et al. Time to negative PCR conversion amongst high-risk patients with mild-to-moderate Omicron BA.1 and BA.2 COVID-19 treated with sotrovimab or nirmatrelvir. Clin Microbiol Infect. 2023;29(4):543.e5-e9. https://doi.org/10.1016/j.cmi.2022.12.016.
World Health Organization. Therapeutics and COVID-19: living guideline, 13 January 2023. World Health Organization. 2023. https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2023.1. Accessed 7 June 2023.
Agresti A, Caffo B. Simple and effective confidence intervals for proportions and differences of proportions result from adding two successes and two failures. Am Stat. 2000;54(4):280–8. https://doi.org/10.2307/2685779.
Agresti A, Min Y. Effects and non-effects of paired identical observations in comparing proportions with binary matched-pairs data. Stat Med. 2004;23(1):65–75. https://doi.org/10.1002/sim.1589.
Austin PC. The performance of different propensity-score methods for estimating differences in proportions (risk differences or absolute risk reductions) in observational studies. Stat Med. 2010;29(20):2137–48. https://doi.org/10.1002/sim.3854.
Ho D, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42(8):1–28. https://doi.org/10.18637/jss.v042.i08.
Signorell A, Aho K, Alfons A, Anderegg N, Aragon T, Arachchige C, et al. DescTools: tools for descriptive statistics. R package, version 2021:0.99.40. https://cran.r-project.org/web/packages/DescTools/index.html. Updated Feb 3, 2021. Accessed 19 Aug 2023.
Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Rodrigues Falci D, et al. Effect of Sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;327(13):1236–46. https://doi.org/10.1001/jama.2022.2832.
Acknowledgements
Not applicable.
Funding
This work was supported by the McMaster Medical Specialties Residents/Fellows Research Grant. The funding body had no role in the design of the study, data collection, analysis, interpretation of data or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
CKLL, ASK, ZC and ADB conceived and designed the study. CKLL, CKFL, ASK and ADB performed the data collection and supported project administration. ADB conducted data analysis and prepared all tables. CKLL, CKFL and ADB jointly wrote the first draft of the manuscript. All authors reviewed the full data set prior to publication and take responsibility for its accuracy. All authors reviewed and revised the manuscript and approved a final version to the submitted for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Local research ethics board from each participating centre (St. Paul’s Hospital H22-01209; St. Joseph’s Healthcare Hamilton HiREB#14526; Kingston Health Science Centre TRAQ#6036035) approved this study. Informed consent was waived by the before mentioned ethics committees in view of the retrospective nature of the study, which analyzed retrospective aggregated deidentified data. All methods were carried out in accordance with relevant and local guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary material including: Text S1. Criteria for immunocompromised conditions and risk factors for progression to severe COVID-19, based on local Canadian guidelines. Table S1. Balance of prognostic factors before and after matching by propensity scores. Figures S1 and S2. Graphical representation of unadjusted and adjusted risk differences of co-primary outcomes 30 days post-sotrovimab.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lo, C.K.L., Lo, C.K.F., Komorowski, A.S. et al. Evaluating in vivo effectiveness of sotrovimab for the treatment of Omicron subvariant BA.2 versus BA.1: a multicentre, retrospective cohort study. BMC Res Notes 17, 37 (2024). https://doi.org/10.1186/s13104-024-06695-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-024-06695-x