Abstract
Background
Both aerobic exercise and whey protein can improve glucose regulation. The purpose of this study was to investigate how a single bout of vigorous-intensity aerobic exercise and whey protein, independently, as well as when combined, influence glycemia during an oral glucose tolerance test in sedentary, young men.
Methods
Healthy males (n = 11) completed four randomized trials: no exercise/no whey protein (R); exercise (EX; walking at 70% VO2max for 60 min); 50 g of whey protein (W); and exercise combined with 50 g of whey protein (EXW). Each trial included a 75 g oral glucose tolerance test (OGTT) that was completed after an overnight fast. Blood samples were collected over a two-hour period during the OGTT. For EX and EXW, the exercise was performed the evening before the OGTT and the 50 g of whey protein was dissolved in 250 mL of water and was consumed as a preload 30 min prior to the OGTT. For R and EX, participants consumed 250 mL of water prior to the OGTT. Plasma samples were analyzed for glucose, insulin, C-peptide, glucagon, gastric inhibitory peptide (GIP) and glucagon like peptide 1 (GLP-1), and postprandial incremental area under the curve (iAUC) was calculated for each.
Results
Glucose iAUC was reduced during W (− 32.9 ± 22.3 mmol/L) compared to R (122.7 ± 29.8 mmol/L; p < 0.01) and EX (154.3 ± 29.2 mmol/L; p < 0.01). Similarly, glucose iAUC was reduced for EXW (17.4 ± 28.9 mmol/L) compared to R and EX (p < 0.01 for both). There were no differences in iAUC for insulin, C-peptide, GIP, GLP-1, and glucagon between the four trials. Insulin, C-peptide, glucagon, GIP, and GLP-1 were elevated during the whey protein preload period for W and EXW compared to EX and R (p < 0.01). There were no differences for insulin, C-peptide, glucagon, GIP, or GLP-1 between trials for the remaining duration of the OGTT.
Conclusions
Glucose responses during an oral glucose tolerance test were improved for W compared to EX. There were no additional improvements in glucose responses when vigorous-intensity aerobic exercise was combined with whey protein (EXW).
Similar content being viewed by others
Background
Aerobic exercise, along with changes in diet, are primary strategies for improving blood glucose management [1]. Acute aerobic exercise improves glycemia through intracellular signaling within skeletal muscle [2]. This results in the activation of 5’-adenosine monophosphate-activated protein kinase (AMPK) and calcium/calmodulin-dependent protein kinase (CaMK) II, each of which influences GLUT4 translocation and expression, promoting an increase in cellular glucose uptake [3,4,5]. This increase in glucose uptake can persist for several hours in response to a single bout of aerobic exercise, and the change in glucose uptake post-exercise is dependent on the intensity, duration, and type of exercise performed [6, 7]. Following exercise, skeletal muscle also exhibits enhanced insulin sensitivity, and this has been shown to persist for 24 to 48 h post-exercise [3, 6, 8,9,10]. This enhanced insulin sensitivity contributes to improved glycemia while also reducing serum insulin values for 12 to 24 h following exercise [5, 11, 12]. The intensity and duration of endurance exercise influences the magnitude of its effects on blood glucose and insulin sensitivity post-exercise. Many studies have attempted to determine the optimal intensity and duration of endurance exercise to maximize its effects on managing blood glucose [11, 13, 14]. Many of these studies report positive effects occur when exercise is performed for 45 to 60 min at moderate to vigorous intensities (≥ 50% of VO2max).
Similar to aerobic exercise, nutritional strategies (e.g., whey protein) are also effective for improving blood glucose. Whey protein, when consumed prior to, or with a meal, improves postprandial glycemia [15,16,17,18,19,20]. These improvements in postprandial glycemia are partially due to whey protein’s ability to enhance insulin secretion [21,22,23]. Whey protein also stimulates the secretion of incretin hormones, glucagon-like peptide-1 (GLP-1) and gastric inhibitory peptide (GIP), which have been demonstrated to potentiate insulin secretion [21, 24,25,26]. Thus, whey protein’s ability to both directly and indirectly increase insulin secretion contributes to improved postprandial glycemia.
The timing of whey protein consumption is important when examining its effects on postprandial glycemia. Consuming whey protein (10 or 20 g) 30 min prior to a meal (i.e., preload) has been demonstrated to slow gastric emptying and increase GLP-1 secretion [18]. In a similar study, Gunnerud et al. (2012) found that 9 g of whey protein consumed as a preload immediately prior to a mixed meal reduced postprandial plasma glucose responses (iAUC) in the first 60 minutes [17]. The authors attributed this effect to increased insulin secretion as a result of the whey protein consumption. In addition to timing, the dose or amount of whey protein consumed appears to influence the magnitude of its effects on blood glucose [21]. Several studies have demonstrated positive effects on insulin and glucose responses with higher doses (20 – 55 g) of whey protein [19, 23, 27, 28]. Our lab has previously examined how differences in the dose of whey protein can influence glycemia. We found greater improvements in glycemic responses during a 75 g oral glucose tolerance test (OGTT) when 30 g of whey protein, compared to 20 g, was consumed 30 min prior to the OGTT [29]. Thus, whey protein’s positive effects on glycemia may be dose dependent, and its effects on postprandial glycemia may be maximized when the whey protein is consumed as a preload (e.g., 30 min prior to a meal or beverage with a high glycemic load). Several studies using similar designs reported improvements in measured variables using 50 g of whey protein [27, 28]. Based upon our observations that whey protein’s effects may be dose-dependent, we hypothesized a large dose of whey protein (i.e., 50 g) would result in greater improvements in postprandial glycemia compared to lower doses.
Both acute aerobic exercise and whey protein consumption improve blood glucose. Each may be effective individually, but their effects when combined are not fully understood within human subjects. Thus, the aim of this study was to investigate the individual and combined effects of a single bout of aerobic exercise and a whey protein preload on glycemic responses following an oral glucose tolerance test. When considering whey protein’s ability to increase insulin secretion and exercise’s effects on insulin sensitivity, we hypothesized that acute vigorous-intensity aerobic exercise (70% of VO2max; performed the previous day, 12 to 14 h before the whey protein consumption) in combination with a 50 g preload of whey protein prior to an oral glucose tolerance test would result in greater improvements in postprandial blood glucose responses when compared to whey protein or acute aerobic exercise alone.
Methods
Study population
Twelve apparently healthy, sedentary males aged 18 to 44 years were recruited for this study. Participants were excluded from data collection if they were prescribed medications that would influence blood glucose concentration (sulfonylurea or metformin), blood pressure medication (thiazide diuretics, angiotensin receptor blockers, angiotensin-converting enzyme inhibitors), or answered “Yes” to any question on the Physical-Activity Readiness Questionnaire (PAR-Q +) (2016) assessment. Participation in this study also required that participants perform less than 3 days per week of exercise, or less than 150 min of moderate-intensity cardiorespiratory exercise for the previous three months to be classified as sedentary according to American College of Sports Medicine criteria [30].
Participant screening
Prior to data collection, this study was approved by the Institutional Review Board of Texas Woman’s University (Protocol #: 19,806). In addition, prior to participation in this study, all participants were informed of the study purpose and procedures and gave their written informed consent. This study and its procedures were performed in accordance with the Declaration of Helsinki. Following explanation of the study and informed consent, a fasted (10–12 h) blood sample was collected from an antecubital vein and analyzed for plasma glucose concentration and hemoglobin A1C. Participants with a fasted blood glucose value ≥ 100 mg/dL were excluded from the study. Throughout the duration of this study, participants were required to keep three-day diet records detailing food and drink consumed prior to each trial. Though a total of twelve participants were recruited for the study, one participant dropped out of the study due to noncompliance, resulting in eleven participants completing the study. Anthropometrics and characteristics for participants are shown in Table 1 and dietary records reflecting caloric and carbohydrate intake 24-h prior to each trial are presented in Table 2.
Anthropometrics
Height was measured using a stadiometer (Perspective Enterprises; Kalamazoo, MI, USA) and weight was measured to the nearest 0.1 kg using a digital scale (Tanita Corp.; Arlington Heights, IL, USA). From these measurements, body mass index (BMI) was calculated (kg/m2). Body composition was analyzed using a dual energy X-ray absorptiometry (DEXA) scan (General Electric Lunar DXA-Prodigy, Madison, WI, USA).
Maximal aerobic capacity test
Participants performed a graded exercise test to determine their cardiorespiratory fitness. This test was performed using the Bruce Protocol on a Quinton ST65 motor driven treadmill (Quinton®-Q Stress, Ventura, CA, USA) until exhaustion [31]. During this test, heart rate and rhythm were continuously monitored using a Quinton Q Stress 12-lead electrocardiograph (Welch Allyn™, Skaneateles Falls, NY, USA). Thirty-second averages of respiratory gas exchange were continuously collected through indirect calorimetry (ParvoMedics, Sandy, UT, USA). Attainment of maximal oxygen consumption (VO2max) was determined by a plateau in VO2 (≤ 150 ml/min) with an increase in workload, or a combination of achieving an RER greater than 1.1 and a maximal heart rate within 10 bpm of age-predicted heart rate max (220-age) [30].
Study design
Participants in this study completed four trials, and each participant performed the sequence of trials in a randomized order (see Fig. 1 for example): Trial 1; no exercise and no whey protein prior to an OGTT (R). Trial 2; 60 min of exercise at 70% of VO2max and no whey protein prior to an OGTT (EX). Trial 3; no exercise and a 50 g whey protein preload consumed prior to an OGTT (W). Trial 4; 60 min of exercise at 70% of VO2max and a 50 g whey protein preload consumed prior to an OGTT (EXW). For trials in which exercise was performed, EX and EXW, the exercise session was performed 12–14 h before the start of the OGTT for that trial (i.e., the evening before). Trials for each participant were separated by a minimum of seven days, and participants were instructed to refrain from exercise or intense physical activity between each trial. Participants were also instructed to consume a meal similar in composition to their three-day diet records the evening prior to each OGTT.
Whey protein protocols
Whey protein isolate (50 g; Isopure® Unflavored WPI, Nature’s Best™, Hauppauge, NY, USA) was consumed as a preload 30 min prior to a 75 g OGTT for both W and EXW trials. The 50 g of whey protein was mixed with 250 ml of water for both W and EXW trials. For R and EX trials, participants were given 250 ml of water only as a control preload prior to their respective OGTTs.
Exercise or rest protocols
Exercise sessions were performed the evening prior to EX and EXW OGTTs (12–14 h before the OGTT). These sessions consisted of walking for 60 min at a speed and grade that achieved 70% of the participant’s VO2max.VO2 was measured for 60 s at three separate time intervals (5 min, 30 min, and 60 min) throughout the exercise duration to ensure the intensity was appropriate, with adjustments made to the speed or grade if necessary to maintain the target VO2. Heart rate was continuously monitored and recorded throughout the exercise session using a Polar H9 heart rate sensor (Polar Electro Inc., Bethpage, NY, USA).
Oral glucose tolerance test (OGTT) procedures
Participants arrived fasted (10–12 h) to the Exercise Biochemistry Lab at Texas Woman’s University between 0600 and 0800 for all OGTTs. For each OGTT, a catheter was placed in an antecubital vein by a trained phlebotomist. A blood sample (5 mL) was collected and analyzed for fasted glucose to verify the participant was in the fasted state. Participants then consumed either the 50 g of whey protein preload (W and EXW) or water as a control beverage (R and EX). Participants were required to consume the whey or control beverage within five minutes. Thirty minutes after consuming the preload or water (i.e., control), a blood sample was collected. Immediately following this blood sample, participants consumed a commercial 75 g glucose tolerance test beverage (Trutol® Dextrose, ThermoFisher Scientific™ Inc., Waltham, MA, USA). Blood samples were then collected 15, 30, 60, 90, and 120 min following the consumption of the glucose tolerance test beverage (Fig. 2). A sterile saline drip was used to flush the catheter following each blood sample, with a drip rate of 1 drop per 4 s. Participants remained seated in a thermoneutral environment and were able to read or watch television throughout the duration of the OGTT.
Biochemical analysis
Blood samples were collected into 6 mL EDTA tubes containing a concentration of 1.25 mg Pefabloc® of blood (Roche Diagnostics, Mannheim, Germany) and 5 μL Protease Inhibitor (EMD Millipore™ Corporation, Billerica, MA, USA) per mL of blood. After collection, blood samples were immediately centrifuged at 3000 rpm for 10 min at 10 ℃. Plasma glucose was analyzed using a YSI 2900D glucose analyzer (Yellow Spring Inc, Yellow Springs, OH, USA). Plasma hormone concentrations of C-peptide, GIP, GLP-1, insulin, and glucagon were analyzed using a Luminex™ Human Metabolic Hormone multiplex assay (HMHEMAG-34, EMD Millipore™, Billerica, MA, USA).
Statistical analysis
Postprandial incremental area under the curve (iAUC) was calculated from timepoints 0 to 120 min (OGTT) using the trapezoidal method for glucose, insulin, C-peptide, GIP, GLP-1, and glucagon. Repeated measures analysis of variance (ANOVA) was used to examine differences in iAUC for all dependent variables. A two-way repeated measures ANOVA (trial x time) was also used to determine differences for glucose, insulin, glucagon, GIP, GLP-1, and C-peptide between timepoints for each trial. Greenhouse Geisser correction was used if the assumption of sphericity was violated and a Bonferroni post-hoc test was used for making comparisons when appropriate. The level of significance was set at p ≤ 0.05. All statistical analyses were performed using SPSS statistical software (IBM™ SPSS Statistics v.25, Armonk, NY, USA). All data are expressed as mean ± standard error (SE).
Results
Fasting values for C-peptide were higher for W (1217.7 ± 21.8 pg/mL) compared to EX (1034.2 ± 165.4 pg/mL; p = 0.02). There were no other differences for fasting variables across the four trials (Table 3).
Glucose
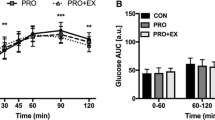
No differences in glucose values were reported between timepoints for each trial (Fig. 3A). There was a main effect observed for glucose iAUC. Glucose iAUC was lower for W (− 32.9 ± 22.3 mmol/L) compared to R (122.7 ± 29.8 mmol/L; p < 0.01) and EX (154.3 ± 29.2 mmol/L;
A Blood glucose response (mmol/L) prior to and during a two-hour oral glucose tolerance test between each trial. B Blood glucose iAUC (mmol/L) during the two-hour oral glucose tolerance test between each trial. R; no exercise, no whey protein, EX; exercise, no whey protein, W; no exercise, whey protein, EXW; exercise, whey protein. aRepresents a significant difference compared to R. bRepresents a significant difference compared to EX
p < 0.01; Fig. 3B). Additionally, glucose iAUC was lower for EXW (17.4 ± 28.9 mmol/L) compared to R (p < 0.01) and EX (p < 0.01). There were no differences between W and EXW.
Insulin and C-peptide
As shown in Fig. 4A, insulin was elevated during the 30-min whey protein preload period (timepoints -30 to 0) for both W (1742.0 ± 238.0 pg/mL) and EXW (1791.4 ± 292.8 pg/mL) compared to R (891.6 ± 165.2 pg/mL; p < 0.01) and EX (789.1 ± 147.2 pg/mL; p < 0.01). After consuming the 75 g glucose tolerance test drink (timepoint 0 from Fig. 2), there were no differences in insulin iAUC between the four trials. C-peptide response was similar to insulin, with a significant increase between timepoints -30 to 0 for W (1979.0 ± 260.1 pg/mL; p = 0.02) and EXW (1984.4 ± 273.6 pg/mL; p < 0.01) compared to EX (982.8 ± 160.6 pg/mL) and R (1183.7 ± 215.6 pg/mL; p < 0.01; Fig. 5A). No differences were observed for C-peptide iAUC between the four trials (Fig. 5B).
A Insulin response (pg/mL) prior to and during a two-hour oral glucose tolerance test between each trial. B Insulin iAUC (pg/mL) during the two-hour oral glucose tolerance test between each trial. R; no exercise, no whey protein, EX; exercise, no whey protein, W; no exercise, whey protein, EXW; exercise, whey protein. a and b represent significant differences for W compared to R and EX between timepoints -30 to 0, respectively. c and d represent significant differences for EXW compared to R and EX between timepoints −30 to 0, respectively
A C-peptide response (pg/mL) prior to and during a two-hour oral glucose tolerance test between each trial. B C-peptide iAUC (pg/mL) during the two-hour oral glucose tolerance test between each trial. R; no exercise, no whey protein, EX; exercise, no whey protein, W; no exercise, whey protein, EXW; exercise, whey protein. a and b represent significant differences for W compared to R and EX between timepoints -30 to 0, respectively. c and d represent significant differences for EXW compared to R and EX between timepoints compared to R and EX between −30 to 0, respectively
Incretins and glucagon
As shown in Fig. 6A, GIP was elevated during the 30-min whey protein preload period (timepoints -30 to 0) for W (169.5 ± 19.5 pg/mL) and EXW (179.3 ± 11.7 pg/mL) compared to R (60.9 ± 8.1 pg/mL; p < 0.01) and EX (66.1 ± 7.6 pg/mL; p < 0.01). Similarly, GLP-1 was elevated during the 30-min whey protein period (timepoints -30 to 0) for W (15.5 ± 2.5 pg/mL) compared to EX (5.3 ± 1.4 pg/mLl p < 0.01), while EXW was increased during this period compared to R (6.2 ± 1.8 pg/mL; p < 0.01) and EX (p < 0.01; Fig. 6C.) There were no significant differences in iAUC between the four trials for GIP (Fig. 6B) or GLP-1 (Fig. 6D).
A GIP response (pg/mL) prior to and during a two-hour oral glucose tolerance test between each trial. B GIP iAUC (pg/mL) during the two-hour oral glucose tolerance test between each trial. C GLP-1 response (pg/mL) prior to and during a two-hour oral glucose tolerance test between each trial. D GLP-1 iAUC (pg/mL) during the two-hour oral glucose tolerance test between each trial. R; no exercise, no whey protein, EX; exercise, no whey protein, W; no exercise, whey protein, EXW; exercise, whey protein. a and b represent significant differences for W compared to R and EX between timepoints -30 to 0, respectively. c and d represent significant differences for EXW compared to R and EX between timepoints −30 to 0, respectively
Glucagon was elevated during the 30-min whey protein preload period (timepoints -30 to 0) for both W (140.9 ± 11.6 pg/mL) and EXW (163.5 ± 14.9 pg/mL) compared to R (67.7 ±
10.3; p < 0.01) and EX (69.2 ± 11.2 pg/mL; p < 0.01; Fig. 7A). There were no significant differences in iAUC between the four trials for glucagon (Fig. 7B).
A Glucagon response (pg/mL) prior to and during a two-hour oral glucose tolerance test between each trial. B Glucagon iAUC (pg/mL) during the two-hour oral glucose tolerance test between each trial. R; no exercise, no whey protein, EX; exercise, no whey protein, W; no exercise, whey protein, EXW; exercise, whey protein. a and b represent significant differences for W compared to R and EX between timepoints -30 to 0, respectively. c and d represent significant differences for EXW compared to R and EX between timepoints -30 to 0, respectively
Discussion
Here, we investigated how a single bout of vigorous-intensity aerobic exercise and whey protein consumption, independent of each other and combined, influence glycemic responses to a 75 g OGTT in young adult men. This study is novel as it is one of few studies that has investigated their combined effects on acute glycemia. Moreover, this study utilized a unique time frame (exercise and whey protein separated by 12–14 h) for investigating how aerobic exercise and whey protein interact to affect glycemia.
We found the combination of vigorous-intensity aerobic exercise and whey protein (EXW) reduced glucose iAUC values during a two-hour OGTT compared to exercise alone (EX). Additionally, we observed a similar effect on glucose iAUC for W compared to EX. Importantly, these effects on glucose iAUC were achieved without significant increases in insulin iAUC during W or EXW compared to R or EX. Despite these effects on glucose and insulin iAUC, there were no differences in C-peptide, glucagon, GIP, or GLP-1 iAUC values throughout the two-hour OGTT between the four trials.
Glucose/insulin
Our results for glucose iAUC when 50 g of whey protein was consumed as a preload (W and EXW) are similar to other studies that have investigated whey protein’s effects at higher doses (50 g) on glycemia [27, 28]. However, these effects on glycemia were not observed when exercise was performed independent of whey protein (EX). Comparably, a previous study reported similar outcomes for glucose and insulin with 31 g of whey protein was consumed as a preload. The authors reported glucose response was reduced during a 75 g OGTT when the whey protein was consumed 15 min prior to a graded exercise test in healthy young men and women, when compared to control and fructose beverages [32]. The improvements in glucose iAUC for W and EXW were accompanied by increases in insulin responses 30 min after whey protein consumption (-30 to 0 timepoint), though insulin iAUC was not different between the four trials. The changes observed for W and EXW may be attributed to this acute spike in insulin following the whey protein preload, which was then followed by a more normalized insulin response throughout the remaining duration of the OGTT.
Whey protein’s positive effects on glucose management are exemplified by our results for EX. EX had similar glucose responses to R at each individual timepoint throughout the OGTT (Fig. 3A), and glucose iAUC values for EX were higher than R (Fig. 3B). Our results for EX are similar to previous studies that have investigated the influence of acute aerobic exercise on glycemia [33, 34]. Though overall insulin responses to the OGTT were lowest for EX, there were no significant effects on insulin iAUC or differences between timepoints compared to the other three trials. We found this surprising, as several studies have reported improvements in insulin responses to aerobic exercise [35, 36]. Our results for EX may be explained most clearly by the fact that the exercise was performed 12–14 h prior to the OGTT, potentially diminishing exercise’s insulin sensitizing effects. As the participants in this study were relatively young, healthy men, it indicates higher intensities and/or durations may be necessary to sustain post-exercise-induced improvements in insulin sensitivity over longer durations (12 + hours) in this population. Moreover, there was large variability in insulin iAUC for EX, which may be explained by elevated insulin concentrations in two participants at several consecutive timepoints during the OGTT for this specific trial. This variability, in addition to a smaller sample size, likely influenced any observed significance of EX on insulin iAUC. Overall, our findings for W in relation to EX indicate whey protein has important applications for managing glycemia. As there were no further improvements with EXW compared to W, our results suggest whey protein may have a larger influence on acute glycemia compared to exercise in this population.
GIP, GLP-1, and glucagon
The incretins, GIP and GLP-1, have important roles in promoting insulin secretion and glucose management, with some suggesting the incretins account for 50–70% of insulin secretion following a meal [37]. In comparison to previous literature on this topic, Ma et al. (2009) reported whey protein had stimulatory effects on both GIP and GLP-1 at doses similar to those used in this study [27]. We found no differences in iAUC for GIP or GLP-1 between the four trials. However, we observed significant increases in GIP and GLP-1 for both W and EXW during the whey protein preload prior to the OGTT (timepoints -30 to 0). These increases in GIP and GLP-1 for W and EXW coincide with observed changes in insulin, which likely contributed to the improvements in glucose iAUC for these trials. Specifically, we speculate that this brief increase in GIP and GLP-1 following whey protein consumption could have potentiated insulin secretion, contributing to the observed glucose responses for W and EXW. Our findings for GIP and GLP-1 following whey protein consumption is similar to results from Jakubowicz et al. (2014). Using a similar study design, they found increases in GLP-1 30 min after consuming whey protein, though also reporting GLP-1 continued to increase following a high glycemic-index meal [15].
In addition to their effects on insulin secretion, the incretin hormones have regulatory effects on glucagon. Specifically, GIP can enhance glucagon secretion, while GLP-1 has been shown to inhibit glucagon secretion [25, 38]. We found no significant differences in glucagon iAUC between the four trials. However, similar to GIP and GLP-1, when examining glucagon’s response prior to the OGTT (timepoints -30 to 0), glucagon was elevated for both W and EXW during this time period. Considering GIP and GLP-1’s opposing effects on glucagon, it appears GIP may have a stronger regulatory effect on glucagon than GLP-1, contributing to the observed increase in glucagon following the whey protein preload. Comparing responses for glucose, insulin, and glucagon throughout the OGTT, an interesting pattern emerges whereby insulin and glucagon are elevated similarly for W and EXW with respect to R and EX. Specifically, for W and EXW, glucagon peaked after the whey protein preload period (timepoints -30 to 0) but remained elevated, albeit to a lesser extent, throughout the duration of the OGTT. Insulin response was similar, increasing after the whey protein was consumed, though its concentration peaked after the OGTT commenced (30-min timepoint). What is interesting is that despite these similar, yet opposing, responses between glucagon and insulin, glucose responses for W and EXW were relatively stagnant, and even decreased below baseline glucose values throughout the OGTT. This observed effect appears to be exclusive to whey protein consumption as well, as glucagon decreased for EX during the OGTT. What this dynamic interaction between glucagon and insulin means in the context of glycemia regulation when whey protein is consumed, as well as how GIP and GLP-1 may influence these observed effects requires further exploration.
Limitations
This study and its results have some limitations that should be considered for prospective investigations. For instance, the participants in this study were limited to young, adult men. As the scope of this study is largely relevant to the management of metabolic disease (i.e., type II diabetes), future studies should consider exploring the relationship between whey protein and exercise in individuals with metabolic disease. Additionally, several studies have explored whey protein’s effects on glycemia at varied doses (e.g., 20 g or 30 g). Based upon our findings in this study at 50 g of whey protein, it may be worth exploring how varied doses of whey protein, in conjunction with vigorous-intensity aerobic exercise, may influence glycemia. Lastly, as participants in this study walked at a vigorous intensity for the exercise intervention, our results are limited to this specific type of exercise. It may be worth investigating how different modes of exercise incorporating mechanical loading (e.g., resistance exercise, or aerobic and resistance exercise combined) when combined with whey protein may influence glycemia.
Conclusions
We found whey protein consumed independent of performing exercise improved glucose responses to a 75 g OGTT. This improvement was also observed when whey protein and vigorous-intensity aerobic exercise were combined, but this effect was lost when exercise was performed alone. These results provide information for how vigorous-intensity aerobic exercise and whey protein individually, and when combined, may affect acute glycemia. This study addressed how the timing between exercise and whey protein may influence these responses as well, which is important when considering the real-world application of these results. To summarize, our results highlight the short-term effectiveness of whey protein, independently and in combination with aerobic exercise, for improving glycemia.
Availability of data and materials
The datasets used and/or analyzed in this current study are available from the corresponding author on reasonable request.
Abbreviations
- DEXA:
-
Dual energy X-ray absorptiometry
- EX:
-
Exercise/no whey protein
- EXW:
-
Exercise/whey protein
- GIP:
-
Gastric inhibitory peptide
- GLP-1:
-
Glucagon-like peptide 1
- iAUC:
-
Incremental area under the curve
- OGTT:
-
Oral glucose tolerance test
- R:
-
No exercise/no whey protein
- W:
-
No exercise, whey protein
References
Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Clevel Clin J Med. 2017;84(7 Suppl 1):S15–21. https://doi.org/10.3949/ccjm.84.s1.03.
McGarrah RW, Slentz CA, Kraus WE. The effect of vigorous- versus moderate-intensity aerobic exercise on insulin action. Curr Cardiol Rep. 2016;18(12):117. https://doi.org/10.1007/s11886-016-0797-7.
Lehnen A, Angelis KD, Markoski MM, Schaan BDA. Changes in the GLUT4 expression by acute exercise, exercise training and detraining in experimental models. J Diabetes Metabol. 2012. https://doi.org/10.4172/2155-6156.S10-002.
Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013. https://doi.org/10.1152/physrev.00038.2012.
Douen AG, Ramlal T, Rastogi S, Bilan PJ, Cartee GD, Vranic M, Klip A. Exercise induces recruitment of the “insulin-responsive glucose transporter.” evidence for distinct intracellular insulin-and exercise-recruitable transporter pools in skeletal muscle. J Biol Chem. 1990;265(23):13427–30. https://doi.org/10.1016/S0021-9258(18)77362-6.
Maarbjerg SJ, Sylow L, Richter EA. Current understanding of increased insulin sensitivity after exercise–emerging candidates. Acta Physiol. 2011;202(3):323–35. https://doi.org/10.1111/j.1748-1716.2011.02267.x.
Jensen TE, Richter EA. Regulation of glucose and glycogen metabolism during and after exercise. J Physiol. 2012;590(5):1069–76. https://doi.org/10.1113/jphysiol.2011.224972.
Kjøbsted R, Munk-Hansen N, Birk JB, Foretz M, Viollet B, Björnholm M, Zierath J, Treeback J, Wojtaszewski JF. Enhanced muscle insulin sensitivity after contraction/exercise is mediated by AMPK. Diabetes. 2017;66(3):598–612. https://doi.org/10.2337/db16-0530.
Cartee GD. Mechanisms for greater insulin-stimulated glucose uptake in normal and insulin-resistant skeletal muscle after acute exercise. Am J Physiol-Endocrinol Metabolism. 2015;309(12):E949–59. https://doi.org/10.1152/ajpendo.00416.2015.
Steenberg DE, Jørgensen NB, Birk JB, Sjøberg KA, Kiens B, Richter EA, Wojtaszewski JF. Exercise training reduces the insulin-sensitizing effect of a single bout of exercise in human skeletal muscle. J Physiol. 2019;597(1):89–103. https://doi.org/10.1113/JP276735.
Hübinger A, Franzen A, Gries FA. Hormonal and metabolic response to physical exercise in hyperinsulinemic and non-hyperinsulinemic type 2 diabetics. Diabetes Res. 1987;4(2):57–61.
Jankowski C, Ben-Ezra V, Kendrick K, Morriss R, Nichols D. Effect of exercise on postprandial insulin responses in Mexican American and non-Hispanic women. Metabolism. 1999;48(8):971–7. https://doi.org/10.1016/S0026-0495(99)90192-0.
Rogers M, Yamamoto C, King D, Hagberg J, Ehsani A, Holloszy J. Improvement in glucose tolerance after 1 wk of exercise in patients with mild NIDDM. Diabetes Care. 1988;11(8):613–8. https://doi.org/10.2337/diacare.11.8.613.
Sylow L, Kleinert M, Richter EA, Jensen TE. Exercise-stimulated glucose uptake—regulation and implications for glycaemic control. Nat Rev Endocrinol. 2017;13(3):133–48. https://doi.org/10.1038/nrendo.2016.162.
Jakubowicz D, Froy O, Ahrén B, Boaz M, Landau Z, Bar-Dayan Y, Ganz T, Barnea M, Wainstein J. Incretin, insulinotropic and glucose-lowering effects of whey protein pre-load in type 2 diabetes: a randomised clinical trial. Diabetologia. 2014;57(9):1807–11. https://doi.org/10.1007/s00125-014-3305-x.
Gunnerud UJ, Östman EM, Björck IME. Effects of whey proteins on glycaemia and insulinaemia to an oral glucose load in healthy adults; a dose–response study. Eur J Clin Nutr. 2013;67(7):749–53. https://doi.org/10.1038/ejcn.2013.88.
Gunnerud UJ, Heinzle C, Holst JJ, Östman EM, Björck IM. Effects of pre-meal drinks with protein and amino acids on glycemic and metabolic responses at a subsequent composite meal. PLoS ONE. 2012;7(9):e44731. https://doi.org/10.1371/journal.pone.0044731.
Akhavan T, Luhovyy BL, Panahi S, Kubant R, Brown PH, Anderson GH. Mechanism of action of pre-meal consumption of whey protein on glycemic control in young adults. J Nutr Biochem. 2014;25(1):36–43. https://doi.org/10.1016/j.jnutbio.2013.08.012.
Petersen BL, Ward LS, Bastian ED, Jenkins AL, Campbell J, Vuksan V. A whey protein supplement decreases post-prandial glycemia. Nutr J. 2009;8(1):1–5. https://doi.org/10.1186/1475-2891-8-47.
Bowen J, Noakes M, Trenerry C, Clifton PM. Energy intake, ghrelin, and cholecystokinin after different carbohydrate and protein preloads in overweight men. J Clin Endocrinol Metab. 2006;91(4):1477–83. https://doi.org/10.1210/jc.2005-1856.
Mignone LE, Wu T, Horowitz M, Rayner CK. Whey protein: the “whey” forward for treatment of type 2 diabetes? World J Diabetes. 2015;6(14):1274.
Stevenson EJ, Allerton DM. The role of whey protein in postprandial glycaemic control. Proc Nutrition Soc. 2018;77(1):42–51. https://doi.org/10.1017/S0029665117002002.
Akhavan T, Luhovyy BL, Brown PH, Cho CE, Anderson GH. Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. The Am J Clinic Nutrition. 2010;91(4):966–75. https://doi.org/10.3945/ajcn.2009.28406.
Nilsson M, Holst JJ, Björck IM. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: studies using glucose-equivalent drinks. Am J Clin Nutr. 2007;85(4):996–1004. https://doi.org/10.1093/ajcn/85.4.996.
Frid AH, Nilsson M, Holst JJ, Björck IM. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am J Clin Nutr. 2005;82(1):69–75. https://doi.org/10.1093/ajcn/82.1.69.
Adams RL, Broughton KS. Insulinotropic effects of whey: mechanisms of action, recent clinical trials, and clinical applications. Ann Nutr Metab. 2016;69(1):56–63. https://doi.org/10.1159/000448665.
Ma J, Stevens JE, Cukier K, Maddox AF, Wishart JM, Jones KL, Clifton PM, Horowitz M, Rayner CK. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care. 2009;32(9):1600–2. https://doi.org/10.2337/dc09-0723.
Hoefle AS, Bangert AM, Stamfort A, Gedrich K, Rist MJ, Lee YM, Daniel H. Metabolic responses of healthy or prediabetic adults to bovine whey protein and sodium caseinate do not differ. J Nutr. 2015;145(3):467–75. https://doi.org/10.3945/jn.114.199190.
Castleberry T, Irvine C, Gordon R, Brisebois M, Deemer S, Henderson A, Sokoloski M, Ben-Ezra V. The dose effect of whey protein on glycemic control in adults with insulin resistance. Int J Sci Biotechnol. 2020;5(4):62–7. https://doi.org/10.11648/j.ijfsb.20200504.12.
Liguori, G., & American College of sports medicine. (2020). ACSM's guidelines for exercise testing and prescription. Lippincott Williams & Wilkins.
Fletcher GF, Balady GJ, Amsterdam EA, Chaitman B, Eckel R, Fleg J, Froelicher VF, Leon AS, Ileana LP, Rodney R, Simons-Morton DA, Williams MA, Bazzarre T. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104(14):1694–740. https://doi.org/10.1161/hc3901.095960.
Kluess HA, Neidert LE. Post-exercise glucose response following whey protein ingestion in healthy young people: a randomized pilot study. Open Diabetes J. 2018. https://doi.org/10.2174/1876524601808010001.
Cononie CC, Goldberg AP, Rogus E, Hagberg JM. Seven consecutive days of exercise lowers plasma insulin responses to an oral glucose challenge in sedentary elderly. J Am Geriatr Soc. 1994;42(4):394–8. https://doi.org/10.1111/j.1532-5415.1994.tb07487.x.
Jankowski CM, Ben-Ezra V, Gozansky WS, Scheaffer SE. Effects of oral contraceptives on glucoregulatory responses to exercise. Metabolism. 2004;53(3):348–52. https://doi.org/10.1016/j.metabol.2003.10.024.
Henriksen EJ. Invited review: effects of acute exercise and exercise training on insulin resistance. J Appl Physiol. 2002;93(2):788–96. https://doi.org/10.1152/japplphysiol.01219.2001.
Frampton J, Cobbold B, Nozdrin M, Oo HT, Wilson H, Murphy KG, Frost G, Chambers ES. The effect of a single bout of continuous aerobic exercise on glucose, insulin and glucagon concentrations compared to resting conditions in healthy adults: a systematic review, meta-analysis and meta-regression. Sports Med. 2021. https://doi.org/10.1007/s40279-021-01473-2.
Holst JJ, Deacon CF. Is there a place for incretin therapies in obesity and prediabetes? Trends Endocrinol Metab. 2013;24(3):145–52. https://doi.org/10.1016/j.tem.2013.01.004.
Holst JJ, Christensen M, Lund A, De Heer J, Svendsen B, Kielgast U, Knop FK. Regulation of glucagon secretion by incretins. Diabetes Obes Metab. 2011;13:89–94. https://doi.org/10.1111/j.1463-1326.2011.01452.x.
Acknowledgements
The authors would like to thank the subjects who participated in this study.
Funding
Funding used for the collection and analysis of data was supported in part by a Texas American College of Sports Medicine Student Research Development Award (SRDA). ELZ is currently supported by a T32 Postdoctoral Award from the National Institutes of Health (T32HL007457). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
TJC and VB conceived and designed research. RAG, ELZ, TJC, MLS, MFB, and CJI conducted experiments. RAG, ELZ, and TJC analyzed data. RAG, ELZ, and MLS drafted the manuscript. MFB, AAD, and VB critically revised the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study includes human participants and data, and prior to data collection, this study was approved by the Institutional Review Board of Texas Woman’s University (Protocol #: 19806). In addition, prior to participation in this study, all participants were informed of the study purpose and procedures and gave their written informed consent. This study and its procedures were performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest in regard to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gordon, R.A., Zumbro, E.L., Castleberry, T.J. et al. Whey protein improves glycemia during an oral glucose tolerance test compared to vigorous-intensity aerobic exercise in young adult men. BMC Sports Sci Med Rehabil 14, 147 (2022). https://doi.org/10.1186/s13102-022-00540-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13102-022-00540-z