Abstract
Purpose
Acute submaximal exercise and whey protein supplementation have been reported to improve postprandial metabolic and appetite responses to a subsequent meal independently. We aimed to examine the combination of these strategies on postprandial responses to a carbohydrate-rich breakfast.
Methods
Twelve centrally obese males (age 41 ± 3 years, waist circumference 123.4 ± 2.9 cm), completed three trials in a single-blind, crossover design. Participants rested for 30 min (CON) or completed 30 min low–moderate-intensity treadmill walking (51 ± 1% \({{\dot{V}O}}_{\text{2peak}}\)) followed immediately by ingestion of 20 g whey protein (EX + PRO) or placebo (EX). After 15 min, a standardised breakfast was consumed and blood, expired gas and subjective appetite were sampled postprandially. After 240 min, an ad libitum lunch meal was provided to assess energy intake.
Results
During EX + PRO, post-breakfast peak blood glucose was reduced when compared with EX and CON (EX + PRO: 7.6 ± 0.4 vs EX: 8.4 ± 0.3; CON: 8.3 ± 0.3 mmol l−1, p ≤ 0.04). Early postprandial glucose AUC0–60 min was significantly lower under EX + PRO than EX (p = 0.011), but not CON (p = 0.12). Over the full postprandial period, AUC0–240 min during EX + PRO did not differ from other trials (p > 0.05). Peak plasma insulin concentrations and AUC0–240 min were higher during EX + PRO than CON, but similar to EX. Plasma triglyceride concentrations, substrate oxidation and subjective appetite responses were similar across trials and ad libitum energy intake was not influenced by prior fasted exercise, nor its combination with whey protein supplementation (p > 0.05).
Conclusion
Following fasted low–moderate-intensity exercise, consuming whey protein before breakfast may improve postprandial glucose excursions, without influencing appetite or subsequent energy intake, in centrally obese males.
Trial registration number
NCT02714309.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well established that obesity is associated with dysregulation of a number of metabolic processes including glucose and lipoprotein metabolism, and thus increased risk of developing type 2 diabetes and cardiovascular disease [1]. Indeed, in developed countries, obesity is by far the most prevalent cause of insulin resistance [2], which is related to chronic, low-grade inflammation and macrophage infiltration in adipose tissue and increased circulating concentrations of pro-inflammatory cytokines [3, 4]. Central (abdominal) obesity is known to be particularly hazardous in the pathogenesis of insulin resistance and type 2 diabetes [5], with visceral fat tissue expressing higher levels of many cytokines [6] and a higher rate of lipolysis [7] than subcutaneous depots. The liver is, therefore, exposed to high levels of both non-esterified fatty acids and pro-inflammatory factors released from visceral fat, leading to elevated hepatic triglyceride and consequent deleterious effects on hepatic insulin sensitivity [8].
In the progressive decline from normal to impaired glucose tolerance that precedes the development of type 2 diabetes, it is postprandial rather than fasting glucose that appears to deteriorate first [9, 10]. Postprandial hyperglycaemia has been identified as an independent risk factor for cardiovascular disease in diabetic and non-diabetic populations [11,12,13], including when fasting glucose is in the normal range [14]. Excessive postprandial glucose excursions are, therefore, a strong predictor of future cardiovascular disease events [15]. Moreover, dysregulated lipoprotein metabolism is prevalent in those with central obesity and insulin resistance, with post-meal hypertriglyceridaemia increasingly recognised as a contributor to cardiovascular disease risk [16].
Exercise is a potent non-pharmacological strategy to reduce the burden of insulin resistance induced postprandial hyperglycaemia [17, 18] and lipidaemia [19]. Acute bouts of moderate-intensity exercise can upregulate a number of pathways that contribute to increased postprandial glucose disposal. This includes expression and translocation of GLUT4 to the cell membrane [20], which persists for several hours after cessation of exercise [21], in addition to some evidence of upregulated insulin signalling pathways following moderate-intensity exercise [22]. Additionally, exercise reduces postprandial lipaemia via increased hydrolysis of intramuscular triglyceride, due to increased lipoprotein lipase activity and reduced hepatic output of very low-density lipoproteins [23]. Regular training appears to impact postprandial glucose disposal via improvements in insulin sensitivity induced by adaptations including upregulation of muscle GLUT4 protein, increased enzyme capacities and muscle capillarization [20].
Nutritional strategies can also impact postprandial hyperglycaemia, and it has recently been demonstrated that prandial whey protein supplementation can significantly reduce subsequent glycaemic excursions in overweight men [24]. Whey protein contains amino acids and bioactive peptides which reduce postprandial glucose excursions via insulin-dependent and independent mechanisms [25]. Numerous studies have investigated the efficacy of whey protein supplementation on subsequent postprandial glycaemia in patients with type 2 diabetes [26,27,28], however, few trials have been conducted in centrally obese, non-diabetic individuals. Such individuals are at risk of being exposed to the adverse effects of postprandial hyperglycaemia [29]. Considering the effectiveness of both exercise and whey protein supplementation for improving postprandial glycaemia, a combination of these strategies may be a more effective approach.
The vast majority of studies investigating the effects of post-exercise whey protein supplementation have been conducted following resistance-type exercise, with beneficial effects on muscle protein synthesis [30] and lean mass maintenance [31, 32] previously described. However, the influence of whey protein supplementation following aerobic exercise on subsequent metabolic and appetite responses has received little attention. Reduced ad libitum energy intake has been observed 60 min after milk [33] or whey protein [34] consumption following prior moderate-intensity cycling exercise in recreationally active participants. Whether postprandial metabolic and appetite responses would be influenced by post-exercise whey protein consumption in habitually inactive obese individuals remains unclear. Given that a single bout of exercise may produce divergent responses in subsequent glucose tolerance [35], and that whey has been observed to influence postprandial glycaemia and insulinaemia consistently [36], the impact of post-exercise whey consumption on postprandial metabolic responses may be of significance.
Therefore, the aim of this study was to investigate the effect of fasted moderate-intensity exercise and subsequent whey protein supplementation on postprandial metabolic and appetite responses in centrally obese males.
Materials and methods
Participants
Male participants, aged 18–55 years, with central obesity and a low physical activity level were recruited. Central obesity was defined as waist circumference above the WHO threshold (102 cm) for abdominal obesity in males [37]. Physical activity level was assessed using the categorical scoring method following completion of the International Physical Activity Questionnaire [38]. Participants were excluded if they suffered from cardiovascular, metabolic or renal disorders, had a current illness, were taking medication that may affect metabolism or were a smoker. Additionally, participants were excluded if they self-reported regularly skipping breakfast (consuming breakfast on two or less occasions in the previous 7 days), having food allergies/intolerances or an eating disorder. All participants provided written informed consent prior to participation. Sample size was determined using a within-subject power analysis using a previous study in an overweight/obese population [39]. Following consumption of a whey protein preload with a mixed-macronutrient breakfast, a 16% reduction in glucose AUC was observed with a within-group variance of 11%. Statistical power was set at 80%, with a two-sided alpha level of 0.05.
Experimental design
In a single-blind within-subject design, participants completed three trials, separated by at least 7 days. Participants were randomly assigned to pre-determined counterbalanced trial schedules that were created using an online randomisation tool for researchers (https://www.randomization.com/). Two exercise trials involved a 30 min bout of brisk treadmill walking followed by consumption of a whey protein (EX + PRO) or placebo (EX) preload beverage. A third trial involved participants remaining sedentary for the same duration followed by consumption of the placebo beverage (CON). A standardised, carbohydrate-rich, breakfast meal was provided 15 min after consumption of test preloads (whey protein or placebo) under all conditions. Participants remained sedentary for a further 240 min, followed by consumption of an ad libitum mixed-macronutrient lunch meal. This research took place within the Faculty of Health and Life Sciences research laboratories, Northumbria University, UK, with recruitment, data collection and analysis taking place between March and August 2016.
Pre-trial procedures
Prior to experimental visits, participants completed a submaximal treadmill walking test to determine their prescribed walking speed for the main trials. Four steady-state walking stages were completed on a motorised laboratory treadmill (Pulsar 3p, h/p/cosmos, Germany). Participants began walking at 3–4 km h−1 with walking speed increased by 0.5 or 1 km h−1 at the end of each 3 min stage according to the discretion of the researcher, and based on the rating of perceived exertion (RPE) in the final 30 s of each stage [40]. Throughout the test, expired gas was sampled using a breath by breath gas analyser (Oxycon Pro, CareFusion, USA) and heart rate was recorded using short-range telemetry (Polar RS400, Polar Electro, Finland). The relationship between oxygen consumption and heart rate was extrapolated to age-predicted maximum heart rate to estimate \({{\dot{V}O}}_{\text{2peak}}\) for each participant. The walking speed eliciting an intensity of 50% \({{\dot{V}O}}_{\text{2peak}}\) was determined from the relationship between oxygen consumption and walking speed, and selected as the prescribed walking speed during main trials.
Participants were instructed to avoid strenuous physical activity in addition to caffeine and alcohol consumption for 24 h prior to each laboratory visit. To standardise pre-trial nutritional intake, an identical mixed-macronutrient evening meal was provided before each visit, and participants were asked to consume this 12 h prior to arrival. This consisted of a beef lasagne meal (Tesco, UK) with a honey-flavoured oat bar (Nature Valley, USA), providing 3501 kJ energy (837 kcal; 37% carbohydrate, 19% protein, 44% fat).
Main trial procedures
Participants arrived at the laboratory following an overnight fast, where a cannula was inserted into an antecubital vein. Baseline venous and capillary blood samples were taken, and measures of subjective appetite and expired gas were collected. During CON participants remained rested, whilst during EX + PRO and EX, a 30 min bout of steady-state brisk treadmill walking at 50% \({{\dot{V}O}}_{\text{2peak}}\) was performed. The mode, intensity and duration of exercise was designed to be achievable for habitually sedentary individuals, while also conforming to UK recommendations for prescribed levels of daily physical activity for prevention of obesity [41].
Within 5 min of exercise completion, participants consumed a test beverage containing whey protein during EX + PRO, and a placebo beverage during EX. During CON, a placebo beverage was consumed at the corresponding time point. The remainder of the trial procedure was identical under all conditions. A standardised breakfast was consumed 15 min after test beverage ingestion, and participants subsequently remained seated and rested for 240 min with blood, expired gas and VAS sampled at regular intervals (Fig. 1). After 240 min, an ad libitum lunch meal was provided to assess energy intake.
Test meals
In the EX + PRO trial, the test beverage consisted of 20 g whey protein isolate (Lacprodan SP-9225 Instant; Arla Food Ingredients Group, Viby, Denmark) combined with 150 ml water and 0.5 ml energy-free strawberry flavouring (FlavDrops, Myprotein, UK). In the EX and CON trials, an isovolumetric bolus of similarly flavoured water was consumed as a placebo. All test drinks were provided in opaque bottles. An additional 200 ml drinking water was administered after each test beverage to eliminate any after taste.
A standardised portion of rolled porridge oats with semi-skimmed milk and honey was provided as breakfast under all conditions, providing 1958 kJ of energy (468 kcal; 70% carbohydrate, 17% fat, 13% protein). Participants were encouraged to consume this meal within 10 min, and 250 ml drinking water was provided alongside the porridge. A timer was started upon completion of this meal.
A homogenous pasta meal was provided ad libitum at lunch to assess energy intake. This consisted of cooked dried pasta, a tomato-based sauce, cheddar cheese and olive oil (Tesco, UK) as described previously [42], providing 53%, 14% and 33% energy from carbohydrate, protein and fat, respectively. Participants were initially provided with a 400 g (2845 kJ, 680 kcal) portion of the pasta and were instructed to eat until they felt ‘comfortably full’ on each occasion. The serving bowl was topped up with fresh pasta prior to completion, thus removing the effect of bowl clearance as a stimulus for food intake termination. All portions of cooked pasta (served or unserved) were weighed immediately before and after consumption to determine energy intake.
Indirect calorimetry
Expired gas was sampled at regular intervals throughout resting and exercise periods of the protocol (Fig. 1) using an online gas analyser (Oxycon Pro, CareFusion, USA). Samples were collected at baseline following 10 min rest, immediately after the test beverage, 20 min post-breakfast, and every subsequent 30 min. Resting expired gas was sampled for 10 min periods, with data from the first and last minute of each period discarded. During treadmill walking, expired gas was collected for 5 min periods at 5, 15 and 25 min during the exercise bout, with the first and last minutes discarded. Resting substrate oxidation rates were calculated as per the equations of Frayn [43] and exercising substrate oxidation rates were calculated as per Jeukendrup and Wallis [44], accounting for the increased glycogen contribution to carbohydrate metabolism during moderate-intensity exercise.
Blood sampling and analysis
A cannula (Vasofix 22G, B.Braun Melsungen AG, Germany) was inserted into a vein in the antecubital fossa upon participant arrival. At regular intervals (Fig. 1), 10 ml of whole venous blood was transferred into EDTA-coated tubes (Vacutainer, Becton Dickinson, USA) and immediately centrifuged for 10 min at 1734 g and 4 °C (Allegra X-22R, Beckman Coulter, USA). Plasma was stored at − 80 °C for subsequent analysis. Fingertip capillary blood was sampled (20 µl) at corresponding time points with blood glucose concentration immediately determined (Biosen C_line analyser, EKF Diagnostics, UK). Additional samples were collected at 5 and 10 min post-meal to increase the resolution of the blood glucose curve. A commercially available ELISA (IBL International, Hamburg, Germany) was used to determine venous plasma insulin concentrations, with intra- and inter-assay variation (CV) of 7.8% and 8.8%, respectively. Enzymatic colorimetric assays were performed on an automated analyser (RX Daytona, Randox Laboratories, UK) to determine plasma glycerol and triglyceride concentrations.
Subjective appetite
Subjective appetite ratings were assessed using VAS, with a combined appetite score subsequently calculated as described previously [45]. Ratings for hunger, fullness, PFC and satisfaction were collected at corresponding time points to venous blood samples (Fig. 1), with a final VAS completed following termination of the lunch meal.
Statistical analysis
Total AUC was calculated from blood analyte and subjective appetite data for the early (0–60 min), intermediate (0–120 min) and full (0–240 min) postprandial periods using the trapezoidal method [46]. Fasting and postprandial concentrations of plasma glucose and insulin were used to calculate the Matsuda Insulin Sensitivity Index (ISI) [47]. Missing data (2.5% of all planned observations) were imputed using the linear interpolation technique. Completer only statistical analysis was performed using SPSS (version 21, IBM, USA). Glucose, insulin, triglyceride, glycerol and subjective appetite responses were analysed using two-way repeated measures analysis of variance (ANOVA) with condition and time as factors. Baseline comparisons between trials, AUC for all variables, measures of energy balance and substrate metabolism were assessed using one-way repeated measures ANOVA. Post hoc comparisons were conducted upon identification of significant main effects and were adjusted for multiple comparisons using the Bonferroni correction. The level of statistical significance was set at p < 0.05 and data are presented as mean ± standard error of the mean (SEM).
Results
Participant characteristics
In total, 15 participants were recruited to take part in the study. Three participants did not complete the protocol, with one dropping out prior to, and two following the pre-trial submaximal walking test. Participant characteristics for all participants who completed the study (n = 12) are displayed in Table 1.
Blood glucose and plasma insulin
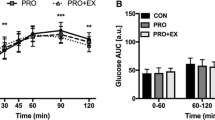
Glucose displayed a significant condition × time interaction effect (p < 0.001), time effect (p < 0.001) and main effect of condition (p = 0.009; Fig. 2a). The post-breakfast increase in glucose was reduced in EX + PRO compared with placebo trials at 15–30 min post-breakfast, and a significantly reduced peak was observed in this condition (EX + PRO: 7.6 ± 0.4 vs EX: 8.4 ± 0.3, CON: 8.3 ± 0.3 mmol l−1, p ≤ 0.04). Early postprandial glucose AUC0–60 min was significantly lower under EX + PRO when compared to EX (Fig. 2b; p = 0.011), but not significantly different from CON (p = 0.12) after correcting for multiple comparisons. Glucose was significantly lower during CON than EX + PRO and EX at 90 min, and lower than EX + PRO at 120 min post-breakfast (all p < 0.05). Values declined significantly below baseline levels after 180 min in all conditions. Over the full postprandial period (0–240 min), glycaemia was greater during EX compared with CON (p = 0.002) but not significantly higher than EX + PRO (Fig. 2b; p = 0.241).
Mean ± SEM (n = 12) temporal changes in blood glucose (a) and plasma insulin (c) concentrations, with associated AUC for glucose (b) and insulin (d). Significant differences (p < 0.05) between conditions at individual time points are defined as follows; a EX + PRO vs CON; b EX vs CON; c EX + PRO vs EX. Significant differences between bars are denoted with an asterisk. Dotted line indicates time of breakfast consumption. EX + PRO exercise with whey protein preload trial, EX exercise trial, CON resting trial
Insulin displayed a significant interaction of condition and time (p = 0.006), and main effects for time (p < 0.001) and condition (p = 0.027; Fig. 2c). Plasma insulin concentrations were not different immediately post-exercise, but were significantly greater immediately prior to breakfast in EX + PRO compared with CON (EX + PRO: 249 ± 32 vs CON: 118 ± 13 pmol l−1, p < 0.001), but not EX (151 ± 44 pmol l−1, p = 0.379). A larger peak in insulin was observed during EX + PRO compared with CON (EX + PRO: 1374 ± 602 vs CON: 1050 ± 420 pmol l−1, p = 0.004) and insulin AUC was greater during EX + PRO than CON, but not EX, during the acute (0–60 min), intermediate (0–120 min) and full (0–240 min) postprandial analyses (Fig. 2d). There were no differences observed between conditions in whole-body insulin sensitivity following breakfast consumption (Matsuda-ISI: EX + PRO: 2.3 ± 0.3, EX: 2.3 ± 0.3, CON: 2.6 ± 0.4; p = 0.344).
Plasma triglyceride and glycerol
There was no effect of condition or condition x time interaction effect on plasma triglyceride responses (Fig. 3a). There were no differences between conditions at baseline, immediately post-exercise, or immediately prior to breakfast in plasma triglyceride concentrations (p > 0.05). Following breakfast, responses were significantly affected by time (p < 0.001), such that triglyceride was significantly increased above baseline at 120–210 min post-breakfast in all conditions (Fig. 3a; all p < 0.05). AUC was similar across conditions (Fig. 3b; all p > 0.05).
Mean ± SEM (n = 12) temporal changes in plasma triglyceride (a) and glycerol (c) concentrations with associated AUC for triglyceride (b) and glycerol (d). Significant differences (p < 0.05) between conditions at individual time points are defined as follows; a EX + PRO vs CON; b EX vs CON; c EX + PRO vs EX. Significant differences between bars are denoted with an asterisk. Dotted line indicates time of breakfast consumption. EX + PRO exercise with whey protein preload trial, EX exercise trial, CON resting trial
Post-breakfast glycerol concentrations were influenced by condition (p = 0.004) and time (p < 0.001), with a condition x time interaction effect also observed (p < 0.001; Fig. 3c). Circulating glycerol concentrations did not differ between conditions at baseline, but were significantly greater in exercise trials than CON immediately post-exercise (Fig. 3b; both p ≤ 0.004), remaining elevated immediately prior to breakfast in EX (p = 0.019). AUC0–240 min was lower in EX + PRO compared to both EX (p = 0.023) and CON (p = 0.005; Fig. 3d).
Energy balance and substrate oxidation
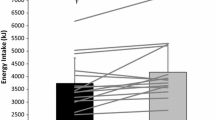
The amount of energy expended did not differ between EX + PRO and EX throughout (p > 0.05), but was greater during the exercise period in EX + PRO and EX than CON, fully accounting for the significantly greater total energy expenditure in exercise trials compared with CON (both p < 0.001; Table 2). No differences were detected between conditions in absolute energy intake at the ad libitum lunch meal (p = 0.886), signifying that participants did not compensate for the excess energy expended in exercise trials at the subsequent lunch meal. When total intake over the whole trial (breakfast, test drink and lunch) was compared, no between-condition differences were present (p = 0.491).
Rates of fat and carbohydrate oxidation did not differ between conditions at baseline (p = 0.593 and p = 0.879, respectively). Greater amounts of fat and carbohydrate were utilised over the course of each exercise trial in comparison to resting control (all p < 0.05), however, substrate metabolism was not influenced by consumption of whey protein, with similar fat and carbohydrate oxidation observed between EX + PRO and EX throughout (all p > 0.05; Table 2).
Subjective appetite ratings
A significant effect of time on combined appetite responses was observed (p < 0.001). Appetite decreased similarly following breakfast in all trials, returning to baseline levels at 90–240 min post-breakfast, before decreasing similarly after the ad libitum lunch meal (Fig. 4a). There was no difference in AUC for combined appetite score (Fig. 4b) or individual components of subjective appetite under all conditions (all p > 0.05).
Discussion
The main finding observed was that fasted exercise followed by pre-breakfast whey protein supplementation improved early postprandial glycaemia. This was illustrated by a reduction in the post-breakfast glucose peak in comparison to both placebo trials, and lower glucose AUC(0–60 min) when compared to exercise alone. In addition, it is shown that fasted exercise in conjunction with post-exercise whey protein supplementation does not influence subsequent energy intake in centrally obese males.
An acute bout of exercise moderately impaired glucose tolerance, indicated by a higher glucose AUC0–240 min in the exercise control compared to resting control, while insulin AUC remained unchanged. Glucose peak and early AUC were not different between these conditions, indicating that this effect was manifested by a more prolonged elevation in blood glucose after prior exercise. When whey protein was ingested following exercise, this impairment appears to be negated, with glycaemia not differing from control overall. Additionally, glycaemia was ~ 9% lower in the early (0–60 min) period following breakfast when compared to exercise without additional protein, whilst peak postprandial glucose was significantly reduced compared to other conditions.
The increased glycaemic response immediately following a bout of exercise is in accordance with previous findings in healthy trained [48] and obese normoglycaemic males [49]. Rose et al. [48] observed a 30% elevation in glucose appearance during an OGTT following 30 min of cycling exercise, albeit at a higher intensity (70% \({{\dot{V}O}}_{\text{2peak}}\)) than the current study. The counterregulatory response to sustained exercise (more than ~ 20 min) involves increases in glucagon, catecholamines, and cortisol secretion, among other hormones [50], which may result in a rate of glucose appearance [51] which exceeds disappearance [52]. Exercise-induced elevation in catecholamine levels has also been shown to enhance the appearance of orally ingested glucose in an animal model via stimulation of sodium-glucose linked transporter type 1 (SGLT1) [53], whilst increased postprandial splanchnic perfusion after exercise may also explain increased glucose absorption [54]. It is currently unclear how long this effect persists for, however, it is likely to be transient in nature [49], and blood glucose levels were similar between exercise and control trials beyond 120 min post-breakfast in the current study. Moreover, should obese individuals take part in regular exercise training, then improvements in aerobic fitness and potential weight loss are likely to have independent effects on improving glucose metabolism [20, 55]. Nevertheless, knowledge of the acute effect of exercise on glucose tolerance when food is ingested immediately or shortly after exercise, is of significance when aiming to ameliorate the adverse effects of postprandial hyperglycaemia on metabolic health [56, 57].
The observed reduction in acute glycaemia following post-exercise whey protein supplementation could be attributed to a combination of mechanisms, including the direct effects of amino acids, particularly leucine, on β-cell stimulation [58] and activation of the incretin response [59]. Circulating insulin was increased 15 min after consumption of the whey preload, coinciding with the timing of breakfast consumption. The post-breakfast rise in insulin secretion also occurred earlier in the EX + PRO trial, with higher concentrations observed at 15 min post-breakfast compared with both non-protein conditions. The post-breakfast elevation in EX + PRO persists for up to 90 min, without further reductions in postprandial glycaemia. Increased postprandial insulinaemia in the absence of a reduction in glycaemia is suggestive of compromised insulin sensitivity, an effect that has previously been observed following acute whey protein ingestion [60]. Thus, there is potential for the chronic exposure to the insulinotrophic effects of whey protein to have a desensitising effect on sites of insulin secretion or action, however, the implementation of longer-term supplementation protocols are required to investigate this potential effect. As the acute glucose response was attenuated in EX + PRO compared with EX, in the context of a potentially increased rate of post-exercise glucose absorption, this is suggestive of delayed gastrointestinal transport of orally ingested glucose. The rate of gastric emptying exerts considerable influence on the magnitude of postprandial glucose excursions [61, 62], accounting for ~ 35% of variance in glycaemic response to carbohydrate-containing meals in healthy individuals [63].
Large fluctuations in circulating glucose concentrations have deleterious effects on endothelial function and oxidative stress in both healthy individuals and T2DM patients [57]. The attenuation of peak glucose excursion compared to both exercise and resting control conditions in the current study may, therefore, indicate a role for pre-meal supplementation of whey protein both at rest, and when consuming meals shortly after bouts of low–moderate-intensity exercise, in obese individuals. The stimulus was not large enough to detect a reduction in glycaemia over the full postprandial period, however, it was sufficient to negate the increase observed in post-exercise glycaemia without additional whey protein.
There was no effect of whey protein supplementation on postprandial triglyceride in the current study. This reflects previous findings when similar doses of whey protein have been administered prior to the same breakfast meal [24] or meals with considerably higher fat loads [64,65,66], albeit without prior exercise. Conversely, studies administering considerably higher doses of whey protein (45 g) alongside high fat loads (80 g) have shown significant reductions in postprandial lipaemia [67, 68]. The present study was designed to reflect practical application, thus the amount of whey protein and the composition of test meals were realistic in the context of habitual eating habits [69].
Elevated glycerol concentrations immediately post-exercise are indicative of increased lipolysis and lipid substrate availability for exercise, confirmed by the observed increase in fat oxidation during exercise. Increased fat oxidation is associated with the postprandial triglyceride-lowering effects of exercise [70], however, postprandial substrate utilisation was not significantly different between exercise and control trials in the present study, and triglyceride responses were similar between conditions. A large body of evidence implicates exercise in the attenuation of postprandial lipaemia [19, 23], however, the clear majority of studies have administered a test meal > 4 h after cessation of exercise. Those studies that have shown a more acute effect have administered meals considerably greater in fat content (> 95 g) and prescribed a greater workload during aerobic exercise bouts than the current study [71, 72]. Furthermore, replacement of the exercise-induced energy deficit via mixed-macronutrient [73] or carbohydrate [74] feeding attenuates or abolishes improvements in postprandial triglyceridaemia. In the current study, participants consumed breakfast containing more than double the amount of energy expended during treadmill walking, which may account for the similar lipaemic responses in exercise and resting conditions. It has also been observed that consumption of a meal immediately after exercise diminishes the shift from carbohydrate to fat oxidation that usually follows exercise [75], which may explain the lack of significant differences in substrate utilisation in resting and exercise conditions in this study. Although it could be speculated that the intensity of the exercise bout was not high enough to have a prolonged metabolic effect, it has previously been established that total energy expenditure rather than intensity is of primary importance to the triglyceride-lowering effect of prior exercise [19].
Appetite was unaffected by prior exercise in the current study, which appears to be consistent with previous evidence suggesting that appetite is not altered by acute moderate-intensity exercise [76]. In accordance with the comparable subjective appetite responses, lunch meal energy intake 4 h post-breakfast was similar between conditions. The fact that participants did not compensate for the deficit created by prior exercise reflects the findings of the majority of exercise studies [77] and brisk walking protocols [78] in lean, healthy individuals, whilst limited evidence exists to support a similar tendency in obese individuals. The moderate energy deficit created by the exercise bout in the present study, in addition to the short duration between exercise and consumption of a standardised breakfast, is likely to have influenced this response. It may also be a possibility that energy expenditure from exercise is gradually compensated for over several meals or even days, however such compensation is likely to only partially account for energy expended [79]. Nevertheless, the difference in energy balance in the exercise control trial compared with resting control was equivalent to the net amount expended during exercise, highlighting the potential efficacy of brisk walking to create acute energy deficits in obese individuals.
A limitation of the current study is the lack of a non-exercise whey protein condition, which makes uncoupling of the effects of whey protein and prior exercise on glycaemia problematic. Whether the blood glucose response would be further reduced by consuming a whey protein preload in the absence of prior exercise can only be speculated, however, we have previously observed reductions in peak glucose and postprandial glycaemia at rest following whey protein consumption prior to the same breakfast meal in centrally obese males [24]. Furthermore, implementation of a longer investigation period may have been warranted, as the transient effects of a single bout of exercise on insulin sensitivity may last for up to 72 h [21], indicating that these beneficial effects may occur beyond the time frame examined here. The timing of post-exercise feeding may have limited the ability to identify significant effects of exercise on postprandial lipaemia and subsequent intake, however, the consumption of a meal or snack following fasted morning exercise is likely, ensuring that these findings hold relevance in a free-living setting. The fact that the study was ran in a single-blind fashion may also be considered a limitation, however, efforts were made to eliminate sources of bias including analysing all samples from a participant on a single run where possible, and employing an additional (blinded) researcher to independently measure appetite responses to verify the measurements made by the primary researcher.
The use of paired t tests to conduct pairwise comparisons for differences within main effects may be considered a limitation due to the assumption that systematic differences in responses between testing visits are absent. This was mitigated as far as possible by including a considerable wash out period between visits of 7 days, in addition to counterbalancing trial order regimens. Additionally, the study was powered to detect differences in the primary outcome of postprandial glycaemia, with the consequent likelihood that secondary outcomes may have been underpowered to detect differences.
The ecological validity of these findings is reinforced by utilising a dose of protein that could realistically be supplemented prior to a meal, along with an exercise load that is realistic and tolerable in the population of interest. Care was also taken to use foods that were typical of those consumed at breakfast and lunch meals across the population. Whilst the effects of high levels of protein intake on health is of interest, current evidence suggests that whey protein supplementation and higher total protein is not detrimental to bone health [80]. Acute trials such as the current study provide valuable information regarding the effects of whey protein consumption on immediate post-meal responses, however, consideration should be given to the fact that prevention of deteriorating metabolic health may require chronic improvements in postprandial glycaemia and other markers, which cannot be observed in the acute laboratory setting. Studies assessing the longer term effects of whey supplementation are relatively few in number, with only a small number in overweight/obese [81,82,83] or diabetic [84] individuals. Although some inconsistencies are apparent, the limited evidence to date appears to show that chronic supplementation of the diet with whey protein is associated with metabolic health benefits including improved fasting lipid profile and insulin sensitivity, with possible effects on food intake and body mass. There is increasing focus on non-pharmaceutical methods and functional foods in this area, and such work may be advanced through development of an optimal chronic supplementation strategy, while development of food products incorporating whey protein may enhance adherence to supplementation.
In summary, an isolated bout of brisk walking exercise moderately impaired post-exercise glucose tolerance, however a whey protein preload consumed immediately post-exercise negates this effect. Furthermore, acute postprandial glycaemia was attenuated following whey protein consumption, whilst energy intake at a later lunch meal was not influenced by the intervention.
References
Atawia RT, Bunch KL, Toque HA, Caldwell RB, Caldwell RW (2019) Mechanisms of obesity-induced metabolic and vascular dysfunctions. Front Biosci (Landmark Ed) 24:890–934
Boden G (2011) Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes 18:139–143
Kwon H, Pessin J (2013) Adipokines mediate inflammation and insulin resistance. Front Endocrinol 4:71
Ouchi N, Parker JL, Lugus JJ, Walsh K (2011) Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11:85–97
Item F, Konrad D (2012) Visceral fat and metabolic inflammation: the portal theory revisited. Obes Rev 13:30–39
Hamdy O, Porramatikul S, Al-Ozairi E (2006) Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev 2:367–373
Bano G (2013) Glucose homeostasis, obesity and diabetes. Best Pract Res Clin Obstet Gynaecol 27:715–726
Tchernof A, Després J-P (2013) Pathophysiology of human visceral obesity: an update. Physiol Rev 93:359–404
Monnier L, Colette C, Dunseath GJ, Owens DR (2007) The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care 30:263–269
Woerle HJ, Pimenta WP, Meyer C et al (2004) Diagnostic and therapeutic implications of relationships between fasting, 2-hour postchallenge plasma glucose and hemoglobin a1c values. Arch Intern Med 164:1627–1632
Bianchi C, Miccoli R, Penno G, Del Prato S (2008) Primary prevention of cardiovascular disease in people with dysglycemia. Diabetes Care 31:S208–S214
Coutinho M, Gerstein HC, Wang Y, Yusuf S (1999) The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 22:233–240
Gerich JE (2003) Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch Intern Med 163:1306–1316
Ning F, Zhang L, Dekker JM, Onat A, Stehouwer CD, Yudkin JS, Laatikainen T, Tuomilehto J et al (2012) Development of coronary heart disease and ischemic stroke in relation to fasting and 2-hour plasma glucose levels in the normal range. Cardiovasc Diabetol 11:76
Fava S (2008) Role of postprandial hyperglycemia in cardiovascular disease. Expert Rev Cardiovasc Ther 6:859–872
Kolovou GD, Mikhailidis DP, Kovar J, Lairon D, Nordestgaard BG, Ooi TC, Perez-Martinez P, Bilianou H et al (2011) Assessment and clinical relevance of non-fasting and postprandial triglycerides: an expert panel statement. Curr Vasc Pharmacol 9:258–270
Francois ME, Baldi JC, Manning PJ, Lucas SJE, Hawley JA, Williams MJA, Cotter JD (2014) ‘Exercise snacks’ before meals: a novel strategy to improve glycaemic control in individuals with insulin resistance. Diabetologia 57:1437–1445
Rynders CA, Weltman JY, Jiang B, Breton M, Patrie J, Barrett EJ, Weltman A (2014) Effects of exercise intensity on postprandial improvement in glucose disposal and insulin sensitivity in prediabetic adults. J Clin Endocrinol Metab 99:220–228
Maraki M, Sidossis L (2013) The latest on the effect of prior exercise on postprandial lipaemia. Sports Med 43:463–481
Borghouts LB, Keizer HA (2000) Exercise and insulin sensitivity: a review. Int J Sports Med 21:1–12
Colberg SR, Albright AL, Blissmer BJ, Braun B, Chasan-Taber L, Fernhall B, Regensteiner JG, Rubin RR et al (2010) Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: Joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc 42:2282–2303
Röhling M, Herder C, Stemper T, Müssig K (2016) Influence of acute and chronic exercise on glucose uptake. J Diabetes Res 2016:33
Freese EC, Gist NH, Cureton KJ (2014) Effect of prior exercise on postprandial lipemia: an updated quantitative review. J Appl Physiol 116:67–75
Allerton DM, Rumbold PLS, West DJ, Stevenson EJ (2019) Effect of supplemental whey protein timing on postprandial glycaemia in centrally obese males. Br J Nutr 121:637–646
Akhavan T, Luhovyy BL, Panahi S, Kubant R, Brown PH, Anderson GH (2014) Mechanism of action of pre-meal consumption of whey protein on glycemic control in young adults. J Nutr Biochem 25:36–43
Jakubowicz D, Froy O, Ahren B, Boaz M, Landau Z, Bar-Dayan Y, Ganz T, Barnea M et al (2014) Incretin, insulinotropic and glucose-lowering effects of whey protein pre-load in type 2 diabetes: a randomised clinical trial. Diabetologia 57:1807–1811
King DG, Stevenson EJ, Walker M, West DJ, Campbell MD, Breen L (2018) A small dose of whey protein co-ingested with mixed-macronutrient breakfast and lunch meals improves postprandial glycemia and suppresses appetite in men with type 2 diabetes: a randomized controlled trial. Am J Clin Nutr 107:550–557
Ma J, Stevens JE, Cukier K, Maddox AF, Wishart JM, Jones KL, Clifton PM, Horowitz M et al (2009) Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 32:1600–1602
Grundy SM (2012) Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol 59:635–643
Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD (2014) Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr 99:86–95
Hayes A, Cribb PJ (2008) Effect of whey protein isolate on strength, body composition and muscle hypertrophy during resistance training. Curr Opin Clin Nutr Metab Care 11:40–44
Naclerio F, Larumbe-Zabala E (2016) Effects of whey protein alone or as part of a multi-ingredient formulation on strength, fat-free mass, or lean body mass in resistance-trained individuals: a meta-analysis. Sports Med 46:125–137
Rumbold P, Shaw E, James L, Stevenson E (2015) Milk consumption following exercise reduces subsequent energy intake in female recreational exercisers. Nutrients 7:293–305
Clayton DJ, Stensel DJ, Watson P, James LJ (2014) The effect of post-exercise drink macronutrient content on appetite and energy intake. Appetite 82:173–179
Gonzalez JT (2014) Paradoxical second-meal phenomenon in the acute postexercise period. Nutrition 30:961–967
Stevenson EJ, Allerton DM (2018) The role of whey protein in postprandial glycaemic control. Proc Nutr Soc 77:42–51
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP et al (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120:1640–1645
Hagströmer M, Oja P, Sjöström M (2006) The international physical activity questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr 9:755–762
Pal S, Ellis V, Ho S (2010) Acute effects of whey protein isolate on cardiovascular risk factors in overweight, post-menopausal women. Atherosclerosis 212:339–344
Borg G (1990) Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health 16:55–58
National Institute for Health and Care Excellence (2014) Obesity: Identification, assessment and management of overweight and obesity in children, young people and adults. National Clinical Guideline Centre, London
Gonzalez JT, Green BP, Brown MA, Rumbold PL, Turner LA, Stevenson EJ (2015) Calcium ingestion suppresses appetite and produces acute overcompensation of energy intake independent of protein in healthy adults. J Nutr 145:476–482
Frayn KN (1983) Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55:628–634
Jeukendrup AE, Wallis GA (2005) Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sports Med 26:S28–S37
Gonzalez JT, Stevenson EJ (2014) Calcium co-ingestion augments postprandial glucose-dependent insulinotropic peptide(1–42), glucagon-like peptide-1 and insulin concentrations in humans. Eur J Nutr 53:375–385
Matthews JN, Altman DG, Campbell MJ, Royston P (1990) Analysis of serial measurements in medical research. BMJ 300:230–235
Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470
Rose AJ, Howlett K, King DS, Hargreaves M (2001) Effect of prior exercise on glucose metabolism in trained men. Am J Physiol Endocrinol Metab 281:E766–771
Knudsen SH, Karstoft K, Pedersen BK, van Hall G, Solomon TPJ (2014) The immediate effects of a single bout of aerobic exercise on oral glucose tolerance across the glucose tolerance continuum. Physiol Rep 2:e12114
Camacho RC, Galassetti P, Davis SN, Wasserman DH (2005) Glucoregulation during and after exercise in health and insulin-dependent diabetes. Exerc Sport Sci Rev 33:17–23
Holloszy JO, Kohrt WM (1996) Regulation of carbohydrate and fat metabolism during and after exercise. Annu Rev Nutr 16:121–138
Wasserman DH (1995) Regulation of glucose fluxes during exercise in the postabsorptive state. Annu Rev Physiol 57:191–218
Aschenbach JR, Borau T, Gäbel G (2002) Glucose uptake via SGLT-1 is stimulated by β2-adrenoceptors in the ruminal epithelium of sheep. J Nutr 132:1254–1257
Maehlum S, Felig P, Wahren J (1978) Splanchnic glucose and muscle glycogen metabolism after glucose feeding during postexercise recovery. Am J Physiol Endocrinol Metab 235:E255–E260
Coker RH, Williams RH, Yeo SE, Kortebein PM, Bodenner DL, Kern PA, Evans WJ (2009) The impact of exercise training compared to caloric restriction on hepatic and peripheral insulin resistance in obesity. J Clin Endocrinol Metab 94:4258–4266
Monnier L, Colette C (2015) Postprandial and basal hyperglycaemia in type 2 diabetes: Contributions to overall glucose exposure and diabetic complications. Diabetes Metab 41:6S9–6S15
Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D (2008) Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 57:1349–1354
Gao Z, Young RA, Li G, Najafi H, Buettger C, Sukumvanich SS, Wong RK, Wolf BA et al (2003) Distinguishing features of leucine and α-ketoisocaproate sensing in pancreatic β-cells. Endocrinology 144:1949–1957
Salehi A, Gunnerud U, Muhammed SJ, Ostman E, Holst JJ, Bjorck I, Rorsman P (2012) The insulinogenic effect of whey protein is partially mediated by a direct effect of amino acids and gip on beta-cells. Nutr Metab 9:48
Smith GI, Yoshino J, Stromsdorfer KL, Klein SJ, Magkos F, Reeds DN, Klein S, Mittendorfer B (2015) Protein ingestion induces muscle insulin resistance independent of leucine-mediated mtor activation. Diabetes 64:1555–1563
Marathe CS, Rayner CK, Jones KL, Horowitz M (2013) Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care 36:1396–1405
Mignone LE, Wu T, Horowitz M, Rayner CK (2015) Whey protein: The “whey” forward for treatment of type 2 diabetes? World J Diabetes 6:1274–1284
Horowitz M, Edelbroek MAL, Wishart JM, Straathof JW (1993) Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 36:857–862
Bjørnshave A, Hermansen K, Holst J (2018) Pre-meal effect of whey proteins on metabolic parameters in subjects with and without type 2 diabetes: a randomized, crossover trial. Nutrients 10:122
Bjørnshave A, Johansen TN, Amer B, Dalsgaard TK, Holst JJ, Hermansen K (2019) Pre-meal and postprandial lipaemia in subjects with the metabolic syndrome: effects of timing and protein quality (randomised crossover trial). Br J Nutr 121:312–321
Bjørnshave A, Holst JJ, Hermansen K (2019) A pre-meal of whey proteins induces differential effects on glucose and lipid metabolism in subjects with the metabolic syndrome: a randomised cross-over trial. Eur J Nutr 58:755–764
Holmer-Jensen J, Mortensen LS, Astrup A, de Vrese M, Holst JJ, Thomsen C, Hermansen K (2013) Acute differential effects of dietary protein quality on postprandial lipemia in obese non-diabetic subjects. Nutr Res 33:34–40
Mortensen LS, Hartvigsen ML, Brader LJ, Astrup A, Schrezenmeir J, Holst JJ, Thomsen C, Hermansen K (2009) Differential effects of protein quality on postprandial lipemia in response to a fat-rich meal in type 2 diabetes: Comparison of whey, casein, gluten, and cod protein. Am J Clin Nutr 90:41–48
Reeves S, Halsey LG, McMeel Y, Huber JW (2013) Breakfast habits, beliefs and measures of health and wellbeing in a nationally representative UK sample. Appetite 60:51–57
Trombold JR, Christmas KM, Machin DR, Van Pelt DW, Chou T-H, Kim I-Y, Coyle EF (2014) Postexercise macronutrient intake and subsequent postprandial triglyceride metabolism. Med Sci Sports Exerc 46:2099–2106
Katsanos CS, Moffatt RJ (2004) Acute effects of premeal versus postmeal exercise on postprandial hypertriglyceridemia. Clin J Sport Med 14:33–39
Plaisance EP, Mestek ML, Mahurin AJ, Taylor JK, Moncada-Jimenez J, Grandjean PW (2008) Postprandial triglyceride responses to aerobic exercise and extended-release niacin. Am J Clin Nutr 88:30–37
Burton FL, Malkova D, Caslake MJ, Gill JMR (2008) Energy replacement attenuates the effects of prior moderate exercise on postprandial metabolism in overweight/obese men. Int J Obes 32:481–489
Harrison M, O'Gorman DJ, McCaffrey N, Hamilton MT, Zderic TW, Carson BP, Moyna NM (2009) Influence of acute exercise with and without carbohydrate replacement on postprandial lipid metabolism. J Appl Physiol 106:943–949
Dionne I, Van Vugt S, Tremblay A (1999) Postexercise macronutrient oxidation: a factor dependent on postexercise macronutrient intake. Am J Clin Nutr 69:927–930
Blundell JE, King NA (2000) Exercise, appetite control, and energy balance. Nutr Diabetes 16:519–522
Martins C, Morgan L, Truby H (2008) A review of the effects of exercise on appetite regulation: an obesity perspective. Int J Obes 32:1337–1347
King JA, Wasse LK, Broom DR, Stensel DJ (2010) Influence of brisk walking on appetite, energy intake, and plasma acylated ghrelin. Med Sci Sports Exerc 42:485–492
Blundell JE, Gibbons C, Caudwell P, Finlayson G, Hopkins M (2015) Appetite control and energy balance: impact of exercise. Obes Rev 16:67–76
Wright CS, McMorrow AM, Weinheimer-Haus EM, Campbell WW (2016) Whey protein supplementation and higher total protein intake do not influence bone quantity in overweight and obese adults following a 36-week exercise and diet intervention. J Nutr 147:179–186
Pal S, Radavelli-Bagatini S, Hagger M, Ellis V (2014) Comparative effects of whey and casein proteins on satiety in overweight and obese individuals: a randomized controlled trial. Eur J Clin Nutr 68:980–986
Pal S, Ellis V, Dhaliwal S (2010) Effects of whey protein isolate on body composition, lipids, insulin and glucose in overweight and obese individuals. Br J Nutr 104:716–723
Gouni-Berthold I, Schulte DM, Krone W, Lapointe JF, Lemieux P, Predel HG, Berthold HK (2012) The whey fermentation product malleable protein matrix decreases tag concentrations in patients with the metabolic syndrome: a randomised placebo-controlled trial. Br J Nutr 107:1694–1706
Ma J, Jesudason DR, Stevens JE, Keogh JB, Jones KL, Clifton PM, Horowitz M, Rayner CK (2015) Sustained effects of a protein ‘preload’ on glycaemia and gastric emptying over 4 weeks in patients with type 2 diabetes: a randomized clinical trial. Diabetes Res Clin Pract 108:e31–e34
Funding
This research was funded through a Northumbria University funded PhD scholarship. Whey protein was provided by Arla Food Ingredients Group, Viby, Denmark.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DJW and EJS have previously received funding from Arla Food Ingredients Group for their research.
Ethics approval
This research was conducted with the prior approval of the Faculty of Health and Life Sciences Research Ethics Committee, Northumbria University. The study was conducted in accordance with the Declaration of Helsinki.
Consent to participate
Written informed consent was provided by all participants prior to participation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Allerton, D.M., West, D.J. & Stevenson, E.J. Whey protein consumption following fasted exercise reduces early postprandial glycaemia in centrally obese males: a randomised controlled trial . Eur J Nutr 60, 999–1011 (2021). https://doi.org/10.1007/s00394-020-02304-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02304-2