Abstract
Background
Abnormalities in glucose and lipid metabolism contribute to the progression and exacerbation of type 2 diabetes mellitus (T2DM). Fish oil and probiotics are dietary supplements that have the potential to improve glucose and lipid metabolism. However, their efficacy remains unclear in T2DM patients.
Methods
PubMed, Embase, and the Cochrane Library were retrieved to collect randomized controlled trials (RCTs) on the efficacy of fish oil or probiotic supplementation in T2DM patients from the database inception to December 13, 2023. Primary outcome indicators encompassed glycated hemoglobin (HbA1c), homeostatic model assessment for insulin resistance (HOMA-IR) and blood lipid profile (triglyceride (TG) and total cholesterol (TC). Secondary outcome indicators included inflammatory markers such as tumor necrosis factor -α (TNF-α) and adipocytokine (including leptin and adiponectin). The R software was used for statistical analysis, and GraphPad Prism was used for figure rendering.
Results
A total of 60 RCTs involving 3845 T2DM patients were included in the analysis. The results showed that the probiotics (Bifidobacterium, Lactobacillus, Lactococcus, Propionibacterium, etc.) were more effective in reducing HOMA-IR than fish oil (Surca = 0.935). Bifidobacterium demonstrated the highest efficacy in reducing HbA1c levels (Surca = 0.963). Regarding lipid metabolism, fish oil was superior to probiotics in lowering TG and TC levels (Surca values of 0.978 and 0.902, respectively). Furthermore, fish oil outperformed probiotics in reducing TNF-α (Surca = 0.839) and leptin (Surca = 0.712), and increasing adiponectin levels (Surca = 0.742). Node-splitting analysis showed good consistency (P > 0.05 for direct, indirect, and network comparison across various interventions).
Conclusions
In T2DM patients, fish oil was more effective than probiotics in regulating lipid metabolism. Probiotics outperformed fish oil in regulating glucose metabolism particularly; specifically, Bifidobacterium showed higher efficacy in reducing blood glucose.
Similar content being viewed by others
Introduction
The global prevalence and mortality rate of type 2 diabetes mellitus (T2DM) have been increasing, partly attributed to high sugar and fat diets. T2DM, if poorly managed, often causes such complications as macrovascular, microvascular, and neuropathies [1]. Therefore, effective management to improve glucose and lipid metabolism is crucial for T2DM patients. Although drug therapies and lifestyle interventions (e.g., low carbohydrate diet [2]) are the main strategies to control T2DM, the demand for dietary supplements increases due to their beneficial effects on maintaining or improving metabolic functions, particularly in patients with diabetes mellitus [3]. Fish oil and probiotics are two major supplements that can improve conditions related to digestive system [4, 5], neurological diseases [6], and T2DM [7,8,9,10,11].
Despite growing interest in dietary supplements, the relative efficacy of fish oil and probiotic supplements on glucose and lipid metabolism remains elusive in people with T2DM. Therefore, this systematic review and network meta-analysis (NMA) aims to evaluate the relative efficacy of fish oil and probiotics in improving glucose and lipid metabolism in T2DM patients based on available randomized controlled trials (RCTs). The primary goals are to close current research gaps, offer more informative guidance for the clinical treatment of T2DM, and provide a scientific basis for the development of dietary supplement therapies for T2DM.
Methods
This study was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for systematic Evaluation and Meta-Analysis [12]. The study protocol has been registered in the International Prospective Systems Evaluation Register (PROSPERO) (Registration no. CRD42023407998).
Search strategies
PubMed, Embase, and Cochrane Library were retrieved to collect relevant RCTs. The search strategy was designed based on the combination of MeSH terms and free words. The detailed search strategy is shown in Additional file 1: Table S1. Additionally, the reference lists of previous systematic reviews and meta-analyses were searched to determine potentially eligible studies.
Research selection
The inclusion criteria were designed in strict accordance with the PICOS principle: (1) Population: adult patients were clearly diagnosed with T2DM based on World Health Organization 1999 and American Diabetes Association criteria [13]. No restrictions were imposed on nationality or race; (2) Intervention: The experimental group received fish oil, docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), one or more probiotics; (3) Comparison: The control group received vegetable oil, mineral oil, or placebo, regardless of doses and course of administration. If a study involved different study duration periods, the longest one was taken as the standard; if different doses were used, the largest one was taken as the standard; (4) Outcome: Studies had to report at least of the following outcome indicators: glycated hemoglobin (HbA1c), homeostatic model assessment for insulin resistance (HOMA-IR), triglyceride (TG), total cholesterol (TC), tumor necrosis factor-α (TNF-α), leptin, adiponectin; (5) Study design: The studies had to be RCTs. The following studies were excluded: (1) review, systematic evaluation, abstract, conference, retrospective study, cross-sectional study; (2) Studies that reported no relevant outcome indicators or no data could be extracted; (3) animal and cell tests; (4) Studies on patients with gestational diabetes; (5) non-English studies.
Two researchers (QF and YO) independently screened the studies. The titles and abstracts were checked to select potentially eligible articles. Then, a full-text review was conducted to identify eligible articles. Any dissents were resolved by a third researcher (PN).
Data extraction
Data were independently extracted by two researchers (MZ and FY) using a predesigned spreadsheet. The extracted data included author, publication year, country, sample size, mean age, comparison and treatment details (fish oil and probiotics type, placebo), outcome indicators (HbA1c, HOMA-IR, TG, TC, TNF-α, leptin, adiponectin). Any inconsistencies in their results were adjudicated by a third researcher (PN).
Risk-of-bias assessment
Two researchers (HW and HC) independently employed the Cochrane Risk Bias Tool version 2.0 to evaluate the risk of bias in the included studies, involving random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias. The study quality was rated as low risk of bias, some concerns, and high risk of bias. Any dissents between the two researchers were resolved by a third researcher (JZ).
Data analysis
R version 4.3.1 (R Core Team, Vienna, Austria) was used for statistical analysis, and GraphPad Prism version 9.4.1 (GraphPad Software, San Diego, USA) was used for figure plotting. A network diagram was drawn to show all the available evidence for each intervention. The heterogeneity was determined by the I2 statistic. An I2 ≤ 50% indicated small or no heterogeneity between studies, and the fixed-effects model was employed; otherwise, the random-effects model was adopted [14]. The number of tuning and simulation iterations was set at 5000 and 20,000, respectively. The results were presented as mean difference (MD) with 95% confidence intervals (CIs), and the data were not statistically significant when the 95% CI value contained 0. The surface under the cumulative ranking curve (SUCRA) was used to calculate the probability of each intervention becoming the best intervention. The SUCRA value ranges from 0 to 1. A higher SUCRA value indicated a greater possibility of a treatment method becoming the most effective intervention [15]. To establish a network closed-loop structure, a node-splitting analysis was employed to evaluate the consistency of direct, indirect, and network comparisons across various interventions. The included studies were categorized into two groups based on intervention duration: < 12 weeks and ≥ 12 weeks. Then, network meta-analysis was performed within the groups. A value of α = 0. 05 was considered statistically significant.
Results
Research selection and characteristics
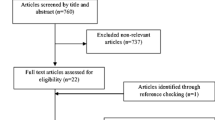
A total of 828 articles were identified in the initial database search from the database inception to December 13, 2023. Besides, the reference lists of previous systematic reviews and meta-analyses were also manually searched, and additional 3 eligible articles were found. Furthermore, 555 papers were left after eliminating duplicate papers. Then, we excluded 15 nonhuman studies or non-English studies, 240 non-RCT or cross-RCT studies, 228 articles that did not conform to the PICO principles of the study, and 12 duplicate publications. Finally, 60 RCTs (Fig. 1) involving 3845 T2DM patients were included in the analysis. The baseline characteristics of the included studies are shown in Tables 1 and 2.
Risk of bias in studies
The risk of bias in the included RCTs was assessed. Among the 60 included studies, 31 studies did not specifically describe the generation of random sequences; 26 studies did not specifically describe the allocation concealment; 9 did not specifically describe the method of blinding participants and implementers; 13 did not specifically describe the blinding of the outcome measurement; 8 studies contained incomplete data; 6 studies selectively reported their results; and 12 studies possessed other biases. The risk of bias in the included studies is illustrated in Fig. 2.
Glucose metabolism
A total of 20 RCTs investigated the effect of fish oil and probiotics on HOMA-IR. The network diagram is shown in Fig. 3A. Due to low heterogeneity, the fixed effect model was used (I2 = 24%). According to SURCA analysis (ranking Additional file 1: Table S2 and column chart Fig. 4A) and League Table 3A), the combination of Bifidobacterium, Lactobacillus, Lactococcus, and Propionibacterium was the most effective in reducing HOMA-IR (SURCA = 0.935), followed by the combination of Bifidobacterium and Lactobacillus (Surca = 0.722) and Lactobacillus alone (Surca = 0.675). Additionally, all types of probiotics were more effective than fish oil in lowering HOMA-IR. Furthermore, 41 RCTs explored the effects of fish oil and probiotics on HbA1C. The network diagram is shown in Fig. 3B. Due to low heterogeneity (I2 = 45%), the fixed effect model was adopted. According to SUCRA analysis (ranking Additional file 1: Table S2 and column chart Fig. 4B) and league table (Table 3B), Bifidobacterium (Surca = 0.963) was the most effective in reducing HbA1c, followed by the combination of Bifidobacterium and Lactobacillus (Surca = 0.840) and the combination of Bifidobacterium, Lactobacillus, and Streptococcus (Surca = 0.729).
Lipid metabolism
A total of 41 RCTs reported TG. The network diagram is shown in Fig. 3C. Owing to low heterogeneity (I2 = 37%), the fixed effect model was employed. According to SUCRA analysis (ranking Additional file 1: Table S2, column chart Fig. 4C) and league Table 3C, fish oil was the most effective in reducing TG (Surca = 0.978), followed by DHA (Surca = 0.844) and the combination of Bifidobacterium, Lactobacillus, and Lactococcus (Surca = 0.783). Furthermore, 43 RCTs analyzed the effects of fish oil and probiotics on TC. The network diagram is shown in Fig. 3D. Due to low heterogeneity (I2 = 30%), the fixed effect model was adopted. According to SUCRA results (ranking Additional file 1: Table S2 and column chart Fig. 4D) and League Table 3D), mineral oil (Surca = 0.902) had the best efficacy in reducing TC, followed by fish oil (Surca = 0.857) and the combination of Bifidobacterium and Lactobacillus (Surca = 0.803).
Inflammatory markers
Seven RCTs reported inflammatory markers TNF-α, as shown in Additional file 1: Figure S1A. With low heterogeneity (I2 = 33%), the fixed effect model was adopted. According to SUCRA analysis (ranking Additional file 1: Table S2 and column chart Additional file 1: Fig. S2A) and League Additional file 1: Table S3A), fish oil (Surca = 0.839) was the most effective in reducing TNF-α, followed by mineral oil (Surca = 0.611) and Lactobacillus (Surca = 0.495).
Adipocytokine
A total of 3 RCTs investigated the effects of fish oil and probiotics on leptin. The network diagram is shown in Additional file 1: Fig. S1B. With low heterogeneity (I2 = 16%), the fixed effect model was adopted. According to SUCRA analysis (ranking Additional file 1: Table S2 and column chart Fig. S2B) and League Additional file 1: Table S3B, fish oil (Surca = 0.712) was the most effective in reducing leptin levels, followed by mineral oil (Surca = 0.514) and Lactobacillus (Surca = 0.401). Moreover, a total of 8 RCTs explored the effects of fish oil and probiotics on adiponectin. The network diagram is shown in Additional file 1: Fig. S1C. With no heterogeneity (I2 = 0%), the fixed-effect model was adopted. According to SUCRA analysis (ranking Additional file 1: Table S2 and column chart Additional file 1: Fig. S2C) and League Additional file 1: Table S3C, fish oil (Surca = 0.742) was the most effective in increasing adiponectin levels, followed by Lactobacillus (Surca = 0.566) and vegetable oil (Surca = 0.469).
Consistency check
Due to the absence of a network closed-loop structure for all secondary outcome measures, node-splitting analysis was employed only for the primary outcome measure. The analysis was conducted on selected interventions, including fish oil, EPA, mineral oil, vegetable oil, placebo, the combination of Bifidobacterium and Lactobacillus, the combination of Lactobacillus and Streptococcus, the combination of Bifidobacterium, Lactobacillus, and Streptococcus. The analysis showed that the P-values for direct, indirect, and network comparisons were greater than 0.05 (Fig. 5).
Subgroup analysis
Subgroup analysis was conducted based on the intervention duration. Specifically, the intervention duration was < 12 weeks in 31 studies and ≥ 12 weeks in 29 studies. The heterogeneity analysis found that I2 in all studies was ≤ 50%, so a fixed effect model was adopted. In the group with an intervention duration of < 12 weeks, the combination of Bifidobacterium, Lactobacillus, Lactococcus, and Propionibacterium was the most effective in reducing HOMA-IR (Surca = 0.892). Furthermore, Lactobacillus demonstrated the highest efficacy in lowering HbA1c (Surca = 0.907), followed by the combination of Bifidobacterium, Lactobacillus, and Streptococcus (Surca = 0.734), and the combination of Bifidobacterium and Lactobacillus (Surca = 0.654). Fish oil was the most effective in reducing TG (Surca = 0.957). Vegetable oil was the most effective in reducing TC (Surca = 0.729), followed by mineral oil (Surca = 0.712) and EPA(Surca = 0.708). In the subgroup with intervention duration ≥ 12 weeks, the combination of Bifidobacterium and Lactobacillus demonstrated the highest efficacy in decreasing HOMA-IR (Surca = 0.910). Bifidobacterium exhibited the most effective in reducing HbA1c (Surca = 0.970). The combination of Bifidobacterium, Lactobacillus and Lactococcus (Surca = 0.945) was the most effective in reducing TG, followed by Bifidobacterium (Surca = 0.790) and fish oil (Surca = 0.627). Fish oil had the best effect on TC reduction (Surca = 0.799).
Discussion
This study is the first to compare the efficacy of fish oil and probiotic supplementation on glucose and lipid metabolism in T2DM patients. Overall, the results demonstrated that fish oil (in the form of omega-3 fatty acids) was superior to EPA and DHA alone. Furthermore, fish oil significantly reduced both TG and TC levels in T2DM patients. Moreover, probiotics significantly ameliorated insulin resistance compared with fish oil. In addition, Bifidobacterium had a better effect on reducing HbA1c than other probiotic supplements and fish oil.
Regarding the glucose metabolism in T2DM patients, our NMA revealed that probiotics significantly reduced HOMA-IR levels compared to fish oil. Probiotics may improve insulin sensitivity by different mechanisms. First, probiotics are able to regulate the composition and function of gut microbiota [76]. For instance, probiotic-fermented blueberry juice improves insulin resistance in mice with a high-fat diet by regulating gut microbiota [77]. Another probiotic supplement, Lactobacillus casei, plays an antidiabetic role by reshaping the intestinal flora in T2DM rats [78]. Second, probiotics can ameliorate inflammation by secreting anti-inflammatory factors to reduce pro-inflammatory cytokines and lipopolysaccharide (LPS) levels, thereby improving insulin resistance and preserving the integrity of the intestinal epithelial cell wall. Proinflammatory cytokines induce the phosphorylation of insulin receptor substrate-1 serine and impede the insulin signaling pathway [79, 80]. LPS, as a component of the outer membrane of gram-negative bacteria, binds to the Toll-like receptor 4 (cluster of differentiation 14) to trigger the production of proinflammatory cytokines [81]. Third, probiotics can produce short-chain fatty acids (SCFAs), such as acetic acid, propionic acid, and butyric acid, through fermentation of dietary fiber. In individuals with T2DM, acetic acid can stimulate insulin secretion [82], while propionic and butyric acids inhibit the production of proinflammatory cytokines [83]. SCFAs can bind to the G protein-coupled receptor [84] and stimulate the production of downstream glucagon-like peptide-1 (GLP-1) and peptide yy [85], both of which improve insulin resistance. Fourth, probiotics can synthesize antioxidants to reduce oxidative stress, thus improving insulin sensitivity. Antioxidants can inhibit chain reactions by scavenging free radical intermediates and neutralizing free radicals [86]. Probiotics can significantly increase serum antioxidant indexes, such as glutathione, and reduce the expression of malondialdehyde in patients with diabetes [87]. Fifth, certain types of probiotics strengthen the mucus barrier by increasing the expression of mucin and stimulating mucus secretion. The intestinal barrier is crucial for preventing bacterial endotoxin from entering the blood and inducing inflammation and insulin resistance, which are important contributors to T2DM [88]. Pediococcus acidilactici pA1c increases the number of cupped cells, promotes the secretion of mucoglycoprotein, and maintains the appropriate length of intestinal villi [89]. Bifidobacterium longum and Lactobacillus reuteri can enhance mucus layer thickness [90]. Lactobacillus spp. can upregulate the expression of Mucin 2 and Mucin 3 [91] to enhance the intestinal mucosal barrier function.
Furthermore, our study found that T2DM patients who consumed Bifidobacterium had lower HbA1c than those who consumed other probiotics or fish oil. The mechanism by which Bifidobacterium lowers blood glucose may be similar to the mechanism just mentioned. It has been reported that Bifidobacterium can also decompose dietary fiber and produce metabolites such as SCFAs [92]. Meanwhile, Bifidobacterium can also indirectly increase the level of GLP-1 secreted by intestinal L cells by increasing the level of SCFAs [93]. Moreover, Bifidobacterium regulates the immune system and reduces chronic low-level inflammatory response, which can reduce blood glucose levels [94]. This finding may provide insights into the hypoglycemic mechanism of Bifidobacterium and the development of target drugs. Although it has been shown that fish oil can affect glucose metabolism, the role of omega-3 fatty acids in regulating blood glucose remains debatable [95]. Our NMA also found that fish oil was less effective than probiotics in regulating glucose metabolism in patients with T2DM.
Regarding lipid metabolism, fish oil was more effective in reducing TG and TC levels in T2DM patients than all probiotics. Similar results have also been documented in several meta-analyses [96,97,98]. Fish oil regulates TG levels through four possible mechanisms. First, omega-3 fatty acids can inhibit the expression of sterol regulatory element binding protein-1C in the liver. Consequently, this leads to a decrease in fatty acid synthase, resulting in reduced fatty acids in the liver. Ultimately, these mechanisms contribute to a reduction in triglyceride levels [99]. Second, omega-3 fatty acids promote fatty acid oxidation by increasing the metabolic rate of fatty acids to produce energy [100]. Third, omega-3 fatty acids reduce triglyceride synthesis by inhibiting phosphatidic acid phosphatase and diacylglycerol acyltransferase [101]. Fourth, omega-3 fatty acids can increase the expression of lipoprotein lipase (LPL). LPL is a key enzyme involved in the removal of triglycerides from circulating triglyceride-rich lipoproteins such as very low density lipoprotein and chylomicron. Increased LPL expression promotes the conversion and clearance of triglycerides, thereby reducing their levels in the blood [102]. These regulatory effects can affect the synthesis, oxidation and clearance of triglycerides. However, the exact mechanisms are still being studied and may be influenced by individual differences.
Despite the role of fish oil in reducing total cholesterol levels, the mechanism of the relationship between fish oil and cholesterol remains to be elucidated. Previous meta-analyses have found that omega-3 fatty acids in fish oil could elevate the concentration of high density lipoprotein cholesterol (HDL-c) in blood [96, 97]. HDL-c facilitates the transportation of cholesterol in the blood and tissues back to the liver for metabolism and excretion. A previous study showed that EPA could lower low density lipoprotein cholesterol (LDL-c) concentrations in blood [98]. Interestingly, based on our SUCRA results, paraffin oil, as a mineral oil, was the most effective intervention for reducing TC levels. However, long-term oral administration of mineral oil can lead to increased intestinal permeability, possibly have proinflammatory effects, and cause reduced TC levels. It may raise concerns about the use of mineral oil as a placebo in clinical studies [103]. The number of available studies on this topic is limited, and further research on the effectiveness of fish oil in reducing cholesterol levels is needed.
In terms of the inflammatory response, our results revealed that fish oil was more effective than probiotics in reducing TNF-α in T2DM patients. TNF-α, a proinflammatory cytokine, is primarily secreted by macrophages and monocytes and is involved in inflammatory and immune responses. Several studies showed that fish oil could not only reduce TNF-α but also inhibit nuclear factor-κB activation, one of the major inflammatory transcription factors [104, 105]. In vitro and in vivo studies demonstrated that EPA and DHA could inhibit the production of TNF-α [106]. Furthermore, our study also found that fish oil supplementation also reduced leptin concentration in blood and increased adiponectin levels. This result is consistent with a previous meta-analysis, which indicates that omega-3 fatty acids reduce leptin levels and increase adiponectin levels in T2DM patients [107]. Leptin is an adipocyte-derived hormone that regulates appetite and energy metabolism to control body weight and energy balance [108]. An increased leptin concentration in blood is associated with insulin resistance and obesity in T2DM individuals [109]. However, the effect of fish oil on leptin levels remains debatable [110, 111]. Adiponectin is a hormone secreted by fat cells and it can promote glucose utilization, inhibit fatty acid oxidation and inflammation, and thus increase insulin sensitivity [112]. Adiponectin synthesis begins when the omega-3 fatty acids in fish oil bind to peroxisome proliferator-activated receptor-γ (PPAR-γ). When PPAR-γ exerts an antagonistic effect, the effect of omega-3 fatty acids on adiponectin is blocked [113]. Alternatively, omega-3 fatty acids inhibit transient receptor potentials in mature adipocytes to regulate calcium channels and thus, enhance adiponectin production [114]. It is important to note that the results should be cautiously interpreted due to the limited number of the included RCTs.
Node-splitting analysis for consistency showed P > 0.05 in direct, indirect, and network comparison of various interventions, suggesting that the included studies had good consistency. No heterogeneity was found in the network analysis (both I2 ≤ 50%), and thus sensitivity analysis was not performed. In the subgroup analysis, the results in the subgroup of intervention duration ≥ 12 weeks were different from the overall analysis results in TG reduction. The SURCA analysis showed that fish oil was more effective than probiotics in regulating lipid metabolism in T2DM patients, and probiotics were superior to fish oil in regulating glucose metabolism. In the subgroup with intervention duration ≥ 12 weeks, the combination of Bifidobacterium, Lactobacillus, and Lactococcus was the most effective in reducing TG, followed by Bifidobacterium and fish oil. However, it is important to note that in this subgroup, the combination of Bifidobacterium, Lactobacillus and Lactococcus was only used in the study by Sabico [58], and Bifidobacterium was only reported in the study by Chaiyasut [73]. Since the two interventions was only used in one study, respectively, our results may be biased due to limited data, and publication bias may exist. It may also indicate that fish oil was more effective in reducing TC only at the early stage (< 12 weeks). Therefore, high-quality RCTs with a large and diverse population are required to validate these findings.
Our findings provide crucial insights into the clinical effects of fish oil and probiotics on T2DM. Fish oil is found to outperform probiotics in reducing triglycerides and total cholesterol levels, thereby mitigating the risk of cardiovascular disease in T2DM. Proper supplementation of fish oil may improve cardiovascular conditions among these patients. Furthermore, probiotics, especially those containing strains like bifidobacteria, are effective in lowering blood sugar levels compared to fish oil. This could contribute to the control of blood sugar and the overall management of diabetes. These findings may assist clinicians in designing more effective and personalized treatment plans. This study not only introduces new insights into the management of T2DM but also provides a deeper understanding of this disease.
There are several limitations in this study. Firstly, other inflammatory markers, such as interleukin-6, procalcitonin, and erythrocyte sedimentation rate, are more widely used than TNF-α in clinical practice. Unfortunately, due to limited data, not all interventions were linked in a network, so further analysis could not be conducted. Secondly, the inconsistency of fish oil and probiotic dosages across studies may lead to biased results. Finally, adherence to interventions is important, especially for those implemented outside hospitals. However, the description of patient compliance in the included RCTs was relatively limited, which may affect the full assessment of the treatment effects. Although we tried to take this into account in the study design, data on patient compliance remain limited. Future studies should consider the potential impact of patient compliance on outcomes.
Conclusions
Regarding lipid metabolism in T2DM patients, fish oil significantly reduced TG and TC levels compared to probiotics. Probiotics were more effective than fish oil in improving insulin resistance. Particularly, Bifidobacterium was more effective in reducing blood glucose levels than other probiotic supplements and fish oil. Nevertheless, high-quality and large-scale RCTs are required to validate these results. Further research should also explore the specific mechanisms and optimal treatment options of probiotics and fish oil to improve glucose and lipid metabolism in T2DM patients.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request.
References
Yun JS, Ko SH. Current trends in epidemiology of cardiovascular disease and cardiovascular risk management in type 2 diabetes. Metabolism. 2021;123:154838.
Goldenberg JZ, Day A, Brinkworth GD, et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ. 2021;372:m4743.
Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731–54.
Dale HF, Rasmussen SH, Asiller ÖÖ, Lied GA. Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients. 2019;11(9):2048.
Musazadeh V, Karimi A, Malekahmadi M, Ahrabi SS, Dehghan P. Omega-3 polyunsaturated fatty acids in the treatment of non-alcoholic fatty liver disease: an umbrella systematic review and meta-analysis. Clin Exp Pharmacol Physiol. 2023;50(5):327–34.
Rahimlou M, Nematollahi S, Husain D, Banaei-Jahromi N, Majdinasab N, Hosseini SA. Probiotic supplementation and systemic inflammation in relapsing-remitting multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Front Neurosci. 2022;16:901846.
Kumar M, Pal N, Sharma P, et al. Omega-3 fatty acids and their interaction with the gut microbiome in the prevention and amelioration of type-2 diabetes. Nutrients. 2022;14(9):1723.
Xiao Y, Zhang Q, Liao X, Elbelt U, Weylandt KH. The effects of omega-3 fatty acids in type 2 diabetes: a systematic review and meta-analysis. Prostaglandins Leukot Essent Fatty Acids. 2022;182:102456.
Zarezadeh M, Musazadeh V, Faghfouri AH, Roshanravan N, Dehghan P. Probiotics act as a potent intervention in improving lipid profile: an umbrella systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2023;63(2):145–58.
Zarezadeh M, Musazadeh V, Faghfouri AH, et al. Probiotic therapy, a novel and efficient adjuvant approach to improve glycemic status: an umbrella meta-analysis. Pharmacol Res. 2022;183:106397.
Faghfouri AH, Afrakoti LGMP, Kavyani Z, et al. The role of probiotic supplementation in inflammatory biomarkers in adults: an umbrella meta-analysis of randomized controlled trials. Inflammopharmacology. 2023;31(5):2253–68.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
American Diabetes Association. 2 Classification and Diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15–33.
Julian Higgins JT, Chandler J, Cumpston M, Li T, Page M, Welch V. Cochrane handbook for systematic reviews of interventions version 6.3. Updated 4 August, 2022. London: The Cochrane Collaboration; 2022.
Mbuagbaw L, Rochwerg B, Jaeschke R, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev. 2017;6(1):79.
Morgan WA, Raskin P, Rosenstock J. A comparison of fish oil or corn oil supplements in hyperlipidemic subjects with NIDDM. Diabetes Care. 1995;18(1):83–6.
Sirtori CR, Crepaldi G, Manzato E, et al. One-year treatment with ethyl esters of n-3 fatty acids in patients with hypertriglyceridemia and glucose intolerance: reduced triglyceridemia, total cholesterol and increased HDL-C without glycemic alterations. Atherosclerosis. 1998;137(2):419–27.
Patti L, Maffettone A, Iovine C, et al. Long-term effects of fish oil on lipoprotein subfractions and low density lipoprotein size in non-insulin-dependent diabetic patients with hypertriglyceridemia. Atherosclerosis. 1999;146(2):361–7.
Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr. 2002;76(5):1007–15.
Pedersen H, Petersen M, Major-Pedersen A, et al. Influence of fish oil supplementation on in vivo and in vitro oxidation resistance of low-density lipoprotein in type 2 diabetes. Eur J Clin Nutr. 2003;57(5):713–20.
Mita T, Watada H, Ogihara T, et al. Eicosapentaenoic acid reduces the progression of carotid intima-media thickness in patients with type 2 diabetes. Atherosclerosis. 2007;191(1):162–7.
Satoh N, Shimatsu A, Kotani K, et al. Purified eicosapentaenoic acid reduces small dense LDL, remnant lipoprotein particles, and C-reactive protein in metabolic syndrome. Diabetes Care. 2007;30(1):144–6.
Kabir M, Skurnik G, Naour N, et al. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr. 2007;86(6):1670–9.
Shidfar F, Keshavarz A, Hosseyni S, Ameri A, Yarahmadi S. Effects of omega-3 fatty acid supplements on serum lipids, apolipoproteins and malondialdehyde in type 2 diabetes patients. East Mediterr Health J. 2008;14(2):305–13.
Wong CY, Yiu KH, Li SW, et al. Fish-oil supplement has neutral effects on vascular and metabolic function but improves renal function in patients with Type 2 diabetes mellitus. Diabet Med. 2010;27(1):54–60.
Malekshahi Moghadam A, Saedisomeolia A, Djalali M, Djazayery A, Pooya S, Sojoudi F. Efficacy of omega-3 fatty acid supplementation on serum levels of tumour necrosis factor-alpha, C-reactive protein and interleukin-2 in type 2 diabetes mellitus patients. Singapore Med J. 2012;53(9):615–9.
Crochemore IC, Souza AF, de Souza AC, Rosado EL. ω-3 polyunsaturated fatty acid supplementation does not influence body composition, insulin resistance, and lipemia in women with type 2 diabetes and obesity. Nutr Clin Pract. 2012;27(4):553–60.
Ogawa S, Abe T, Nako K, et al. Eicosapentaenoic acid improves glycemic control in elderly bedridden patients with type 2 diabetes. Tohoku J Exp Med. 2013;231(1):63–74.
Sarbolouki S, Javanbakht MH, Derakhshanian H, et al. Eicosapentaenoic acid improves insulin sensitivity and blood sugar in overweight type 2 diabetes mellitus patients: a double-blind randomised clinical trial. Singapore Med J. 2013;54(7):387–90.
Toupchian O, Sotoudeh G, Mansoori A, et al. Effects of DHA-enriched fish oil on monocyte/macrophage activation marker sCD163, asymmetric dimethyl arginine, and insulin resistance in type 2 diabetic patients. J Clin Lipidol. 2016;10(4):798–807.
Zheng JS, Lin M, Fang L, et al. Effects of n-3 fatty acid supplements on glycemic traits in Chinese type 2 diabetic patients: a double-blind randomized controlled trial. Mol Nutr Food Res. 2016;60(10):2176–84.
Mazaherioun M, Djalali M, Koohdani F, et al. Beneficial effects of n-3 fatty acids on cardiometabolic and inflammatory markers in type 2 diabetes mellitus: a clinical trial. Med Princ Pract. 2017;26(6):535–41.
Mazaherioun M, Saedisomeolia A, Javanbakht MH, Koohdani F, Eshraghian MR, Djalali M. Beneficial effects of n-3 polyunsaturated fatty acids on adiponectin levels and AdipoR gene expression in patients with type 2 diabetes mellitus: a randomized, placebo-controlled, double-blind clinical trial. Arch Med Sci. 2017;13(4):716–24.
Jacobo-Cejudo MG, Valdés-Ramos R, Guadarrama-López AL, Pardo-Morales RV, Martínez-Carrillo BE, Harbige LS. Effect of n-3 polyunsaturated fatty acid supplementation on metabolic and inflammatory biomarkers in type 2 diabetes mellitus patients. Nutrients. 2017;9(6):573.
Wang F, Wang Y, Zhu Y, et al. Treatment for 6 months with fish oil-derived n-3 polyunsaturated fatty acids has neutral effects on glycemic control but improves dyslipidemia in type 2 diabetic patients with abdominal obesity: a randomized, double-blind, placebo-controlled trial. Eur J Nutr. 2017;56(7):2415–22.
Fayh APT, Borges K, Cunha GS, et al. Effects of n-3 fatty acids and exercise on oxidative stress parameters in type 2 diabetic: a randomized clinical trial. J Int Soc Sports Nutr. 2018;15:18.
Raygan F, Taghizadeh M, Mirhosseini N, et al. A comparison between the effects of flaxseed oil and fish oil supplementation on cardiovascular health in type 2 diabetic patients with coronary heart disease: a randomized, double-blinded, placebo-controlled trial. Phytother Res. 2019;33(7):1943–51.
Rampally P, Koduganti RR, Ganapathi SN, Panthula VR, Surya PJ. Comparison of effectiveness of low-dose aspirin versus omega-3 fatty acids as adjuvants to nonsurgical periodontal therapy in Type II diabetic patients with chronic periodontitis. J Indian Soc Periodontol. 2019;23(3):249–56.
Thota RN, Acharya SH, Garg ML. Curcumin and/or omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance and blood lipids in individuals with high risk of type 2 diabetes: a randomised controlled trial. Lipids Health Dis. 2019;18(1):31.
Golzari MH, Javanbakht MH, Ghaedi E, Mohammadi H, Djalali M. Effect of eicosapentaenoic acid supplementation on paraoxonase 2 gene expression in patients with type 2 diabetes mellitus: a randomized double-blind clinical trial. Clin Nutr Res. 2019;8(1):17–27.
Hua L, Lei M, Xue S, Li X, Li S, Xie Q. Effect of fish oil supplementation combined with high-intensity interval training in newly diagnosed non-obese type 2 diabetes: a randomized controlled trial. J Clin Biochem Nutr. 2020;66(2):146–51.
Naeini Z, Toupchian O, Vatannejad A, et al. Effects of DHA-enriched fish oil on gene expression levels of p53 and NF-κB and PPAR-γ activity in PBMCs of patients with T2DM: a randomized, double-blind, clinical trial. Nutr Metab Cardiovasc Dis. 2020;30(3):441–7.
Golpour P, Nourbakhsh M, Mazaherioun M, Janani L, Nourbakhsh M, Yaghmaei P. Improvement of NRF2 gene expression and antioxidant status in patients with type 2 diabetes mellitus after supplementation with omega-3 polyunsaturated fatty acids: a double-blind randomised placebo-controlled clinical trial. Diabetes Res Clin Pract. 2020;162:108120.
Liu H, Wang F, Liu X, et al. Effects of marine-derived and plant-derived omega-3 polyunsaturated fatty acids on erythrocyte fatty acid composition in type 2 diabetic patients. Lipids Health Dis. 2022;21(1):20.
Kuang X, Shao X, Li H, et al. Lipid extract from blue mussel (Mytilus edulis) improves glycemic traits in Chinese type 2 diabetic mellitus patients: a double-blind randomized controlled trial. J Sci Food Agric. 2023;103(6):2970–80.
Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, et al. Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J Dairy Sci. 2011;94(7):3288–94.
Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28(5):539–43.
Asemi Z, Zare Z, Shakeri H, Sabihi SS, Esmaillzadeh A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab. 2013;63(1–2):1–9.
Tajadadi-Ebrahimi M, Bahmani F, Shakeri H, et al. Effects of daily consumption of synbiotic bread on insulin metabolism and serum high-sensitivity C-reactive protein among diabetic patients: a double-blind, randomized, controlled clinical trial. Ann Nutr Metab. 2014;65(1):34–41.
Shakeri H, Hadaegh H, Abedi F, et al. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids. 2014;49(7):695–701.
Mohamadshahi M, Veissi M, Haidari F, Shahbazian H, Kaydani GA, Mohammadi F. Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts. 2014;4(2):83–8.
Mohamadshahi M, Veissi M, Haidari F, Javid AZ, Mohammadi F, Shirbeigi E. Effects of probiotic yogurt consumption on lipid profile in type 2 diabetic patients: a randomized controlled clinical trial. J Res Med Sci. 2014;19(6):531–6.
Ostadrahimi A, Taghizadeh A, Mobasseri M, et al. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Iran J Public Health. 2015;44(2):228–37.
Feizollahzadeh S, Ghiasvand R, Rezaei A, Khanahmad H, Sadeghi A, Hariri M. Effect of probiotic soy milk on serum levels of adiponectin, inflammatory mediators, lipid profile, and fasting blood glucose among patients with type II diabetes mellitus. Probiotics Antimicrob Proteins. 2017;9(1):41–7.
Rezaei M, Sanagoo A, Jouybari L, Behnampoo N, Kavosi A. The effect of probiotic yogurt on blood glucose and cardiovascular biomarkers in patients with type II diabetes: a randomized controlled trial. Evidence Based Care J. 2017;6(4):26–35.
Tonucci LB, Olbrich Dos Santos KM, Licursi de Oliveira L, Rocha Ribeiro SM, Duarte Martino HS. Clinical application of probiotics in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Clin Nutr. 2017;36(1):85–92.
Firouzi S, Majid HA, Ismail A, Kamaruddin NA, Barakatun-Nisak MY. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: a randomized controlled trial. Eur J Nutr. 2017;56(4):1535–50.
Sabico S, Al-Mashharawi A, Al-Daghri NM, et al. Effects of a multi-strain probiotic supplement for 12 weeks in circulating endotoxin levels and cardiometabolic profiles of medication naïve T2DM patients: a randomized clinical trial. J Transl Med. 2017;15(1):249.
Sato J, Kanazawa A, Azuma K, et al. Probiotic reduces bacterial translocation in type 2 diabetes mellitus: a randomised controlled study. Sci Rep. 2017;7(1):12115.
Mobini R, Tremaroli V, Ståhlman M, et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2017;19(4):579–89.
Abbasi B, Mirlohi M, Daniali M, Ghiasvand R. Effects of probiotic soy milk on lipid panel in type 2 diabetic patients with nephropathy: a double-blind randomized clinical trial. Prog Nutr. 2018;20(Suppl 2):70–8.
Kobyliak N, Falalyeyeva T, Mykhalchyshyn G, Kyriienko D, Komissarenko I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: randomized clinical trial. Diabetes Metab Syndr. 2018;12(5):617–24.
Hsieh MC, Tsai WH, Jheng YP, et al. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: a randomized, double-blinded, placebo-controlled trial. Sci Rep. 2018;8(1):16791.
Raygan F, Rezavandi Z, Bahmani F, et al. The effects of probiotic supplementation on metabolic status in type 2 diabetic patients with coronary heart disease. Diabetol Metab Syndr. 2018;10:51.
Madempudi RS, Ahire JJ, Neelamraju J, Tripathi A, Nanal S. Efficacy of UB0316, a multi-strain probiotic formulation in patients with type 2 diabetes mellitus: a double blind, randomized, placebo controlled study. PLoS ONE. 2019;14(11):e0225168.
Lestari LA, Ratnasari D, Azizah EF, et al. Short-term consumption of probiotic yogurt improved HDL-C of type 2 diabetes mellitus patients: a double-blind randomized controlled trial. Rom J Diabetes Nutr Metab Dis. 2019;26(4):381–92.
Razmpoosh E, Javadi A, Ejtahed HS, Mirmiran P, Javadi M, Yousefinejad A. The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: a randomized placebo controlled trial. Diabetes Metab Syndr. 2019;13(1):175–82.
Khalili L, Alipour B, Asghari Jafar-Abadi M, et al. The effects of Lactobacillus Casei on Glycemic response, serum sirtuin1 and Fetuin-a levels in patients with type 2 diabetes mellitus: a randomized controlled trial. Iran Biomed J. 2019;23(1):68–77.
Palacios T, Vitetta L, Coulson S, et al. Targeting the intestinal microbiota to prevent type 2 diabetes and enhance the effect of metformin on glycaemia: a randomised controlled pilot study. Nutrients. 2020;12(7):2041.
Perraudeau F, McMurdie P, Bullard J, et al. Improvements to postprandial glucose control in subjects with type 2 diabetes: a multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. BMJ Open Diabetes Res Care. 2020;8(1):e001319.
Jiang H, Zhang Y, Xu D, Wang Q. Probiotics ameliorates glycemic control of patients with diabetic nephropathy: a randomized clinical study. J Clin Lab Anal. 2021;35(4):e23650.
Mirjalili M, Salari Sharif A, Sangouni AA, Emtiazi H, Mozaffari-Khosravi H. Effect of probiotic yogurt consumption on glycemic control and lipid profile in patients with type 2 diabetes mellitus: a randomized controlled trial. Clin Nutr ESPEN. 2023;54:144–9.
Chaiyasut C, Sivamaruthi BS, Lailerd N, et al. Influence of Bifidobacterium breve on the glycaemic control, lipid profile and microbiome of type 2 diabetic subjects: a preliminary randomized clinical trial. Pharmaceuticals. 2023;16(5):695.
Savytska M, Kyriienko D, Komisarenko I, Kovalchuk O, Falalyeyeva T, Kobyliak N. Probiotic for pancreatic β-cell function in type 2 diabetes: a randomized, double-blinded placebo-controlled clinical trial. Diabetes Ther. 2023;14(11):1915–31.
Zikou E, Dovrolis N, Dimosthenopoulos C, Gazouli M, Makrilakis K. The effect of probiotic supplements on metabolic parameters of people with type 2 diabetes in Greece-a randomized, double-blind, placebo-controlled study. Nutrients. 2023;15(21):4663.
Scheithauer TPM, Rampanelli E, Nieuwdorp M, et al. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front Immunol. 2020;11:571731.
Zhong H, Abdullah DL, et al. Probiotic-fermented blueberry juice prevents obesity and hyperglycemia in high fat diet-fed mice in association with modulating the gut microbiota. Food Funct. 2020;11(10):9192–207.
Qu L, Ren J, Huang L, et al. Antidiabetic effects of Lactobacillus casei fermented yogurt through reshaping gut microbiota structure in type 2 diabetic rats. J Agric Food Chem. 2018;66(48):12696–705.
Cruz NG, Sousa LP, Sousa MO, Pietrani NT, Fernandes AP, Gomes KB. The linkage between inflammation and Type 2 diabetes mellitus. Diabetes Res Clin Pract. 2013;99(2):85–92.
Jia L, Li D, Feng N, et al. Anti-diabetic effects of clostridium butyricum CGMCC0313.1 through promoting the growth of gut butyrate-producing bacteria in Type 2 diabetic mice. Sci Rep. 2017;7(1):7046.
Allin KH, Nielsen T, Pedersen O. Mechanisms in endocrinology: gut microbiota in patients with type 2 diabetes mellitus. Eur J Endocrinol. 2015;172(4):R167–77.
Perry RJ, Peng L, Barry NA, et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature. 2016;534(7606):213–7.
Mandaliya DK, Seshadri S. Short chain fatty acids, pancreatic dysfunction and type 2 diabetes. Pancreatology. 2019;19(2):280–4.
Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145(2):396-406 e1 10.
Christiansen CB, Gabe MBN, Svendsen B, Dragsted LO, Rosenkilde MM, Holst JJ. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol. 2017;315(1):G53–65.
Mishra V, Shah C, Mokashe N, Chavan R, Yadav H, Prajapati J. Probiotics as potential antioxidants: a systematic review. J Agric Food Chem. 2015;63(14):3615–26.
Zheng HJ, Guo J, Jia Q, et al. The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2019;142:303–13.
Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020;69(12):2232–43.
Cabello-Olmo M, Oneca M, Pajares MJ, et al. Antidiabetic effects of Pediococcus Acidilactici pA1c on HFD-Induced Mice. Nutrients. 2022;14(3):692.
Ahl D, Liu H, Schreiber O, Roos S, Phillipson M, Holm L. Lactobacillus reuteri increases mucus thickness and ameliorates dextran sulphate sodium-induced colitis in mice. Acta Physiol (Oxf). 2016;217(4):300–10.
Sicard JF, Le Bihan G, Vogeleer P, Jacques M, Harel J. Interactions of intestinal bacteria with components of the intestinal mucus. Front Cell Infect Microbiol. 2017;7:387.
Cani PD, Neyrinck AM, Fava F, et al. Selective increases of Bifidobacterium in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50(11):2374–83.
Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem. 2013;288(35):25088–97.
Geurts L, Neyrinck AM, Delzenne NM, Knauf C, Cani PD. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Benef Microbes. 2014;5(1):3–17.
Bhat S, Sarkar S, Zaffar D, Dandona P, Kalyani RR. Omega-3 fatty acids in cardiovascular disease and diabetes: a review of recent evidence. Curr Cardiol Rep. 2023;25(2):51–65.
Gao C, Liu Y, Gan Y, et al. Effects of fish oil supplementation on glucose control and lipid levels among patients with type 2 diabetes mellitus: a Meta-analysis of randomized controlled trials. Lipids Health Dis. 2020;19(1):87.
Alhassan A, Young J, Lean MEJ, Lara J. Consumption of fish and vascular risk factors: a systematic review and meta-analysis of intervention studies. Atherosclerosis. 2017;266:87–94.
Zhang HJ, Gao X, Guo XF, et al. Effects of dietary eicosapentaenoic acid and docosahexaenoic acid supplementation on metabolic syndrome: a systematic review and meta-analysis of data from 33 randomized controlled trials. Clin Nutr. 2021;40(7):4538–50.
Shearer GC, Savinova OV, Harris WS. Fish oil—how does it reduce plasma triglycerides? Biochim Biophys Acta. 2021;1821(5):843–51.
Bays HE, Tighe AP, Sadovsky R, Davidson MH. Prescription omega-3 fatty acids and their lipid effects: physiologic mechanisms of action and clinical implications. Expert Rev Cardiovasc Ther. 2008;6(3):391–409.
Harris WS, Bulchandani D. Why do omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol. 2006;17(4):387–93.
Backes J, Anzalone D, Hilleman D, Catini J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016;15(1):118.
Pieterman EJ, Princen HMG, Jarke A, et al. Chronic Oral administration of mineral oil compared with corn oil: effects on gut permeability and plasma inflammatory and lipid biomarkers. Front Pharmacol. 2021;12:681455.
Kavyani Z, Musazadeh V, Fathi S, Hossein Faghfouri A, Dehghan P, Sarmadi B. Efficacy of the omega-3 fatty acids supplementation on inflammatory biomarkers: an umbrella meta-analysis. Int Immunopharmacol. 2022;111:109104.
Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2017;45(5):1105–15.
Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851(4):469–84.
Farimani AR, Hariri M, Azimi-Nezhad M, Borji A, Zarei S, Hooshmand E. The effect of n-3 PUFAs on circulating adiponectin and leptin in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2018;55(7):641–52.
Fan X, Yuan W, Huang W, Lin Z. Recent progress in leptin signaling from a structural perspective and its implications for diseases. Biochimie. 2023;212:60–75.
Zhao S, Kusminski CM, Elmquist JK, Scherer PE. Leptin: less is more. Diabetes. 2020;69(5):823–9.
Hariri M, Ghiasvand R, Shiranian A, Askari G, Iraj B, Salehi-Abargouei A. Does omega-3 fatty acids supplementation affect circulating leptin levels? A systematic review and meta-analysis on randomized controlled clinical trials. Clin Endocrinol (Oxf). 2015;82(2):221–8.
Sepidarkish M, Rezamand G, Qorbani M, et al. Effect of omega-3 fatty acids supplementation on adipokines: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2022;62(27):7561–75.
Maeda N, Funahashi T, Matsuzawa Y, Shimomura I. Adiponectin, a unique adipocyte-derived factor beyond hormones. Atherosclerosis. 2020;292:1–9.
Brown LH, Mutch DM. Mechanisms underlying N3-PUFA regulation of white adipose tissue endocrine function. Curr Opin Pharmacol. 2020;52:40–6.
Sukumar P, Sedo A, Li J, et al. Constitutively active TRPC channels of adipocytes confer a mechanism for sensing dietary fatty acids and regulating adiponectin. Circ Res. 2012;111(2):191–200.
Acknowledgements
We would like to thank Toedit for its English editing during the preparation of this manuscript.
Funding
This project was supported by the Medical Scientific Research Project of Chengdu (No.2021272), the Scientific Research Fund of Chengdu Fifth People’s Hospital (No.GSPZX2022-19).
Author information
Authors and Affiliations
Contributions
MZ and FY have authors contributed equally. MZ, FY and PN conceived and designed the study. MZ and PN discussed and drafted the study protocol. QF, YO and PN screened and selected the articles. MZ, FY and PN extracted the data. JZ, HW and HC assessed the risk of bias of included trials. PN analysed the data. MZ, FY, JZ and PN interpreted the results. MZ and FY drafted the manuscript. FY, QF, YO, JZ and PN critically revised the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. HW and HC supervised the study. PN is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Complete list of 3 electronic library search terms. Table S2. SUCRA ranking table for each outcome indicator. Table S3. The league table of mean difference and 95% confidence intervals for secondary outcome indicators. Fig. S1. The network plots. Fig. S2. SUCRA analysis column chart.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, M., Yang, F., Feng, Q. et al. Comparison of the efficacy of fish oil and probiotic supplementation on glucose and lipid metabolism in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetol Metab Syndr 16, 25 (2024). https://doi.org/10.1186/s13098-024-01266-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-024-01266-3