Abstract
Objective
Diabetes is a major cause of the progression of acute kidney injury (AKI). Few prediction models have been developed to predict the renal prognosis in diabetic patients with AKI so far. The aim of this study was to develop and validate a predictive model to identify high-risk individuals with non-recovery of renal function at 90 days in diabetic patients with AKI.
Methods
Demographic data and related laboratory indicators of diabetic patients with AKI in the First Affiliated Hospital of Guangxi Medical University from January 31, 2012 to January 31, 2022 were retrospectively analysed, and patients were followed up to 90 days after AKI diagnosis. Based on the results of Logistic regression, a model predicting the risk of non-recovery of renal function at 90 days in diabetic patients with AKI was developed and internal validated. Consistency index (C-index), calibration curve, and decision curve analysis were used to evaluate the differentiation, accuracy, and clinical utility of the prediction model, respectively.
Results
A total of 916 diabetic patients with AKI were enrolled, with a male to female ratio of 2.14:1. The rate of non-recovery of renal function at 90 days was 66.8% (612/916). There were 641 in development cohort and 275 in validation cohort (ration of 7:3). In the development cohort, a prediction model was developed based on the results of Logistic regression analysis. The variables included in the model were: diabetes duration (OR = 1.022, 95% CI 1.012–1.032), hypertension (OR = 1.574, 95% CI 1.043–2.377), chronic kidney disease (OR = 2.241, 95% CI 1.399–3.591), platelet (OR = 0.997, 95% CI 0.995–1.000), 25-hydroxyvitamin D3 (OR = 0.966, 95% CI 0.956–0.976), postprandial blood glucose (OR = 1.104, 95% CI 1.032–1.181), discharged serum creatinine (OR = 1.003, 95% CI 1.001–1.005). The C-indices of the prediction model were 0.807 (95% CI 0.738–0.875) and 0.803 (95% CI 0.713–0.893) in the development and validation cohorts, respectively. The calibration curves were all close to the straight line with slope 1. The decision curve analysis showed that in a wide range of threshold probabilities.
Conclusion
A prediction model was developed to help predict short-term renal prognosis of diabetic patients with AKI, which has been verified to have good differentiation, calibration degree and clinical practicability.
Similar content being viewed by others
Background
Due to overweight, obesity and unhealthy lifestyles, the global prevalence of diabetes is increasing year by year. There are currently about 537 million adults worldwide with diabetes, and the number is expected to rise to 643 million by 2030 and 783 million by 2045 [1, 2]. Diabetes, as a highly prevalent disease with complications affecting multiple systems of the human body, has become an important burden affecting global health. The kidney is one of the important target organs of diabetic vascular damage. It is estimated that more than 60% of patients with diabetes in Asia will develop renal complications [3]. Therefore, it is very important for diabetic patients to recognize and control their renal complications.
Acute kidney injury (AKI) has been recognized as one of the common renal complications of diabetes [4]. Diabetes patients have high prevalence of AKI, and diabetic patients with AKI have worse prognosis than those without diabetes [5]. Diabetes can not only further increase the risk of AKI progress to chronic renal failure (CRF) or end stage renal disease (ESRD), but also increase the risk of mortality, cardiovascular complication and other adverse consequences [6,7,8]. A retrospective cohort showed that the prevalence of AKI in diabetic patients was as high as 48.6%, and diabetic patients were more likely to have chronic kidney disease (CKD) and CRF than non-diabetic patients (46.3% vs. 17.2%) [9]. Data from the Centers for Disease Control and Prevention also indicate that diabetes is present in up to 40% of all hospitalized patients with AKI [10]. In addition, diabetes complicated with AKI not only increases the length of hospitalization and treatment expenditure of patients, but also seriously wastes social and medical resources [11]. Therefore, there is an urgent need for a simple and easy method to rapidly assess the risk of non-recovery of renal function in diabetic patients with AKI.
A nomogram is a user-friendly tool with a graphical representation that can be used to calculate the probability of a specific event for each individual [12, 13]. Early prediction, prevention and treatment of AKI should be benefit for improving the prognosis of diabetes. In addition, a prospective cohort study showed that non-recovery of renal function at 90 days after AKI was an independent risk factor for progression of CKD (OR = 28.03, 95% CI 5.37–146.31) [14]. However, there are few studies on short-term renal outcomes in diabetic patients with AKI, and risk prediction tools are lacking to identify high-risk individuals with non-recovery of renal function at 90 days. This study aims to provide a practical risk stratification method for early prediction of adverse renal outcomes in diabetic patients with AKI.
Materials and methods
Research subjects
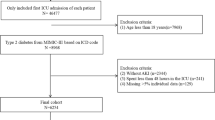
Patients diagnosed with diabetes and AKI in the First Affiliated Hospital of Guangxi Medical University from January 31, 2012 to January 31, 2022 were selected as the research subjects. Inclusion criteria: (1) diabetes was diagnosed before AKI; (2) changes in serum creatinine (Scr) consistent with the diagnostic criteria for AKI; (3) the follow-up time was no less than 90 days. Exclusion criteria: (1) age < 18 years; (2) CKD stage 5 or renal replacement therapy (RRT); (3) death or loss to follow-up within 90 days of AKI diagnosis; (4) patients with missing important baseline data and the Scr values of 90-day following up. This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University [Approval number: 2019(KY-E-028)]. Participants were required to sign (or have a proxy sign) informed consent for data collection. This study was conducted in accordance with the tenets of the Declaration of Helsinki.
Research groupings
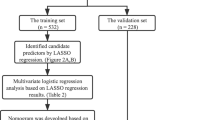
Using a retrospective cohort study, the patients were followed up from the diagnosis of AKI to 90 days. The observed clinical outcome was the recovery of renal function. All subjects were randomly divided into training cohort and validation cohorts by a ratio of 7:3. The development cohort was used to construct the prediction model, and validation cohort was used to verify the model’s prediction ability to predict non-recovery of renal function at 90 days in diabetic patients with AKI.
Clinical data collection
Demographic data and baseline clinical data were collected, including age, gender, duration of diabetes (months), underlying diseases (hypertension, CKD), body mass index (BMI), blood pressure, blood routine, liver function, renal function, electrolytes, myocardial enzymes, 25-hydroxyvitamin D3 [25(OH)D3], blood glucose, and discharged Scr, dialysis, etc. The baseline Scr was defined as a stable Scr within the last 3 months or longer if none was available within 3 months [15]. The discharged Scr was the last inpatient Scr value measured before AKI hospital discharge [16]. Other laboratory indicators were the first time of values after hospitalization.
Data preprocessing
Several steps were used in data preprocessing. Before starting the model building process, we have checked each variable for missing, outliers, or implausible values. As only a small number of missing values were present (< 5% of each variable), the missing data and outliers were removed.
Diagnostic criteria
AKI was defined referring to the diagnostic criteria of AKI in the Kidney Disease: Improving Global Outcomes guideline: Scr increased by ≥ 26.5 µmol/L within 48 h or increased by > 50% of the baseline value within 7 days. The criteria of AKI stage: stage 1: Scr increase to 1.5–1.9 times of baseline value or increased ≥ 0.3 mg/dL, stage 2: Scr increase to 2.0–2.9 times of base value, stage 3: Scr increase to 3 times or ≥ 4.0 mg/ dL or begin RRT [17].
Diagnosis of diabetes conforms to World Health Organization criteria as follows: (1) random blood glucose ≥ 11.1 mmol/L, (2) fasting blood glucose (FBG) ≥ 7.0 mmol/L, or (3) postprandial blood glucose (PBG) ≥ 11.1 mmol/L [18].
Renal function recovery was defined as a return of Scr to less than 1.25 times the baseline value or Scr decrease to normal lab range (Scr ≤ 104 µmol/L in males or Scr ≤ 84 µmol/L in females) or removal of RRT [19]. Otherwise, it is considered to be non-recovery of renal function.
Statistical analysis
All statistical analyses were performed on SPSS 22.0 and R 3.6.3 software. For continuous variables, data are presented as the mean ± standard deviation; for dichotomous variables, data are presented as whole numbers and proportions [n(%)]. Comparisons among two groups were performed by χ2 test. The univariate Logistic regression model was used to screen the risk factors, the variables with P < 0.05 were included in the multivariate Logistic regression (the forward LR method). Based on the multivariate Logistic analysis results, a nomogram was constructed based on the odds ratios of risk factors. The concordance index (C-index) and receiver operating characteristic curve (ROC) were used to evaluate the discrimination of the prediction model in the development and validation cohorts. The Hosmer-Lemeshow goodness of fit test was used for the calibration degree to compare the difference between the predicted probability and the actual probability, and P > 0.05 indicated that the model calibration degree was reliable. The Brier score was also calculated to evaluate the calibration degree of the model, and the better the calibration of the model with a Brier score close to 0. The Bootstrap method was used to draw the calibration curve, and the closer the slope is to 1, the higher the accuracy of the prediction model. Decision curve analysis was conducted to determine the clinical usefulness of the nomogram by quantifying the net benefit at different threshold probabilities in the primary dataset. P < 0.05 was regarded as a statistically significant difference.
Results
Characteristics of patients in the development and validation cohorts
Table 1 shows the characteristics of the patients in the development and validation cohorts. Among 1280 diabetic patients with AKI, 52 were younger than 18 years old, 98 had stage 5 CKD or had regular RRT, 36 had incomplete baseline data, 152 died during follow-up, and 26 were lost to follow-up. Finally, a total of 916 patients were included in this study, with an average age of 61.31 ± 13.4 years and a male to female ratio of 2.14:1. About 39.7% of the patients had history of CKD (364/916); 402 patients (43.9%) with stage 1 AKI, 337 patients (36.8%) with stage 2 AKI, and 177 cases (19.3%) in stage 3 AKI; the 90-day non-recovery rate of renal function was 66.8% (612/916). Diabetic patients with AKI were randomly divided into 641 cases in the development cohort and 275 cases in the validation cohort. The 90-day renal function recovery rate in the development cohort was 34.9% (224/641), and the 90-day renal function recovery rate in the validation cohort was 29.1% (80/275).
Compared with the validation cohort, the development cohort had a longer diabetes duration (p < 0.05). There was no significant difference in gender, age, length of hospital stay, history of hypertension, CKD, BMI, blood pressure, white blood cell count (WBC), hemoglobin, platelet (PLT), albumin, 25(OH)D3, N-terminal prohormone of brain natriuretic peptide (NT-proBNP), FBG, PBG, glycated hemoglobin A1c (HbA1c), blood urea nitrogen (BUN), baseline Scr, uric acid (UA), serum potassium level, AKI stage, RRT rate, discharge Scr and renal function non-recovery rate in development and validation cohorts.
Risk factors affecting 90-day renal outcomes
Logistic regression analysis was used to construct the prediction model, as shown in Table 2. Univariate logistic regression analysis of the development cohort showed that, the diabetes duration, history of hypertension, CKD, mean arterial pressure, WBC, PLT, 25(OH)D3, NT-proBNP, serum potassium, PBG, discharge Scr, and RRT rate were risk factors that affect the renal function of diabetic patients with AKI (p < 0.05). The above variables were included in logistic regression analysis. The results showed that increase of diabetes duration (OR = 1.022, 95% CI 1.012–1.032), history of hypertension (OR = 1.574, 95% CI 1.043–2.377), history of CKD (OR = 2.241, 95% CI 1.399–3.591), decrease of PLT (OR = 0.997, 95% CI 0.995–1.000), lower 25(OH)D3 (OR = 0.966, 95% CI 0.956–0.976), increase of PBG (OR = 1.104, 95% CI 1.032–1.181), increase of discharged Scr (OR = 1.003, 95% CI 1.001–1.005) are the independent risk factors affecting the 90-day non-recovery of function of diabetic patients with AKI (as shown in Table 3).
Construction and validation of predictive models
Based on the above results, a predictive model for 90-day renal prognosis risk in diabetic patients with AKI was constructed. The nomogram is shown in Fig. 1. By calculating the scores corresponding to the risk factors, the individual’s non-recovery rate of renal function at 90 days after the diagnosis of AKI was estimated. Taking a patient as an example, when predicting the 90-day non-recovery rate of renal function, the score of each influencing factor is calculated first. For example, 14 points for the diabetes duration of 30 months, 10 points for history of hypertension, 0 point for having no history of CKD, 22.5 points for PLT of 150 × 109/L, 68.75 points for 25(OH)D3 of 50 nmol/L, 18.75 points for PBG of 12 mmol/L, 20 points for discharge Scr of 300 µmol/L. The total score of this patient was 154 points (14 + 10 + 0 + 22.5 + 68.75 + 18.75 + 20), the risk of non-recovery rate of renal function at 90 days in diabetic patients with AKI was about 72.5%.
The C-index of the prediction model in the development cohort was 0.807 (95% CI 0.738–0.875), the area under the ROC curve (AUROC) of the prediction model for predicting 90-day non-recovered renal function in diabetic patients with AKI was 0.809, and the Youden index was 0.619, the sensitivity and specificity were 0.746 and 0.873, respectively (Fig. 2A). The Hosmer-Lemeshow test of the model in the development cohort was P = 0.326, the Brier score was 0.065, and the calibration curve was a straight line with a slope close to 1 (Fig. 3A). The C-index of the prediction model in the validation cohort was 0.803 (95% CI 0.713–0.893). As shown in Fig. 2B, the AUROC for predicting the 90-day non-recovered renal function in diabetic patients with AKI was 0.748, and the Youden index was 0.598, the sensitivity and specificity were 0.709 and 0.889, respectively. The Hosmer-Lemeshow test of the model in the validation cohort was P = 0.117, the Brier score was 0.082, and the calibration curves were all close to a straight line with a slope of 1 (Fig. 3B).
Clinical use of the model
Decision curve analysis demonstrated the clinical utility of the prediction model based on a continuous potential risk threshold (x-axis) and the net benefit of using the model to risk stratify patients (y-axis). The black line represents that all patients have recovery of renal function, and the gray line represents that all patients have non-recovery of renal function. The threshold probability of the predictive model in the development cohort was 25–100% to produce the greatest benefit (Fig. 4A), and the threshold probability of the predictive model in the validation cohort was in the range of 20–96% to yield the greatest benefit (Fig. 4B), indicating that the model has good clinical practicability in predicting the 90-day non-recovery of renal function in diabetic patients with AKI. For example, if diabetic patients with AKI has an individual threshold probability of 40% (i.e., if he has a > 40% risk of non-recovery of renal function at 90 days, he will choose to undergo further detailed screening), use the nomogram to decide whether to proceed on further detailed screening, its net benefit was 0.35 in the development cohort (0.50 in the validation cohort).
Discussion
In this study, the influencing factors of short-term renal prognosis were evaluated based on the demographic characteristics and clinical data of diabetic patients with AKI, and a prediction model of prognostic risk was constructed. The results showed that diabetes duration, history of hypertension and CKD, increase of PBG and discharged Scr were the risk factors for non-recovery of renal function at 90-day in diabetic patients with AKI; while decrease of PLT and 25(OH)D3 were the protective factors. The prediction model has good discrimination and accuracy through internal verification of Bootstrapping resampling techniques with 1,000 replications and validation cohort. The decision curve analysis also showed good clinical utility. In addition, the predictors used in this model are readily available at discharge and can be used to predict short-term renal prognosis in diabetic patients with AKI, and patients are individually assessed and followed up using this risk stratification [20]. Moreover, early intervention in high-risk patients who had non-recovery of renal function at 90 days may improve renal outcomes and survival in diabetic patients with AKI.
Numerous AKI prediction models have been reported in the previous literature. However, most of prognostic models were developed for specific patient populations (e.g., septic shock, post-cardiac surgery, or critically ill patients) [21,22,23]. Previous study by our team had constructed a prediction model for all-cause mortality in diabetic patients with AKI based on advanced age, low pulse pressure difference, low 25(OH)D3 and multiple organ dysfunction syndrome, and the C-indices were 0.880 and 0.798 in development and validation cohorts, respectively [24]. Diabetes increases the risk of poor renal prognosis in patients with AKI. Then, the clinical question arises: what are the risk factors of poor renal prognosis for diabetic patients with AKI? At present, there are still few predictive models for the non-recovery of renal function in diabetic patients with AKI. In this study, based on the multivariate Logistic stepwise regression analysis results, a predictive model for 90-day non-recovery of renal function in diabetic patients with AKI was constructed. The final variables included in the model were: duration of diabetes, history of CKD, history of hypertension, PLT, 25(OH)D3, PBG, discharged Scr. We hoped that this prediction model could be used to guide clinicians in their decisions to aid prognostication or to stratify disease severity and treatment.
A prospective study showed that, compared with patients with diabetes duration of less than 5 years, patients with diabetes duration of more than 20 years had a faster decline in renal function [25]. Patients with diabetes with a longer duration generally had poorer glycemic control and were more likely to have other comorbidities. Moreover, metabolic disease, and microvascular disease may be more severe [26]. These adverse risk factors may make it more difficult to recovery of renal function in diabetic patients with AKI, which is consistent with the results of high diabetes duration and PBG in our prediction model. History of CKD and hypertension are traditional risk factors for AKI in diabetic patients, as well as risk factors for the progression of AKI to CKD and ESRD [27, 28]. An observational study showed that critically ill patients with a history of CKD to admission had a significantly increased risk of death [29]. Patients with AKI in addition to CKD are at very high risk for ESRD [27]. Diabetic patients with 25(OH)D < 20 ng/ml and 25(OH)D < 30 ng/ml had a higher risk of developing proteinuria (OR = 2.8, 95% CI 1.6–4.9; OR = 2.1, 95% CI 1.3–4.6) [30]. Vitamin D may reduce oxidative stress by enhancing renal antioxidant capacity [31, 32], prevent damage to podocytes by inhibiting hyperglycemia-induced apoptosis, promote anti-inflammatory effects, and improve endothelial function, thereby exerting renal protection [33]. These findings were also confirmed by our regression model, with low 25(OH)D being an independent risk factor for non-recovery of renal function in diabetic patients with AKI. A predictive model of AKI progression to CKD included externally validated study also showed that higher discharge Scr was an independent risk factor for progression to advanced CKD in AKI patients (OR = 37.01, 95% CI 19.46–70.37) [20].
Although AKI is generally reversible, some patients may experience incomplete recovery of renal function, while others experience subsequent accelerated renal loss leading to an increased risk of CKD [34]. In a 10-year study of 3679 patients, AKI episode was an important risk factor for the progression of renal function to stage 4 CKD (HR = 3.56, 95% CI 2.76–4.71), and each episode of AKI doubled that risk (HR = 2.02, 95% CI 1.78–2.30) [35]. Our study showed that the 90-day renal function failure rate was 66.8%, while a retrospective cohort study showed that the 7-day failure rate of renal function recovery in diabetes patients with AKI was about 46.9% [36]. It can be seen that the short-term renal prognosis of diabetic patients with AKI is poor. However, the differences in renal function recovery rates between previous studies and this study may be related to differences in basic characteristics of patients, disease severity, follow-up time and treatment strategies [37].
Incomplete recovery from AKI can lead to chronic dysfunction and progressive decline in renal function, especially in patients with pre-existing CKD [38]. Poorly healed of tubules have been reported to cause disproportionate scarring, leading to loss of peritubular capillaries, resulting in volume-dependent salt-sensitive hypertension and subsequent glomerular damage [39]. Renal damage affecting structure and function could be classified as acute or chronic at 3 months. Clinical practice guidelines also recommend a 3-month follow-up to assess whether patients with AKI develop CKD [40, 41]. Therefore, 90 days after the occurrence of AKI was selected as the time cutoff to observe the recovery of renal function in this study, and a clinical prediction model for short-term non-recovery of renal function in diabetic patients with AKI was developed.
However, this study has several limitations. First, this is a single-center retrospective study with limited sample size and rather short-term following up. Second, the diagnosis of AKI was only based on changes in Scr, and only cases of AKI with decreased urine output might be missed. Third, Scr values were only collected at baseline and 90 days after AKI, which lacked dynamic variation values. Therefore, it is not possible to determine whether patients develop AKI more than once. Fourth, this study only carried out internal verification and did not include external verification. It is still necessary to further expand the sample size for external verification of the predictive performance of the model.
Conclusion
Increase of diabetes duration, history of hypertension, CKD, decrease of PLT, 25(OH)D3, increase of PBG and discharged Scr are independent risk factors for non-recovery of renal function in diabetic patients with AKI within 90 days. Based on the above variables, a nomogram can be used to establish a prediction model to help predict the short-term renal prognosis, which has been verified to have good discrimination, calibration and good clinical practicability.
Availability of data and materials
Considering the privacy of patients, if readers have similar research and want to obtain data related to the article, they can contact the corresponding author, the corresponding research data can be obtained with permission.
References
IDF Diabetes atlas 10th edition 2021. https://www.diabetesatlas.org/en/. Accessed 20 Dec 2021.
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119.
Vupputuri S, Kimes TM, Calloway MO, Christian JB, Bruhn D, Martin AA, et al. The economic burden of progressive chronic kidney disease among patients with type 2 diabetes. J Diabetes Complicat. 2014;28(1):10–6.
Advani A. Acute kidney injury: a bona fide complication of diabetes. Diabetes. 2020;69(11):2229–37.
James MT, Grams ME, Woodward M, Elley CR, Green JA, Wheeler DC, et al. A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis. 2015;66(4):602–12.
Go AS, Hsu CY, Yang J, Tan TC, Zheng S, Ordonez JD, et al. Acute kidney injury and risk of heart failure and atherosclerotic events. Clin J Am Soc Nephrol. 2018;13(6):833–41.
Monseu M, Gand E, Saulnier PJ, Ragot S, Piguel X, Zaoui P, et al. Acute kidney Injury predicts major adverse outcomes in diabetes: synergic impact with low glomerular filtration rate and albuminuria. Diabetes Care. 2015;38(12):2333–40.
Arbel Y, Fuster V, Baber U, Hamza TH, Siami FS, Farkouh ME. Incidence, determinants and impact of acute kidney injury in patients with diabetes mellitus and multivessel disease undergoing coronary revascularization: results from the FREEDOM trial. Int J Cardiol. 2019;293:197–202.
Harding JL, Li Y, Burrows NR, Bullard KM, Pavkov ME. US trends in hospitalizations for dialysis-requiring acute kidney injury in people with versus without diabetes. Am J kidney Dis. 2020;75(6):897–907.
Pavkov ME, Harding JL, Burrows NR. Trends in hospitalizations for acute kidney injury—United States, 2000–2014. Morbidity Mortal Wkly Rep. 2018;67(10):289–93.
Tan L, Chen L, Jia Y, Li L, Wang J, Huang X, et al. Impact of diabetes mellitus on short-term prognosis, length of stay, and costs in patients with acute kidney injury: a nationwide survey in China. PLoS ONE. 2021;16(5):e0250934.
Li W, Xie B, Qiu S, Huang X, Chen J, Wang X, et al. Non-lab and semi-lab algorithms for screening undiagnosed diabetes: a cross-sectional study. EBioMedicine. 2018;35:307–16.
Xu L, Li C, Zhao L, Zhou B, Luo C, Man X, et al. Acute kidney injury after nephrectomy: a new nomogram to predict postoperative renal function. BMC Nephrol. 2020;21(1):181.
Horne KL, Packington R, Monaghan J, Reilly T, Selby NM. Three-year outcomes after acute kidney injury: results of a prospective parallel group cohort study. BMJ open. 2017;7(3):e015316.
Chao CT, Tsai HB, Wu CY, Lin YF, Hsu NC, Chen JS, et al. The severity of initial acute kidney injury at admission of geriatric patients significantly correlates with subsequent in-hospital complications. Sci Rep. 2015;5:13925.
Sawhney S, Marks A, Fluck N, Levin A, McLernon D, Prescott G, et al. Post-discharge kidney function is associated with subsequent ten-year renal progression risk among survivors of acute kidney injury. Kidney Int. 2017;92(2):440–52.
Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–30.
Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53.
Pan L, Mo M, Huang A, Li S, Luo Y, Li X, et al. Coagulation parameters may predict clinical outcomes in patients with septic acute kidney injury. Clin Nephrol. 2021;96(5):253–62.
James MT, Pannu N, Hemmelgarn BR, Austin PC, Tan Z, McArthur E, et al. Derivation and external validation of prediction models for advanced chronic kidney disease following acute kidney injury. JAMA. 2017;318(18):1787–97.
Yue S, Li S, Huang X, Liu J, Hou X, Wang Y, et al. Construction and validation of a risk prediction model for acute kidney injury in patients suffering from septic shock. Dis Markers. 2022;2022:9367873.
Koyner JL, Adhikari R, Edelson DP, Churpek MM. Development of a multicenter ward-based AKI prediction model. Clin J Am Soc Nephrol. 2016;11(11):1935–43.
Malhotra R, Kashani KB, Macedo E, Kim J, Bouchard J, Wynn S, et al. A risk prediction score for acute kidney injury in the intensive care unit. Nephrol Dial Transplant. 2017;32(5):814–22.
Mo M, Pan L, Huang Z, Liang Y, Liao Y, Xia N. Development and validation of a prediction model for survival in diabetic patients with acute kidney injury. Front Endocrinol. 2021;12:737996.
Buyadaa O, Salim A, Morton JI, Magliano DJ, Shaw JE. Rate of decline in kidney function and known age-of-onset or duration of type 2 diabetes. Sci Rep. 2021;11(1):14705.
Yeung RO, Zhang Y, Luk A, Yang W, Sobrepena L, Yoon KH, et al. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): a cross-sectional study of a prospective cohort. Lancet Diabetes Endocrinol. 2014;2(12):935–43.
Yang L. How acute kidney injury contributes to renal fibrosis. Adv Exp Med Biol. 2019;1165:117–42.
Safadi S, Hommos MS, Enders FT, Lieske JC, Kashani KB. Risk factors for acute kidney injury in hospitalized non-critically Ill patients: a population-based study. Mayo Clin Proc. 2020;95(3):459–67.
Fortrie G, de Geus HRH, Betjes MGH. The aftermath of acute kidney injury: a narrative review of long-term mortality and renal function. Crit Care. 2019;23(1):24.
Felício JS, de Rider Britto HA, Cortez PC, de Souza Resende F, de Lemos MN, de Moraes LV, et al. Association between 25(OH)vitamin D, HbA1c and albuminuria in diabetes mellitus: data from a population-based study (VIDAMAZON). Front Endocrinol. 2021;12:723502.
Deng X, Cheng J, Shen M. Vitamin D improves diabetic nephropathy in rats by inhibiting renin and relieving oxidative stress. J Endocrinol Investig. 2016;39(6):657–66.
Franca Gois PH, Wolley M, Ranganathan D, Seguro AC. Vitamin D deficiency in chronic kidney disease: recent evidence and controversies. Int J Environ Res Public Health. 2018;15(8):1773.
Lei M, Liu Z, Guo J. The emerging role of vitamin D and vitamin D receptor in diabetic nephropathy. Biomed Res Int. 2020;2020:4137268.
Kellum JA, Romagnani P, Ashuntantang G, Ronco C. Acute kidney injury. Nat Rev Dis Primers. 2021;7(1):52.
Thakar CV, Christianson A, Himmelfarb J, Leonard AC. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol. 2011;6(11):2567–72.
Jiang G, Luk AO, Tam CHT, Ozaki R, Lim CKP, Chow EYK, et al. Clinical predictors and long-term impact of acute kidney injury on progression of diabetic kidney disease in chinese patients with type 2 diabetes. Diabetes. 2022;71(3):520–9.
Forni LG, Darmon M, Ostermann M, Oudemans-van Straaten HM, Pettilä V, Prowle JR, et al. Renal recovery after acute kidney injury. Intensive Care Med. 2017;43(6):855–66.
Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371(1):58–66.
Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK, Failed Tubule Recovery AKI-CKD. Transition, and kidney disease progression. J Am Soc Nephrol. 2015;26(8):1765–76.
Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR, Thakar CV, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J kidney Dis. 2013;61(5):649–72.
Godin M, Macedo E, Mehta RL. Clinical determinants of renal recovery. Nephron Clin Pract. 2014;127(1–4):25–9.
Funding
This work was supported by the National Natural Science Foundation of China (NO. 8176030057), the Guangxi Natural Science Foundation (No. 2018GXNSFBA050040), Guangxi Clinical Research Center for Urology and Nephrology (No. 2020AC03006), and the Scientific Research and Technological Development Program of Guangxi (No. GuiKeGong 1598011-6).
Author information
Authors and Affiliations
Contributions
MM: study design, data analysis, writing the manuscript; ZH: study design, data analysis, data collection, and writing the manuscript; TG, YLU, XP: study design, revised the manuscript; ZY: data collection and data analysis; NX, LP, YLI: data collection and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University [approval number: 2019(KY-E-028)]. Participants were required to sign (or have a proxy sign) informed consent for data collection. This study was conducted in accordance with the tenets of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mo, M., Huang, Z., Gao, T. et al. Development and validation of short-term renal prognosis prediction model in diabetic patients with acute kidney injury. Diabetol Metab Syndr 14, 197 (2022). https://doi.org/10.1186/s13098-022-00971-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-022-00971-1