Abstract
Background
There is no predictive tool for type 2 diabetes mellitus (T2DM) patients with acute kidney injury (AKI). Our study aimed to establish an effective nomogram model for predicting mortality in T2DM patients with AKI.
Method
Data on T2DM patients with AKI were obtained from the Medical Information Mart for Intensive Care III. 70% and 30% of the patients were randomly selected as the training and validation cohorts, respectively. Univariate and multivariate logistic regression analyses were used to identify factors associated with death in T2DM patients with AKI. Factors significantly associated with survival outcomes were used to construct a nomogram predicting 90-day mortality. The nomogram effect was evaluated by receiver operating characteristic curve analysis, Hosmer‒Lemeshow test, calibration curve, and decision curve analysis (DCA).
Results
There were 4375 patients in the training cohort and 1879 in the validation cohort. Multivariate logistic regression analysis showed that age, BMI, chronic heart failure, coronary artery disease, malignancy, stages of AKI, white blood cell count, blood urea nitrogen, arterial partial pressure of oxygen and partial thromboplastin time were independent predictors of patient survival. The results showed that the nomogram had a higher area under the curve value than the sequential organ failure assessment score and simplified acute physiology score II. The Hosmer‒Lemeshow test and calibration curve suggested that the nomogram had a good calibration effect. The DCA curve showed that the nomogram model had good clinical application value.
Conclusion
The nomogram model accurately predicted 90-day mortality in T2DM patients with AKI. It may provide assistance for clinical decision-making and treatment, thereby reducing the medical burden.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disease caused by various etiologies leading to dysfunction of insulin secretion or action. A study [1] predicted that the number of patients with diabetes will gradually increase, and the economic burden will also further increase. T2DM and diabetes-related complications are also major causes of hospitalization, disability, and death [2, 3]. Diabetes increases the risk of acute kidney injury (AKI), which can sometimes be regarded as an acute complication of diabetes [4]. AKI is a sudden renal dysfunction syndrome with a high incidence rate and mortality, is common in patients with critical illness and cardiac surgery and is associated with genetic susceptibilities [5,6,7]. Studies have found that AKI affects more than 13 million people per year, 80% of patients live in the developing world, and AKI contributes to 1.7 million deaths annually [8, 9]. Several studies [10, 11] have found that approximately 50% of critically ill patients develop AKI, and 11.0% of patients with severe AKI die in intensive care units (ICU). A study [12] found that 40% of AKI patients had diabetes. In acutely unwell patients with AKI who have underlying diabetes, there is a serious risk of medical complications that have significant financial implications. Therefore, it is necessary to pay attention to the prognosis of T2DM patients with AKI.
Li et al. [13] constructed a predictive model for the occurrence of AKI in the ICU, and the area under curve (AUC) of the AKI prognostic model was 0.716. Fan et al. [14] constructed a nomogram to predict the risk of AKI in patients with diabetic ketoacidosis in the ICU. In these AKI prognostic models, the results in diabetes patients were not considered. A study [15] used machine learning to find the best model for predicting the death of diabetic patients in the ICU, but that study did not further explore the prognosis of this model in diabetic patients with AKI. Acute physiology chronic health evaluation (APACHE) II, simplified acute physiology score (SAPS) III, and sequential organ failure assessment (SOFA) scores are commonly used to predict patient prognosis in the ICU [16,17,18]. Interestingly, how valuable these predictive models will be in T2DM patients with AKI. In addition, we aimed to establish a nomogram that integrated multiple independent significant factors to better predict 90-day mortality in T2DM patients with AKI to further provide some help for medical decision-making.
Materials and methods
Data source
After relevant training, we obtained access to the Medical Information Mart for Intensive Care III (MIMIC-III) (https://physionet.org/content/mimiciii/1.4/). MIMIC-III is a publicly available ICU database that contains data on approximately 50,000 patients, including general information, clinical information, and related medical insurance data of patients [19]. Access to the database was approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center (Boston, MA) and the Massachusetts Institute of Technology (Cambridge, MA). The patient’s information in the database had been standardized, and the establishment of these data did not affect clinical care and was thus exempted from the requirement of individual informed consent.
Inclusion and exclusion criteria
There are 58,976 hospitalizations in the MIMIC-III database. The inclusion criteria for this study were as follows: (1) patients admitted to the ICU for the first time and (2) patients with an ICD code for T2DM. The exclusion criteria were: (1) younger than 18 years of age; (2) without AKI. For inclusion, the patients had to be diagnosed with AKI after entering in ICU, in which the diagnosis was based on the kidney disease: improving global outcomes guidelines [20]. (3) less than 48 h in the ICU; and (4) variables that had missing data for more than 5% of the patients. Finally, 6254 patients were included in this study. The participants were randomly divided into a training cohort (70%) and a validation cohort (30%) (Fig. 1).
Data extraction
We extracted data from the database with structured query language in PostgreSQL. We mainly extracted demographic information, clinical laboratory data and related scoring information (Supplementary Table 1).
Statistical analysis
Continuous variable data were expressed as the mean ± standard deviation for normal distribution; interquartile ranges (IQRs) were used for variables with nonnormal distribution. The categorical variables were expressed as the total and percentage, and the chi-square test was used to evaluate categorical data for two group comparisons. Student’s t test was used for comparison between two groups of data with normal distribution, and Wilcoxon rank-sum test was used for comparison between two groups of data with nonnormal distribution. Univariate and multivariate logistic regression were used to identify predictors of 90-day mortality in the training cohort. These predictors were further applied to build a nomogram for estimating 90-day mortality. Finally, the nomogram was verified using data from the validation cohort.
Receiver operating characteristic curve (ROC) analysis, the Hosmer‒Lemeshow test, and calibration curves were used to evaluate the accuracy of nomogram prediction. The clinical value of the nomogram was verified based on decision curve analysis (DCA). P < 0.05 was considered statistically significant. All statistical analyses were carried out using Stata version 16.0.
Results
Baseline characteristics
A total of 6254 patients were enrolled and randomly allocated to a training cohort (n = 4375) and a validation cohort (n = 1879) in our study (Fig. 1). The training cohort included 1832 (41.9%) females and 2543 (58.1%) males with a median age of 69.6 years (IQR = 60.6–78.3 years) with an average body mass index (BMI) of 30 (IQR = 25.5–35.2), whereas the validation cohort included 799 (42.5%) females and 1080 (57.5%) males with a median age of 69.9 years (IQR = 61.1–78.6 years) and an average BMI of 29.8 (IQR = 25.4–34.5). Most of the patients in the training and validation cohorts were white (> 60%). The median length of hospital stay was 2.9 days (IQR = 1.5–5.3 days) in the training cohort and 3.0 days (IQR = 1.7–5.4 days) in the validation cohort. The 30- and 90-day mortality rates in the training cohort and validation cohort were 15% (n = 656) and 19% (n = 833) and 14% (262) and 18.4% (346), respectively. The 90-day mortality rate was selected for further analyses. The baseline characteristics of the training and validation cohorts did not differ significantly (Table 1).
Nomogram construction
Univariate logistic regression analyses showed that the significant predictors of 90-day mortality were age, BMI, chronic heart failure (CHF), coronary artery disease(CAD), hypertension, RRT, malignancy, stage of AKI, SOFA score, SAPS II score, white blood cell (WBC) count, platelet count, hemoglobin (HGB), sodium, phosphate, calcium (Ca), creatinine, blood urea nitrogen (Bun), arterial partial pressure of oxygen (PaO2), lactate (Lac) and partial thromboplastin time (PTT) in the training group (Table 2). The predictors differing significantly in the univariate analyses (P < 0.05) were included in a multivariable logistic regression model with forward stepwise selection. The multivariate analysis showed that the factors predictive of improved 90-day survival included BMI (OR = 0.960, P < 0.001), CAD (OR = 0.494, P < 0.001) and PaO2 (OR = 0.997, P < 0.001), whereas risk factors included age (OR = 1.031, P < 0.001), CHF (OR = 1.287, P = 0.004), malignancy (OR = 1.714, P < 0.001), stage of AKI (OR = 1.642, P < 0.001), WBC count (OR = 1.035, P < 0.001), PTT (OR = 1.005, P < 0.001) and Bun (OR = 1.015, P < 0.001) (Table 3). A nomogram was established based on the significant variables identified in the multivariate analyses (Fig. 2). The nomogram showed that BMI had the greatest impact on prognosis, followed by age, Bun, PaO2, stages of AKI, WBC count, CAD, PTT, malignancy and CHF.
Assessment and validation of the nomogram performance
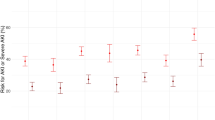
According to the ROC analysis, the AUC value of the training cohort was 0.768 (95% CI = 0.751–0.785), which showed a significantly higher AUC value than the SOFA and SAPS II score systems (Fig. 3). The Hosmer‒Lemeshow test (χ2 = 11.75, P = 0.302) and calibration curves indicated good calibration of the model in the training cohort (Fig. 4). The AUC value of the validation was 0.779 (95% CI = 0.754–0.804), which showed significantly higher AUC values than the SOFA and SAPS II score systems (Fig. 3). The Hosmer‒Lemeshow test (χ2 = 11.22, P = 0.478) and calibration curves also indicated good calibration of the model in the validation cohorts (Fig. 4). The DCA curves showed that the nomogram had favorable clinical validity in predicting 90-day mortality (Fig. 5).
DCA curves of the training and validation cohorts. The horizontal line indicates that all samples were negative and were not treated, with a net benefit of 0. The oblique line indicates that all samples were positive. The brown line shows the net benefit of SOFA score, the orange line shows the net benefit of the SAPS II score, and the blue line shows the net benefit of the nomogram
Discussion
Studies have shown that diabetes is an independent risk factor for the incidence of AKI [21, 22]. AKI was significantly associated with increased mortality in critically ill patients [23]. We attempted to establish a convenient and objective scoring model to predict the risk of 90-day mortality in T2DM patients with AKI and for further individualized treatment.
As age increases, the risk of death will increase, owing to the weakened capacity of kidney reserve in all DM patients [24]. Another study [25] found that age was positively correlated with all-cause mortality in all T2DM patients. Similarly, in our study, we also found that age was significantly associated with an increased risk of 90-day mortality in T2DM patients with AKI. Heart failure led to worsening of clinical outcomes and was significantly associated with an increased risk of death in T2DM patients [26]. We also concluded that T2DM patients with CHF have a higher risk of 90-day mortality, which is similar to the opinion that the interaction between DM, heart failure and kidney dysfunction, which forms a vicious cycle and can increase the occurrence of poor prognosis [27]. A study [28] found that a higher WBC count was a predictor of death in DM patients with heart failure. A higher WBC count was associated with an increased risk of death in T2DM patients [29]. Our model showed that WBC count was a significant independent risk prognostic factor for T2DM patients with AKI. It is well known that elevated WBC counts indicate an inflammatory state, which can cause cell damage and further induce organ dysfunction, resulting in patient death [30]. Elevated Bun can further increase the risk of poor prognosis in T2DM patients [31]. A study [32] found that a high Bun level was a risk factor for death in patients with AKI. Similarly, we concluded that elevated Bun was significantly associated with an increased risk of mortality in T2DM patients with AKI. CAD that indicates the coronary artery stenosis is greater than 50% is an independent risk factor for death in T2DM patients [33]. We concluded that CAD was favorable for the prognosis of T2DM patients with AKI, which differs from past opinions. This may be because patients had taken preventive and therapeutic measures to improve the prognosis of CAD prior to hospital. Coagulation disorders, including thrombocytopenia, elevated INR and prolonged APTT, may predict adverse clinical outcomes in patients with septic AKI [34]. In our model, prolonged PTT also increased the risk of death in T2DM patients with AKI. A study [35] found that malignancy patients with diabetes had higher all-cause mortality than those without diabetes. Cancer was an independent risk factor for T2DM with AKI [36]. Our study also found that malignancy was associated with an increased risk of 90-day mortality. A study [37] found that obesity was not only a risk factor for AKI but also a risk factor for death in AKI patients. However, a large multicenter cohort of critically ill patients reported that overweight patients had a lower risk of 60-day mortality [38]. In addition, a meta-analysis reported that overweight and obese patients could more easily improve their prognosis compared with normal BMI patients [39]. Similarly, in our model, we also found that higher BMI can reduce the risk of death. Critically ill patients are often in a state of consumption. Patients with moderately high BMI may have a relatively good compensatory capacity, thereby reducing the risk of death. Moreover, adipokines secreted by adipocytes may weaken the inflammatory response, thereby potentially improving the survival rate of critically ill patients [40]. Based on the KDIGO criteria, AKI stage represents the degree of kidney function damage. A study [41] showed that the risk of death in hospitalized patients was positively correlated with the stage of AKI, with the highest mortality in patients with stage 3 AKI. This was consistent with our findings that the stage of AKI was associated with an increased risk of 90-day mortality. SpO2 reflects the body’s oxygen supply and degree of hypoxia, which is a factor related to critical illness [42]. We also concluded that T2DM patients with low PaO2 have a higher risk of 90-day mortality.
We often use a series of scoring systems to predict the prognosis of patients, such as SOFA scores and SAPS II scores. SOFA and SAPS II scores are the most commonly used clinical scoring systems and can effectively evaluate the prognosis of severe patients in the ICU [43, 44]. However, the predictive value of these scoring systems is different in different diseases. The main advantage of our study was the establishment of a nomogram based on objective indicators to predict the prognosis of T2DM patients with AKI. The AUC value of our model was higher than that of the SOFA and SAPS II scores, and the Hosmer‒Lemeshow test and correction curve confirmed that the model had good discrimination power in both the training cohort and validation cohort.
There are several limitations of the study. First, our study was a single-center retrospective study, and there was selection bias. Second, there were uncontrollable confounding factors affecting the results, such as the use of drugs and unspecified comorbidities. Third, the database was relatively old, and our model needs to be validated by using external data from a recent multicenter study.
Conclusion
In this study, we developed and validated a nomogram model for predicting 90-day mortality in T2DM patients with AKI. The model included 10 indicators that were easily obtained in clinical practice, showing good clinical applicability. We hope that our model can help clinicians better distinguish patients with high risk of death, and timely formulate treatment plans and interventions to reduce the death of patients.
Availability of data and materials
Original data used in this study is from the MIMIC-III database: MIMIC III (https://physionet.org/content/mimiciii/1.4/, version 1.4). The author (S.L.) obtained access to this database (certification number: 42883491) and was responsible for extracting the data. If needed, related data can be provided by contacting G.H. and S.L.
References
Roden M, Shulman GI. The integrative biology of type 2 diabetes. Nature. 2019;576(7785):51–60.
Bhutani J, Bhutani S. Worldwide burden of diabetes. Indian J Endocrinol Metab. 2014;18:868–70.
Monge L, Gnavi R, Carnà P, et al. Incidence of hospitalization and mortality in patients with diabetic foot regardless of amputation: a population study. Acta Diabetol. 2020;57(2):221–8.
Advani A. Acute kdney injury: a bona fide complication of diabetes. Diabetes. 2020;69(11):2229–37.
Casanova AG, Sancho-Martínez SM, Vicente-Vicente L, et al. Diagnosis of cardiac surgery-associated acute kidney injury: state of the art and perspectives. J Clin Med. 2022;11(15):4576.
Ortega-Loubon C, Martínez-Paz P, García-Morán E, et al. Genetic susceptibility to acute kidney injury. J Clin Med. 2021;10(14):3039.
Chen JJ, Chang CH, Wu VC, et al. Long-term outcomes of acute kidney injury after different types of cardiac surgeries: a population-based study. J Am Heart Assoc. 2021;10(9):e019718.
Lewington AJ, Cerdá J, Mehta RL. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 2013;84:457–67.
Mehta RL, Cerdá J, Burdmann EA, et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385:2616–43.
Liborio AB, Leite TT, Neves FM, et al. AKI complications in critically ill patients: Association with mortality rates and RRT. Clin J Am Soc Nephrol. 2015;7(1):21–8. 10(.
Kaddourah A, Basu RK, Bagshaw SM, et al. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;5(1):11–20. 376(.
Pavkov ME, Harding JL, Burrows NR. Trends in hospitalizations for acute kidney injury-United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018;67:289–93.
Li M, Yang H, Yang W, et al. Development of acute kidney injury prognostic model for critically ill patients based on MIMIC-III database. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021;33(8):949–54.
Fan T, Wang H, Wang J, et al. Nomogram to predict the risk of acute kidney injury in patients with diabetic ketoacidosis: an analysis of the MIMIC-III database. BMC Endocr Disord. 2021;21(1):37.
Anand RS, Stey P, Jain S, et al. Predicting mortality in diabetic ICU patients using machine learning and severity indices. AMIA Jt Summits Transl Sci Proc. 2018;2017:310–9.
Niewinski G, Starczewska M, Kanski A. Prognostic scoring systems for mortality in intensive care units–the APACHE model. Anaesthesiol Intensive Ther. 2014;46(1):46–9.
Moreno RP, Metnitz PGH, Almeida E, et al. SAPS 3-From evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2006;32(5):1345–55.
Vincent J-L, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707–10.
Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;24:3:160035.
Kellum JA, Lameire N, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care. 2013;4(1):204. 17(.
Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3(3):844–61.
James MT, Grams ME, Woodward M, et al. A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis. 2015;66(4):602–12.
Perinel S, Vincent F, Lautrette A, et al. Transient and persistent acute kidney injury and the risk of hospital mortality in critically ill patients: results of a multicenter cohort study. Crit Care Med. 2015;43(8):e269-75.
Kam MW, Lau G. IT, et al. Clinical predictors and long-term impact of acute kidney injury on progression of diabetic kidney disease in chinese patients with type 2 diabetes. Diabetes. 2022;1(3):520–9. 71(.
Tancredi M, Rosengren A, Svensson AM, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373(18):1720–32.
Birkeland KI, Bodegard J, Eriksson JW, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes Obes Metab. 2020;22(9):1607–18.
Braunwald E. Diabetes, heart failure, and renal dysfunction: the vicious circles. Prog Cardiovasc Dis. 2019;62(4):298–302.
Kawabe A, Yasu T, Morimoto T. T et al. WBC count predicts heart failure in diabetes and coronary artery disease patients: a retrospective cohort study. ESC Heart Fail. 2021;8(5):3748–59.
Chang YK, Huang LF, Shin SJ, et al. A point-based mortality prediction system for older adults with diabetes. Sci Rep. 2017;4(1):12652. 7(.
Amulic B, Cazalet C, Hayes GL, et al. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–89.
Liu F, Ma G, Tong C, et al. Elevated blood urea nitrogen-to-creatinine ratio increased the risk of coronary artery disease in patients living with type 2 diabetes mellitus. BMC Endocr Disord. 2022;28(1):50. 22(.
Uchino S, Bellomo R, Goldsmith D. The meaning of the blood urea nitrogen/creatinine ratio in acute kidney injury. Clin Kidney J. 2012;5(2):187–91.
Htay T, Soe K, Lopez-Perez A, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. Curr Cardiol Rep. 2019;22(6):45. 21(.
Pan L, Mo M, Huang A, et al. Coagulation parameters may predict clinical outcomes in patients with septic acute kidney injury. Clin Nephrol. 2021;96(5):253–62.
Kiburg KV, Ward GM, Vogrin S, et al. Impact of type 2 diabetes on hospitalization and mortality in people with malignancy. Diabet Med. 2020;37(2):362–8.
Juwon L, Jang G, Kim S, et al. Outcomes of acute kidney injury patients with and without cancer. Ren Fail. 2015;37(10):332–7.
Danziger J, Chen KP, Lee J, et al. Obesity, acute kidney injury, and mortality in critical illness. Crit Care Med. 2016;44(2):328–34.
Sakr Y, Alhussami I, Nanchal R, et al. Being overweight is associated with greater survival in ICU patients: results from the intensive care over nations audit. Crit Care Med. 2015;43(12):2623–32.
Hogue CW Jr, Stearns JD, Colantuoni E, et al. The impact of obesity on outcomes after critical illness: a meta-analysis. Intensive Care Med. 2009;35(7):1152–70.
Hauner H. Secretory factors from human adipose tissue and their functional role. Proc Nutr Soc. 2005;64(2):163–9.
Khadzhynov D, Schmidt D, Hardt J, et al. The incidence of acute kidney injury and associated hospital mortality. Dtsch Arztebl Int. 2019;31(22):397–404. 116(.
de Jonge E, Peelen L, Keijzers PJ, et al. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care. 2008;12(6):R156.
Godinjak A, Iglica A, Rama A, et al. Predictive value of SAPS II and APACHE II scoring systems for patient outcome in a medical intensive care unit. Acta Med Acad. 2016;45(2):97–103.
Raith EP, Udy AA, Bailey M, et al. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017;317(3):290–300.
Acknowledgements
We thank AJE for the linguistic editing and proofreading of the manuscript.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualization, S.L. and G.H.; methodology, S.L., C.Q., F.L., and G.H.; software, S.L., Z.Y., and C.Q; validation, S.L., C.Q., and F.L.; formal analysis, X.L., S.L., and C.Q.; investigation, S.L., and X.L.; resources, S.L.; data curation, Z.Y., S.L. and C.Q.; writing—original draft preparation, all authors; writing—review and editing, C.Q., S.L., and G.H.; visualization, S.L.; supervision, G.H. and F.L.; project administration, S.L.; All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The use of the database was approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center (Boston, MA) and the Massachusetts Institute of Technology (Cambridge, MA). The patient’s information has been standardized and the project did not affect clinical care, so requirement for individual patient consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Extracted variables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, S., Qiu, C., Li, X. et al. A nomogram for predicting the mortality of patients with type 2 diabetes mellitus complicated with acute kidney injury in the intensive care unit. BMC Anesthesiol 23, 4 (2023). https://doi.org/10.1186/s12871-022-01961-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-022-01961-6