Abstract
Background
Diabetic hepatopathy is a serious complication of poorly controlled diabetes mellitus. An efficient antidiabetic drug which keeps normal liver tissues is not available. The renin-angiotensin system has been reported to be involved in both diabetic state and liver function. Aliskiren is a direct renin inhibitor and a recently antihypertensive drug with poly-pharmacological properties. The aim of the current study is to explore the possible hepatoprotective effects and mechanisms of action of aliskiren against streptozotocin (STZ) induced liver toxicity.
Methods
Mice were distributed to 3 groups; first: the normal control group, second: the diabetic control group, third: the diabetic group which received aliskiren (25 mg/kg; oral) for 4 weeks. At the end of the treatment period, plasma glucose, insulin, lipid profile, oxidative stress, and liver function tests were evaluated spectrophotometrically. ELISA technique was used to measure the expression levels of TNF-α and adiponectin. Furthermore, a Histopathological examination of liver samples was done.
Results
It was shown that aliskiren treatment ameliorated the STZ-induced oxidative stress and elevated inflammatory biomarkers, hypercholesterolemia, serum aminotransferases and alkaline phosphatase levels in diabetic mice. In addition, hepatocellular necrosis, and fibrosis were improved by aliskiren treatment.
Conclusion
aliskiren protects against the liver damage caused by STZ-induced diabetes. This can be explained by its ability to block angiotensin-II, and its anti-diabetic, hypocholesterolemic, antioxidant and anti-inflammatory effects. Aliskiren could be a novel therapeutic strategy to prevent liver diseases associated with hypertension and diabetes mellitus.
Graphical Abstract

Similar content being viewed by others
Background
Diabetes mellitus (DM) is a chronic severe metabolic endocrine disorder. It is considered one of the most common and serious chronic disorders worldwide [1, 2]. Previous studies have reported that diabetic patients with poorer glycemic control suffer hepatomegaly, elevated levels of serum alanine transaminase (ALT) and aspartate transaminase (AST) [3, 4], high oxidative stress [5], hepatocellular necrosis and fibrosis [6]. Thus indicates liver impairment associated with DM [7, 8].
Blocking of the renin angiotensin aldosterone system (RAAS) by different ways has reported to be involved in hepatic protective impacts [9,10,11]. Aliskiren (C30H53N3O6, Molecular weight: 551.8 g/mol) is the first approved drug in the recent antihypertensive class; direct renin inhibitors [12]. Aliskiren acts by down-regulation of the RAAS, lower plasma renin activity, plasma angiotensin I (Ang I), and angiotensin II (Ang II) [13, 14]. Ang II is produced through endothelial cleavage of Ang I. Ang I is produced from angiotensinogen by hepatocytes. Renin cleaves angiotensinogen to form Ang I which is then converted by ACE to the active Ang II [15]. Furthermore, the antidiabetic effects of aliskiren have been proven previously [16]. A previous study has reported that aliskiren reduced portal pressure and intrahepatic resistance in cirrhotic rat liver [17]. The current study aimed to estimate the hepatoprotective impacts and possible mechanisms of action of aliskiren in an experimental model of induced diabetes in mice using STZ.
Methods
Animals and experimental design
Male Swiss albino mice (25–30 g) were maintained at 20–25 °C in a 12 h light/dark cycle, with a commercial normal rodent diet and water freely available. They were divided into three groups, 8 mice each: 1st; normal control group (received citrate buffer, IP for 1 month), 2nd; diabetic control group (STZ-induced diabetic mice and received normal saline, IP, for 1 month). 3rd; diabetic mice were treated with aliskiren; three days after STZ injection, this group of mice received daily aliskiren (25 mg /kg oral, dissolved in sterile normal saline) for 1 month. The volume of injection was 0.25 ml/20 g mice. Different dose levels of aliskiren have been tested before in experimental studies [18, 19]. We have selected the least effective dose correlated to the clinical dose used in patients [20]. We did not try different dose levels to reduce the number of used animals according to the standard research ethics.
Diabetes induction
Mice for the second and third groups were overnight fasted and then injected intraperitoneally with a single dose of 50 mg/kg streptozotocin dissolved in a freshly prepared citrate buffer (pH 4.5), 15 min after nicotinamide (NA) 110 mg/kg (dissolved in normal saline), to partially prevents the harmful effects of STZ to develop a model of non-insulin-dependent DM. Three days later; blood glucose was checked by a one-touch Glucometer, using blood from the tail. Mice with hyperglycemia (≥ 250 mg/dl) were selected as diabetic [21].
At the end of the treatment period, mice were fasted overnight. Blood samples were collected into tubes containing EDTA. Plasma was separated by centrifugation (10,000 rpm/min, for 10 min, 4 °C). Liver samples were separated, washed with saline, and kept in 10% formalin.
STZ, aliskiren, and ELISA kits were purchased from Sigma Aldrich Co (St Louis, MO, USA). Other chemicals and reagents were of analytical grade and were supplied by Biodiagnostic company, Egypt.
Biochemical analysis
Blood glucose and serum insulin
Fasting blood glucose was assessed spectrophotometrically using blood from the lateral tail vein on the last day of the experiment; by a commercial kit (Biodiagnostic, Egypt) [22]. Serum insulin was assessed using the ELISA technique.
Oxidative stress biomarkers
Serum oxidative stress biomarkers and antioxidant enzymes [reduced Glutathione (GSH), Superoxide dismutase (SOD), Malondialdehyde (MDA), and Nitric oxide (NO)] were measured spectrophotometrically using commercial reagent kits. SOD was assessed in line with the pyrogallol autoxidation technique [23]. MDA was estimated according to Satoh [24]. GSH was measured in agreement with Beutler et al. [25].
Liver functions markers and lipid profile
Serum albumin level was assessed in agreement with the modified bromocresol green technique [26]. Serum ALT and AST were assayed according to Piyachaturawat et al. [27].
The level of serum alkaline phosphatase was assayed according to Reitman and Frankel [28]. CPK-total was assayed according to Bishop et al. [29]. Cholesterol and triglyceride were determined enzymatically using a commercial kit according to Cox and García-Palmieri [30].
Adiponectin
Adiponectin was quantitatively measured in serum using a commercial ELISA kit (Biovision, Inc., San Francisco). The principle of the assay is that polyclonal antibody specific for adiponectin has been pre-coated into 96 well microplate. Standards and samples were pipetted into the wells and any adiponectin present is bound by immobilized antibody. The bound adiponectin is then captured by anti-adiponectin monoclonal antibody. With adding HRP conjugated anti-mouse IgG and HRP substrate, the colors developed in proportion to the bound adiponectin, can be easily measured by Elisa plate reader [31].
Tumor necrosis factor-alpha (TNF-α)
TNF-α was measured using BioVision’s ELISA Kit. This assay employs a monoclonal antibody specific for mouse TNF-α coated on a 96-well plate [32].
Histological preparation
Liver samples were flushed and fixed in 10% neutral buffered formalin for 72 h. Samples were trimmed and processed in serial grades of ethanol, cleared in Xylene, synthetic wax infiltration and embedding into Paraplast tissue embedding media. 5μn tissue sections were cut by rotatory microtome then fixed into glass slides and stained by Hematoxylin and Eosin (H and E) stain. Then examined by experienced histologist in blinded manner by using Full HD microscopic imaging system “(Leica Microsystems GmbH, Wetzlar, Germany)”. All standard procedures for samples fixation, processing and staining were done according to Culling, C.F.A. [33].
Statistical analysis
The data were obtained from at least 3 different experiments and were expressed as means ± S.E. Results were calculated by one-way ANOVA, followed by Tukey’s test as a post hoc test. P < 0.05 was considered significant. All the analyses were carried out by using SPSS software (version 22.0) for Windows 8.1 (SPSS, Inc., Chicago, IL, USA).
Results
Blood glucose and serum insulin level
STZ leads to significant hyperglycemia associated with a significant decrease in serum insulin. Treatment with aliskiren leads to a significant lowering in blood glucose and increase in serum insulin as related to STZ only treated group, P < 0.05 (Table 1).
Oxidative stress markers
STZ treated group showed a significant elevation in oxidative stress biomarkers (MDA and NO). This was accompanied by a significant reduction in plasma antioxidants (GSH, SOD). Treatment with aliskiren demonstrated a significant reduction of oxidative stress markers (MDA and NO) and significant elevation in GSH and SOD, P < 0.05 (Table 1).
Liver functions markers and Lipid profile
STZ resulted in impairment of liver function markers that was demonstrated by the significant elevation in AST, ALT, ALP, and CPK-total. This was accompanied by a significant reduction in albumin content. In addition to a significant elevation in lipid profile as demonstrated by the significant increase in TG and C, P < 0.05 (Figs. 1, 2, 3, Table 2).
Effect of one month treatment with aliskiren on Albumin level in STZ-induced diabetes in mice. Each value represents mean of 8 mice. Statistical analysis was carried out using one way analysis of variance (ANOVA) followed by Tukey Kramer multiple comparisons test. *Significantly different from Normal control at P < 0.05. #Significantly different from Diabetic control at P < 0.05
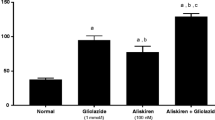
Effect of one month treatment with aliskiren on Cholesterol level in STZ-induced diabetes in mice. Each value represents mean of 8 mice. Statistical analysis was carried out using one way analysis of variance (ANOVA) followed by Tukey Kramer multiple comparisons test. *Significantly different from Normal control at P < 0.05. #Significantly different from Diabetic control at P < 0.05
Effect of one month treatment with aliskiren on Triglyceride in STZ-induced diabetes in mice. Each value represents mean of 8 mice. Statistical analysis was carried out using one way analysis of variance (ANOVA) followed by Tukey Kramer multiple comparisons test. *Significantly different from Normal control at P < 0.05. #Significantly different from Diabetic control at P < 0.05
Treatment of the diabetic group with aliskiren for 1 month resulted in a significant reduction of TG and C and liver function tests (AST, ALT, ALP, CPK-total). In addition to, a significant increase in plasma albumin content, P < 0.05 (Figs. 1, 2, 3, Table 2).
Adiponectin level
A significant reduction in serum adiponectin was observed in the diabetic group. However, a significant increase in serum adiponectin was observed in aliskiren treated group (Fig. 4).
Effect of one month treatment with aliskiren on adiponectin level in STZ-induced diabetes in mice. Each value represents mean of 8 mice. Statistical analysis was carried out using one way analysis of variance (ANOVA) followed by Tukey Kramer multiple comparisons test. *Significantly different from Normal control at P < 0.05. #Significantly different from Diabetic control at P < 0.05
Tumor necrosis factor-α (TNF-α)
The diabetic control group showed a significant elevation in serum TNF-α. Aliskiren treatment leads to a significant reduction in TNF-α in comparison to STZ diabetic mice (Fig. 5).
Effect of one month treatment with aliskiren on Tumor necrosis factor—alpha in STZ-induced diabetes in mice. Each value represents mean of 8 mice. Statistical analysis was carried out using one way analysis of variance (ANOVA) followed by Tukey Kramer multiple comparisons test. *Significantly different from Normal control at P < 0.05. #Significantly different from Diabetic control at P < 0.05
Histological examination
Microscopic examination of different hepatic tissue sections showed; normal controls demonstrated normal morphological features of hepatic parenchyma with many apparent intact hepatocytes having large vesicular nuclei, and intact hepatic vasculatures were observed with minimal degenerative changes. Diabetic model samples showed significant periportal and perivascular inflammatory cell infiltrates with mild vacuolar degenerative changes of hepatocytes with many nucleocytomegaly records with prominent nucleoli. Aliskiren treated group showed minimal inflammatory cell infiltrates records with more apparent intact hepatocytes with occasional nucleocytomegaly and or binucleation alternated with fewer degenerated cells accompanied with activated kupffer cells (Fig. 6).
Effect of one month treatment with aliskiren on Microscopic examination of different hepatic tissue sections in STZ-induced diabetes in mice (H and E-stained) (× 400). a: normal control, b: diabetic control and c: aliskiren treated group. a Normal controls demonstrated normal morphological features of hepatic parenchyma with many apparent intact hepatocytes having large vesicular nuclei, intact hepatic vasculatures were observed with minimal degenerative changes. b Diabetic model samples showed significant periportal and perivascular inflammatory cells infiltrates with mild vacuolar degenerative changes of hepatocytes with many nucleocytomegaly records with prominent nucleoli. c Aliskiren treated group showed minimal inflammatory cells infiltrates records with more apparent intact hepatocytes with occasional nucleocytomegaly and or binucleation alternated with fewer degenerated cells accompanied with activated kupffer cells
Discussion
Diabetes Mellitus (DM) is a common endocrine and metabolic disorder. It is considered a major health care threat worldwide due to its severe complications. Streptozotocin (STZ); a pancreatic cytotoxic induces irreversible necrosis of cells. It is used in the induction of diabetes experimentally as it mimics the endogenous chronic tissue damage and oxidative stress as a result of hyperglycemia [34,35,36]. The liver is the major organ of glucose metabolism in response to insulin. Liver is responsible for detoxification, clearance of oxidative stress bioproducts, regulating glycolysis, and gluconeogenesis [37, 38]. Long term hyperglycemia in poorly controlled DM may lead to liver disease. Moreover, liver impairment can predispose prediabetes or type 2 DM [7].
Diabetic mice, in the current study, had demonstrated significant hyperglycemia associated with a decrease in insulin level. Aliskiren treatment interrupted the observed increase in glucose level and decrease in insulin when compared to the diabetic group. This result is supported by previous studies demonstrating that aliskiren stimulates insulin secretion in-vitro from isolated β-cells and decreases insulin resistance in-vivo [16]. The decreased plasma glucose after aliskiren treatment can be explained by its ability to increase insulin secretion or enhance insulin sensitivity [39]. Aliskiren also upregulates glucose transporters expression levels in the liver (GLUT 2) and muscle (GLUT 4), these confirm the improvement of insulin resistance by aliskiren [40, 41].
Furthermore, aliskiren increases the plasma level of adiponectin. Adiponectin is a novel adipocytokine produced mainly in adipose tissue and is involved in regulating glucose levels, fatty acid breakdown, and insulin metabolism. It plays a role in the suppression of metabolic abnormalities that may result in type 2 diabetes. Adiponectin therapy has been shown to induce beneficial metabolic effects in animals by decreasing both hepatic gluconeogenesis and plasma triglyceride levels and has also antiatherogenic and anti-inflammatory effects [42]. Adiponectin enhances insulin sensitivity primarily through upregulation of fatty acid oxidation and suppression of hepatic glucose production [43, 44]. So adiponectin stimulation by aliskiren leads to increasing insulin sensitivity. In addition to, its insulin stimulation and hypoglycemic properties.
In line, high oxidative stress is involved in the bad prognosis of DM and worsens its complications on the liver [45, 46]. A possible explanation may be that reactive oxygen species (ROS) could interact with proteins, lipids, and DNA, resulting in the dysfunction of these important macromolecules [47]. ROS generates oxidative stress, which accelerates the damage and destruction of many organs [48, 49]. So, antioxidant enzymes including GSH, and SOD have potential protective impacts against tissue damage by their ability to decompose ROS and block lipid peroxidation [49, 50].
Lipid peroxidation is induced by the interaction of ROSand polyunsaturated fatty acids and results in the formation of MDA, which represents cellular damage and cytotoxicity [50]. Evidence has reported that high lipid peroxidation leads to the progression of DM by altering the normal functions of membrane-bound enzymes and receptors. Oxidative stress leads to the development of microvascular and cardiovascular diabetic complications. Hyperglycemia causes mitochondrial superoxide overproduction in endothelial cells of both large and small vessels. This increased superoxide production causes the activation of five major pathways involved in the pathogenesis of diabetes complications: polyol pathway flux, increased formation of advanced glycation end-products (AGEs), increased expression of the receptor for AGEs and its activating ligands, activation of protein kinase C isoforms, and overactivity of the hexosamine pathway. It also directly inactivates two critical antiatherosclerotic enzymes, eNOS and prostacyclin synthase [51].
In the current study, we observed a significant reduction in the activities of SOD, and GSH and an increase in MDA, and NO serum levels in STZ-induced diabetic mice. However, a significant elevation in antioxidants and a remarkable lowering in lipid peroxidation were observed in the aliskiren treated group. This was indicated by the elevation of SOD, GSH, and the reduction in MDA, NO. The antioxidant potential of aliskiren was in line with previous studies. Ang II may possess a role in phosphorylation and rise of ROS in the liver [52] and contributed to the bad prognosis of non-alcoholic fatty liver disease by elevating hepatic ROS [53]. Oxidative stress also triggers the development of steatohepatitis by stimulating inflammatory response [54]. So, the blockade of Ang II by aliskiren resulted in its antioxidant potential which also contributes to its potency to decrease insulin resistance and protects hepatocytes from high oxidative stress induced by DM.
In addition, hepatic ROS, as well as proinflammatory cytokines (TNF-α, IL-1β, and IL-6) are reduced with Ang-II inhibition [55]. As observed in the current study that aliskiren ameliorated the STZ-induced elevation in TNF-α. Aliskiren possesses anti-inflammatory properties leads to the preservation of liver function markers.
Increased activities of serum aminotransferases are also diagnostic markers of liver disorder and are occurred more frequently in diabetic patients [56, 57]. The present study demonstrated that serum ALT and AST levels were significantly elevated in STZ-treated animals, these were following previous studies [58,59,60] and indicated hepatocellular necrosis [61]. It has also been reported that high serum ALT and AST are involved in increasing insulin resistance and defective utilization of glucose by the liver [62]. Insulin deficiency leads to the breakdown of protein and enhances amino acid catabolism to provide substrates for gluconeogenesis [63].
Administration of aliskiren significantly lowered AST and ALT activities, indicating that it had potential effects on improving liver function. These results agreed with those of Hsieh et al. [17] who demonstrated that there is a beneficial effect of aliskiren on portal pressure and intrahepatic resistance through reduction of Ang II production in the cirrhotic liver. These authors concluded that direct renin inhibition may serve as a potential and effective therapeutic strategy for the management of portal hypertension.
A high level of ALP was demonstrated in the STZ diabetic group. Increased ALP has been reported in pathological conditions which involve the kidney and liver [64, 65]. In addition, diabetic mice showed serious lipid dysfunction, which was experimentally validated by the increased levels of TG and TC similar to the clinical properties of human DM [66]. TC and TG are important parameters for evaluating blood viscosity, and the risk of atherosclerosis. The administration of aliskiren significantly decreased TG and TC, this indicated its potential impacts on improving lipid metabolism and was in accordance with a previous study that demonstrated increased hepatic turnover of triglycerides with an upregulation in fatty acid transport and breakdown after aliskiren treatment [9]. So, the RAAS influences hepatic fatty acid metabolism.
Finally, the microscopic examination of liver tissue from STZ-treated mice revealed loss of hepatic architecture, hepatomegaly, dilatation of hepatic sinusoid capillaries close to the central vein, apoptotic hepatocytes, and hepatocytes with lipid droplets in their cytoplasm indicating increased adipogenesis and cell death as well as signs of inflammation, which were all mitigated by aliskiren treatment [58, 67].
From the shown data, liver fibrosis and its serious complications such as portal hypertension and hepatocellular carcinoma are evidenced to be related to the bad prognosis of diabetes [68, 69]. Plasma renin activity and angiotensins were reported to be increased in advanced liver disease [70,71,72] which indicates the role of RAAS in diabetes-induced liver impairment. So, inhibition of Ang II synthesis by aliskiren may attenuate hepatic fibrosis as observed in the current and previous studies [73, 74]. Aliskiren has been proven as an effective way to block renin activity and restrain liver fibrosis in experimental models. In addition to its explained hepatoprotective mechanisms by lowering the induced high blood glucose, lipid profile, liver enzymes, oxidative stress, and inflammatory biomarkers.
Conclusion
Aliskiren is a direct renin inhibitor, that prevents the formation of Ang II by blocking renin from converting to Ang I. Our study is the first to demonstrate the hepatoprotective impacts and mechanisms of aliskiren in a model of STZ-induced diabetes in mice. The evidenced hepatoprotective effect was a result of improvement of RAAS by inhibition of Ang II production, Ang II action, glycemic control, reduction of insulin resistance, changes in lipid metabolism, anti-inflammatory and antioxidant effects elicited by aliskiren treatment. Aliskiren is considered a promising treatment for the underlying conditions associated with hypertension, hypercholesterolemia, and diabetes. Clinical studies are essential to ensure its impact on the management of hypertensive or diabetic patients with liver diseases.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AGEs:
-
Advanced glycation end-products
- ALT:
-
Alanine transaminase
- ALP:
-
Alkaline phosphatase
- ACE:
-
Angiotensin converting enzyme
- Ang I:
-
Angiotensin I
- Ang II:
-
Angiotensin II
- AST:
-
Aspartate transaminase
- CPK-total:
-
Creatine phosphokinase
- DM:
-
Diabetes mellitus
- eNOS:
-
Endothelial nitric oxide synthase
- H and E:
-
Hematoxylin–eosin
- IL-1β:
-
Interleukin-1β
- IP:
-
Intraperitoneal
- MDA:
-
Malondialdehyde
- NO:
-
Nitric oxide
- NF-κB:
-
Nuclear factor kappa B
- GSH:
-
Reduced glutathione
- RAAS:
-
Renin angiotensin aldosterone system
- ROS:
-
Reactive oxygen species
- STZ:
-
Streptozotocin
- SOD:
-
Superoxide dismutase
- TNF- α:
-
Tumor necrosis factor-alpha
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
References
Liang T, Zhang Q, Sun W, Xin Y, Zhang Z, Tan Y, et al. Zinc treatment prevents type 1 diabetes-induced hepatic oxidative damage, endoplasmic reticulum stress, and cell death, and even prevents possible steatohepatitis in the OVE26 mouse model: important role of metallothionein. Toxicol Lett. 2015;233(2):114–24. https://doi.org/10.1016/j.toxlet.2015.01.010.
Brinkman AK. Management of type 1 diabetes. Nurs Clin North Am. 2017;52(4):499–511. https://doi.org/10.1016/j.cnur.2017.07.001.
Harrison SA. Liver disease in patients with diabetes mellitus. J Clin Gastroenterol. 2006;40(1):68–76. https://doi.org/10.1097/01.mcg.0000190774.91875.d2.
Torbenson M, Chen YY, Brunt E, Cummings OW, Gottfried M, Jakate S, et al. Glycogenic hepatopathy: an underrecognized hepatic complication of diabetes mellitus. Am J Surg Pathol. 2006;30(4):508–13. https://doi.org/10.1097/00000478-200604000-00012.
Ingaramo PI, Ronco MT, Francés DE, Monti JA, Pisani GB, Ceballos MP, et al. Tumor necrosis factor alpha pathways develops liver apoptosis in type 1 diabetes mellitus. Mol Immunol. 2011;48(12–13):1397–407. https://doi.org/10.1016/j.molimm.2011.03.015.
Afrin R, Arumugam S, Wahed MI, Pitchaimani V, Karuppagounder V, Sreedhar R, et al. Attenuation of endoplasmic reticulum stressmediated liver damage by mulberry leaf diet in streptozotocin-induced diabetic rats. Am J Chin Med. 2016;44(1):87–101. https://doi.org/10.1142/S0192415X16500063.
DPV Initiative and the German BMBF Competence Network Diabetes mellitus, Stadler M, Bollow E, Fritsch M, Kerner W, Schuetz-Fuhrmann I, Krakow D, et al. Prevalence of elevated liver enzymes in adults with type 1 diabetes: a multicentre analysis of the German/Austrian DPV database. Diabetes Obes Metab. 2017;19(8):1171–8. https://doi.org/10.1111/dom.12929.
Taurino F, Stanca E, Vonghia L, Siculella L, Sardanelli AM, Papa S, et al. Short-term type-1 diabetes differentially modulates 14-3-3 proteins in rat brain and liver. Eur J Clin Invest. 2014;44(4):350–8. https://doi.org/10.1111/eci.12241.
Lee KC, Chan CC, Yang YY, Hsieh YC, Huang YH, Lin HC. Aliskiren attenuates steatohepatitis and increases turnover of hepatic fat in mice fed with a methionine and choline deficient diet. PLoS ONE. 2013;8(10):e77817. https://doi.org/10.1371/journal.pone.0077817.
Kishina M, Koda M, Kato J, Tokunaga S, Matono T, Sugihara T, et al. Therapeutic effects of the direct renin inhibitor, aliskiren, on non-alcoholic steatohepatitis in fatty liver Shionogi ob/ob male mice. Hepatol Res. 2014;44(8):888–96. https://doi.org/10.1111/hepr.12186.
Aihara Y, Yoshiji H, Noguchi R, Kaji K, Namisaki T, Shirai Y, et al. Direct renin inhibitor, aliskiren, attenuates the progression of non-alcoholic steatohepatitis in the rat model. Hepatol Res. 2013;43(11):1241–50. https://doi.org/10.1111/hepr.12081.
Gradman AH, Kad R. Renin inhibition in hypertension. J Am Coll Cardiol. 2008;51:519e28.
Nussberger J, Wuerzner G, Jensen C, Brunner HR. Angiotensin II suppression in humans by the orally active renin inhibitor Aliskiren (SPP100): comparison with enalapril. Hypertension. 2002;39:E1e8.
Fisher ND, Jan Danser AH, Nussberger J, Dole WP, Hollenberg NK. Renal and hormonal responses to direct renin inhibition with aliskiren in healthy humans. Circulation. 2008;117:3199e205. https://doi.org/10.1161/CIRCULATIONAHA.108.767202.
Allikmets K. Aliskiren—an orally active renin inhibitor. Review of pharmacology, pharmacodynamics, kinetics, and clinical potential in the treatment of hypertension. Vasc Health Risk Manag. 2007;3(6):809–15.
Mahfoz AM, El-Latif HA, Ahmed LA, Hassanein NM, Shoka AA. Anti-diabetic and renoprotective effects of aliskiren in streptozotocin-induced diabetic nephropathy in female rats. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(12):1315–24. https://doi.org/10.1007/s00210-016-1299-2.
Hsieh YC, Chan CC, Lee KC, Huang YT, Lee FY, Yang YY, Lin HC. Aliskiren reduces portal pressure and intrahepatic resistance in biliary cirrhotic rats. J Chin Med Assoc. 2012;75:501–8.
Dong YF, Liu L, Kataoka K, et al. Aliskiren prevents cardiovascular complications and pancreatic injury in a mouse model of obesity and type 2 diabetes. Diabetologia. 2010;53:180. https://doi.org/10.1007/s00125-009-1575-5.
Aziz TA, Kareem AA, Othman HH, Ahmed ZA. The anti-inflammatory effect of different doses of aliskiren in rat models of inflammation. Drug Des Devel Ther. 2020;20(14):2841–51. https://doi.org/10.2147/DDDT.S255607.PMID:32764883;PMCID:PMC7381093.
Yamamoto E, Kataoka K, Dong YF, Nakamura T, Fukuda M, Tokutomi Y, et al. Aliskiren enhances the protective effects of valsartan against cardiovascular and renal injury in endothelial nitric oxide synthase-deficient mice. Hypertension. 2009;54(3):633–8. https://doi.org/10.1161/HYPERTENSIONAHA.109.133884.
Ahangarpour A, Heidari H, Oroojan AA, Mirzavandi F, Esfehani KN, Mohammadi ZD. Antidiabetic, hypolipidemic and hepatoprotective effects of Arctium lappa root’s hydro-alcoholic extract on nicotinamide-streptozotocin induced type 2 model of diabetes in male mice. Avicenna J Phytomed. 2017;7(2):169–79.
Trinder P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol. 1969;22(2):158–61. https://doi.org/10.1136/jcp.22.2.158.
Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47(3):469–74. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x.
Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90(1):37–43. https://doi.org/10.1016/0009-8981(78)90081-5.
Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8.
Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 1971;31(1):87–96. https://doi.org/10.1016/0009-8981(71)90365-2.
Piyachaturawat P, Poprasit J, Glinsnkon T, Wanichon C. Gastric mucosal in STZ-diabetic rats. Cell Biol Int Rep. 1988;12(1):53–63. https://doi.org/10.1016/0309-1651(88)90111-7.
Reitman S, Frankel S. Glutamic—pyruvate transaminase assay by colorimetric method. Am J Clin Pathol. 1957;28:56. https://doi.org/10.1093/ajcp/28.1.56.
Bishop ML, Fody EP, Schoeff LE. Clinical chemistry: principles, procedures, correlations. Philadelphia: Lippincott Williams and Wilkins; 2004. p. 243.
Cox RA, García-Palmieri MR. Cholesterol, triglycerides, and associated lipoproteins. In: Walker HK, Hall WD, Hurst JW, editors. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Boston: Butterworths; 1990.
Mehany HA, Abo-youssef AM, Ahmed LA, Arafa ES, Abd El-Latif HA. Protective effect of vitamin E and atorvastatin against potassium dichromate-induced nephrotoxicity in rats. Beni Suef Univ J Basic Appl Sci. 2013;2(2):96–102. https://doi.org/10.1016/j.bjbas.2013.02.002.
Petrovas C, Daskas SM, Lianidou ES. Determination of tumor necrosis factor-alpha (TNF-alpha) in serum by a highly sensitive enzyme amplified lanthanide luminescence immunoassay. Clin Biochem. 1999;32(4):241–7. https://doi.org/10.1016/S0009-9120(99)00004-1.
Culling CFA. Handbook of histopathological and histochemical techniques. 3rd ed. London: Butterworths; 2013.
Arora S, Ojha S, Vohora D. Characterisation of streptozotocin induced diabetes mellitus in swiss albino mice. Glob J Pharmacol. 2009;3:81–4.
Mollazadeh H, Sadeghnia HR, Hoseini A, Farzadnia M, Boroushaki MT. Effects of pomegranate seed oil on oxidative stress markers, serum biochemical parameters and pathological findings in kidney and heart of streptozotocin-induced diabetic rats. Ren Fail. 2016;38(8):1256–66. https://doi.org/10.1080/0886022X.2016.1207053.
Lin L, Cui F, Zhang J, Gao X, Zhou M, Xu N, et al. Antioxidative and renoprotective effects of residue polysaccharides from Flammulina velutipes. Carbohydr Polym. 2016;146:388–95. https://doi.org/10.1016/j.carbpol.2016.03.071.
Roden M, Bernroider E. Hepatic glucose metabolism in humans—its role in health and disease. Best Pract Res Clin Endocrinol Metab. 2003;17(3):365–83. https://doi.org/10.1016/S1521-690X(03)00031-9.
Naso FC, Dias AS, Porawski M, Marroni NA. Exogenous superoxide dismutase: action on liver oxidative stress in animals with streptozotocin-induced diabetes. Exp Diabetes Res. 2011;2011:754132.
Kang YS, Lee MH, Song HK, Hyun YY, Cha JJ, Ko GJ, et al. Aliskiren improves insulin resistance and ameliorates diabetic vascular complications in db/db mice. Nephrol Dial Transplant. 2011;26(4):1194–204. https://doi.org/10.1093/ndt/gfq579.
Gandhi S, Srinivasan B, Akarte AS. Aliskiren improves insulin resistance and ameliorates diabetic renal vascular complications in STZ-induced diabetic rats. J Renin Angiotensin Aldosterone Syst. 2013;14(1):3–13. https://doi.org/10.1177/1470320312452766.
Frantz ED, Crespo-Mascarenhas C, Barreto-Vianna AR, Aguila MB, Mandarim-de-Lacerda CA. Renin-angiotensin system blockers protect pancreatic islets against diet-induced obesity and insulin resistance in mice. PLoS ONE. 2013;8(7):e67192. https://doi.org/10.1371/journal.pone.0067192.PMID:23894285;PMCID:PMC3718820.
Ukkola O, Santaniemi M. Adiponectin: a link between excess adiposity and associated comorbidities? J Mol Med. 2002;80(11):696–702. https://doi.org/10.1007/s00109-002-0378-7.
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941–6. https://doi.org/10.1038/90984.
Fisman EZ, Tenenbaum A. Adiponectin: a manifold therapeutic target for metabolic syndrome, diabetes, and coronary disease? Cardiovasc Diabetol. 2014;13(1):103. https://doi.org/10.1186/1475-2840-13-103.
Kaur G, Padiya R, Adela R, Putcha UK, Reddy GS, Reddy BR, et al. Garlic and resveratrol attenuate diabetic complications, loss of β-cells, pancreatic and hepatic oxidative stress in streptozotocin-induced diabetic rats. Front Pharmacol. 2016;7:360. https://doi.org/10.3389/fphar.2016.00360.
Mutlu HH, Caklili OT, Coskunpinar E. Serum concentrations of cyclophilin A in patients with nonalcoholic fatty liver disease. Acta Gastroenterol Belg. 2017;80(1):3–7.
Cheeseman KH, Slater TF. An introduction to free radical biochemistry. Br Med Bull. 1993;49(3):481–93. https://doi.org/10.1093/oxfordjournals.bmb.a072625PMID:82210Hsieh.
Sarrafchi A, Bahmani M, Shirzad H, Rafieian-Kopaei M. Oxidative stress and Parkinson’s disease: new hopes in treatment with herbal antioxidants. Curr Pharm Des. 2016;22(2):238–46. https://doi.org/10.2174/1381612822666151112151653.
Nyam KL, Chow CF, Tan CS, Ng ST. Antidiabetic properties of the tiger’s milk medicinal mushroom, Lignosus rhinocerotis (Agaricomycetes), in streptozotocin-induced diabetic rats. Int J Med Mushrooms. 2017;19(7):607–17. https://doi.org/10.1615/IntJMedMushrooms.2017021186.
Wang J, Wang C, Li S, Li W, Yuan G, Pan Y, et al. Anti-diabetic effects of Inonotus obliquus polysaccharides in streptozotocin-induced type 2 diabetic mice and potential mechanism via PI3K-Akt signal pathway. Biomed Pharmacother. 2017;95:1669–77. https://doi.org/10.1016/j.biopha.2017.09.104.
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–70. https://doi.org/10.1161/CIRCRESAHA.110.223545.
Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112(9):1383–94. https://doi.org/10.1172/JCI18212.
Wei Y, Clark SE, Morris EM, Thyfault JP, Uptergrove GM, Whaley-Connell AT, et al. Angiotensin II-induced non-alcoholic fatty liver disease is mediated by oxidative stress in transgenic TG(mRen2)27(Ren2) rats. J Hepatol. 2008;49(3):417–28. https://doi.org/10.1016/j.jhep.2008.03.018.
Sutti S, Jindal A, Locatelli I, Vacchiano M, Gigliotti L, Bozzola C, et al. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology. 2014;59(3):886–97. https://doi.org/10.1002/hep.26749.
McAllister-Lucas LM, Ruland J, Siu K, Jin X, Gu S, Kim DS, et al. CARMA3/Bcl10/MALT1-dependent NF-kappaB activation mediates angiotensin II-responsive inflammatory signaling in nonimmune cells. Proc Natl Acad Sci USA. 2007;104(1):139–44. https://doi.org/10.1073/pnas.0601947103.
Ahmed O, Mahmoud A, Abdel-Moneim A, Ashour M. Antidiabetic effects of hesperidin and naringin in type 2 diabetic rats. Diabetol Croat. 2012;41:53–67.
Kim JJ, Choi J, Lee MK, Kang KY, Paik MJ, Jo SK, et al. Immunomodulatory and antidiabetic effects of a new herbal preparation (HemoHIM) on streptozotocin induced diabetic mice. Evid Based Complement Alternat Med. 2014;2014:461685. https://doi.org/10.1155/2014/461685.
Degirmenchi I, Kalender S, Ustuner MC, Kalender Y, Gunes HV, Unal N, et al. The effects of acarbose and Rumex patientia on liver ultrastructure in streptozotocin-induced diabetic (type II) rats. Drugs Exp Clin Res. 2002;28(6):229–34.
Talaat IM, Nasr A, Alsulaimani AA, Alghamdi H, Alswat KA, Almalki DM, et al. Association between type 1, type 2 cytokines, diabetic autoantibodies and 25-hydroxyvitamin D in children with type 1 diabetes. J Endocrinol Invest. 2016;39(12):1425–34. https://doi.org/10.1007/s40618-016-0514-9.
Gomes RM, de Paulo LF, Bonato Panizzon CP, Neves CQ, Cordeiro BC, Zanoni JN, et al. Anti-diabetic effects of the ethyl-acetate fraction of Trichilia catigua in streptozotocin-induced type 1 diabetic rats. Cell Physiol Biochem. 2017;42(3):1087–97. https://doi.org/10.1159/000478761.
Senthil D, Choudhury GG, McLaurin C, Kasinath BS. Vascular endothelial growth factor induces protein synthesis in renal epithelial cells: a potential role in diabetic nephropathy. Kidney Int. 2003;64(2):468–79. https://doi.org/10.1046/j.1523-1755.2003.00135.x.
Gómez-Sámano MA, Cuevas-Ramos D, Mehta R, Brau-Figueroa H, Meza-Arana CE, Gulias-Herrero A. Association of alanine aminotransferase levels (ALT) with the hepatic insulin resistance index (HIRI): a cross-sectional study. BMC Endocr Disord. 2012;12(1):16. https://doi.org/10.1186/1472-6823-12-16.
Murali R, Srinivasan S, Ashokkumar N. Antihyperglycemic effect of fraxetin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Biochimie. 2013;95(10):1848–54. https://doi.org/10.1016/j.biochi.2013.06.013.
Ramesh B, Pugalendi KV. Impact of umbelliferone (7-hydroxycourmarin) on hepatic marker enzymes in Streptozotocin diabetic rats. Indian J Pharmacol. 2006;38(3):209–10. https://doi.org/10.4103/0253-7613.25813.
Salimuddin U, Upadhyaya KC, Baquer NZ. Effects of vanadate on expression of liver arginase in experimental diabetic rats. IUBMB Life. 1999;48(2):237–40. https://doi.org/10.1080/152165499307297.
Stein O, Stein Y. Atheroprotective mechanisms of HDL. Atherosclerosis. 1999;144(2):285–301. https://doi.org/10.1016/S0021-9150(99)00065-9.
Welt K, Weiss J, Martin R, Dettmer D, Hermsdorf T, Asayama K, et al. Ultrastructural, immunohistochemical and biochemical investigations of the rat liver exposed to experimental diabetes und acute hypoxia with and without application of Ginkgo extract. Exp Toxicol Pathol. 2004;55(5):331–45. https://doi.org/10.1078/0940-2993-00337.
Yoshiji H, Noguchi R, Ikenaka Y, Kaji K, Aihara Y, Fukui H. Impact of renin-angiotensin system in hepatocellular carcinoma. Curr Cancer Drug Targets. 2011;11(4):431–41. https://doi.org/10.2174/156800911795538084.
Herath CB, Grace JA, Angus PW. Therapeutic potential of targeting the renin angiotensin system in portal hypertension. World J Gastrointest Pathophysiol. 2013;4(1):1–11. https://doi.org/10.4291/wjgp.v4.i1.1.
Arroyo V, Colmenero J. Ascites and hepatorenal syndrome in cirrhosis: pathophysiological basis of therapy and current management. J Hepatol. 2003;38(Suppl 1):S69-89. https://doi.org/10.1016/S0168-8278(03)00007-2.
Arroyo V, Terra C, Ginès P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46(5):935–46. https://doi.org/10.1016/j.jhep.2007.02.001.
Vilas-Boas W, Oliveira A, Pereira R, Ribeiro R, Almeida J, Nadu A, Silva A, Santos R. Relationship between angiotensin and angiotensin-(1–7) II correlates with hemodynamic changes in human liver cirrhosis. World J Gastroenterol. 2009;15(20):2512–9.
Shaaban AA, Shaker ME, Zalata KR, El-kashef HA, Ibrahim TM. Modulation of carbon tetrachloride-induced hepatic oxidative stress, injury and fibrosis by olmesartan and omega-3. Chem Biol Interact. 2014;207:81–91. https://doi.org/10.1016/j.cbi.2013.10.008.
El Maleky W, Mahfoz AM, Osman AO, Abd El-Latif HA. Investigation of the impacts of zamzam water on streptozotocin-induced diabetic nephropathy in rats In-vivo and in-vitro study. Biomed Pharmacother. 2021;138:111474. https://doi.org/10.1016/j.biopha.2021.111474.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Science and Technology Development Fund (STDF OA Agreement).
Author information
Authors and Affiliations
Contributions
AM and AG designed the study and conducted the systematic review. Conducted experiments, and statistical analysis and drafted the paper. All authors read and approved the manuscript. The authors declare that all data were generated in-house and that no paper mill was used. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol of the current study is in line with the international guidelines for use of laboratory animals; published by the US NIH. It is also approved by the research ethics committee for experimental and clinical studies at the Faculty of Pharmacy, Modern University for Technology and Information (Permit number: ES 882).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mahfoz, A.M., Gawish, A.Y. Insight into the hepatoprotective, hypolipidemic, and antidiabetic impacts of aliskiren in streptozotocin-induced diabetic liver disease in mice. Diabetol Metab Syndr 14, 163 (2022). https://doi.org/10.1186/s13098-022-00935-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-022-00935-5