Abstract
Background
The hemoglobin glycation index (HGI) is the difference between measured and estimated glycation of hemoglobin. However, there is limited evidence to investigate the HGI and the clinical outcomes of acute coronary syndrome patients. This study aimed to evaluate the association between HGI and the clinical outcomes of acute coronary syndrome (ACS) in a China cohort.
Method
This single-center retrospective study was carried out in the Cardiovascular Center of Beijing Friendship Hospital, a total of 11004 consecutive patients with ACS from Dec 2012–Dec 2020 were enrolled in this study. Patients were divided into quintiles according to their HGI levels. The incidence of major adverse cardiac and cerebrovascular events (MACCEs) was recorded.
Result
HGI were divided into five quintiles quintiles: −0.906 (−7.188, −0.663), −0.491 (−0.663, −0.343), −0.196 (−0.342, −0.039), 0.170 (−0.039, 0.485), and 1.156 (0.485, 7.875), respectively.
Competing risk regression revealed that HGI was positively related to all-cause death, CV death, and composite MACCEs. Multivariate Cox proportional hazards regression analysis indicated that hypertension (HR:1.109, P = 0.013), previous stroke (HR:1.208, P < 0.001), past PCI (HR: 1.268, P < 0.001), age (HR: 1.011, P < 0.001), BMI (HR: 0.987, P = 0.012), heart rate (HR: 1.004, P = 0.001), NSTEMI (HR: 1.205, P < 0.001), WBC (HR: 1.020, P = 0.008), eGFR (HR: 0.993, P < 0.001), HDL-C (HR: 0.809, P = 0.002), LVEF (HR:0.240, P < 0.001), LM/three-vessel or proximal LAD involved (HR: 1.208 P < 0.001; HR:0.914, P = 0.019, respectively), and antiplatelet agents during hospitalization (HR:0.806, P = 0.020) independently predicted the incidence of MACCEs in ACS patients. Restricted cubic spline indicated a U-shaped association between the HGI and risk of MACCEs.
Conclusion
Both low HGI and high HGI was associated with an increased risk of adverse outcomes in patients with acute coronary syndrome, compared with moderate HGI.
Similar content being viewed by others
Introduction
Acute coronary syndrome (ACS) is the leading cause of death worldwide [1]. Diabetes mellitus (DM) is considered to confer equal risk to that of coronary artery disease for cardiovascular mortality [2, 3]. According to recent evidence, approximately 20–25% of patients with ACS reportedly also have DM [4]. A large body of evidence indicates that proper glucose control contributes to long-term cardiovascular benefits. Patients with ACS and DM suffer higher mortality than those with only ACS [5, 6].
Haemoglobin A1c (HbA1c) is an indicator reflecting the glucose level over the past 3 months and has been the most commonly used marker of glucose control [7]. The latest guideline for diabetes recommended HbA1c > 6.5% as a diagnostic criterion for diabetes [8]. However, as HbA1c is an important parameter of average glucose levels, and there is a significant linear correlation between blood glucose and HbA1c, there are interindividual variations in the rate of haemoglobin glycation caused by factors other than mean blood glucose levels among patients with or without diabetes [9, 10]. In addition, some studies found that only 60–80% of patients demonstrated consistent HbA1c and average blood glucose levels [11]. various factors such as glucose metabolism, genetic factors and passive hemoglobin glycation rates affected HbA1c largely. Recently, a new indicator, haemoglobin glycation index (HGI), was introduced to quantify this variation [12].
HGI is defined as the disparity between the observed and predicted HbA1c according to a linear regression between HbA1c and fasting plasma glucose (FPG) [13]. Some evidence has investigated the clinical applications of HGI in diabetes. Daiji and colleagues [14] reported a positive correlation between HGI and systemic arterial stiffening independent of hyperglycaemia. Another study from Yuesong presented a U-shaped relationship between HGI and the risk of diabetic patients with stroke, indicating that both low and high HGI is associated with a higher risk of poor prognosis [15]. However, there are rare clinical studies evaluating the applications of HGI among ACS patients.
This study aimed to assess the association between HGI and the prognosis in ACS subjects with or without diabetes using single-centre cohort data.
Methods
Study population
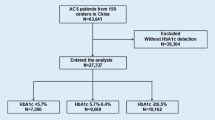
All participants were included from the Cardiovascular Center of Beijing Friendship Hospital. A total of 15172 consecutive patients diagnosed with ACS from Dec 2012–Dec 2020 were enrolled. According to the flow chart (Fig. 1), 4168 patients were excluded according to the exclusion criteria: (1) 1166 patients lacked HbA1c or FBG data, (2) 85 patients were diagnosed with severe valvular diseases or cardiomyopathy, (3) 382 patients were suffering from infectious disease, rheumatic disease, or neoplastic disease, (4) 134 patients were diagnosed with severe renal disease, (5) 1909 patients lacked coronary angiography data, and (6) 492 patients had missing clinical or follow-up data. The final follow-up ends up at Dec 31, 2021, with a median follow-up of 36.5 months. All enrolled patients were followed-up by phone interview, clinical visiting, or hospital records.
Data collections and definitions
This study was approved by the ethics committee of Beijing Friendship Hospital and conducted in accordance with the Declaration of Helsinki.
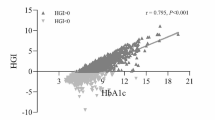
All the basic information was recorded by two independent persons. The concentrations of fasting plasma glucose (FPG) and HbA1c was tested after hospitalization at the clinical laboratory of our hospital. Predicted HbA1c was calculated by inserting the corresponding FPG value into the linear regression equation (HbA1c [%] = 4.036 + 0.399 FPG [mmol/L], P < 0.001, adjusted r = 0.691). HGI was the difference between the predicted HbA1c and the observed HbA1c, the correlation is shown (Fig. 2). The population was then divided into five quintiles according to their HGI levels. Major adverse cardiac and cerebral events were recorded during follow-up periods.
Criteria for diabetes mellitus included (1) currently receiving anti-diabetic medication, (2) typical diabetic symptoms plus an FPG ≥ 7.0 mmol/L, (3) typical diabetic symptoms with random blood glucose (RBG) ≥ 11.1 mmol/L, and (3) a positive oral glucose tolerance test (OGTT) (2-h plasma glucose level ≥ 11.1 mmol/L). Hypertension was defined as currently receiving antihypertensive agents or blood pressure equal to or greater than 140/90 mmHg three times on different days. The criteria for dyslipidaemia were identified as one of the following conditions: (1) fasting total cholesterol (TC) > 5.18 mmol/L, (2) low-density lipoprotein cholesterol (LDL-C) > 3.36 mmol/L, (3) high-density lipoprotein cholesterol (HDL-C) < 1.03 mmol/L, (4) triglyceride (TG) > 1.69 mmol/L, and (5) previous use of lipid-lowering agents. Acute coronary syndrome included ST-elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI), and unstable angina (UA).
MACCEs include all-cause death (including CV death and non-CV death), CV death, nonfatal MI, cardiac rehospitalization (rehospitalization due to heart failure or ACS), revascularization, and nonfatal stroke (ischaemic and haemorrhagic stroke). Nonfatal stroke was identified as neural dysfunction due to vascular sudden rupture or obstruction and diagnosed according to the signs of brain dysfunction or imaging evidence.
Statistical analyses
Mean ± standard deviation (SD) or median (IQR) were applied to present continuous variables. Student’s test or Mann–Whitney U-test were used to analyze the comparisons among these groups. Categorical variables were shown as numbers and percentages, which were compared via the Pearson chi-square test or Fisher’s exact test. First, Kaplan–Meier plots were generated to estimate the cumulative incidence of the outcomes in different HGI groups. To figure out the factors related to MACCEs, then, baseline variables were enrolled to correlate with MACCEs by univariate analysis, and the related factors entered the multivariate model with the use of Cox hazards regression models. Considering the competitive risk between all-cause death and other endpoints, Competing risk model was employed to analyze the incidence of MACCEs in different HGI levels and evaluate the predictive effect of the HGI on clinical outcomes. To further examine the continuous association between HGI levels and mortality, Restricted cubic spline (RCS) was used to analyze the relationship between HGI value and types of MACCEs. Subgroup analyses were also performed according to the hazard ratios of MACCEs after adjusting for confounding factors. Statistical tests were performed with SPSS statistics 26, Stata MP 15.1, and the R Programming Language. A two-tailed P value < 0.05 was considered as statistically significant.
Results
Baseline characteristics of patients
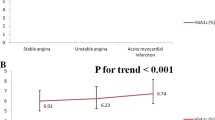
The baseline characteristics of the enrolled patients in different HGI groups are illustrated (Table 1). The median HGI of the 11004 patients was −0.196 (−7.188, 7.875). The five quintiles are −0.906 (−7.188, −0.663), −0.491 (−0.663, −0.343), −0.196 (−0.342, −0.039), 0.170 (−0.039, 0.485), and 1.156 (0.485, 7.875), respectively. In the Q2-Q3 groups, the prevalence of diabetes, dyslipidaemia, and stroke were significantly lower than those in the Q1, Q4 and Q5 groups. The systolic blood pressure and medication usage on admission (antiplatelet agents, ACEIs/ARBs, beta-blockers, and statins) increased with the HGI levels. In addition, BMI, HbA1c, and FPG are positively associated with HGI. Male patients are more likely to have a lower HGI.
HGI predicted the occurrence of MACCEs
The incidence of composite MACCEs was calculated (Table 2). MACCEs occurred in 3298 (30.0%) patients [784 (7.1%) all-cause death, 420 (3.8%) CV death, 457 (4.2%) nonfatal MI, 164 (1.5%) nonfatal stroke, 2638 (24.0%) cardiac rehospitalization, 739 (6.7%) revascularizations]. Low and high HGI leaded to increased risk of all-cause death, CV death, and composite MACCEs significantly increased along with HGI levels (p < 0.001), while patients with moderate HGI (Q2: −0.491 (−0.663, −0.343)) presented the lowest rate of the above outcomes. During the median of 36.5 months of follow-up, Kaplan–Meier analysis of event-free survival indicated that there was a significant difference of survival rate among HGI groups (Figs. 3 and 4). Cox regression analyses and predictors for subvarieties of MACCEs is presented (Table 3). Univariate analysis found that the predictors associated with MACCEs occurrence were HGI, age, hypertension, diabetes, previous stroke/MI, past PCI/CABG, BMI, blood pressure, heart rate, diagnosis with NSTEMI, UA, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB) usage at admission, laboratory data including WBC, haemoglobin, Hs-CRP, RBG at admission, FPG, HbA1c, albumin, creatinine, eGFR, TC, LDL-C, HDL-C, left ventricular ejection fraction, LM/three-vessel or proximal LAD involved, medication during hospitalization including antiplatelet agents, ACEI/ARB and statins, alpha-glucosidase inhibitor and insulin usage (P < 0.05). After adjusting for confounding factors, multivariate Cox proportional hazards regression analysis indicated that age, hypertension, previous stroke, past PCI, BMI, heart rate, NSTEMI, WBC, eGFR, HDL-C, LVEF, LM/three-vessel or proximal LAD involved, and antiplatelet agents during hospitalization independently predicted the incidence of MACCEs in ACS patients. Finally, competing risk regression analysis was employed to compare the endpoints in different groups. The results indicate that the cumulative occurrence of CV death, nonfatal MI, revascularization, and nonfatal MACCEs were significantly correlated with HGI levels on unadjusted competing risk modelling. Notably, after adjusting for confounding factors, the multivariate-adjusted hazard ratio (HR) also increased with increasing HGI for CV death (P < 0.05). It is reported that patients with HGI of Q2-Q3 may suffer the lowest incidence of CV death and nonfatal stroke [CV death: Q2: 0.547 (0.403–0.742); Q3: 0.466 (0.340,0.640); nonfatal stroke: Q2: 0.512 (0.305,0.860); Q3: 0.625 (0.387,1.011)] (Table 4). To further investigate this issue, RCS were employed to analyze the relationship between HGI and the incidence of MACCEs. An HGI between −1.32 and 0.12 positively impacted the composite MACCEs after adjusting for confounding factors (χ2 = 12.7, P = 0.005) (Fig. 5). Similar results were also found for all-cause death (HGI between −1.32 and 0.46) (χ2 = 25.3, P < 0.001) and CV death (HGI between −1.32 and −0.08) (χ2 = 11.9, P = 0.008) (Additional file 1: Figure S1).
Unadjusted and adjusted RCS of HGI and the incidence of composite MACCEs. Adjusted model included age, BMI, heart rate, hypertension, previous stroke, past PCI, NSTEMI, WBC, eGFR, HDL-C, LVEF, LM/three-vessel or proximal LAD involved, and antiplatelet agents during hospitalization. RCS restricted cubic spline, HGI haemoglobin glycation index, HR hazard ratio, MACCEs major adverse cardiac and cerebral events, BMI body mass index, PCI percutaneous coronary intervention, NSTEMI non-ST segment elevation myocardial infarction, WBC white blood cells, eGFR estimated glomerular filtration rate, HDL-C high-density lipoprotein cholesterol, LVEF left ventricular ejection fraction, LM left main vessel, LAD left anterior descending artery
Independent association of HGI with MACCEs in different subgroups
Subgroup analysis was carried out according to age, sex, BMI, smoker, hypertension, diabetes, eGFR, and LVEF, demonstrating a predictive effect of HGI on MACCEs in many subgroups (Fig. 6). For patients aged ≥ 65 years, moderate HGI (Q2, Q3, Q4) usually comes with a lower incidence of MAACEs. Male patients with Q2 HGI and female patients with Q2-Q4 HGI suffered a lower risk of MACCEs. For patients with BMI ≥ 25 and hypertension, HGI within Q2-Q3 was correlated with a lower incidence of MACCEs. Patients with LVEF < 55 or without diabetes had a lower risk of MACCEs in the Q1-Q4 HGI groups than in the Q5 HGI group.
Forest plot of composite MACCEs according to different subgroups. Adjusted model included age, BMI, heart rate, hypertension, previous stroke, past PCI, NSTEMI, WBC, eGFR, HDL-C, LVEF, LM/three-vessel or proximal LAD involved, and antiplatelet agents during hospitalization. HR hazard ratio, MACCEs major adverse cardiac and cerebral events, BMI body mass index, PCI percutaneous coronary intervention, NSTEMI non-ST segment elevation myocardial infarction, WBC white blood cells, eGFR estimated glomerular filtration rate, HDL-C high-density lipoprotein cholesterol, LVEF left ventricular ejection fraction, LM left main vessel, LAD left anterior descending artery, Ref. reference(Q5, 0.485 ≤ HGI < 7.875)
Discussion
This study aimed to elucidate the predictive effects of HGI levels on the outcomes in ACS patients with or without diabetes, to the best of our knowledge. The main findings include the following: (1) A U-shaped relationship was reported between HGI levels and incidence of MACCEs. Both lower and higher HGI could cause an increased risk of poor outcomes in ACS patients. This finding was consistent with Yuesong’s work in diabetic patients with ischaemic stroke [15]. They identified that in diabetic patients coexisting with ischaemic stroke, low HGI and high HGI contributed to an increased risk of stroke recurrence and poor outcome. (2) The predictive effect of HGI on MACCEs is powerful in Q2-Q3 (−0.491 to −0.196). (3) Several clinical factors, such as age, hypertension, previous stroke, past PCI, BMI, and heart rate, independently predicted the incidence of MACCEs in ACS patients.
There are no clear mechanisms of the variation between the actual and predicted levels oof HbA1c. HbA1c is glycated haemoglobin formed by an intracellular nonenzymatic reaction, while FPG reflects the plasma glucose condition [16]. Considering this situation, lower erythrocyte turnover rates may contribute to the accumulation of HbA1c [17]. According to existing evidence, glycation is a complex biological process affected by various factors, factors that influence intracellular glucose concentrations or nonenzymatic haemoglobin glycation (such as the intracellular pH value) may also affect the degree of haemoglobin glycation [16, 17]. From this perspective, all factors regulated glucose metabolism may contribute to the individual HGI variations.
A large body of evidence has focused on the association of HGI with clinical situations [13, 18, 19]. In the Diabetes Control and Complications Trial (DCCT), Twomey et al. found that in patients with type 1 diabetes, the increased rate of retinopathy and nephropathy usually comes after higher HGI [13]. In addition, the Control Cardiovascular Risk in Diabetes (ACCORD) trial [18] reported that patients with low and moderate HGI levels could benefit from intensive treatment, which is not observed in high HGI group. Unlike the linear relation between HGI and vascular complications reported in previous studies, our study reported a U-shaped association of HGI with the prognosis of ACS patients. Both patients with low and high HGI had poorer prognoses than those with moderate HGI. One potential explanation is stress hyperglycemia [20], a feature of ACS. Stress hyperglycaemia could contribute to high FPG followed by low HGI, but more research is needed to confirm this point.
To better understand the predictive power of HGI for ACS, this study analyzed the correlation between HGI and each type of MACCE and found that HGI was closely related to CV death. In addition, we determined the predictive value of HGI on the composite of MACCEs in different subgroups, such as sex, age, and medical history, indicating that HGI is a good predictor for MACCEs. There is limited evidence showing clinical factors affecting the effect of the HGI value. Di-Shuang et al. [21]. found that higher HGI increased the incidence of hepatic steatosis when adjusted for age, sex, and BMI. Several studies also reported a close relationship between HGI and sex [18, 22], while others did not show any sex differences in HGI [23, 24]. In the future, more research is required to assess the findings. Although HGI is a complex parameter to measure the differences between predict and actual levels of glycation of haemoglobin, current evidence indicated that HGI is helpful to evaluate the prognosis of ACS patients, which may help to develop personalized treatment strategies.
In addition, Table 1 demonstrated that there is significant difference of the usage of ACEI/ARB, statin among different HGI groups. This promoted us to investigate the interactions between drugs and HGI levels. Currently, there are few studies focused on this issue. However, we could study from some indirect evidence. A study focused on the efficacy of HGI on non-diabetes patients, and it came out that high HGI were likely to come with obese, higher levels of TG and lower levels of HDL-C [19]. From this aspect, statin treatment may contribute to lower HGI levels. Various researches have reported the efficacy of ACEI/ARBs or SGLT2 inhibitors in diabetes. HGI and HbAc1 shared some commons, indicating that these agents may also influence HGI levels. To further digging the relationship between HGI levels and drug usage.
Limitations
First, although this study included a large sample size, there were still bias due to the single-center and retrospective design issue. Second, laboratory parameters were only measured once during the hospitalization period, which could cause potential bias. Third, we enrolled patients with or without diabetes, which may weaken the confidence of our findings. In the following step, we will enroll ACS patients with diabetes to further investigate the predictive value of HGI. Finally, more prospective cohort studies are necessary to confirm our results.
Conclusion
Conclusively, this study firstly demonstrated the relationship between hemoglobin glycation index and outcomes in patients diagnosed with ACS. Both low HGI and high HGI was reported to attribute higher risk of poor prognosis in ACS patients compared with moderate HGI.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to the provisions of the CBD Bank but are available from the corresponding author on reasonable request.
Abbreviations
- HGI:
-
Haemoglobin glycation index
- ACS:
-
Acute coronary syndrome
- MACCEs:
-
Major adverse cardiac and cerebral events
- HR:
-
Hazard ratio
- CV death:
-
Cardiovascular death
- PCI:
-
Percutaneous coronary intervention
- BMI:
-
Body mass index
- NSTEMI:
-
Non-ST segment elevation myocardial infarction
- WBC:
-
White blood cells
- eGFR:
-
Estimated glomerular filtration rate
- HDL-C:
-
High-density lipoprotein cholesterol
- LVEF:
-
Left ventricular ejection fraction
- LM:
-
Left main vessel
- LAD:
-
Left anterior descending artery
- DM:
-
Diabetes mellitus
- HbA1c:
-
Haemoglobin A1c
- FPG:
-
Fast plasma glucose
- CBD:
-
Center of Beijing Friendship Hospital Database
- RBG:
-
Random blood glucose
- OGTT:
-
Oral glucose tolerance test
- TC:
-
Total cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- TG:
-
Triglyceride
- STEMI:
-
ST segment elevation myocardial infarction
- UA:
-
Unstable angina
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- CIs:
-
Confidence intervals
- RCS:
-
Restricted cubic spline
- CABG:
-
Coronary Artery Bypass Grafting
- ACEI:
-
Angiotensin-converting enzyme inhibitors
- ARB:
-
Angiotensin receptor blockers
- Hs-CRP:
-
Hypersensitive c-reactive protein
- DCCT:
-
Diabetes Control and Complications Trial
- ACCORD:
-
Control Cardiovascular Risk in Diabetes trial
References
Xin YG, Zhang HS, Li YZ, Guan QG, Guo L, Gao Y, Yu HJ, Zhang XG, Xu F, Zhang YL, et al. Efficacy and safety of ticagrelor versus clopidogrel with different dosage in high-risk patients with acute coronary syndrome. Int J Cardiol. 2017;228:275–9.
Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57(8):1542–51.
Wang H, Ba Y, Cai RC, Xing Q. Association between diabetes mellitus and the risk for major cardiovascular outcomes and all-cause mortality in women compared with men: a meta-analysis of prospective cohort studies. BMJ Open. 2019;9(7): e024935.
Zhou M, Liu J, Hao Y, Liu J, Huo Y, Smith SC Jr, Ge J, Ma C, Han Y, Fonarow GC, et al. Prevalence and in-hospital outcomes of diabetes among patients with acute coronary syndrome in China: findings from the Improving Care for Cardiovascular Disease in China-Acute Coronary Syndrome Project. Cardiovasc Diabetol. 2018;17(1):147.
Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–97.
Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–15.
Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–7.
Classification and Diagnosis of Diabetes. Standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13-s28.
Balasubramanian S, Carnagarin R, Arcot Jagdeeshwaran M, Subhashree. Effect of iron deficiency on glycation of haemoglobin in nondiabetics. J Clin Diagn Res. 2013;7(1):15–7.
Rodríguez-Segade S, Rodríguez J, García Lopez JM, Casanueva FF, Camiña F. Estimation of the glycation gap in diabetic patients with stable glycemic control. Diabetes Care. 2012;35(12):2447–50.
Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473–8.
van Steen SC, Woodward M, Chalmers J, Li Q, Marre M, Cooper ME, Hamet P, Mancia G, Colagiuri S, Williams B, et al. Haemoglobin glycation index and risk for diabetes-related complications in the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial. Diabetologia. 2018;61(4):780–9.
Twomey PJ, Viljoen A, Reynolds TM, Wierzbicki AS. Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care. 2004;27(10):2569–70.
Nagayama D, Watanabe Y, Yamaguchi T, Saiki A, Shirai K, Tatsuno I. High hemoglobin glycation index is associated with increased systemic arterial stiffness independent of hyperglycemia in real-world Japanese population: a cross-sectional study. Diab Vasc Dis Res. 2020;17(9):1479164120958625.
Pan Y, Jing J, Wang Y, Liu L, Wang Y, He Y. Association of hemoglobin glycation index with outcomes of acute ischemic stroke in type 2 diabetic patients. Neurol Res. 2018;40(7):573–80.
Hempe JM, Gomez R, McCarter RJ Jr, Chalew SA. High and low hemoglobin glycation phenotypes in type 1 diabetes: a challenge for interpretation of glycemic control. J Diabetes Complications. 2002;16(5):313–20.
Chalew SA, McCarter RJ, Thomas J, Thomson JL, Hempe JM. A comparison of the Glycosylation Gap and Hemoglobin Glycation Index in patients with diabetes. J Diabetes Complications. 2005;19(4):218–22.
Hempe JM, Liu S, Myers L, McCarter RJ, Buse JB, Fonseca V. The hemoglobin glycation index identifies subpopulations with harms or benefits from intensive treatment in the ACCORD trial. Diabetes Care. 2015;38(6):1067–74.
Marini MA, Fiorentino TV, Succurro E, Pedace E, Andreozzi F, Sciacqua A, Perticone F, Sesti G. Association between hemoglobin glycation index with insulin resistance and carotid atherosclerosis in non-diabetic individuals. PLoS ONE. 2017;12(4): e0175547.
Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373(9677):1798–807.
Hu DS, Zhu SH, Li X, Chen QF, Lin CJ, Fang DH, Wu JS. Association between Hemoglobin Glycation Index and NAFLD in Chinese Nondiabetic Individuals. Can J Gastroenterol Hepatol. 2019;2019:8748459.
Ahn CH, Min SH, Lee DH, Oh TJ, Kim KM, Moon JH, Choi SH, Park KS, Jang HC, Ha J, et al. Hemoglobin Glycation Index is associated with cardiovascular diseases in people with impaired glucose metabolism. J Clin Endocrinol Metab. 2017;102(8):2905–13.
Lee B, Heo YJ, Lee YA, Lee J, Kim JH, Lee SY, Shin CH, Yang SW. Association between hemoglobin glycation index and cardiometabolic risk factors in Korean pediatric nondiabetic population. Ann Pediatr Endocrinol Metab. 2018;23(4):196–203.
van Steen SC, Schrieks IC, Hoekstra JB, Lincoff AM, Tardif JC, Mellbin LG, Rydén L, Grobbee DE, DeVries JH. The haemoglobin glycation index as predictor of diabetes-related complications in the AleCardio trial. Eur J Prev Cardiol. 2017;24(8):858–66.
Acknowledgements
We gratefully acknowledge the contributions of all staffs who work on the CBD Bank, especially the assistance with data acquisition by Dr. Guoliang Zhao.
Funding
This work was supported by the National Key R&D Program of China (Grant No.2021ZD0111004), the National Natural Science Foundation of China (Grant No.82070357) and Beijing Key Clinical Subject Program.
Author information
Authors and Affiliations
Contributions
JL and YX draft the manuscript and carried out the statistical analysis, JL participated in study data collection, LZ and HQ contributed discussion and edited manuscript. AS and HC revised manuscript. HL designed and supervised the project. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study data collections were approved by the Institutional Review Board of Beijing Friendship Hospital affiliated to Capital Medical University, and informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Additional file 1: Figure S1.
Adjusted RCS of HGI and the incidence of all-cause death (A), CV death (B), non-fatal MI (C), cardiac rehospitalization (D), revascularization (E), non-fatal stroke (F). Adjusted model included age, BMI, heart rate, hypertension, previous stroke, past PCI, NSTEMI, WBC, eGFR, HDL-C, LVEF, LM/three-vessel or proximal LAD involved, and antiplatelet agents during hospitalization. RCS, restricted cubic spline; HGI, haemoglobin glycation index; HR, hazard ratio; MACCEs, major adverse cardiac and cerebral events; CV death, cardiovascular death; MI, myocardial infarction; BMI, body mass index; PCI, percutaneous coronary intervention; NSTEMI, non-ST segment elevation myocardial infarction; WBC, white blood cells; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; LM, left main vessel; LAD, left anterior descending artery.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, J., Xin, Y., Li, J. et al. Association of haemoglobin glycation index with outcomes in patients with acute coronary syndrome: results from an observational cohort study in China. Diabetol Metab Syndr 14, 162 (2022). https://doi.org/10.1186/s13098-022-00926-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-022-00926-6