Abstract
Background
Epidemiological observational studies have elucidated a correlation between rheumatoid arthritis (RA) and bronchiectasis. However, the causal nature of this association remains ambiguous. To clarify this potential causal linkage, we conducted a two-sample Mendelian randomization (MR) analysis to explore the bidirectional causality between RA and bronchiectasis.
Methods
Summary statistics for RA and bronchiectasis were obtained from the IEU OpenGWAS database We employed various methods, including inverse variance weighting (IVW), MR-Egger, weighted median, weighted mode, and simple mode, to explore potential causal links between RA and bronchiectasis. Additionally, a series of sensitivity studies, such as Cochran’s Q test, MR Egger intercept test, and leave-one-out analysis, were conducted to assess the MR analysis’s accuracy further.
Results
In the forward MR analysis, the primary analysis indicated that a genetic predisposition to RA correlated with an increased risk of bronchiectasis in European populations (IVW odds ratio (OR): 1.28, 95% confidence interval (CI): 1.20–1.37, p = 1.18E-13). Comparable results were noted in the East Asian subjects (IVW OR: 1.55, 95% CI: 1.30–1.34, p = 8.33E-07). The OR estimates from the other four methods were consistent with those obtained from the IVW method. Sensitivity analysis detected no evidence of horizontal pleiotropy or heterogeneity. Conversely, in the reverse MR analysis, we found no evidence to support a genetic causality between bronchiectasis and RA in either European or East Asian populations.
Conclusion
This study indicates that genetic predisposition to RA correlates with a heightened risk of bronchiectasis in both European and East Asian populations. These results imply that routine screening for bronchiectasis in RA patients could be beneficial, and effective management of RA may contribute to a reduced risk of bronchiectasis. Future research should aim to clarify the underlying mechanisms linking these two conditions.
Similar content being viewed by others
Background
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disorder characterized by progressive joint damage and extra-articular manifestations, potentially leading to permanent disability and heightened mortality [1]. A meta-analysis of 67 rheumatoid arthritis cohort studies across 41 countries indicated a pooled prevalence of 0.46% [2]. The most common extra-articular manifestation of RA is lung involvement, which can affect up to 60% of patients with RA during the disease course [3]. Bronchiectasis is an established extra-articular manifestation of RA, which presents as irreversible damage to the bronchi along with widening and thickening resulting in exuberant mucus production [4]. Approximately 30% of RA patients demonstrate bronchiectasis on High-Resolution Computed Tomography, which can be asymptomatic and may precede or follow the development of RA [5,6,7]. RA-associated bronchiectasis significantly reduces quality of life and increases the risk of mortality [4, 8]. Consequently, it is imperative to investigate the relationship and pathogenesis between RA and bronchiectasis.
The link between bronchiectasis and RA has been a subject of extensive research over several decades. Numerous studies indicate that RA may contribute to the risk of developing bronchiectasis. For instance, a systematic review and meta-analysis revealed an elevated prevalence of both radiological and symptomatic bronchiectasis among RA patients relative to the general population [9]. Conversely, evidence also suggests that bronchiectasis could be a risk factor for RA. A prospective study observed that 50% of bronchiectasis patients with positive rheumatoid factor and high anti-cyclic citrullinated peptide levels developed RA within a 12-month period [10].
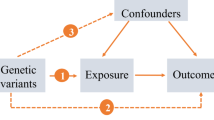
These observational studies could be influenced by potential confounders and reverse causation, which makes it difficult to ascertain the causality between bronchiectasis and RA. Mendelian randomization (MR), a statistical approach employing genetic variation as an instrumental variable (IV), evaluates whether the observational association between exposure factors and outcomes aligns with a causal effect [11]. It avoided confounding and reverse causality because genetic variants were identified at the time of conception [12]. Consequently, our study took advantage of MR and investigated the bidirectional causal relationship between bronchiectasis and RA.
Materials and methods
Study design
An overview of the bidirectional MR study design is shown in Fig. 1. First, a two-sample MR analysis was conducted, treating RA as the exposure and bronchiectasis as the outcome. Subsequently, the analysis was inverted to investigate bronchiectasis as the exposure and RA as the outcome. The validity of MR analysis hinges on three critical assumptions [13]: (1) There is a strong association between the instrumental variables and the exposure; (2) Each IV is not associated with any confounding variables; (3) Each IV is associated with the outcome solely through the exposure, and there are no alternative pathways for the association.
GWAS data sources
The datasets for this MR study were obtained from the publicly accessible IEU Open GWAS database (https://gwas.mrcieu.ac.uk/, accessed on 19 November 2023). Summary statistics for RA were obtained from a GWAS including 417,256 European subjects (8,255 cases and 409,001 controls) and another encompassing 178,616 individuals (2,888 cases and 440,263 controls) from East Asian populations. Similarly, bronchiectasis data were derived from a GWAS including 443,151 European subjects (5,348 cases and 173,268 controls) and an additional study involving 162,044 East Asian individuals (241 cases and 161,803 controls). Comprehensive details of all GWAS used in our study are detailed in Table 1.
Instrumental variable selection
To ensure the validity of our genetic IVs and satisfy the three core MR assumptions, we employed a comprehensive set of quality control techniques. Firstly, single nucleotide polymorphisms (SNPs) associated with the exposure were identified using a genome-wide significance threshold of p < 5E–08. In cases where the number of SNPs meeting this criterion is inadequate, SNPs with a relaxed significance threshold (p < 5E–06) may be considered as IVs. Secondly, to reduce linkage disequilibrium, the identified exposure SNPs were clumped (r² = 0.001, window = 10 Mb, p -value threshold = 5E-8) utilizing the 1000 Genomes European dataset as a reference. Thirdly, we also excluded SNPs with palindromic intermediate allele frequencies and incompatible alleles [14]. Fourthly, each SNP’s secondary phenotype was assessed using the PhenoScanner database (http://www.phenoscanner.medschl.cam.ac.uk/) with a p -value threshold of p < 1E–05, and SNPs linked to confounders were excluded to mitigate pleiotropic effects. Finally, we ensured that the F statistic of all IVs was greater than the threshold 10 to minimize weak instrument bias. The F statistic was calculated using the formula F = R2(N-K-1)/K(1-R2) [15].
Statistical analysis
This study employed multiple complementary methods to explore the causal relationship between bronchiectasis and RA. These methods included the inverse variance weighted method, the weighted median approach, the MR-Egger regression, the simple mode and weighted mode. The IVW method, used as the primary analysis for causal estimates, was most precise when all instrumental variables are valid [16]. The weighted median approach offered a consistent estimate of the causal effect when at least half of the SNPs serve as effective IVs [17]. The MR-Egger regression was used to confirm whether horizontal pleiotropy of IVs exists, and its intercept represents the effect estimate of horizontal pleiotropy [18]. The simple mode and weighted mode were performed as complementary analyses.
Additionally, we performed sensitivity analyses to assess the dependability of our findings. The MR-Egger intercept was used to determine directional horizontal pleiotropy [19]. Subsequently, the Mendelian randomization pleiotropy residual sum and outlier (MR-PRESSO) test was applied to detect potential horizontal pleiotropy and correct it by removing outliers [20]. The Cochrane Q test was used to evaluate heterogeneity between SNPs [21]. Furthermore, the leave-one-out analysis was used to investigate whether the genetic causal relationship between exposures and outcomes was influenced by a single SNP [22]. All statistical analyses were performed using the “TwoSampleMR” and “MR-PRESSO” packages in R software (version 4.3.1).
Results
Forward MR analyses of the effects of RA on bronchiectasis
Following a rigorous selection process for eligible IVs and the exclusion of potential pleiotropic SNPs, 24 SNPs for RA in European populations and 31 SNPs for RA in East Asian populations were finally identified as IVs in the MR analysis. The F-statistics of the screened SNPs were all > 10 to avoid bias caused by weak IVs. The information of these genetic variants utilized in the MR analyses is detailed in Supplementary Tables 1–2.
Figure 2 illustrates that the MR results support a causal link between genetic susceptibility to RA and an increased risk of bronchiectasis. Among individuals of European ancestry, the primary analysis revealed a significant causal association (IVW odds ratio (OR): 1.28, 95% confidence interval (CI): 1.20–1.37, p = 1.18E-13). Similar findings were observed in the East Asian population (IVW OR: 1.55, 95% CI: 1.30–1.34, p = 8.33E-07). This result was broadly consistent with results obtained using other MR methods including the weighted median approach, the MR-Egger regression, the simple mode and weighted mode.
In the sensitivity analysis, the Cochran Q test revealed no heterogeneity (p -value > 0.05), and the MR-Egger regression indicated no evidence of pleiotropy (intercept p -value > 0.05), as detailed in Table 2. Additionally, the leave-one-out analysis suggested that the causal effect of RA on bronchiectasis was not driven by any single SNP (Supplementary Figs. 1–2).
Reverse MR analyses of the effects of bronchiectasis on RA
After using strict criteria to rule out SNPs, 7 SNPs for bronchiectasis in European populations and 5 SNPs for bronchiectasis in Asian populations were identified as IVs in the MR analysis. The F-statistics of the screened SNPs were all > 10 to avoid bias caused by weak IVs. The information of these genetic variants utilized in the MR analyses is detailed in Supplementary Tables 3–4.
As shown in Fig. 3, there were no causal effects of bronchiectasis on RA either in European ancestry (IVW OR: 0.93, 95% CI: 0.86–1.02, p = 0.113) or East Asian populations (IVW OR: 0.99, 95% CI: 0.97–1.03, p = 0.74). This absence of causal effects was consistent across various MR methods including the weighted median approach, the MR-Egger regression, the simple mode and weighted mode. The sensitivity analysis did not find heterogeneity (p -value of Cochran Q > 0.05) and pleiotropy (p -value for intercept > 0.05) (Table 2). Leave-one-out analysis showed that no outlier SNP was detected. (Supplementary Figs. 3–4).
Discussion
For the first time, we conducted a bidirectional two-sample MR analysis to investigate the causal effect between RA and bronchiectasis. The results revealed a causal association between genetic susceptibility to RA and an increased risk of bronchiectasis. Conversely, no evidence was found to suggest a causal relationship between bronchiectasis and an increased risk of RA. The findings of this study offer significant insights into the potential causal connection between these two conditions.
Epidemiological research has consistently shown a link between rheumatoid arthritis and bronchiectasis. For instance, a cross-sectional study indicated that RA might contribute to more severe and extensive bronchiectasis [23]. Additionally, evidence from a retrospective cohort study suggested that RA disease duration is associated with higher risk of having respiratory symptoms (not specific to bronchiectasis) [24]. These results are consistent with our forward MR analysis. However, our reverse MR analysis provided contrasting evidence, diverging from observational studies. A recent meta-analysis revealed that bronchiectasis preceded RA in 53 of 69 cases, indicating that bronchiectasis may contribute to the development of RA [9]. Furthermore, a prospective study observed that 50% of bronchiectasis patients with positive rheumatoid factor and high anti-cyclic citrullinated peptide levels developed RA within a 12-month period [10]. A potential explanation for the differences between the results of observational studies and our reverse MR analysis might be that the previously observed results were either coincidental or influenced by unidentified confounding factors.
Several hypothesis have been put forward to explain the mechanisms underlying the development of bronchiectasis in RA [25]. Primarily, the onset of bronchiectasis in RA may be linked to RA-related autoantibodies. Aubart et al. demonstrated that elevated levels of anti-cyclic citrullinated peptide antibodies (ACPA) correlated with the occurrence of pulmonary diseases like bronchiectasis in RA patients [26]. Additionally, Attar et al. reported that patients with RA who were positive for ACPA exhibited an increased risk of bronchiectasis [27]. Collectively, these studies implied that autoantibodies substantially may contribute to bronchiectasis in RA patients. Secondly, the likelihood that recurrent infections related to chronic immune suppression may predispose patients with rheumatoid arthritis to bronchiectasis [8]. For instance, patients with RA and bronchiectasis have frequent lower respiratory tract infections, and these infections were associated with the utilization of biologic disease-modifying antirheumatic drugs (DMARD) [28]. Consequently, it is hypothesized that the recurrent infections linked to DMARD use in RA patients might contribute to an increased risk of concurrent bronchiectasis. Lastly, genetic factors may also play a role in the association between RA and bronchiectasis. A family-based association study highlighted that cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations in RA patients are indicators of associated bronchiectasis risk [29]. However, research indicates that only 15.4% of patients diagnosed with both bronchiectasis and rheumatoid arthritis display mutations in the CFTR gene [30], suggesting that these mutations may have a minimal impact.
There are several advantages to this study. Firstly, we used a MR analysis that is not affected by confounders and reverse causation compared with observational studies. Secondly, the bidirectional approach facilitates the exploration of causal relationships in both directions between RA and bronchiectasis. Additionally, the validation of our findings across European and East Asian datasets enhances the credibility of our conclusions. However, there are still limitations. Firstly, the MR results are primarily based on European and East Asian populations, and the applicability of these findings to other populations requires further investigation. Secondly, the absence of demographic data such as gender and age in the public databases precluded the possibility of conducting additional subgroup analyses. Furthermore, the SNPs used in the analysis may be associated with other traits due to genetic polymorphisms, potentially impacting the accuracy of causal inference.
Conclusions
In summary, this study utilized a bidirectional two-sample MR approach to assess the causal relationship between RA and bronchiectasis. It established a causal link between genetic predisposition to RA and an elevated risk of bronchiectasis, while not finding a similar association for genetic predisposition to bronchiectasis increasing the risk of RA. These findings suggest that routine screening for bronchiectasis in RA patients, coupled with effective RA management, could help mitigate the risk of developing bronchiectasis. Additionally, it is crucial to carry out a placebo-controlled randomized trial in patients with RA associated bronchiectasis to identify treatment strategies that improve prognosis for this subgroup. Furthermore, future research should focus on clarifying the underlying mechanisms that link RA and bronchiectasis.
Data availability
Publicly available datasets can be found here: the Integrative Epidemiology Unit (IEU) GWAS database (https://gwas.mrcieu.ac.uk).
Abbreviations
- RA:
-
Rheumatoid arthritis
- MR:
-
Mendelian randomization
- SNP:
-
Single-nucleotide polymorphism
- GWAS:
-
Genome-wide association
- IVW:
-
Inverse-variance-weighted
- OR:
-
Odds ratio
- CI:
-
confidence interval
- MRPRESSO:
-
Mendelian randomization pleiotropy residual sum and outlier
- ACPA:
-
anti-cyclic citrullinated peptide antibodies
- CFTR:
-
cystic fibrosis transmembrane conductance regulator study
References
Finckh A, Gilbert B, Hodkinson B, Bae S-C, Thomas R, Deane KD, et al. Global epidemiology of rheumatoid arthritis. Nat Rev Rheumatol. 2022;18(10):591–602.
Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C. The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int. 2021;41(5):863–77.
Laria A, Lurati AM, Zizzo G, Zaccara E, Mazzocchi D, Re KA, et al. Interstitial lung disease in rheumatoid arthritis: a practical review. Front Med (Lausanne). 2022;9:837133.
Martin LW, Prisco LC, Huang W, McDermott G, Shadick NA, Doyle TJ, et al. Prevalence and risk factors of bronchiectasis in rheumatoid arthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2021;51(5):1067–80.
Cortet B, Flipo RM, Rémy-Jardin M, Coquerelle P, Duquesnoy B, Rêmy J, et al. Use of high resolution computed tomography of the lungs in patients with rheumatoid arthritis. Ann Rheum Dis. 1995;54(10):815–19.
Wilczynska MM, Condliffe AM, McKeon DJ. Coexistence of bronchiectasis and rheumatoid arthritis: revisited. Respir Care. 2013;58(4):694–701.
Cohen M, Sahn SA. Bronchiectasis in systemic diseases. Chest. 1999;116(4):1063–74.
Matson SM, Demoruelle MK, Castro M. Airway Disease in Rheumatoid Arthritis. Ann Am Thorac Soc. 2022;19(3):343–52.
Wiater R, Håkansson KEJ, Ulrik CS. A causal relationship between rheumatoid arthritis and bronchiectasis? A systematic review and meta-analysis. Chron Respir Dis. 2021;18:1479973121994565.
Perry E, Stenton C, Kelly C, Eggleton P, Hutchinson D, De Soyza A. RA autoantibodies as predictors of rheumatoid arthritis in non-cystic fibrosis bronchiectasis patients. Eur Respir J. 2014;44(4):1082–85.
Emdin CA, Khera AV, Kathiresan S, Mendelian Randomization. JAMA. 2017;318(19):1925–26.
Duan L, Chen D, Shi Y, Ye S, Dou S, Feng Y. Rheumatoid arthritis and hypothyroidism: a bidirectional mendelian randomization study. Front Immunol. 2023;14:1146261.
Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG. Using published data in mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30(7):543–52.
Cao Z, Wu Y, Li Q, Li Y, Wu J. A causal relationship between childhood obesity and risk of osteoarthritis: results from a two-sample mendelian randomization analysis. Ann Med. 2022;54(1):1636–45.
Dan Y-L, Wang P, Cheng Z, Wu Q, Wang X-R, Wang D-G, et al. Circulating adiponectin levels and systemic lupus erythematosus: a two-sample mendelian randomization study. Rheumatology (Oxford). 2021;60(2):940–46.
Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35(11):1880–906.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some Invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14.
Burgess S, Thompson SG. Interpreting findings from mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–89.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25.
Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–98.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7.
Su Y, Hu Y, Xu Y, Yang M, Wu F, Peng Y. Genetic causal relationship between age at menarche and benign oesophageal neoplasia identified by a mendelian randomization study. Front Endocrinol (Lausanne). 2023;14:1113765.
Metafratzi ZM, Georgiadis AN, Ioannidou CV, Alamanos Y, Vassiliou MP, Zikou AK, et al. Pulmonary involvement in patients with early rheumatoid arthritis. Scand J Rheumatol. 2007;36(5):338–44.
Remy-Jardin M, Remy J, Cortet B, Mauri F, Delcambre B. Lung changes in rheumatoid arthritis: CT findings. Radiology. 1994;193(2):375–82.
Bakheet SM, Hammami MM, Powe J. Radioiodine uptake in rheumatoid arthritis-associated lung disease mimicking thyroid cancer metastases. Clin Nucl Med. 1998;23(5):319–20.
Aubart F, Crestani B, Nicaise-Roland P, Tubach F, Bollet C, Dawidowicz K, et al. High levels of anti-cyclic citrullinated peptide autoantibodies are associated with co-occurrence of pulmonary diseases with rheumatoid arthritis. J Rheumatol. 2011;38(6):979–82.
Reynisdottir G, Karimi R, Joshua V, Olsen H, Hensvold AH, Harju A, et al. Structural changes and antibody enrichment in the lungs are early features of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheumatol. 2014;66(1):31–9.
Geri G, Dadoun S, Bui T, Del Castillo Pinol N, Paternotte S, Dougados M, et al. Risk of infections in bronchiectasis during disease-modifying treatment and biologics for rheumatic diseases. BMC Infect Dis. 2011;11:304.
Puéchal X, Génin E, Bienvenu T, Le Jeunne C, Dusser DJ. Poor survival in rheumatoid arthritis associated with bronchiectasis: a family-based cohort study. PLoS ONE. 2014;9(10):e110066.
Puéchal X, Fajac I, Fau - Bienvenu T, Bienvenu T, Fau - Desmazes-Dufeu N, Desmazes-Dufeu N, Fau - Hubert D, Hubert D. Fau - Kaplan JC, Kaplan Jc Fau - Menkès CJ, Increased frequency of cystic fibrosis deltaF508 mutation in bronchiectasis associated with rheumatoid arthritis. (0903–1936 (Print)).
Acknowledgements
We appreciate a lot for the investigators who shared the GWAS data.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
JL and YYH designed the study. ZHC and HLS acquired and analyzed the data. XGL and ZHC drafted the article. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The summary statistics used in the present study are aggregated level of data which do not contain any personal information. The original GWAS have obtained ethical approval from relevant ethics review committees.
Consent for publication
All authors read and approved the fnal manuscript. And agree to the publication of the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, Z., Li, X., Shi, H. et al. Causal relationship between rheumatoid arthritis and bronchiectasis: a bidirectional mendelian randomization study. Arthritis Res Ther 26, 104 (2024). https://doi.org/10.1186/s13075-024-03336-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-024-03336-3