Abstract
Background
Previous observational studies have shown an association between asthma, atopic dermatitis (AD) and rheumatoid arthritis (RA). However, the bidirectional cause-effect chain between asthma and AD and RA has not been proven yet.
Methods
We performed bidirectional two-sample Mendelian randomization (TSMR) and selected single nucleotide polymorphisms (SNPs) associated with asthma, AD, and RA as instrumental variables. All of the SNPs were obtained from the latest genome-wide association study in Europeans. Inverse variance weighted (IVW) was the main method used in MR analysis. MR-Egger, weighted model, simple model, and weighted median were used for quality control. The robustness of the results was tested by sensitivity analysis.
Results
Asthma was found to be the largest effect size for RA susceptibility using the IVW method (OR, 1.35;95%CI, 1.13–1.60; P, 0.001), followed by AD (OR, 1.10;95%CI, 1.02–1.19; P, 0.019). In contrast, there was no causal relationship between RA and asthma (IVW: P = 0.673) or AD (IVW: P = 0.342). No pleiotropy or heterogeneity was found in the sensitivity analysis.
Conclusion
Findings from this study showed a causal relationship between genetic susceptibility to asthma or AD and increased risk of RA, but do not support a causal relationship between genetic susceptibility to RA and asthma or AD.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by joint inflammation and destruction, followed by systemic inflammation. An estimated 0.4–1.3% of people in the world are affected by this disease, and its incidence rate in women is 2 to 4 times higher [1]. The occurrence of RA will not only affects the quality of life of patients but also brings great economic losses to society [2, 3]. RA is a disease caused by multiple factors, involving a complex interaction between genetic predisposition and environmental triggers. Smoking has been implicated as a major risk factor for RA as a result of the production of anti-citrullinated peptide antibodies (ACPA) [4, 5]. Moreover, the lifetime risk of RA is significantly higher for individuals who carry certain human leukocyte antigen (HLA) alleles [6].
To prevent the occurrence and development of RA, other risk factors are receiving increasing attention from scholars, such as allergic diseases. An observational study has shown that patients with allergic diseases, particularly asthma and allergic rhinitis, are at significantly increased risk for RA [7]. However, a cross-sectional study noted a higher prevalence of RA in non-asthmatic patients compared to asthmatic patients [8]. Moreover, Lu et al. [9] noted in a study that atopic dermatitis (AD) increases the risk of developing RA. While in contrast, Hilliquin et al. [10] suggested that atopic reactivity may have a preventive effect on the development of RA. Meanwhile, several reports have suggested that patients with RA have less atopic disease than patients in normal controls [11,12,13]. In contrast, Kero et al. [14] reported a significantly higher cumulative incidence of asthma in children with RA than in non-RA children. A study showed an association between RA and an increased risk of allergic diseases such as asthma and allergic rhinitis [15]. In light of the controversial association between the results of these studies, and the limitations of observational studies, further research is needed to determine this relationship. At the same time, studies examining the link between allergic diseases and RA at the genetic level have yet to emerge.
Mendelian randomization (MR), an epidemiological method, has been widely executed to evaluate the potential causal associations between exposures and outcomes [16, 17]. In MR analyses, using genetic variants as instrumental variables (IVs) could minimize confounders’ inverse causations or effects [18, 19]. Of note, a large number of recent Genome Wide Association Study (GWAS) data on allergic disease [20] and RA allow the use of MR analysis to explore associated disease causality [21,22,23]. Therefore, to fill the limitations of the current study, we performed a bidirectional TSMR analysis aimed at examining whether there is a causal association between allergic diseases (asthma and AD) and RA, as well as determining the direction of causality.
Data and Methods
Study design overview
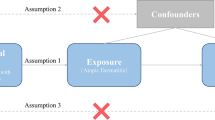
We executed a bidirectional TSMR analysis to assess the bidirectional causal effects of asthma or AD and RA. We applied previously identified genetic variants (single-nucleotide polymorphisms, SNPs) from published data or the Genome Reference Consortium to estimate their potential causal effects of exposures on outcomes. The valid MR analyses were structured to define the following three key assumptions: [1] genetic variants are strongly associated with the studied exposures, [2] exposures and outcomes are independent of any known confounders, and [3] genetic variants do not affect the outcomes through alternative pathways except the relevant assumption. The relationships between the exposures and outcomes are illustrated in Fig. 1.
Date sources
The summary statistics of the GWAS for asthma, AD, and RA were currently obtained from the IEU OpenGWAS (https://gwas.mrcieu.ac.uk/). There are 56,167 cases and 352,255 controls for asthma; 7,024 cases and 198,740 controls for AD; and 2,843 cases and 5,540 controls for RA. All cases were confirmed by clinical laboratory testing, physician diagnosis, or self-reported. To reduce outcome bias from race-related confounders, the study was limited to the European population. Table S1 shows detailed information on our data used.
Instrumental variables
At the beginning of our study design, we selected appropriate SNPs as IVs, which must be robustly associated with exposures (P < 5 × 10− 8). SNPs should be restricted by low linkage disequilibrium (LD, r2 < 0.01, 5,000 kb) using clumping. In addition, we excluded palindromic SNPs whose minor allele frequency (MAF) of outcomes was less than 0.01.
Mendelian randomization analyses
All analyses were executed in R version 4.2.0 using the TwoSampleMR package 0.5.6. After selecting appropriate SNPs of exposures, the inverse-variance weighted (IVW) analysis was chosen as the primary approach to evaluate the causal-and-effect relationship. Then, we added a series of MR analyses including Mendelian randomization-egger (MR-Egger), Weighted median (WM), Weighted mode, and Simple mode methods. When performing MR approaches, P < 0.05 was regarded as suggestive evidence for potential association. The odd ratio (OR) and standard error (SE) were calculated to show effect sizes. In addition, the IVW method and MR-Egger regression were used to investigate the presence of heterogeneity in the results, which was quantified using Cochran’s Q-test [24]. MR-Egger regression is also used to determine the likelihood of pleiotropy, with the intercept term indicating potential horizontal pleiotropy [25]. Meanwhile, we also used the “leave-one-out” method to remove single SNP which has a significant independent influence on the MR method [26].
Results
Strength of genetic instruments
Based on the above criteria for MR analyses, 53 SNPs associated with asthma, 25 SNPs associated with AD, and 40 SNPs correlated with RA were used as instrumental variables for subsequent analysis. All of these IVs had F-values greater than 10, indicating that the bias of these instrumental variables did not directly affect the assessment of causal effects (Table S2).
Effect of asthma and AD on RA
The effects of each SNPs in asthma and AD on RA can be found in Table 1. We found that the causal relationship between asthma or AD and RA differed among the five MR methods. MR results for the IVW, MR-Egger, and WM methods showed a significant connection between asthma and RA (IVW, OR = 1.35, 95% CI = 1.13–1.60, P = 0.001; MR-Egger, OR = 2.62, 95% CI = 1.20–5.72, P = 0.020; WM, OR = 1.29, 95% CI = 1.01–1.66, P = 0.042) (Table 1). For AD, the results of IVW method can support this causal relationship (OR = 1.10, 95% CI = 1.02–1.19, P = 0.019) (Table 1). The MR-Egger’s analysis showed no underlying horizontal pleiotropy (asthma: P = 0.094, AD: P = 0.450) (Table 2). Cochran’s Q test (Table 2) showed no heterogeneity in the risk of asthma or AD and RA. Furthermore, we conducted a “leave-one-out analysis” and found that none of the SNPs strongly influenced the overall effect of asthma or AD and RA (Fig. S1).
Effect of RA on asthma and AD
As shown in Tables 3 and 40 instrumental variables were included in the reverse MR analysis. To determine the correlation between RA and asthma or AD, we performed a reverse MR analysis and the IVW method showed no correlation between RA and asthma (IVW: P = 0.673). Likewise, there is no causal relationship between RA and AD (IVW: P = 0.342). Sensitivity analyses and tests of heterogeneity did not indicate potential horizontal pleiotropy and significant heterogeneity (Table 4). No single SNP strongly affected the overall outcome of RA on asthma and AD as demonstrated by the “leave-one-out” sensitivity analysis (Fig. S2).
Discussion
In the present study, we assessed the bidirectional causal relationship between asthma, AD, and RA. By using a two-sample MR approach and using GWAS summary statistics, the results showed a positive causal relationship between asthma, AD and RA. In reverse MR analysis, no causal relationship was observed between RA and asthma or atopic dermatitis. These findings suggested that the three diseases may have similar pathogenesis.
Previous epidemiological studies have examined the associations between asthma, AD, and RA. Several case-control studies have identified asthma as a possible risk factor for RA, but with the potential for recall bias [27,28,29]. In addition, retrospective cohorts have analyzed the overall risk of asthma and RA [7, 8, 30,31,32]. Studies using administrative datasets have also reported an association between asthma and RA risk, but lack data on smoking or serum status with RA [30,31,32]. Certainly, in a large national study in Taiwan based on billed claims data, RA was associated with a 2-fold increased risk of asthma compared to controls [11]. Furthermore, studies on AD have published different views, and several previous epidemiological studies have shown no association between AD and RA [10, 33, 34]. However, recent studies point to a correlation between AD and RA and that AD may promote the development of RA [15, 29, 35]. Our study was based on the largest available GWAS dataset and restricted the population to individuals of European ancestry to avoid bias on account of small sample size or ethnic differences. The results showed that both asthma and AD are related to an increased risk of RA and that there is no reverse causality between them, suggesting that the previously observed controversial results may be attribute to confounding factors or ethnic differences.
The underlying mechanisms of these associations are poorly understood, but there are still some mechanisms that can be used to explain their possible connection. In the first place, some common immune pathogenesis between not only asthma but also AD and RA. T helper 17 (TH17) cells are one of the pro-inflammatory T helper cell subsets and their increased activity plays a big role in the development of RA [36]. Interleukin 17 (IL-17), an inflammatory factor produced by TH17 cells, has increased expression in AD patients compared to healthy subjects [37]. Of course, studies also suggested that increased TH17 activity and increased IL-17 expression play an important role in the development of airway inflammation in asthma by inducing Th2-interrelated eosinophilia and airway Mucin 5AC expression, as well as increased airway hyperresponsiveness [38,39,40,41,42]. This immune pathway is also thought to be involved in the pathogenesis of RA, as IL-17 expression and TH17 activity are increased in RA patients compared to non-RA individuals [43, 44]. In the second place, studies have pointed out that asthma and RA may have overlapping genetic predispositions, and certain genetic variants in immune-related genes are associated with increased susceptibility to asthma and RA, such as HLA-DRB1 [45, 46], CD40L, and CD86 [47]. Interestingly, certain genes (such as HLA-DRB1 and PTPN22) have been confirmed to be associated with AD and RA [48,49,50], but no common susceptibility locus have been identified for these two diseases. Therefore, further studies are needed to explore this potential explanation.
In addition, several environmental factors can be used to explain this association of asthma or AD and RA, for example, smoking can contribute to increased inflammation in the lower respiratory tract through a variety of mechanisms and is a predisposing factor for asthma [51]. Smoking also induces the release of peptidylarginine deiminase 2 and enzyme 4 from lung phagocytes, which can convert endogenous proteins into guanylated autoantigens [5]. These guanylated autoantigens, in turn, stimulate the development of anti-citrullinated peptide antibodies in genetically susceptible individuals and may eventually trigger a chronic inflammatory response in synovial junctions, leading to RA development [6, 52]. Toxic substances produced by smoking (e.g., nicotine and carbon monoxide) disrupt skin barrier function, and these substances disrupt blood flow and oxygenation of the skin [53]. These skin disorders and associated subcutaneous structures allow the penetration of allergens into the skin, leading to AD [54]. Thus, smoking is a causative factor in their communication. These possible assumptions can provide theoretical support for our research results.
The main strength of this study is MR analysis; while randomized controlled trials (RCTs) can provide the most convincing evidence, they involve many ethical issues and have high financial costs. For observational studies, which are adjusted for other relative variables, the undetected bias cannot be ignored. Hence, MR provides the most convincing results. Bias due to confounding and reverse sources can be reduced by MR. To minimize potential violations of MR assumptions, we also performed continuous sensitivity analyses and detected any outliers by radial MR analysis.
Nevertheless, the limitations of our MR analyses also need to be acknowledged. Firstly, our results of MR analyses were mainly focused on the European population to reduce the ethnic confounding. Hence, reliable datasets on non-European or mixed population are urgently needed, because we need to break the link correlated with particular genes of the local environment. Secondly, potential pleiotropy could not be fully ruled out, resulting in three hypotheses that could not be accurately evaluated. However, sensitivity analyses were performed using multiple methods to obtain consistent results, which makes the results of this study reassuring.
Conclusion
Overall, we used two-sample MR analyses to describe the bidirectional causality between asthma, AD, and RA, focusing on filling a gap in knowledge of this causal chain. The results of MR Analysis provide strong evidence for a positive causal relationship between asthma and AD and RA. Nevertheless, more large-scale studies are needed to support our findings and explore the specific mechanisms involved.
Data Availability
As data for this study was acquired via a database (244,991,502,576 genetic associations from 42,335 GWAS summary datasets), the authors applied a broad consent to allow research participants to query and download a broad range of their data (https://gwas.mrcieu.ac.uk/).
References
Lin Y, Anzaghe M, Schülke S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis.Cells. 2020;9(4).
Guo Q, Wang Y, Xu D, Nossent J, Pavlos N, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15.
Safiri S, Kolahi A, Hoy D, Smith E, Bettampadi D, Mansournia M, et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis. 2019;78(11):1463–71.
Klareskog L, Stolt P, Lundberg K, Källberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54(1):38–46.
Anderson R, Meyer P, Ally M, Tikly M. Smoking and Air Pollution as pro-inflammatory triggers for the development of rheumatoid arthritis. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2016;18(7):1556–65.
Charoenngam N, Ponvilawan B, Rittiphairoj T, Tornsatitkul S, Wattanachayakul P, Rujirachun P, et al. Patients with asthma have a higher risk of rheumatoid arthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2020;50(5):968–76.
Lai N, Tsai T, Koo M, Lu M. Association of rheumatoid arthritis with allergic diseases: A nationwide population-based cohort study. Allergy and asthma proceedings. 2015;36(5):99–103.
Tirosh A, Mandel D, Mimouni FB, Zimlichman E, Shochat T, Kochba I. Autoimmune diseases in asthma. Ann Intern Med. 2006;144(12):877–83.
Lu Z, Zeng N, Cheng Y, Chen Y, Li Y, Lu Q, et al. Atopic dermatitis and risk of autoimmune diseases: a systematic review and meta-analysis. Allergy, asthma, and clinical immunology: official. J Can Soc Allergy Clin Immunol. 2021;17(1):96.
Hilliquin P, Allanore Y, Coste J, Renoux M, Kahan A, Menkès CJ. Reduced incidence and prevalence of atopy in rheumatoid arthritis. Results of a case-control study. Rheumatology (Oxford). 2000;39(9):1020–6.
Shen TC, Lin CL, Wei CC, Tu CY, Li YF. The risk of asthma in rheumatoid arthritis: a population-based cohort study. QJM: monthly journal of the Association of Physicians. 2014;107(6):435–42.
Rabin RL, Levinson AI. The nexus between atopic disease and autoimmunity: a review of the epidemiological and mechanistic literature. Clin Exp Immunol. 2008;153(1):19–30.
Hajdarbegovic E, Thio B, Nijsten T. Lower lifetime prevalence of atopy in rheumatoid arthritis. Rheumatol Int. 2014;34(6):847–8.
Kero J, Gissler M, Hemminki E, Isolauri E. Could TH1 and TH2 diseases coexist? Evaluation of asthma incidence in children with coeliac disease, type 1 diabetes, or rheumatoid arthritis: a register study. J Allergy Clin Immunol. 2001;108(5):781–3.
Jeong HE, Jung SM, Cho SI. Association between Rheumatoid Arthritis and respiratory allergic Diseases in korean adults: a propensity score matched Case-Control Study. Int J Rheumatol. 2018;2018:3798124.
Emdin CA, Khera AV, Kathiresan S, Mendelian Randomization. JAMA. 2017;318(19):1925–6.
Davies NM, Holmes MV, Davey Smith G. Reading mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601.
Boef AG, Dekkers OM, le Cessie S. Mendelian randomization studies: a review of the approaches used and the quality of reporting. Int J Epidemiol. 2015;44(2):496–511.
Yavorska OO, Burgess S. MendelianRandomization: an R package for performing mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734–9.
Ahn K, Penn RB, Rattan S, Panettieri RA Jr, Voight BF, An SS. Mendelian randomization analysis reveals a Complex Genetic Interplay among atopic dermatitis, Asthma, and GERD. American journal of respiratory and critical care medicine; 2022.
Kawai V, Shi M, Feng Q, Chung C, Liu G, Cox N et al. Pleiotropy in the Genetic Predisposition to Rheumatoid Arthritis: A Phenome-Wide Association Study and Inverse Variance-Weighted Meta-Analysis. Arthritis & rheumatology (Hoboken, NJ). 2020;72(9):1483–92.
Zhang K, Jia Y, Wang R, Guo D, Yang P, Sun L et al. Rheumatoid arthritis and the risk of major cardiometabolic diseases: a Mendelian randomization study.Scandinavian journal of rheumatology. 2022:1–7.
Inamo J, Kochi Y, Takeuchi T. Is type 2 diabetes mellitus an inverse risk factor for the development of rheumatoid arthritis? J Hum Genet. 2021;66(2):219–23.
Gao RC, Sang N, Jia CZ, Zhang MY, Li BH, Wei M, et al. Association between Sleep Traits and Rheumatoid Arthritis: a mendelian randomization study. Front public health. 2022;10:940161.
Chen D, Zhang Y, Yidilisi A, Xu Y, Dong Q, Jiang J. Causal Associations between circulating Adipokines and Cardiovascular Disease: a mendelian randomization study. J Clin Endocrinol Metab. 2022;107(6):e2572–e80.
Park S, Lee S, Kim Y, Cho S, Kim K, Kim YC, et al. A mendelian randomization study found causal linkage between telomere attrition and chronic kidney disease. Kidney Int. 2021;100(5):1063–70.
de Roos AJ, Cooper GS, Alavanja MC, Sandler DP. Personal and family medical history correlates of rheumatoid arthritis. Ann Epidemiol. 2008;18(6):433–9.
Sheen Y, Rolfes M, Wi C, Crowson C, Pendegraft R, King K, et al. Association of Asthma with Rheumatoid Arthritis: a Population-Based case-control study. J allergy Clin Immunol Pract. 2018;6(1):219–26.
Kronzer V, Crowson C, Sparks J, Vassallo R, Davis J. Investigating Asthma, Allergic Disease, Passive Smoke Exposure, and Risk of Rheumatoid Arthritis. Arthritis & rheumatology (Hoboken, NJ). 2019;71(8):1217–24.
Yun HD, Knoebel E, Fenta Y, Gabriel SE, Leibson CL, Loftus EV Jr et al. Asthma and proinflammatory conditions: a population-based retrospective matched cohort study. Mayo Clinic proceedings. 2012;87(10):953 – 60.
Hemminki K, Li X, Sundquist J, Sundquist K. Subsequent autoimmune or related disease in asthma patients: clustering of diseases or medical care? Ann Epidemiol. 2010;20(3):217–22.
Hou Y, Hu H, Liu I, Chang Y, Wu C. The risk of autoimmune connective tissue diseases in patients with atopy: A nationwide population-based cohort study. Allergy and asthma proceedings. 2017;38(5):383-9.
Hartung AD, Bohnert A, Hackstein H, Ohly A, Schmidt KL, Bein G. Th2-mediated atopic disease protection in Th1-mediated rheumatoid arthritis. Clin Exp Rheumatol. 2003;21(4):481–4.
Olsson AR, Wingren G, Skogh T, Svernell O, Ernerudh J. Allergic manifestations in patients with rheumatoid arthritis. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 2003;111(10):940–4.
Karsh J, Chen Y, Lin M, Dales R. The association between allergy and rheumatoid arthritis in the canadian population. Eur J Epidemiol. 2005;20(9):783–7.
Roeleveld DM, Koenders MI. The role of the Th17 cytokines IL-17 and IL-22 in rheumatoid arthritis pathogenesis and developments in cytokine immunotherapy. Cytokine. 2015;74(1):101–7.
Tan Q, Yang H, Liu EM, Wang H. Establishing a role for Interleukin-17 in atopic dermatitis-related skin inflammation. J Cutan Med Surg. 2017;21(4):308–15.
Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, et al. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003;28(1):42–50.
Oda N, Canelos PB, Essayan DM, Plunkett BA, Myers AC, Huang SK. Interleukin-17F induces pulmonary neutrophilia and amplifies antigen-induced allergic response. Am J Respir Crit Care Med. 2005;171(1):12–8.
Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, et al. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med. 2008;178(10):1023–32.
Finkelman FD, Hogan SP, Hershey GK, Rothenberg ME, Wills-Karp M. Importance of cytokines in murine allergic airway disease and human asthma. Journal of immunology (Baltimore, Md: 1950). 2010;184(4):1663-74.
Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem. 2003;278(19):17036–43.
van Hamburg JP, Asmawidjaja PS, Davelaar N, Mus AM, Colin EM, Hazes JM, et al. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2011;63(1):73–83.
Ziolkowska M, Koc A, Luszczykiewicz G, Ksiezopolska-Pietrzak K, Klimczak E, Chwalinska-Sadowska H et al. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. Journal of immunology (Baltimore, Md: 1950). 2000;164(5):2832-8.
van Drongelen V, Holoshitz J. Human Leukocyte Antigen-Disease Associations in Rheumatoid Arthritis. Rheum Dis Clin North Am. 2017;43(3):363–76.
Mahdi BM, Al-Hadithi ATR, Raouf H, Zalzala HH, Abid LA, Nehad Z. Effect of HLA on development of asthma. Annals of medicine and surgery (2012). 2018;36:118 – 21.
Lee SH, Lee EB, Shin ES, Lee JE, Cho SH, Min KU, et al. The Interaction between allelic variants of CD86 and CD40LG: a common risk factor of allergic asthma and rheumatoid arthritis. Allergy Asthma Immunol Res. 2014;6(2):137–41.
Roque JB, O’Leary CA, Kyaw-Tanner M, Duffy DL, Gharahkhani P, Vogelnest L, et al. PTPN22 polymorphisms may indicate a role for this gene in atopic dermatitis in West Highland white terriers. BMC Res Notes. 2011;4:571.
Kurkó J, Besenyei T, Laki J, Glant TT, Mikecz K, Szekanecz Z. Genetics of rheumatoid arthritis - a comprehensive review. Clin Rev Allergy Immunol. 2013;45(2):170–9.
Margolis DJ, Mitra N, Kim B, Gupta J, Hoffstad OJ, Papadopoulos M, et al. Association of HLA-DRB1 genetic variants with the persistence of atopic dermatitis. Hum Immunol. 2015;76(8):571–7.
Stapleton M, Howard-Thompson A, George C, Hoover RM, Self TH. Smoking and asthma. J Am Board Family Medicine: JABFM. 2011;24(3):313–22.
Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30(11):1205–13.
Leow YH, Maibach HI. Cigarette smoking, cutaneous vasculature, and tissue oxygen. Clin Dermatol. 1998;16(5):579–84.
Yi O, Kwon HJ, Kim H, Ha M, Hong SJ, Hong YC, et al. Effect of environmental tobacco smoke on atopic dermatitis among children in Korea. Environ Res. 2012;113:40–5.
Acknowledgements
We appreciate the Key Research and Development (R&D) Projects of Shaanxi Province for funding our project. We thank all individuals providing and conducting data collection for this work.
Funding
This work was supported by the Key Research and Development (R&D) Projects of Shaanxi Province (No. 2022SF-074).
Author information
Authors and Affiliations
Contributions
All authors have substantially contributed to the design, performance, analysis, and reporting of the work. Yucai Wang and Chuiji Chen designed the study. Le Su, Wenhao Duan, and Yansen Zheng collected and analyzed the data. Dianzhong Zhang was responsible for check of the data. All authors revised the paper critically for intellectual content and approved the final version. All authors agree to be accountable for the work and to ensure that any questions relating to the accuracy and integrity of the paper are investigated and properly resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research has been conducted using published studies and consortia providing publicly available summary statistics. All original studies have been approved by the corresponding ethical review board, and the participants have provided informed consent. In addition, no individual-level data was used in this study. Therefore, no new ethical review board approval was required.
Consent for publication
Not applicable.
Competing Interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, C., Su, L., Duan, W. et al. Asthma and atopic dermatitis as risk factors for rheumatoid arthritis: a bidirectional mendelian randomization study. BMC Med Genomics 16, 41 (2023). https://doi.org/10.1186/s12920-023-01461-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-023-01461-7