Abstract
Our understanding of the organization of the chromatin fiber within the cell nucleus has made great progress in the last few years. High-resolution techniques based on next-generation sequencing as well as optical imaging that can investigate chromatin conformations down to the single cell level have revealed that chromatin structure is highly heterogeneous at the level of the individual allele. While TAD boundaries and enhancer–promoter pairs emerge as hotspots of 3D proximity, the spatiotemporal dynamics of these different types of chromatin contacts remain largely unexplored. Investigation of chromatin contacts in live single cells is necessary to close this knowledge gap and further enhance the current models of 3D genome organization and enhancer–promoter communication. In this review, we first discuss the potential of single locus labeling to study architectural and enhancer–promoter contacts and provide an overview of the available single locus labeling techniques such as FROS, TALE, CRISPR–dCas9 and ANCHOR, and discuss the latest developments and applications of these systems.
Similar content being viewed by others
Introduction
Human diploid cells need to handle a genome consisting of little more than 6 Gigabases in a way that protects it against damage and can be propagated unchanged over generations. Genes need to be activated and silenced depending on external signals, cell-fate decisions and to maintain cell identity. This depends on a tightly controlled access of gene-regulatory proteins to the genome. At the same time, the whole genome undergoes major reorganization in proliferating cells. The entire functional genome is duplicated during replication and then highly compacted upon entry into mitosis to allow segregation of sister chromatids and equal distribution of the genome onto the daughter cells.
The packing of the DNA with its bound proteins (chromatin fiber) within the cell nucleus is fundamental for these processes. In interphase, the chromatin of individual chromosomes locates in distinct chromosome territories with little intermingling. Genome-wide conformation capture studies such as Hi-C revealed that chromosomes can be divided into more transcriptionally active regions (A compartment) and inactive regions (B compartment). These are marked by specific histone modifications and interact with the same type of regions while avoiding the other compartment. The driving forces between this compartmentalization are complex and involve the folding of the chromatin fiber, general transcriptional activity and clustering of proteins and DNA, invoking mechanisms of phase separation. Liquid–liquid phase separation (LLPS) is a little understood multifactorial process underlying the self-organization of membraneless chromatin compartments, driven by intrinsic properties of the chromatin fiber, associated RNA and chromatin-binding proteins via disordered domains (e.g., BRD4) and/or by accumulation of transcription activators (transcription factories, RNA Pol II) (for a review see [1]).
Chromatin conformation capture approaches revealed regions with strong directionality of chromatin contacts within these compartments, visible as characteristic triangles in contact maps and referred to as topologically associating domains (TADs) (Fig. 1). Contact maps obtained with higher resolution show that TADs consist of single or multiple contacts formed by distinct chromatin loops [2], which we refer to as “structural loops”, because they are very similar in different cell types. Methods like Hi-C generally process millions of cells and hence contact maps represent an average of a large cell population and do not yield information about the presence of the individual loops in individual cells.
Organizational layers of chromatin and their reflection in Hi-C maps. Organizational layers of the chromatin fiber in the cell nucleus (top) aligned with the representation of those features in Hi-C interaction maps (bottom). Chromosomes reside in distinct territories with little intermingling. Therefore, chromatin contacts occur predominantly within the same chromosome (left panels). Within the chromosomes compartments with different chromatin properties (A compartment—active chromatin, B compartment—inactive chromatin) can be observed that interact with regions belonging to the same compartment type (blue box) (middle panels). Underlying these features in the folding of the chromatin fiber into chromatin loops, to a large extend mediated by the cohesin complex and CTCF, visible in the 1 Mb region shown for chr19 as regions with strong interactions (right panels). (Hi-C maps were generated using data for HCT116 cells by Rao et al. 2017 [5] and Juicebox.)
Key proteins for the formation of structural loops are the cohesin complex and the chromatin insulator protein CTCF. Depletion of critical cohesin complex subunits such as RAD21 or CTCF leads to the apparent loss of chromatin loops [3,4,5]. Models for the establishment of chromatin loops by cohesin and CTCF suggest an intriguing mechanism of ATP-driven loop extrusion by the cohesin complex that is halted when the extrusion complex encounters two convergently oriented CTCF sites [6, 7]. Within these structural loops more loops are formed between enhancers and promoters which we refer to as “functional loops”. Among the players involved in the formation and positioning of such loops are RNA polymerase II [8], the Mediator complex [9] transcription factors and cohesin. A reorganization of chromatin loops occurs during cell division. During chromatin compaction in the prophase and prometaphase of mitosis, the interphase loops are replaced by a more homogeneous type of loop compaction generated by condensin I and condensin II, loop-extruding complexes that are structurally and mechanistically similar to cohesin [10]. During entry into G1-phase the cohesin-dependent loops are re-established [11] with cohesin-mediated loop extrusion ongoing during the entire interphase. Thus, all types of chromatin loops are dynamic and are re-organized during the cell cycle and by transcriptional cues during differentiation and development.
The loops provide a dynamic structural scaffold to chromatin and impact gene expression, by directly recruiting enhancers to promoters or indirectly through confining the search space of enhancers.
A general depletion of cohesin or CTCF in cells only leads to mild misexpression of a few hundred genes [12], indicating that TAD chromatin loops are only one layer for controlling gene expression. However, disruption or reorganization of specific loops has been shown to impair development in mouse models [13] and to be linked to diseases, including cancer (e.g., enhancer hijacking in AML [14]) and to developmental defects [15].
Chromatin conformation capture methods (such as 4C, Hi-C, Micro-C or GAM) cannot show the dynamics of chromatin loops since they analyze millions of cells simultaneously; while available single cell Hi-C data [16] as well as super-resolution chromatin tracing with DNA-FISH [17, 18] indicate cell-to-cell variability with respect to presence and position of loops. Moreover, all these methods miss out the fourth dimension—time—since they are carried out on fixed cells.
The observation and eventual manipulation of chromatin contacts in live cells by imaging techniques provides a new perspective on chromatin dynamics. Fundamental questions can be approached from a different angle, for example the dynamics of promoter–enhancer interactions, the stability and duration of looping interactions, the role of proteins (in addition to CTCF and cohesin) and protein aggregates/droplets (e.g., transcription factories) for stabilizing or destabilizing specific loops and chromatin-reorganizing processes (replication and cell division). These observations require the labeling of DNA loci with fluorophores and live-cell imaging at high resolution. Major challenges include the selection of suitable labeling approaches to obtain a high signal-to-background ratio that permits visualization and tracking of the signals, to allow imaging over long periods of time (see also the review by the Hansen lab) [19]. In this review, we will discuss the different approaches successfully used to label DNA loci in live cells and highlight novel insights gained with these techniques.
Single locus labeling approaches
Fluorescent repressor operator systems (FROS)
The first approach used to visualize specific sequences in mammalian cell nuclei was the lac operator–repressor system. In a pioneer study, Robinett et al. [20] inserted a mammalian DHFR expression vector with 256 lac repeats randomly into the genome of Chinese hamster ovary cells and demonstrated that the genetically amplified operator array gives a comparable chromatin staining after in situ hybridization, immunostaining with exogenous lac repressor protein after fixation and live cells by expressing the lac repressor protein fused to a fluorescent protein and a nuclear localization signal. Moreover, in the absence of gene amplification but with appropriate nuclear expression levels, a single copy insertion of 256 lac repeats provided sufficient signal-to-background signal to detect the locus as a diffraction limited spot (Table 1) [20].
The use of the Lac repressor–operator system was followed by the successful adaption of several other repressor–operator combinations (TetR, λcI, MalI and CymR) [21, 22] which allowed researchers to simultaneously visualize multiple genomic loci at diverse locations in live cells for the first time.
In these initial FROS experiments, repressor proteins were used because their high affinity to the operator binding sites would create a spot with a detectable signal-to-background ratio. However, it was shown later that the tight binding of LacR and TetR molecules to their operator sequences can have undesired biological consequences such as gene silencing. In the human osteosarcoma U2OS cell line, insertions of lac repeats were shown to induce an arrest in early S-phase, possibly by stalling DNA polymerase [23]. The same replication roadblock effect was induced by the introduction of a tet operator array in Escherichia coli [24]. In mouse embryonic stem cells, it was reported that the insertion of lac operator arrays next to the DHJH elements and the Eμ enhancer of the immunoglobin locus failed to result in germline transmission. The integration of the lac operator array in mouse embryonic stem cells resulted in an abnormal chromosome count of 70–80 chromosomes, indicating that the insertion of the lac operator array can lead to chromosome instability [25].
As shown by these examples, FROS labeling might induce genomic instability, and the effect of genomic insertion of FROS arrays needs to be carefully controlled.
Before the advent of CRISPR-based genome editing, the use of operator–repressor arrays to image specific loci of interest was largely limited to species with high rates of homologous recombination such as Saccharomyces cerevisiae [26]. In mammalian cells, FROS arrays were typically randomly inserted into the genome. With the increased possibilities of genome editing by homologous recombination and more recently CRISPR–Cas9 directed insertion of different operator–repressor assays within the same locus or chromosome became a possibility. However, the large size and repetitive nature of the operator arrays (typically 196–256 operator binding sites making up a total of 7–12 kb) still posed an experimental hurdle for facile CRISPR-based genome integration. Alexander et al. circumvented this problem by first integrating two AttP landing sites recognized by the bacteriophage integrases PhiC31 and Bbx1. This was followed by the integration of a plasmid containing the operator array sequence and an antibiotic resistance gene, which was removed later by CRE-lox or FLP-FRT recombination [27]. Tasan et al. developed a method of synthesizing a 96-mer TetO array with varying intermittent spacer sequences. This optimized TetO array of approximately 3 kb length can be inserted via CRISPR–Cas9 in a genomic location of interest and no perturbation of the nuclear localization or chromatin state of the targeted loci was observed. By employing a mutant TetR protein and mutated TetO sequence, even the multiplexing of two TetR type FROS systems in mammalian cells became possible [28].
Transcription activator-like effectors (TALEs)
Transcription activator-like effectors (TALEs), discovered in the plant pathogenic bacteria Xanthomonas, are proteins that bind to specific DNA sequences [29]. TALEs bind to DNA through a domain of 32 to 35 amino acids called repeat-variable di-residues (RVDs) with two hypervariable amino acids, each of them binding to a specific base pair. Through DNA cloning, the amino acid code can be reprogrammed to bind specific DNA sequences. This versatility made TALEs a popular platform for applications such as gene editing and transcriptional modulation [30, 31].
Since TALEs can be modified to bind specific DNA sequences, it has also been an attractive method to fluorescently label such sequences (Table 1). Several studies have successfully labeled repetitive sequences like telomeres and centromeres in different circumstances. For example, Miyanari et al. demonstrated that TALEs can be used to study chromatin dynamics throughout the cell cycle [32]. Ren et al. have studied age-associated genomic alterations at telomeres and centromeres in premature aging models in both human and mouse. Others have labeled major satellite repeat regions and followed the dynamics of chromosomes through the cell cycle or were able to differentially label both parental chromosomes in hybrid mouse cells [31, 33].
While studies on repetitive sequences have provided valuable insights, it is still limited to a set of specific repetitive regions. To research TAD boundaries or enhancer–promoter interactions, it would be necessary to label non-repetitive single loci. Ma et al. demonstrated that TALEs can be used to visualize single DNA loci by mapping HIV-1 proviral DNA sequences in the human genome using TALEs labeled with quantum dots (QDs), which have unique optical properties including excellent brightness and photostability compared with traditional organic dyes [34]. The QDs greatly improve the signal-to-background ratio, reducing the number of fluorescent particles necessary to be detectable. However, a disadvantage is that TALE-QD particles need to be delivered to cells by transfection for each experiment, which limits their application.
While the other methods mentioned in this review are currently more prevalent, TALEs can be a valuable tool for DNA visualization. It might be interesting to develop a repetitive array, similar to FROS arrays, that can recruit a set of the same TALEs without the need to develop many different TALEs each targeting a specific DNA sequence. This would yield another system that can be used in combination with other DNA labeling systems such as FROS, dCas9 or ANCHOR. In addition, in cases were the binding of repressors to operator arrays induces replication roadblock effects, TetO targeting TALEs could be an alternative. An advantage of TALEs is that the residence time of TALEs can be adjusted by varying the length of the DNA binding domain, whereas repressors can currently only be altered by previously described mutations [35].
CRISPR/deadCas9 (dCas9)
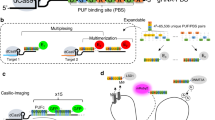
The CRISPR/Cas9 (Cas9 for short) genome editing tool that revolutionized biomedical research can be repurposed for labeling of genomic regions. Originally an antiviral defense system of prokaryotes, the Cas9 protein together with a guide RNA (sgRNA) sequence can induce a double stranded break with near base pair precision [36]. This made Cas9 the gold standard of genome editing [37]. Catalytic inactive mutants of Cas9 (deadCas9 or dCas9) turned out to be also quite versatile tools [38]. Since the dCas9 cannot cleave the target DNA, it is not released from chromatin. This long residence time makes it a very attractive tool to recruit proteins or protein domains, such as fluorescent tags or gene silencing domains, to specific genomic loci (Fig. 2). A clear advantage of dCas9-mediated labeling over other methods covered in this review (see Table 1) is that genome editing is in general not necessary, making it relatively straightforward to use on many different loci as well as in cell lines that are difficult to edit.
Example image of ANCHOR and dCas9 labeling A: Image of a live HCT116 cell with two labeled loci, the D4Z4 repeats and a neighboring TAD boundary that are roughly 15 Mbp apart on chromosome X. The D4Z4 repeats were labeled with and a repeat-specific gRNA and dCas9-eGFP(green), the neighboring TAD boundary was visualized with an ANCH3 insertion in combination with the OR3-HaloTag protein and JF-549 dye (red). The image was taken on a SP5 Leica confocal microscope. B: Image of a live mouse embryonic stem cell with a locus on chromosome 4 visualized by an ANCH3 insertion and OR3-Halo/JF-646. The image was taken on a SpinSR SoRa Olympus spinning disk microscope. Note that the locus was already replicated and the two sister chromatids are visible
Chen et al. were the first to describe imaging of telomeres with dCas9-eGFP in combination with one sgRNA [39]. They also labeled the non-repetitive regions of the Muc4 gene with combinations of 26 or more sgRNAs. A similar approach was used to label enhancers and promoters with 36 sgRNAs each [40]. An alternative approach involving genome editing is the CRISPR-tag, developed by Chen and colleagues [41]. They inserted a repeat of short CRISPR target sites derived from the Caenorhabditis elegans genome (up to 6 repeats with at least 24 sgRNA sites) in the genomic region of interest [41] to recruit sufficient dCas9 molecules.
While it is very attractive to use dCas9 to label a specific locus, a major problem of dCas9-FP-based methods remains the low signal due to the limited number of fluorophores that can be recruited to the locus. Some groups succeeded in amplifying the signal by fusing the supernova tagging system (SunTag) to dCas9 [42,43,44]. The SunTag is a peptide scaffold with multiple GCN4 epitopes (e.g., 24) that can be bound by a single-chain variable fragment of an anti-GCN4 antibody. The nanobody can be tagged with fluorophores and expressed in mammalian cells (scFv-GCN4-GFP). When the SunTag system is fused to dCas9, this enables a substantial amplification of the fluorescent signal. This technique has been shown to work with guideRNAs for repetitive sequences as well as on non-repetitive regions such as the MUC4 gene [39, 45,46,47].
Alternative approaches that allow the recruitment of a large number of fluorophores per dCas9 molecule involve extending the sgRNA with RNA aptamer repeats such as repeats of the MS2 RNA binding sequence, bound by fluorophore-fusions of the MS2 bacteriophage coat protein, or other analogues [48,49,50,51]. Each RNA aptamer recruits two fluorescent proteins, leading to an accumulation of fluorescent signal at the target sequence. Combining different RNA aptamer analogues enables multi-color imaging. Maass et al. successfully labeled two different alleles in 129S1/CAST hybrid mouse embryonic stem cells (mESCs) and mouse embryonic fibroblasts (MEFs) by using single nucleotide polymorphisms in PAM sites [52]. This allowed them to confirm that single loci as well as chromosomes are stably positioned in space and time. Kamiyama et al. developed a splitGFP-system consisting of strands 1–10 of the GFP beta-barrel structure that only becomes fluorescent when it is complemented by the 11th beta-strand, which can be fused as a tagging sequence to a protein of interest [45]. Ghosh et al. [46] developed the GFP enhancer nanobody array (ArrayG) that can be attached to dCas9. Wild-type monomeric GFP molecules are initially dim but increase about 26 times in brightness when bound to the nanobody array. This system was used to track a repetitive genomic locus in live cells [47]. Clow et al. successfully adapted a dCas9-based platform that they previously used to label repetitive sequences (Casilio) [50, 53]. This system uses PumilioFBF (PUF)-tethered factors which can be engineered to bind to a specific 8-mer at the 3’end of a gRNA. By multiplexing the 8-mer sequences, PUF domains fused with fluorescent domains can accumulate at the dCas9. With this approach, they successfully labeled three different non-repetitive loci with three different colors. They demonstrate the applicability of Casilio for chromatin interaction studies by labeling the IER5L promoter and its super-enhancer, a known ~ 500-kb chromatin interaction dependent on RAD21 [5]. After degradation of RAD21, they indeed detected an increased distance between the labeled loci. The adaptations mentioned above use fluorophores (antibody-FP, MCP-FP and PUF-FP) which stochastically bind to the dCas9 fusion protein. This renders these systems inherently more resistant to photobleaching than dCas9-FP labeling schemes, paving the way for acquiring longer time tracks.
The advances on the dCas9 labeling techniques have significantly improved its signal-to-background ratio, resistance to photobleaching and ability to visualize loci over long time periods. Successful dCas9 labeling in these systems requires the expression of multiple genetic components (dCas9, binder and sgRNA(s)) either via transient transfection or the generation of stable cell lines. While dCas9 imaging systems can be relatively easily established on telomeres or other repetitive genomic loci, the step to label a single, non-repetitive locus is often large. This can be explained by the delicate balance of getting both a sufficient fluorescent protein level to label the locus and avoiding a high background signal that could obstruct the single locus signal. Expressing the fluorescent moiety at a low but sufficient level is therefore almost always an advantage in obtaining a high signal-to-background ratio, because expressing the fluorescent molecule at high levels increases the number of molecules in the background while the number of locus-binding molecules remains the same (small) number, certainly when single non-repetitive loci are targeted. At best the binding equilibrium at the target is somewhat improved, but this will be negligible relative to the increase in background fluorescent molecules, particularly when the fluorescent molecule has a long residence time at the target sequence. The flexibility of the dCas9 system has a great appeal for single locus tracking techniques, and ultimately a combination of solutions as described above might improve robust labeling of single loci.
The ANCHOR DNA labeling system
In 2014, Saad et al. introduced a novel protein–DNA imaging approach called the ANCHOR DNA labeling system [54]. This labeling approach leverages the ParB–ParS binding of the ParABS partitioning system naturally occurring in bacteria. In the ANCHOR DNA labeling approach, fluorescently labeled ParB molecules (ORs) recognize a small set of ParS sites within a short non-repetitive INT sequence (ANCH) which can be integrated next to the locus of interest. Upon recognition of the ParS site by a ParB homodimer, a conformational change of the ParB molecules leads to N-terminal mediated oligomerization and spreading of ParB along the adjacent DNA strand via non-specific interactions. Together, the binding and spreading of fluorescent ParB proteins creates a diffraction limited spot at the locus of interest (Fig. 2) [55, 56]. A key advantage of the ANCHOR DNA labeling system is that it has been reported to not inhibit transcription even when present directly adjacent to a promoter or an intronic enhancer [55, 57].
Since different bacteria strains and replicons (e.g., genomes and plasmids) within the same bacterium contain different ParB-INT combinations, multiplexing non-crossreactive ANCHORs to label multiple loci became possible. For example, the ANCHOR1 and ANCHOR2 system were used in combination to visualize the dynamics of double strand break repair in Saccharomyces cerevisiae. Later, the ANCHOR1–ANCHOR3 pair was used in mouse embryonic stem cells to investigate the heterogeneity of chromatin dynamics [58]. Recently, significant differences between the labeling efficiency of different ANCHORs have been reported. A survey of single locus tracking techniques in Drosophila showed that ANCHOR2 labeled two regions of interest (ROIs) equally efficiently as LacI-LacO labeling, whereas ANCHOR1 showed less efficient labeling at both ROIs. In addition, both ANCHOR systems showed sensitivity to the design of the ParB-FP construct and cellular context [57]. This suggests that the extent of binding and/or cis-spreading of different ANCHORs might be tissue-, cell- and locus-dependent.
The ANCHOR DNA labeling system shows great promise as the latest addition to the available single locus tracking techniques. The ease of CRISPR-based genomic insertion of the short ANCH sequences (~ 1 kb) in combination with the ability to non-invasively label genomic regions of interest make the ANCHOR DNA labeling system a promising tool for studying locus dynamics (see Table 1). In the future, optimizations to the ParB-FP fusion design might further improve the labeling efficiency and robustness of the existing ANCHOR systems and novel ANCHOR types and combinations may be developed.
Insights into the dynamics of higher order chromatin structures using single locus labeling approaches
Several groups have demonstrated that the single locus labeling approaches described above are very powerful to study the dynamics of chromatin interactions, including also promoter–enhancer interactions.
Gabriele et al. studied a 505 kb TAD containing only the Fbn2 gene, which is inactive in mESCs. They used a combination of ANCH3 and TetO array integrations to achieve dual color labeling [59]. Interestingly, they found that the Fbn2 loop is rare and highly dynamic. The fully looped state was present in only ~ 3–6,5% of the analyzed cells and the median loop lifetime of the Fbn2 loop is 10 to 30 min. Given that the Fbn2 structural loop appears as a relatively strong interaction in Hi-C, suggesting a frequent occurrence of the loop, this unexpected result shows that live-cell imaging of genomic loci gives a very different perspective on the interpretation of Hi-C results.
Mach et al. used live-cell imaging to specifically study the effect of CTCF and cohesin on TAD dynamics [60]. They introduced LacO and TetO arrays containing removable 3xCTCF sites separated by 150 kb in a TAD (560 kb) without active or inactive genes. In presence of the 3xCTCF sites and RAD21, they found that the arrays spent 78% of the time in close contact, while this was reduced to 33% when the 3xCTCF sites were removed and 23% after RAD21 depletion. The average contact time and formation rate decreased from a contact lasting 16 min and reforming every 5 min to an average contact duration of 6 min reoccurring every 10 min after removal of CTCF sites, and 2 min encounters with an average reformation rate of 22 min upon RAD21 depletion. Thus, CTCF and cohesin greatly impact the stability and frequency of contacts with a genomic separation distance of 150 kb and 500 kb [59]. Both studies suggest that CTCF–CTCF contacts are transient and that CTCF-dependent TAD structures in single cells are highly dynamic entities.
Active transcription has been widely associated with 3D enhancer–promoter proximity. Using a dual locus labeling of the Sox2 promoter and Sox2 control region in mouse embryonic stem cells with the tetO/TetR system as well as the novel cuO/CymR pair (repressor system from Pseudomonas putida), Alexander et al. have excluded the presence of a long-lived state in which the enhancer and promoter physically interact [27]. Similar observations have been reported by Platania et al. for the same locus using ANCHOR1/3 dual color labeling [61]. This is actually in line with earlier observations at the ß-globin locus in which the locus control region can dynamically flip-flop between downstream promoters at a time scale of minutes [62]. We anticipate that future studies will quantify the spatiotemporal dynamics of enhancer–promoter pairs similar to recently published studies on the dynamics of architectural loops [59, 60].
Several studies show an interplay between transcription activity and locus mobility. For example, Gu et al. have shown with a dCas9-based labeling approach that enhancers and promoters diffuse at a faster rate when a locus is transcriptionally active in comparison to an inactive locus [40]. Using a combination of ANCHOR3 labeling at the promoter and visualization of nascent transcripts with MS2 repeats, Germier et al. showed that transcriptional activation of a transgene leads to the confinement of its promoter [55]. At the Sox2 locus, not only the promoter and the Sox2 control region (SCR) displayed confined motion, also the intermittent and downstream genomic region showed a higher level of confinement [61] than a transcriptionally inactive region (HoxA locus) measured in the same cell type [58].
The abovementioned results indicate that transcriptional activation of a locus can have effects on the mobility of regulatory and non-regulatory elements. However, nascent transcription has been shown to occur in transcriptional bursts [62]. Studies which simultaneously visualize mRNA and chromatin in live cells show that whereas for some loci the bursting gene is on average more confined than the non-bursting gene, no differences in mobility were observed for other loci [63, 64]. Similarly, it was shown that the Sox2 enhancer diffuses more freely at transcriptionally poised alleles compared to transcribing alleles, whereas the level of confinement of the Sox2 promoter remains similar [61].
In the future, experiments in which the movement of single loci are tracked across a longer time window spanning multiple transcriptional events might increase our understanding of the relationship between active transcription and chromatin dynamics.
Selection and optimization of single locus labeling approaches
As the insertion of a fluorogenic array or the fluorescent labeling of an endogenous genomic region has the potential to disrupt the genomic feature of interest, the choice of the place for insertion and labeling a region is a careful balancing act between minimizing the linkage error without causing perturbation. In a recent article, Brandão et al. simulated the effect of label placement and localization error on the ability to detect a 500 kb region in a looped and nonlooped state. When the linkage and localization error become too large, looped and nonlooped states can no longer be detected by locus tracking experiments [19].
In endogenous contexts, loop anchors such as enhancers, promoters and CTCF sites have been successfully tracked by FROS, dCas9-based and ANCHOR approaches with linkage distances ranging from several kilobases to 10 kb [27, 40, 57, 59, 65].
Another important consideration for selecting the type and the position of the label is the impact on the chromatin context for gene regulatory elements and gene expression. For example, Delker et al. found that in Drosophila cells, the presence of LacI molecules at a 20mer LacO array placed at the Ubx intronic enhancer decreased Ubx protein levels, whereas no perturbation was observed when using the ANCHOR1 and ANCHOR2 labeling technique [57]. In line with this observation, strong DNA binding of wild-type TetR to a 96 × TetO repeat array reduced transcription when it was inserted into an intron of the Nanog gene as part of the STREAMING tag system [64]. Therefore, arrays involving strong DNA binding proteins have disadvantages when placed within genes.
Approaches based on dCas9 can avoid interference with ongoing transcription by targeting the template strand, since guide RNAs targeting the non-template strand have a higher gene silencing effect compared to those targeting the template strand [64, 66].
Important limitations of all approaches are the signal-to-background ratio as discussed above and the stability of the selected fluorophores against fluorescence bleaching. Depending on the imaging setup, these factors limit the experiment and complicate image analysis. One system that efficiently increases the signal-to-background ratio are fluorogenic tags, green fluorescent protein–nanobody arrays, and monomeric wild-type green fluorescent protein binders that are initially dim but brighten ~ 26-fold on binding with the array [46, 47] (see also Lu et al. [67] for an in-depth discussion of signal amplification methods). The advantage of such multicomponent approach is also that bleached fluorophores can be replenished at the target site over time.
Perspective
The single locus labeling approaches covered here will contribute to solving major questions around the dynamics of the chromatin fiber. Success depends on the right choice of the labels (reviewed here, see also [68]) and equally on the available imaging acquisition setup and the image analysis tools (reviewed in [19]). The ongoing continuous improvement of imaging technology in combination with the improvement of the fluorescent labels and protein manipulation tools (e.g., rapid protein depletion systems and optogenetics) will overcome some of the limitations faced by the current studies, making currently impossible experiments feasible in the near future.
Looking ahead, one important problem is the correlation of enhancer–promoter proximity with transcription, which was suggested in many biological systems [69,70,71,72,73] but is mechanistically not understood yet. The ability to visualize an enhancer, promoter and nascent RNA in single live cells has led to the notion that live-cell imaging approaches could be used to test models of enhancer–promoter communication. In Drosophila, transcriptional activation by a distal enhancer only occurred when the enhancer and promoter were in a proximal state (< 340 nm) [74]. In contrast, in mouse embryonic stem cells, tracking of the Sox2 enhancer control region (SCR), promoter and nascent RNA revealed no correlation between enhancer–promoter proximity and nascent transcription. This study of Alexander et al. revealed no evidence of sustained physical contact between enhancers and promoters. The authors propose that the absence of a correlation between enhancer–promoter proximity and Sox2 transcription might be the result of long-lived activation of the promoter, long-range gene activation, for example by formation of large transcription factor condensates, or by limitations in the spatiotemporal resolution of the experiment [27]. We anticipate that continuous improvements on the precision of single locus tracking techniques by (fluorogenic) signal amplification strategies and the increase in length and temporal resolution of dual locus tracks obtained by light sheet imaging and point spread function (PSF) engineering ([47], reviewed in [75]) will lead to the first estimates on the frequency and duration of enhancer–promoter contacts in mammalian cells. A number of observations reinforce the importance of contextual chromatin contacts on enhancer–promoter interactions and transcription. At the SOX9 locus, interactions between structural elements positioned between the enhancer cluster and the promoter facilitate enhancer–promoter communication and transcription [76]. At the mouse α-globin cluster, deletion of CTCF binding sites led to ectopic contacts and aberrant activation of neighboring genes by α-globin enhancers [77]. Similarly, in some chromatin contexts promoters seem to compete for the same enhancer [62], whereas at other loci multiple promoters co-assemble in regulatory hubs [78].
Revisiting these observations by investigating chromatin contacts in live single cells and eventually the combination of DNA, RNA and protein labeling to visualize all components contributing to the nanoscale organization of the cell nucleus, will lead to a deeper understanding of gene regulatory mechanisms.
Many proteins have been shown to accumulate in transient (seconds) and stable foci (minutes to hours) in which the individual molecules exchange within seconds [79,80,81,82]. Dual imaging of foci and single molecules enables the detection of mobility patterns of these molecules inside and outside these foci [83], but much more information can be gained when single particle tracking is combined with the genomic context (e.g., promoters, enhancers) by labeling of genomic loci. The latter experimental setup opens the possibility of determining the biophysical properties of proteins visiting individual genomic loci, for example during a target search [84].
In addition, recent advances in super-resolution techniques such as STED have enabled the detection of sub-diffraction sized clusters of regulatory factors proximal to transcription sites [64, 65, 73, 85]. Manipulation of biomolecules by mutations, their rapid nuclear depletion and/or small molecules are used to reveal which players and protein domains are essential for foci formation and stabilization [65, 86,87,88].
In the future, similar perturbation experiments can be performed to evaluate foci formation and stabilization at specific genomic locations. Alterations, deletions, inversions and rearrangements of DNA sequences powered by novel synthetic regulatory genomic approaches [89] combined with super-resolution imaging of foci and single loci might provide insights in the role of individual regulatory factors and enhancer elements in the formation of foci at enhancer clusters [90].
Ultimately, these experiments might not only reveal to what extent foci influence the biophysical behavior of individual molecules, but also how foci and the number of individual molecules within foci are related to the output of local molecular processes such as DNA damage repair, DNA replication and transcription.
Availability of data and materials
This is a review article, there are no data linked to the manuscript that can be made publically available.
References
Rippe K. Liquid-liquid phase separation in chromatin. Cold Spring Harb Perspect Biol. 2022;14(2): a040683.
Rao SS, et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159(7):1665–80.
Zuin J, et al. Cohesin and CTCF differentially affect chromatin architecture and gene expression in human cells. Proc Natl Acad Sci U S A. 2014;111(3):996–1001.
Nora EP, et al. Targeted degradation of CTCF decouples local insulation of chromosome domains from genomic compartmentalization. Cell. 2017;169(5):930–44.
Rao SSP, et al. Cohesin loss eliminates all loop domains. Cell. 2017;171(2):305–20.
Sanborn AL, et al. Chromatin extrusion explains key features of loop and domain formation in wild-type and engineered genomes. Proc Natl Acad Sci U S A. 2015;112(47):E6456–65.
de Wit E, et al. CTCF binding polarity determines chromatin looping. Mol Cell. 2015;60(4):676–84.
Zhang S, et al. RNA polymerase II is required for spatial chromatin reorganization following exit from mitosis. Sci Adv. 2021;7(43):eabg8205.
Kagey MH, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467(7314):430–5.
Gibcus JH, et al. A pathway for mitotic chromosome formation. Science. 2018. https://doi.org/10.1126/science.aao6135.
Zhang H, Emerson DJ, Gilgenast TG, et al. Chromatin structure dynamics during the mitosis-to-G1 phase transition. Nature. 2019;576:158–62.
Wendt KS, et al. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature. 2008;451(7180):796–801.
Rodriguez-Carballo E, et al. The HoxD cluster is a dynamic and resilient TAD boundary controlling the segregation of antagonistic regulatory landscapes. Genes Dev. 2017;31(22):2264–81.
Ottema S, et al. The leukemic oncogene EVI1 hijacks a MYC super-enhancer by CTCF-facilitated loops. Nat Commun. 2021;12(1):5679.
Lupianez DG, et al. Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell. 2015;161(5):1012–25.
Nagano T, et al. Single-cell Hi-C reveals cell-to-cell variability in chromosome structure. Nature. 2013;502(7469):59–64.
Bintu B, et al. Super-resolution chromatin tracing reveals domains and cooperative interactions in single cells. Science. 2018;362(6413):1783.
Beckwith KS, et al. Nanoscale 3D DNA tracing in single human cells visualizes loop extrusion directly in situ. BioRxiv. 2021. https://doi.org/10.1101/2021.04.12.439407.
Brandao HB, Gabriele M, Hansen AS. Tracking and interpreting long-range chromatin interactions with super-resolution live-cell imaging. Curr Opin Cell Biol. 2021;70:18–26.
Robinett CC, et al. In vivo localization of DNA sequences and visualization of large-scale chromatin organization using lac operator/repressor recognition. J Cell Biol. 1996;135(6 Pt 2):1685–700.
Sellars LE, et al. Development of a new fluorescent reporter:operator system: location of AraC regulated genes in Escherichia coli K-12. BMC Microbiol. 2017;17(1):170.
Lassadi I, et al. Differential chromosome conformations as hallmarks of cellular identity revealed by mathematical polymer modeling. PLoS Comput Biol. 2015;11(6): e1004306.
Jegou T, et al. Dynamics of telomeres and promyelocytic leukemia nuclear bodies in a telomerase-negative human cell line. Mol Biol Cell. 2009;20(7):2070–82.
Possoz C, et al. Tracking of controlled Escherichia coli replication fork stalling and restart at repressor-bound DNA in vivo. EMBO J. 2006;25(11):2596–604.
Lucas JS, et al. 3D trajectories adopted by coding and regulatory DNA elements: first-passage times for genomic interactions. Cell. 2014;158(2):339–52.
Heun P, et al. Chromosome dynamics in the yeast interphase nucleus. Science. 2001;294(5549):2181–6.
Alexander JM, et al. Live-cell imaging reveals enhancer-dependent Sox2 transcription in the absence of enhancer proximity. Elife. 2019. https://doi.org/10.7554/eLife.41769.
Tasan I, et al. Two-Color Imaging of Nonrepetitive Endogenous Loci in Human Cells. ACS Synth Biol. 2020;9(9):2502–14.
Boch J, et al. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326(5959):1509–12.
Baker M. Gene-editing nucleases. Nat Methods. 2012;9(1):23–6.
Zhang F, et al. Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription. Nat Biotechnol. 2011;29(2):149–53.
Miyanari Y, Ziegler-Birling C, Torres-Padilla ME. Live visualization of chromatin dynamics with fluorescent TALEs. Nat Struct Mol Biol. 2013;20(11):1321–4.
Thanisch K, et al. Targeting and tracing of specific DNA sequences with dTALEs in living cells. Nucleic Acids Res. 2014;42(6): e38.
Ma Y, et al. Live cell imaging of single genomic loci with quantum dot-labeled TALEs. Nat Commun. 2017;8:15318.
Popp AP, Hettich J, Gebhardt JCM. Altering transcription factor binding reveals comprehensive transcriptional kinetics of a basic gene. Nucleic Acids Res. 2021;49(11):6249–66.
Jinek M, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21.
Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–23.
Fu Y, et al. CRISPR-dCas9 and sgRNA scaffolds enable dual-colour live imaging of satellite sequences and repeat-enriched individual loci. Nat Commun. 2016;7:11707.
Chen B, et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell. 2013;155(7):1479–91.
Gu B, et al. Transcription-coupled changes in nuclear mobility of mammalian cis-regulatory elements. Science. 2018;359(6379):1050–5.
Chen B, et al. Efficient labeling and imaging of protein-coding genes in living cells using CRISPR-Tag. Nat Commun. 2018;9(1):5065.
Ye H, Rong Z, Lin Y. Live cell imaging of genomic loci using dCas9-SunTag system and a bright fluorescent protein. Protein Cell. 2017;8(11):853–5.
Tanenbaum ME, et al. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell. 2014;159(3):635–46.
See K, et al. Histone methyltransferase activity programs nuclear peripheral genome positioning. Dev Biol. 2020;466(1–2):90–8.
Kamiyama D, et al. Versatile protein tagging in cells with split fluorescent protein. Nat Commun. 2016;7:11046.
Ghosh RP, et al. A fluorogenic array for temporally unlimited single-molecule tracking. Nat Chem Biol. 2019;15(4):401–9.
Gustavsson AK, et al. Fast and parallel nanoscale three-dimensional tracking of heterogeneous mammalian chromatin dynamics. Mol Biol Cell. 2022;33(6):47.
Shechner DM, et al. Multiplexable, locus-specific targeting of long RNAs with CRISPR-Display. Nat Methods. 2015;12(7):664–70.
Ma H, et al. Multiplexed labeling of genomic loci with dCas9 and engineered sgRNAs using CRISPRainbow. Nat Biotechnol. 2016;34(5):528–30.
Cheng AW, et al. Casilio: a versatile CRISPR-Cas9-Pumilio hybrid for gene regulation and genomic labeling. Cell Res. 2016;26(2):254–7.
Shao S, et al. Long-term dual-color tracking of genomic loci by modified sgRNAs of the CRISPR/Cas9 system. Nucleic Acids Res. 2016;44(9): e86.
Maass PG, et al. Spatiotemporal allele organization by allele-specific CRISPR live-cell imaging (SNP-CLING). Nat Struct Mol Biol. 2018;25(2):176–84.
Clow PA, et al. CRISPR-mediated multiplexed live cell imaging of nonrepetitive genomic loci with one guide RNA per locus. Nat Commun. 2022;13(1):1871.
Saad H, et al. DNA dynamics during early double-strand break processing revealed by non-intrusive imaging of living cells. PLoS Genet. 2014;10(3): e1004187.
Germier T, et al. Real-time imaging of a single gene reveals transcription-initiated local confinement. Biophys J. 2017;113(7):1383–94.
Germier T, et al. Real-time imaging of specific genomic loci in eukaryotic cells using the ANCHOR DNA labelling system. Methods. 2018;142:16–23.
Delker RK, et al. Fluorescent labeling of genomic loci in Drosophila imaginal discs with heterologous DNA-binding proteins. Cell Rep Methods. 2022;2(3): 100175.
Oliveira GM, et al. Precise measurements of chromatin diffusion dynamics by modeling using Gaussian processes. Nat Commun. 2021;12(1):6184.
Gabriele M, et al. Dynamics of CTCF- and cohesin-mediated chromatin looping revealed by live-cell imaging. Science. 2022;376(6592):496–501.
Mach P, et al. Live-cell imaging and physical modeling reveal control of chromosome folding dynamics by cohesin and CTCF. Nat Genet. 2022;54(12):1907–18.
Platania A, et al. Competition between transcription and loop extrusion modulates promoter and enhancer dynamics. BioRxiv. 2023. https://doi.org/10.1101/2023.04.25.538222.
Wijgerde M, Grosveld F, Fraser P. Transcription complex stability and chromatin dynamics in vivo. Nature. 1995;377(6546):209–13.
Ochiai H. Real-time observation of localization and expression (ROLEX) system for live imaging of the transcriptional activity and nuclear position of a specific endogenous gene. In: Shav-Tal Y, editor. Imaging gene expression methods in molecular biology. New York: Humana; 2019.
Ohishi H, et al. STREAMING-tag system reveals spatiotemporal relationships between transcriptional regulatory factors and transcriptional activity. Nat Commun. 2022;13(1):7672.
Li J, et al. Single-gene imaging links genome topology, promoter-enhancer communication and transcription control. Nat Struct Mol Biol. 2020;27(11):1032–40.
Zuin J, et al. Regulation of the cohesin-loading factor NIPBL: Role of the lncRNA NIPBL-AS1 and identification of a distal enhancer element. PLoS Genet. 2017;13(12): e1007137.
Lu S, et al. Live cell imaging of DNA and RNA with fluorescent signal amplification and background reduction techniques. Frontiers Cell Develop Biol. 2023. https://doi.org/10.3389/fcell.2023.1216232.
Viushkov VS, et al. Visualizing the genome: experimental approaches for live-cell chromatin imaging. Cells. 2022;11(24):4086.
Dillon N, et al. The effect of distance on long-range chromatin interactions. Mol Cell. 1997;1(1):131–9.
Bartman CR, et al. Enhancer regulation of transcriptional bursting parameters revealed by forced chromatin looping. Mol Cell. 2016;62(2):237–47.
Zuin J, et al. Nonlinear control of transcription through enhancer-promoter interactions. Nature. 2022;604(7906):571–7.
Rinzema NJ, et al. Building regulatory landscapes reveals that an enhancer can recruit cohesin to create contact domains, engage CTCF sites and activate distant genes. Nat Struct Mol Biol. 2022;29(6):563–74.
Cheng L, et al. Mechanisms of transcription control by distal enhancers from high-resolution single-gene imaging. BioRxiv. 2023. https://doi.org/10.1101/2023.03.19.533190.
Chen H, et al. Dynamic interplay between enhancer-promoter topology and gene activity. Nat Genet. 2018;50(9):1296–303.
von Diezmann L, Shechtman Y, Moerner WE. Three-dimensional localization of single molecules for super-resolution imaging and single-particle tracking. Chem Rev. 2017;117(11):7244–75.
Chen L-F, et al. Structural elements facilitate extreme long-range gene regulation at a human disease locus. Mol Cell. 2023;83(9):1446–61.
Hanssen LLP, et al. Tissue-specific CTCF-cohesin-mediated chromatin architecture delimits enhancer interactions and function in vivo. Nat Cell Biol. 2017;19(8):952–61.
Oudelaar AM, et al. A revised model for promoter competition based on multi-way chromatin interactions at the alpha-globin locus. Nat Commun. 2019;10(1):5412.
Ghamari A, et al. In vivo live imaging of RNA polymerase II transcription factories in primary cells. Genes Dev. 2013;27(7):767–77.
Cho WK, et al. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science. 2018;361(6400):412–5.
Liu Z, et al. 3D imaging of Sox2 enhancer clusters in embryonic stem cells. Elife. 2014;3: e04236.
Farla P, et al. The androgen receptor ligand-binding domain stabilizes DNA binding in living cells. J Struct Biol. 2004;147(1):50–61.
Paul MW, et al. Distinct mobility patterns of BRCA2 molecules at DNA damage sites. BioRxiv. 2023. https://doi.org/10.1101/2023.02.20.527475.
Lovely GA, et al. Direct observation of RAG recombinase recruitment to chromatin and the IgH locus in live pro-B cells. BioRxiv. 2020. https://doi.org/10.1101/2020.09.07.286484.
Li J, et al. Single-molecule nanoscopy elucidates RNA polymerase II transcription at single genes in live cells. Cell. 2019;178(2):491–506.
Stortz M, et al. Unraveling the molecular interactions involved in phase separation of glucocorticoid receptor. BMC Biol. 2020;18(1):59.
Garcia DA, et al. An intrinsically disordered region-mediated confinement state contributes to the dynamics and function of transcription factors. Mol Cell. 2021;81(7):1484–98.
Hannon CE, Eisen MB. Intrinsic protein disorder is insufficient to drive subnuclear clustering in embryonic transcription factors. BioRxiv. 2023. https://doi.org/10.1101/2023.03.27.534457.
Brosh R, et al. Synthetic regulatory genomics uncovers enhancer context dependence at the Sox2 locus. Mol Cell. 2023;83(7):1140–52.
Blobel GA, et al. Testing the super-enhancer concept. Nat Rev Genet. 2021;22(12):749–55.
Peng Q, et al. Engineering inducible biomolecular assemblies for genome imaging and manipulation in living cells. Nat Comm. 2022;13:7933.
Acknowledgements
We thank Hélène Kabbech and Selçuk Yavuz for critical reading and input into the manuscript.
Funding
Work in the labs of F.G and K.S.W was supported by a grant of the Dutch Research Council (NWO, Nederlandse Organisatie voor Wetenschappelijk Onderzoek) NWO-BBOL Grant 737.016.014.
Author information
Authors and Affiliations
Contributions
Writing—original draft: JvS; TvS; writing—review and editing: KSW, FG. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
van Staalduinen, J., van Staveren, T., Grosveld, F. et al. Live-cell imaging of chromatin contacts opens a new window into chromatin dynamics. Epigenetics & Chromatin 16, 27 (2023). https://doi.org/10.1186/s13072-023-00503-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13072-023-00503-9