Abstract
Background
Surveillance data documenting tick and tick-borne disease (TBD) prevalence is needed to develop risk assessments and implement control strategies. Despite extensive research in Africa, there is no standardized, comprehensive review.
Methods
Here we tackle this knowledge gap, by producing a comprehensive review of research articles on ticks and TBD between 1901 and 2020 in Chad, Djibouti, Ethiopia, Kenya, Tanzania, and Uganda. Over 8356 English language articles were recovered. Our search strategy included 19 related MeSH terms. Articles were reviewed, and 331 met inclusion criteria. Articles containing mappable data were compiled into a standardized data schema, georeferenced, and uploaded to VectorMap.
Results
Tick and pathogen matrixes were created, providing information on vector distributions and tick–pathogen associations within the six selected African countries.
Conclusions
These results provide a digital, mappable database of current and historical tick and TBD distributions across six countries in Africa, which can inform specific risk modeling, determine surveillance gaps, and guide future surveillance priorities.
Graphical Abstract

Similar content being viewed by others
Background
Tick-borne disease (TBD) represents a growing threat to both human and animal health around the world. Increases in TBD burden over the last century may in part reflect improved surveillance and diagnostic capability. However, climate change and other ecological disturbances are also contributing to the displacement and/or expansion of tick habitats, which has further increased pathogen prevalence within tick populations [1, 2]. Global travel and international animal trade further facilitate the expanded distribution of certain tick species, as highlighted by the recent introduction and establishment of Haemaphysalis longicornis in the United States [3,4,5].
Sub-Saharan Africa is particularly vulnerable to the growing threat of ticks and TBD. An estimated 50% of the African continent’s livestock is found within the East Africa region, with livestock accounting for at least 20% of the agricultural gross domestic product (GDP) within Ethiopia, Kenya, and Uganda alone [6]. In Tanzania, around two-thirds of rural populations are reported to keep livestock [7]. Furthermore, roughly 1.5 million people maintain a pastoralist lifestyle within Kenya, Tanzania, and Uganda, creating ample opportunity for transboundary movement of ticks and TBD [8].
Previously reported TBDs in Eastern and Central sub-Saharan Africa include African tick bite fever (ATBF), Boutonneuse fever (BF), Crimean Congo hemorrhagic fever (CCHF), East Coast fever (ECF), Nairobi sheep disease (NSD), Coxiella burnetii (Q-fever), and tick-borne relapsing fever (TBRF) [9]. While some of these diseases are characterized by febrile illness and skin rashes, others have more severe manifestations, such as CCHF, which is characterized by severe hemorrhagic disease, with reported fatality rates of up to 40% [10]. Diseases like East Coast fever, NSD, and Q-fever can also result in significant economic losses due to their effects on livestock health. In certain areas of Uganda, East Coast fever is responsible for as much as 50% of calf death within cattle production systems [11].
Despite the large number of tick collection studies that have been published over the last century and the known health risks and economic impacts of TBD, there is still no comprehensive resource for data on ticks or TBD presence in Africa. This lack of centralized knowledge undermines TBD mitigation and vector control efforts, leading to increased disease burden to humans and animals. To address this knowledge gap, our team conducted an expansive systematic literature review to describe the distribution of ticks and associated TBDs that have been reported from studies in Chad, Djibouti, Ethiopia, Kenya, Tanzania, and Uganda. Our primary objectives were to (1) identify peer-reviewed publications that contain high-quality, mappable tick collection data, (2) standardize data and georeference collection events, (3) submit data from systematic review to the VectorMap dashboard, a comprehensive country-specific database of tick species and pathogen distributions, and (4) identify surveillance gaps and other knowledge vacuums.
This open-access dataset will enhance various future analyses, as the data can be easily integrated with new collection data to model disease risk to humans and animals. By analyzing these data within a geographic information system (GIS) such as VectorMap, environmental and population data can be easily correlated with surveillance results, providing an opportunity to better characterize the risk profile of TBDs under current and future environmental and demographic conditions. Such information would prove highly beneficial to the regional economy and to veterinary and public health in sub-Saharan Africa.
Methods
Nineteen Medical Subject Headings (MeSH) terms were used to search the PubMed, Scopus, Web of Science, and CABI VetMed databases. The details of all Boolean operators used across the databases are found in Table 1. Independent searches were conducted for all six countries in each of the selected databases. Searches targeted articles published from January 1901 through August 2020 and articles written in English. While our search strategy targeted articles written in English, we also translated any articles written in French identified during our search. The reference sections of each article meeting our inclusion criteria were also reviewed to accrue additional articles to evaluate that were not captured from the initial search results; these additional articles were reviewed in the same manner as those returned during database searches.
Eligibility criteria
To determine whether an article should be included, the following exclusion criteria were used: studies conducted outside of geographical targets or with insufficient geographical data, laboratory studies (e.g., vector competency, insectaries), and review articles (note: cited references were reviewed for original research). The inclusion criteria of articles were as follows: original research studies on tick species in the target countries of Central and East Africa including all life stages, original research articles reporting TBD prevalence in humans/animals, and studies that included mappable collection data (e.g., sufficient geographical data). Included articles were combined and broken down by country (Additional file 3: Table S1).
Our team held weekly meetings to discuss questionable articles and to ensure that scrutiny was applied evenly when determining which articles met the set criteria. Articles were reviewed and tracked using guidance from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (Additional file 1: Figure S1). All articles that met our inclusion criteria were original research studies reporting results of tick collections and/or screening of TBDs in vectors or hosts. Included studies also reported sufficient information to determine the geographical origin of the collection events with measurable accuracy and precision. Our exclusion criteria identified non-peer-reviewed articles, articles reporting previously published results (reviews), or articles reporting ambiguous or dubious collection site descriptions. Any articles meeting our exclusion criteria were eliminated from our review.
Data management
The process of assessing articles for inclusion eligibility was documented using separate MS Excel spreadsheets for each country. All articles captured using the Boolean search terms across all four databases were compiled into a single document for each country. The lists of returned articles then underwent the criteria stages outlined in PRISMA, with each stage documented as a separate sheet. First, duplicate articles were removed to provide a single list of unique articles returned from all four databases. After duplicate removal, articles were reviewed first by title and then by abstract to assess for article relevance. Any articles that did not meet the inclusion criteria were removed and documented during these steps. The remaining articles underwent a full review, and any that met the a priori inclusion criteria were used for data mining, as described below. A reference library containing all articles meeting our inclusion criteria was built and maintained using Zotero citation management software.
Data mining
Data mining of eligible articles was conducted using a customized data schema established by the VectorMap project, which captures 93 fields of information, and a spreadsheet collection form, prompting different pieces of information to be extracted for each collection event entry. The extensive design ensured that as much information as possible was collected verbatim from the article as well as offering sections for unique aspects of the collection events. Data collected included tick/host identification methods and taxonomy, collection event locality descriptions, elevation and geographical coordinates, individual tick count, sex and life stage, collection methods, collection event habitat, and pathogen detection methods and results. Concerning the collection event locality, the most specific location information made available for each tick collection event was recorded. New entries were made for each unique collection event reported within the articles, separating by collection method and collection date whenever possible. Separate data entries were also created for different tick species collected during the same collection event. The extracted dataset for this work, including all references, Global Positioning System (GPS) points, pathogen detection results, and details on individual manuscripts, can be found within Additional file 2: Dataset S1.
Georeferencing
Geographical data associated with each collection event entry were divided into two categories: those reporting geo-coordinates and those reporting named places only. For locations with geo-coordinates, we converted the latitude and longitude values to decimal degrees and calculated a spatial uncertainty measurement using the point-radius method [12, 13]. Locations described solely as named places were georeferenced using keyword searches retrieved from an online gazetteer (GeoNames.org). The spatial uncertainty of named places was calculated by measuring the distance between the locality’s centroid provided by the gazetteer to the farthest edge of the administrative border of that geographical entity [13]. If the collection event locality information was unclear, georeferencing was performed using the next most specific location information (e.g., province- or country-level information). Geographical data visualizations were generated using ArcGIS Pro 2.7.3 (Esri) and country shapefiles from the Database of Global Administrative Areas (GADM, version 4.0, https://gadm.org/).
Results
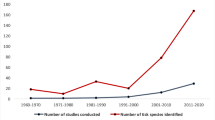
A total of 8357 articles, reporting data from our six target countries, were initially captured using the four search engines (CABI = 3176, PubMed = 2172, Scopus = 1754, and Web of Science = 1242), of which 315 articles met the final inclusion criteria and underwent data extraction and georeferencing. The stepwise results of the inclusion/exclusion process for the entire systematic review can be found in Fig. 1. A breakdown of the inclusion/exclusion process by country can be found in Table 2. A graphical timeline summary of all publications that met the final inclusion criteria is presented in Fig. 2. A total of 91 articles identified during our search were not able to be located after exhausting the interlibrary loan systems at each of our affiliated institutions. A full list of work contributing to this dataset can be found in Supplemental Table 1. There were 315 articles which met inclusion criteria for data extraction from Chad [14,15,16,17,18,19], Djibouti [15, 20,21,22,23,24,25,26], Ethiopia [15, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138, 154, 156, 175, 176], Kenya [15, 138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258], Tanzania [141, 177, 243, 259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298], and Uganda [15, 26, 145, 156, 175, 299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324,325,326].
Study results
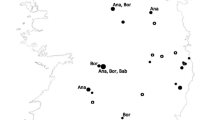
Across the six countries, collection records for six hard tick and two soft tick genera were captured. Additionally, the Nuttalliella ticks (Ixodoidae: Nuttalliellidae), which share morphological features of both hard and soft tick genera, were identified in reports from Tanzania [327]. Ticks of the genus Rhipicephalus were found to be the most diverse, with 43 species reported, while the Dermacentor and Nuttalliella genera were only mentioned once in the literature. Amblyomma variegatum was commonplace, with 414 unique collection event entries, followed by Rhipicephalus evertsi evertsi (n = 354) and Rhipicephalus decoloratus (n = 335). Among a total of 4305 unique collection entries, 3909 (90.8%) described ticks that were removed from an animal, which may lead to skewed reporting of tick species of veterinary importance when compared to tick diversity from environment sampling. A total of 10 different genera of bacterial pathogens, four different genera of protozoa, and 16 viruses were identified in ticks across all six countries. Rickettsia spp. was the most frequently reported bacterial genus overall, with 128 unique collection event entries reporting Rickettsia africae. Other commonly reported medically relevant bacteria included Anaplasma bovis (n = 56), Anaplasma platys (n = 77), Ehrlichia canis (n = 50), and Ehrlichia ruminantium (n = 89). The most frequently reported viruses were CCHF virus (CCHFV) (n = 15) and Dugbe virus (DUGV) (n = 19). Of the 825 unique collection event entries that included an associated pathogen, 85.2% of these described ticks which were collected from an animal. Maps displaying all unique collection sites are provided in Fig. 3, with a summary of tick species captured by country in Table 3.
Chad
A total of 144 unique papers were captured for Chad from the four literature databases, of which 36 articles met the inclusion criteria for a full review, and only six articles reported collection data that met our quality inclusion criteria. These articles produced surveillance records for five unique genera: Amblyomma, Haemaphysalis, Hyalomma, Ornithodoros, and Rhipicephalus. Twelve unique tick species were identified from these reports, including six Rhipicephalus species. Rhipicephalus guilhoni had the greatest number of unique collection event entries within Chad, followed by unspecified Ornithodoros species. Genetic material from DUGV was detected within Hyalomma impeltatum ticks, alluding to the presence of DUGV in Chad [328]. Other microbial species identified within reports from Chad included Rickettsia aeschlimannii detected in Hyalomma rufipes collected from camels and Theileria spp. from Rh. decoloratus collected from Chadian saddle horses (Fig. 4). Most collection events occurred near N’Djamena, while many other collection events occurred within the Batha Ouaddai and Wadi Fira regions.
Djibouti
A total of 129 unique titles were initially identified from Djibouti during our review. Of those, eight articles met our inclusion criteria and reported quality collection data. In total, 23 species of ticks spanning five different genera were reported. Ticks of the genera Hyalomma and Rhipicephalus had the greatest number of unique collection events. Rhipicephalus camicasi and Rhipicephalus sanguineus were the most common species reported. Pathogens detected from Djibouti include Alkhurma virus (AHF), CCHFV, R. aeschlimannii, and R. africae (Fig. 5). Most ticks reported were removed from animals (97/106 data mining event entries), including all instances of tick specimens or pools screened for pathogens. The most common locations reporting tick collection events in Djibouti were Dikhil, Tadjourah, and Ali Sabieh regions and the capital Djibouti City.
Ethiopia
A total of 113 articles reporting tick and TBD collections in Ethiopia met our inclusion criteria. A total of 44 species representing seven genera of ticks were identified including five hard tick genera (Amblyomma, Haemaphysalis, Hyalomma, Ixodes, and Rhipicephalus) and two soft tick genera (Argas and Ornithodoros). Sixteen species of Rhipicephalus ticks were reported, followed by nine different species/subspecies of Hyalomma ticks. The most frequently reported tick species was Am. variegatum, with approximately 73.3% of articles detecting this species. Rhipicephalus decoloratus and Rh. evertsi evertsi were reported in 63.8% and 56.9% of studies, respectively. A total of 26 microbial organisms were reported from Ethiopia, including four viruses: CCHFV, DUGV, Jos (JOSV), and Thogoto (THOV) viruses. Additional microbial organisms include the medically important genera Anaplasma, Borrelia, Coxiella, Ehrlichia, Rickettsia, Babesia, Theileria, and Trypanosoma (Fig. 6). Amblyomma and Rhipicephalus ticks were the most frequently reported genera with associated pathogens. Some 97.8% of tick collections were from animals, most of which were livestock species such as cattle, sheep, goats, and camels. The most commonly surveyed areas include the regions surrounding Addis Ababa (North, East and West Shewa districts) and Dire Dawa (East Hararghe district).
Kenya
Data were extracted from 121 articles reporting tick surveillance in Kenya that met our inclusion criteria. Over 60 unique tick species were compiled during data mining, representing eight different genera: six hard ticks (Amblyomma, Dermacentor, Haemaphysalis, Hyalomma, Ixodes, Rhipicephalus) and three soft ticks (Argas, Carios, Ornithodoros). The tick species with the largest number of unique data extraction entries were Rh. appendiculatus, Rh. pulchellus, Rh. evertsi evertsi, and Am. variegatum. Of these, 88.6% of georeferenced tick entries were removed from domestic or wild animals. Of note, 36.7% of data extraction event entries reported a microbial organism associated with the tick. Bacteria, viruses, and protozoa were observed within the collection entries compiled from these articles, including over 400 instances of Rickettsia.
Other microbial species reported from collected ticks include viruses such as Bhanja (BHAV), CCHFV, Dhori (DHOV), DUGV, Kadam (KADV), Kupe (KUPV), NSD, Ngari (NRIV), and THOV viruses, as well as Anaplasma spp., Borrelia spp., Coxiella spp., Ehrlichia spp., Babesia spp., Hepatozoon spp., and Theileria spp., (Figs. 7, 8). The tick species most frequently reported with an associated microbial organism included Rh. appendiculatus, Rh. pulchellus, Rh. evertsi evertsi, and Am. variegatum. Some 84.9% (558/657) of ticks reported with associated pathogens were removed from animals. Areas of Kenya that were more frequently surveyed include the area surrounding Nairobi, Garissa, Isiolo, Samburu, Laikipia, and Homa Bay counties.
Tanzania
A total of 43 articles reporting tick surveillance from Tanzania met our inclusion criteria during this review. The articles were published between 1950 and 2020, with most entries published in the 1970s. Across all included articles, 68 species spanning nine tick genera were reported, including the only record of Nuttalliella spp. compiled during this review. A total of 24 species were identified within the genus Rhipicephalus, with Rh. appendiculatus reporting the highest number of unique collection event entries (n = 78), followed by Am. variegatum (n = 48) and Rh. evertsi evertsi (n = 44). Over 90% of the unique collection event entries described ticks that were collected from wild or domestic animals. Microbial species that were detected within collection events included members from the following genera: Borrelia, Anaplasma, and Theileria (Fig. 9). In contrast to the other countries included in this review, only 7.7% of pathogen-positive ticks were collected from animals. The collections occurred primarily in the northern, central, eastern, and western regions, with the most collections occurring within the Arusha and Singida regions.
Uganda
A total of 28 unique articles from Uganda met our inclusion criteria. Included articles were published between 1952 and 2020, with an increase in publications occurring between 2015 and 2020. Five articles were published in 2020, which represents the most articles in a single year for Uganda. Ticks from 35 species representing five genera were collected across all articles, including Amblyomma, Haemaphysalis, Hyalomma, and Rhipicephalus hard ticks, and soft ticks of the genera Carios and Ornithodoros. The largest number of species identified within a single genus was Rhipicephalus, with 15 species reported from Uganda. Amblyomma variegatum (n = 20) was the most frequently reported tick species, followed by Rh. appendiculatus (n = 18) and Rh. decoloratus (n = 15). Microbial groups reported from collected ticks include species of Anaplasma, Ehrlichia, Rickettsia, Babesia, and Theileria (Fig. 10). In total, 92.4% of tick entries were those collected from animals, with cattle being the most common. All tick collection event entries with an associated pathogen group describe a tick collected off either a cow or dog. Most collection events occurred in the southwest and northeast regions of Uganda, as well as around the country’s capital Kampala. A final list of all pathogens detected within ticks from this literature review can be found in Table 4.
Discussion
The ability for public health stakeholders to create policies supporting TBD prevention and control is dependent on a thorough understanding of the epidemiology and distribution of tick species and detailed information regarding the clinically relevant pathogens they carry. While gaps remain, such information is available but hard to access, being scattered among numerous published articles. Without incorporation into a single, standardized database, it is difficult to integrate historical baseline data with current surveillance efforts. We sought to address this critical knowledge gap by integrating over 100 years' worth of literature into a singular, standardized database that continues to grow as new surveillance data are generated. Information collected from our review can be used to predict tick and pathogen distribution and create pathogen risk profiles, which can inform appropriate preventative measures for medical and veterinary health care professionals while also identifying gaps where additional surveillance is needed.
By systematically collating and georeferencing data from the past century on TBDs, we have developed a single dataset that can be used by both researchers and policymakers in Africa. While this database will prove useful for future risk assessment analyses, it also identifies major gaps in tick and TBD surveillance within the region, and thus can be used to guide future surveillance studies. Most data reported in the literature document collections of ticks from animals. Although this is a valuable source of data, these studies do not capture the full range of tick species that threaten human and animal health or the ecological dynamics of pathogens with their vectors. Environmental sampling is needed to further understand the dynamic relationships between ticks, their hosts, TBD, and the environment, and future studies should target analyzing the pathogens present within ticks from the environment and the blood meals they may have obtained from feeding on wild animal hosts. Our database clearly identifies the need for enhanced environmental sampling within all countries included in the study. Overall, only 7.9% of data entries represented tick collection events from the environment, with country-level proportions ranging from 2.3% (Ethiopia) to 30.4% (Chad).
Other gaps in surveillance relate to tick identification and the reporting of tick life stage. Only 2008 tick collection event entries report what life stage of each species was captured, which undermines identification confidence for collection events that relied solely on morphological identification methods. It is also important to note the numerous taxonomic changes that have impacted the species names reported in the literature over the last century. While verbatim species names were updated to reflect current valid names, the component taxa within species complexes (e.g., Rh. sanguineus sensu lato) are in flux and distributions are likely to change [329]. Data presented here will be invaluable in determining historical species ranges, informing forthcoming taxonomic revisions. Given the temporal nature of this review, these data may also provide insights into the impact of land use and landscape change, global warming, and epidemiological shifts in disease presence, especially within the last two decades.
Chad
This review highlighted the limited understanding of tick distributions in Chad. Most collection events reported in the literature were opportunistic tick surveys conducted on domestic animals, livestock, and wildlife, with very few collection events coming from vegetation and/or animal burrows. Of the ticks screened for pathogens, DUGV, R. aeschlimannii, and an unspecified Theileria spp. were detected. Equine piroplasmosis (EP), caused by Babesia caballi or Theileria equi, has been associated with up to a 50% mortality rate, although in endemic settings the mortality may be lower. Given the pathogenicity of EP agents, they represent a major threat to communities in Chad, as Dongola horses play a significant role in everyday operations for both the Chadian National Guard and nomadic community members.
Djibouti
Like Chad, the results of the literature review demonstrate a limited characterization of the ticks found within Djibouti. Over 90% of collection events reported described ticks removed from animals. This indicates a major gap in the characterization of ticks within Djibouti, as studies detailing local environmental tick sampling are sparse. Pathogens detected within the sampled ticks included CCHFV in Amblyomma spp., Dermacentor spp., Hyalomma spp., and Rhipicephalus spp. ticks, AHF in Amblyomma lepidum, R. africae in Amblyomma spp. and Rhipicephalus spp., and R. aeschlimannii in Hy. rufipes. Both AHF and CCHFV have been reported to cause non-specific flu-like symptoms that can develop hemorrhagic manifestations, with case fatality rates over 15% [330]. Of note, several collection event entries documenting ticks collected from cattle at slaughterhouses within Djibouti were captured, and in some instances these cattle had been brought into Djibouti from neighboring countries. While sampling at slaughterhouses represents a convenient way to collect ticks, complications arise as to whether any identified ticks or TBD represent species endemic to Djibouti or endemic to the cattle’s country of origin. Given the sustained movement of both humans and animals from Africa to the Middle East through Djibouti, there is enhanced risk for the import or establishment of non-endemic TBDs within the country.
Ethiopia
Numerous pathogens were detected in multiple species collected in Ethiopia. Agents detected included CCHFV, DUGV, JOSV, THOV, Anaplasma centrale, Anaplasma marginale, Anaplasma ovis, Anaplasma phagocytophilum, Borrelia anserina, Borrelia theileri, Coxiella burnetii, E. ruminantium, R. africae, Rickettsia conorii, B. caballi, Theileria orientalis, Theileria mutans, Theileria velifera, and Trypanosoma theileri. The presence of microbes from the Anaplasmataceae family and Borrelia species warrants concern regarding the well-being of livestock and the potential economic impact on pastoralists as it relates to the organisms responsible for human and animal anaplasmosis and ehrlichiosis [331]. Anaplasma phagocytophilum causes multiple diseases in different hosts: granulocytic anaplasmosis, ruminant tick-borne fever, and human granulocytic anaplasmosis (HGA) [332]. Humans with HGA experience symptoms that are like animal anaplasmosis, including anemia, hepatic injury, septic shock, and acute respiratory distress syndrome (ARDS) [333]. Borrelia spp. known to cause disease in domestic animals were also reported, including B. anserina, the causative agent of avian spirochetosis, which is characterized by high mortality in commercial bird species, and B. theileri, which is known to cause bovine borreliosis, a mild disease characterized by anemia and fever [334, 335] . Rickettsia conorii causes Mediterranean spotted fever, which typically presents as spotted fever, although complications such as vasculitis and multiple organ failure have also been reported [336, 337]. In addition, infections with certain Theileria spp. such as T. annulata and T. parva can cause anemia in cattle, impacting milk and meat production. One group estimated that the annual economic loss resulting from T. parva infections alone may cause losses as high as $168 million across regions of eastern, central, and southern Africa [338]. The detection of these numerous TBDs demonstrates a major threat to the people of Ethiopia. The collection records help establish baseline data. However, there is still a need for further surveillance in numerous regions in order to fully characterize the presence of endemic tick species and TBDs of Ethiopia.
Kenya
Data for tick and TBD research conducted within Kenya was abundant compared to other countries targeted during this review. From these studies, 167 unique georeferenced collection sites and 1787 collection event entries were documented. Some 107 animal species were sampled, including livestock, domestic animals, large carnivores, herbivores, rodents, and reptiles. Of the six countries reviewed, Kenya had the highest number of publications reporting data on ticks and the pathogens they carry, with 68 documented tick species from eight genera (Amblyomma, Argas, Dermacentor, Haemaphysalis, Hyalomma, Ixodes, Ornithodoros, and Rhipicephalus). The genera with the highest number of unique species reported were Rhipicephalus and Amblyomma.
Within the collected data, etiological agents detected included CCHFV, DUGV, DHOV, BHAV, Karai virus (KSIV), KADV, NSD virus, NRIV, THOV, A. bovis, A. ovis, A. phagocytophilum, A. platys, Bartonella spp., Borrelia burgdorferi, Borrelia dipodilli, C. burnetii, E. canis, Ehrlichia chaffeensis, E. ruminantium, Paracoccus spp., Proteus mirabilis, R. aeschlimannii, R. africae, Rickettsia akari, R. conorii, Rickettsia rhipicephali, B. caballi, Babesia microti, Hepatozoon canis, Hepatozoon fitzsimonsi, T. parva, Theileria taurotragi, and T. velifera. Rickettsia africae was the most frequently detected pathogen, which as stated above is the causative agent of African tick bite fever in humans [339]. CCHFV, DHOV, NRIV, and THOV can be transmitted from ticks to humans and present as hemorrhagic fever, and occasionally meningoencephalitis [340,341,342]. Like other Anaplasma spp., A. bovis and A. platys cause disease characterized by anemia and weight loss within affected animals [343]. Babesia microti, the primary cause of human babesiosis, produced a malaria-like illness with more severe manifestations in immunocompromised patients such as ARDS, anemia, and disseminated intravascular coagulopathy (DIC) [344]. BHAV is associated with febrile illness and central nervous system manifestations [345]. Rickettsial pox, caused by infection with R. akari, is characterized by flu-like symptoms and vesicular lesions on the trunk and extremities [346]. The NSD virus has a mortality rate of up to 90% in non-immune animals and is characterized by hemorrhagic gastroenteritis and abortion [347]. Ehrlichia canis causes canine ehrlichiosis, which can progress to severe disease with symptoms of hemorrhage, epistaxis, and shock [348]. Ehrlichia chaffeensis is the causative agent of human monocytic ehrlichiosis (HME), with symptoms ranging from vomiting and diarrhea to multiple organ failure [349]. Finally, B. burgdorferi, the pathogen responsible for Lyme borreliosis (Lescot et al., 2008), was also detected in Kenya [350]. Multiple pathogens reported within this systematic review are of clinical relevance to both humans and animals [350, 351], impacting public health, food security, and local economies. Data generated by this study provide a critical baseline of tick and TBD surveillance data published from Kenya. However, our study reveals critical gaps in surveillance coverage in the eastern regions of Kenya, which should be targeted for future surveillance efforts.
Tanzania
A total of 729 unique tick collection events were reported from Tanzania, with only 375 entries including specific geographical information concerning the collection event. Ticks were collected primarily from animals, with over 100 different host species sampled. In total, nine genera of ticks were recorded: Amblyomma, Argas, Dermacentor, Haemaphysalis, Hyalomma, Ixodes, Nuttalliella, Ornithodoros, and Rhipicephalus.
Only 10 articles conducted screening for microbial species present within ticks. Agents found included A. marginale, A. bovis, Borrelia duttonii, T. equi, and T. parva. Anaplasma marginale was the most frequently detected pathogen, which as mentioned above can cause bovine anaplasmosis. Tick-borne relapsing fever is associated with Borrelia spp. that are vectored by soft ticks from the genus Ornithodoros, and there is evidence that this is a circulating zoonosis within East Africa [352]. Much of Tanzania’s land use is dedicated to agriculture, with livestock raised predominantly by small-scale independent farmers in rural areas [353]. Given the large number of human–animal interactions and the impact TBD can have on livestock animals, there is concern regarding the possible threat ticks pose for the livestock owners, which demonstrates the need for more tick and TBD surveillance to ensure food and economic security. Of note, there were no detailed records which examined tick species or TBD specifically from Zanzibar. Given this is a high-traffic trade island off the coast of mainland Tanzania, it would be worthwhile to survey this area in the future and compare the tick diversity between the two regions. There were few collection records which referenced the southernmost regions of Tanzania—Lindi, Mtwara, and Ruvuma. Further surveillance is needed to address gaps in data between different regions of Tanzania, while further testing of ticks needs to be conducted to understand TBD prevalence.
Uganda
Our results indicate that there are several opportunities to expand our knowledge of ticks in Uganda. Most tick collection events reported from Uganda were collected from a host animal, with only 7.6% of collection event entries documenting environmental samples collected on vegetation or in burrows or caves. Only 10 of the articles reviewed conducted pathogen testing on collected ticks. Yet in these few studies, A. marginale, E. ruminantium, R. africae, R. conorii, and T. parva have all been detected, with E. ruminantium, the causative agent of heartwater, and R. africae, the causative agent of tick bite fever, being the most frequently detected agents. Additional tick surveillance implementing environmental sampling would yield more data characterizing suitable questing habitat within Uganda for each species. In addition, sampling a wider variety of host animals to include more reptiles and birds may yield additional tick taxa not detected in this review. Finally, any future surveillance efforts in Uganda should include pathogen screening and molecular confirmation of tick species identification.
Limitations
By using the search criteria established for this literature review, there may have been articles that were published in languages other than English and French, and thus were not captured in the initial search results. This is of particular importance for Tanzania, given that a few published studies were noted to have been written in German. For example, a published tick record in Tanzania demonstrated the first recording of Babesia trautmanni in 1914, but the language in which the article was published resulted in it not being included. Another limitation of this review was the lack of standardized tick identification methods available. Most studies relied primarily on morphology for tick identification, which may have led to misidentified species. Additionally, since the publication of many of these articles, there have been tick species whose taxonomic classifications have changed over time, which may complicate the accuracy of final species designation within this study. Given frequent movement of animals within the study region, animals surveyed for tick studies may represent animals from other regions, possibly impacting the accuracy of the recorded geographical location of reported tick species and any associated microbial agents. This complicates the assessment of whether the ticks or TBD reported from a given study are representative of the study country or neighboring countries. Additionally, throughout our study we did not differentiate the reported recordings of TBDs according to their endemic or epidemic status.
Conclusions
This systematic review provides a novel dataset on ticks, their associated microbial organisms, and their geographical occurrences within six countries of Africa. This database is made freely available to the public, and its use is encouraged for those working to mitigate the risk of TBD in Africa. Additionally, records generated by this project contain verbatim information that can be independently scrutinized by users to determine their relevance to future studies. The aggregation of such data allows for trends in the distribution of ticks and TBD over time to be correlated with changes in land use, population growth, and the effects resulting from climate change. This study also highlights the substantial gaps in knowledge regarding the distribution of ticks and TBDs in Central and East Africa. While around 120 articles were sourced for Ethiopia and Kenya, considerably fewer were available for Chad, Djibouti, Tanzania, and Uganda, clearly demonstrating the presence of major surveillance gaps within these countries. Improvement of surveillance coverage within these countries requires sustained investment and is contingent on local scientists being adequately trained to conduct rigorous surveillance that produces high-quality tick collection data. Ensuring that local scientists have access to standardized guidelines and protocols to aid in their sampling strategies is equally important. Sampling can differ depending on the environment and situation, with some sampling conducted for more routine surveillance while other sampling can be targeted in response to ongoing outbreaks. Special emphasis also needs to be placed on confirming details of the geographical origin of surveyed animals, when possible, to avoid any uncertainties that may arise from data collected from animals that have been moved across country borders. Databases such as VectorMap can be used as a template for local scientists to inform them and their studies of what data should be recorded with each tick collection event, to continue building this database into the future. Additionally, testing of ticks for microbial agents needs to be encouraged to improve the current knowledge on the presence and distribution of TBD within the region. As research continues, there is a need for capacity-building at the local level, to ensure that the work is carried out in a systematic and effective manner that continues to build on results of the past. Many of the knowledge gaps identified within this systematic review were in relation to quality and quantity of tick surveillance data. This review highlights the need for additional capacity-building within the studied countries in order to promote acquisition of high-quality data which can be used within databases such as VectorMap to obtain a better understanding of the density and diversity of tick populations. This high-quality data will also allow groups to further highlight the challenges certain communities face regarding the impact of TBDs on the health and well-being of humans, livestock, and wildlife.
Availability of data and materials
Tick distribution data points for associated pathogens and details of associated collections are openly shared and downloadable from the VectorMap site (vectormap.si.edu).
Abbreviations
- TBD:
-
Tick-borne disease
- GDP:
-
Gross domestic product
- CCHF:
-
Crimean Congo hemorrhagic fever
- GIS:
-
Geographic information system
- DUGV:
-
Dugbe virus
- JOSV:
-
Jos virus
- THOV:
-
Thogoto virus
- DHOV:
-
Dhori virus
- KADV:
-
Kadam virus
- KUPV:
-
Kupe virus
- NSD:
-
Nairobi sheep disease
- NRIV:
-
Ngari virus
- EP:
-
Equine piroplasmosis
- HGA:
-
Human granulocytic anaplasmosis
- ARDS:
-
Acute respiratory distress syndrome
- DIC:
-
Disseminated intravascular coagulopathy
- HME:
-
Human monocytic ehrlichiosis
- AHF:
-
Alkhurma virus
- BHAV:
-
Bhanja virus
- KSIV:
-
Karai virus
- FMDV:
-
Foot-and-mouth disease virus
- MCOV:
-
Marco virus
- TIMV:
-
Timbo virus
- CHOV:
-
Chaco virus
- TBRF:
-
Tick-borne relapsing fever
- ATBF:
-
African tick bite fever
- BF:
-
Boutonneuse fever
- ECF:
-
East coast fever
References
Wisely SM, Glass GE. Advancing the science of tick and tick-borne disease surveillance in the United States. Insects. 2019;10:361.
Diuk-Wasser MA, VanAcker MC, Fernandez MP. Impact of land use changes and habitat fragmentation on the eco-epidemiology of tick-borne diseases. J Med Entomol. 2021;58:1546–64.
von Fricken ME. Living with the longhorned: a perspective on invasive Haemaphysalis longicornis ticks in the United States. Zoonoses Public Hlth. 2020;67:841–2.
Egizi A, Bulaga-Seraphin L, Alt E, Bajwa WI, Bernick J, Bickerton M, et al. First glimpse into the origin and spread of the Asian longhorned tick, Haemaphysalis longicornis, in the United States. Zoonoses Public Hlth. 2020;67:637–50.
Buczek A, Buczek W. Importation of ticks on companion animals and the risk of spread of tick-borne diseases to non-endemic regions in Europe. Animals. 2020;11:6.
Macmillan S. Northern Kenya-Southern Ethiopia dryland livestock traders gathered in Marsabit for better livestock trade and market links. 2018. ILRI Clippings. https://clippings.ilri.org/2018/06/14/northern-kenya-southern-ethiopia-dryland-livestock-traders-gathered-in-marsabit-for-better-livestock-trade-and-market-links/. 6 Jun 2021.
World Bank. Business and livelihoods in African livestock: investments to overcome information gaps. Washington, DC. 2014. http://hdl.handle.net/10986/17801. 6 Jun 2021.
Fratkin E. East African pastoralism in transition: Maasai, Boran, and Rendille cases. Afr Stud Rev. 2001;44:1–25.
Bram RA. Tick-borne livestock diseases and their vectors. FAO Anim Pr. 1983;36:1–5.
World Health Organization. Crimean-Congo haemorrhagic fever. 2022. https://www.who.int/news-room/fact-sheets/detail/crimean-congo-haemorrhagic-fever. Accessed 6 Jun 2021.
Kasaija PD, Estrada-Peña A, Contreras M, Kirunda H, de la Fuente J. Cattle ticks and tick-borne diseases: a review of Uganda’s situation. Ticks Tick-borne Dis. 2021;12:101756.
Wieczorek JQG, Hijmans R. The point-radius method for georeferencing locality descriptions and calculating associated uncertainty. Int J Geogr Inf Sci. 2004;18:745–67.
Chapman AD, & Wieczorek JR. Georeferencing best practices. 2020. Version 1.0.
Dahmana H, Amanzougaghene N, Davoust B, Normand T, Carette O, Demoncheaux JP, et al. Great diversity of Piroplasmida in Equidae in Africa and Europe, including potential new species. Vet Parasitol. 2019. https://doi.org/10.1016/j.vprsr.2019.100332.
Morel PC, Vassiliads G. Les Rhipicephalus du groupe sanguineus: espèces Africaines (Acariens: Ixodoidea). Rev Elev Med Vet Trop. 1962;15:343–86.
Mura A, Socolovschi C, Ginesta J, Lafrance B, Magnan S, Rolain JM, et al. Molecular detection of spotted fever group rickettsiae in ticks from Ethiopia and Chad. Trans R Soc Trop Med Hyg. 2008;102:945–9. https://doi.org/10.1016/j.trstmh.2008.03.015.
Rodrigues R, Telles JN, Essere K, Ducournau C, Roqueplo C, Levieuge A, et al. Development of a one step real time RT-PCR assay to detect and quantify Dugbe virus. J Virol Methods. 2011;176:74–7. https://doi.org/10.1016/j.jviromet.2011.06.003.
Trape JF, Diatta G, Arnathau C, Bitam I, Sarih M, Belghyti D, et al. The epidemiology and geographic distribution of relapsing fever borreliosis in West and North Africa, with a review of the Ornithodoros erraticus complex (Acari: Ixodida). PLoS One. 2013;8:e78473. https://doi.org/10.1371/journal.pone.0078473.
Zachée B, Mahamat O, Saboune M, Awah-Ndukum J. Prevalence, intensity and risk factors of tick infestation of cattle in N’djamena Chad. Int J Vet Sci Anim Husb. 2020;5:139–46.
Estrada-Peña A, Rhipicephalus camicasi Morel, Mouchet and Rodhain,. (Fig. 126). In ticks of Europe and North Africa: a guide to species identification. Springer International Publishing. 1976;2017:317–9. https://doi.org/10.1007/978-3-319-63760-0_60.
Hoogstraal H. On ticks (Ixodidae) of Southern French Somaliland and the rediscovery of Rhipicephalus longicoxatus Neumann 1905. Ann Entom Soc Am. 1953;46:393–8. https://doi.org/10.1093/aesa/46.3.393.
Horton KC, Fahmy NT, Watany N, Zayed A, Mohamed A, Ahmed AA, et al. Crimean Congo hemorrhagic fever virus and Alkhurma virus in ticks in Djibouti. Vector-borne Zoonot. 2016;16:680–2. https://doi.org/10.1089/vbz.2016.1951.
Horton KC, Jiang J, Maina A, Dueger E, Zayed A, Ahmed AA, et al. Evidence of Rickettsia and Orientia infections among Abattoir workers in Djibouti. Am J Trop Med Hyg. 2016;95:462–5. https://doi.org/10.4269/ajtmh.15-0775.
Mouchet J. Aedes aegypti and potential vectors of yellow fever in the Democratic Republic of Somalia and in the French territory of Afars and Issas. B World Health Organ. 1971;45:383–94.
Rodhain F. Preliminary results of an entomological survey of the potential arbovirus vectors in the French territory of Afars and Issas. B Soc Pathol Exot. 1976;69:169–74.
Socolovschi C, Matsumoto K, Jean-Lou Marie JL, Davoust B, Raoult D, Parola P. Identification of rickettsiae, Uganda and Djibouti. Emerg Infect Dis. 2007;13:1508–9. https://doi.org/10.3201/eid1310.070078.
Nejash A, Ibrahim N, Begna F. Prevalence, risk factors and vectors identification of bovine anaplasmosis and babesiosis in and around Jimma town. Southwestern Ethiopia Acta Trop. 2018;177:9–18. https://doi.org/10.1016/j.actatropica.2017.09.010.
Adem A, Muktar Y, Hiko A. Prevalence and risk factors of ticks infesting cattle reared on the main campus of Haramaya university, Eastern Ethiopia. Ethiop Vet J. 2017;21:16. https://doi.org/10.4314/evj.v21i1.2.
Abebe R, Fantahun T, Abera M, Bekele J. Survey of ticks (Acari: Ixodidae) infesting cattle in two districts of Somali regional state. Ethiopia Vet World. 2010;3:539–43.
Abebe R, Tatek M, Megersa B, Sheferaw D. Prevalence of small ruminant ectoparasites and associated risk factors in selected districts of Tigray region. Ethiopia Globlal Veterinaria. 2011;7:433–7.
Adugna A, Gebrewahd TT. Prevalence and risk factors of ectoparasites in small ruminants in and around Haramaya university, Eastern Oromia region, Ethiopia. Ethiop Vet J. 2019;23:78. https://doi.org/10.4314/evj.v23i1.6.
Abera M, Mohammed T, Abebe R, Aragaw K, Bekele J. Survey of Ixodid ticks in domestic ruminants in Bedelle district. Southwestern Ethiopia Trop Anim Hlth Prod. 2010;42:1677–83. https://doi.org/10.1007/s11250-010-9620-4.
Regassa A, Nesibu A, Birhanu H, Yisehak T, Teshale S. Internal and external parasites of camels (Camelus dromedarius) slaughtered at Addis Ababa Abattoir. Ethiopia J Vet Med Anim Hlth. 2015;7:57–63. https://doi.org/10.5897/JVMAH2014.0346.
Fufa A, Kasasa D, Shelima B, Megersa B, Regassa A, Amenu K. Survey of tick infestation in small ruminants of Miesso District, West Harergie, Oromia region. Ethiopia Trop Anim Hlth Prod. 2009;41:969–72. https://doi.org/10.1007/s11250-008-9286-3.
Fufa A, Tura J, Regassa A. Status of tick infestation in small ruminants of Bedelle district, Oromia region, Ethiopia. Glob Vet. 2012; 8.
Ali M, de Castro JJ. Host resistance to ticks (Acari: Ixodidae) in different breeds of cattle at Bako. Ethiopia Trop Anim Hlth Prod. 1993;25:215–22. https://doi.org/10.1007/BF02250871.
Amante M, Yacob H, Terefe G, Asres K. In-vitro louscidal and acaricidal activities of alkaloid of Calpurnia aurea extracts against Linognathus ovillus and Amblyomma variegatum. Int J Pharm Sci Res. 2019;49:431–7. https://doi.org/10.13040/IJPSR.0975-8232.10(1).431-37.
Amare S, Asfaw Y, Tolossa YH. Ectoparasites of sheep and goats in North-West Amhara regional state, Ethiopia. Ethiop Vet J. 2014;17:55. https://doi.org/10.4314/evj.v17i1.5.
Aragaw K, Abdella A, Fekadu A, Kassaye A, Hindebu B, Sheferaw D. Skin associated problems in working donkeys in three districts of Sidama zone Southern Ethiopia. Ethiop Vet J. 2016. https://doi.org/10.4314/evj.v20i2.8.
Ashenafi H, Yimer E. Ectoparasites of local scavenging chickens of Central Ethiopia. SINET Ethiop J Sci. 2005;28:69–74. https://doi.org/10.4314/sinet.v28i1.18235.
Asrate S, Yalew A. Prevalence of cattle tick infestation in and around Haramaya district, Eastern Ethiopia. J Vet Med Anim Hlth. 2012;4:84–8.
Ayalew T, Tolossa YH, Kumsa B. Ixodid ticks infesting cattle in three agroecological zones in Central Oromia: species composition, seasonal variation, and control practices. Comp Clin Path. 2014;23:1103–10. https://doi.org/10.1007/s00580-013-1748-y.
Ayana D, Eshetu E, Waketole H, Abunna F. In-vitro acaricidal efficacy evaluation trial of Ixodid ticks at Borana, Ethiopia. Ethiop Vet J. 2014;17:85. https://doi.org/10.4314/evj.v17i2.7.
Bayisa D, Berhanu A, Fentahun T, Chanie M. Occurrence of bovine dermatophilosis in Ambo town, West Shoa administrative zone, Ethiopia. Am-Euras J Sci Res. 2012;7:172–5.
Bayisa T, Ibrahim N, Dargie M. Prevalence of ovine ectoparasites in and around Ambo town, Ethiopia. Middle East J Sci Res. 2013;16:62–7.
Bedada H, Terefe G, Tolossa YH. Current status of ectoparasites in sheep and management practices against the problem in ectoparasites controlled and uncontrolled areas of Arsi zone in Oromia region, Ethiopia. J Vet Sci Technol. 2015. https://doi.org/10.4172/2157-7579.1000S10-002.
Bekele J, Tariku M, Abebe R. External parasite infestations in small ruminants in Wolmera district of Oromiya region, Central Ethiopia. J Anim Vet Adv. 2011;10:518–23. https://doi.org/10.3923/javaa.2011.518.523.
Bekele T. Studies on seasonal dynamics of ticks of Ogaden cattle and individual variation in resistance to ticks in Eastern Ethiopia. J Vet Med B Infect Dis Vet Public Hlth. 2002;49:285–8. https://doi.org/10.1046/j.1439-0450.2002.00567.x.
Belihu K, Mamo A, Lobago F, Ayana D. Prevalence of ectoparasites in backyard local chickens in three agroecologic zones of East Shoa, Ethiopia. Rev Med Vet. 2009;160:537–41.
Beyecha K, Kumsa B, Beyene D. Ectoparasites of goats in three agroecologies in Central Oromia, Ethiopia. Comp Clin Path. 2014;23:21–8. https://doi.org/10.1007/s00580-012-1563-x.
Tamerat N, Korso L, Hailu S, Yimer M, & Bezabih M. Prevalence and identification of ectoparasites fauna in small ruminants in and around Adami Tulu, East Shawa zone of Oromia, Ethiopia. Livest Res Rural Dev. 2016; 28.
Hilina B, Berihun A, Yasmin J. Prevalence and identification of ticks in cattle in and around Mekelle. Rev Electron. 2012;13:91–206.
Burgdorfer W, Ormsbee RA, Schmidt ML, Hoogstraal H. A search for the epidemic typhus agent in Ethiopian ticks. B World Hlth Organ. 1973;48:563–9.
Burgdorfer W, Schmidt ML, Hoogstraal H. Detection of Trypanosoma theileri in Ethiopian cattle ticks. Acta Trop. 1973;30:340–6.
Choudhury MK, Shiferaw Y, Hussen A. Toxicity of Millettia ferruginea darasana (Family: Fabaceae) against the larvae and adult ticks of Amblyomma variegatum Fabricius a three-host tick in cattle. J Parasit Dis. 2015;39:298–302. https://doi.org/10.1007/s12639-013-0311-8.
Cutler S, Abdissa A, Adamu H, Tolosa T, Gashaw A. Borrelia in Ethiopian ticks. Ticks Tick-Borne Dis. 2012;3:14–7. https://doi.org/10.1016/j.ttbdis.2011.08.004.
Dabasa G, Jilo K, Zewdie W, Shanko T, Gurmesa G, Ahmed NA. Prevalence of small ruminant gastrointestinal parasites infections and associated risk factors in selected districts of Bale zone, South Eastern Ethiopia. J Parasitol Vector Biol. 2017;9:81–9. https://doi.org/10.5897/JPVB2017.0286.
Sheferaw D. Tick resistance of two breeds of cattle in Wolaita zone, Southern Ethiopia. J Vet Med Anim Hlth. 2017;9:349–55. https://doi.org/10.5897/JVMAH2017.0614.
Dinka A, Bedada B, Yacob HT. Study on major parasitic problems of rural cattle in and around Ambo, Western Oromia, Ethiopia. Niger Vet J. 2011. https://doi.org/10.4314/nvj.v31i3.68973.
Dinka A, Eyerusalem B, Yacob H. A study on major ectoparasites of camel in and around Dire Dawa, Eastern Ethiopia. Rev Med Vet. 2010;161:498–501.
Eddie B, Foster WA, Radovsky FJ, Stiller D. Isolation of a pl agent (Chlamydia, Bedsonia) from ticks (Argas (P.) Arboreus) parasitic on the white-necked cormorant (Phalacrocorax arbo) in Ethiopia. J Med Entomol. 1970;7:745–6. https://doi.org/10.1093/jmedent/7.6.745.
Eyob B, Matios. Preliminary survey on the distribution of ixodid ticks in small ruminants of Dhas district of Borena pastoral area, Southern rangelands of Ethiopia. Adv Biores. 2014;5:87–91.
Fantahun B, Mohamed A. Survey on the distribution of tick species in and around Assosa town, Ethiopia. Res J Vet Sci. 2012;5:32–41. https://doi.org/10.3923/rjvs.2012.32.41.
Feleke A, Petros B, Lemecha L, Wossene A, Mulatu W, Rege E. Study on monthly dynamics of ticks and seroprevalence of Anaplasma marginale, Babesia bigemina and Theileria mutans in four indigenous breeds of cattle in Ghibe Valley, Ethiopia. Ethiop Vet Sci. 2008;31:11–20. https://doi.org/10.4314/sinet.v31i1.18293.
Feleke A, Petros B, Mulatu W, Lemecha H, Wossene A. Resistance of Abigar, Guraghe, Horro and Sheko breeds of cattle to tick infestation in Ghibeand; Mdash; Tolley valley. Bull Anim Hlth Prod Afr. 2007. https://doi.org/10.4314/bahpa.v55i3.32805.
Fentahun T, Woldemariam F, Chanie M, Berhan M. Prevalence of ectoparasites on small ruminants in and around Gondar town. Am-Euras J Sci Res. 2012;7:106–11.
Ferede B, Kumsa B, Hailu AB, Kalayou S. Ticks of donkeys in Central Oromia regional state, Ethiopia. Rev Med Vet. 2010;161:121–6.
Ferede Y, Mola L, Asmare Z. Prevalence and species composition of major internal and external parasites of calves in selected dairy farms of Bahir Dar Milk-Shade. Ethiop Vet J. 2018;22:128. https://doi.org/10.4314/evj.v22i2.10.
Gashaw A. Host preference and seasonal variation of tick (Amblyomma cohaerens Donitz, 1909) on naturally infested cattle in Jimma Zone, Southwestern Ethiopia. J Agr Rural Dev Trop. 2005;106:49–57.
Gebeyehu D, Derso S. Prevalence of major skin diseases in ruminants and its associated risk factors at University of Gondar veterinary clinic, North West Ethiopia. J Vet Sci Technol. 2015. https://doi.org/10.4172/2157-7579.1000S13-002.
Gedilu M, Mohamed A, Kechero Y. Determination of the prevalence of Ixodid ticks of cattle breeds, their predilection sites of variation and tick burden between different risk factors in Bahir Dar, Ethiopia. Glob Vet. 2014;13:520–9.
Hadgu M, Taddele H, Girma A, Abrha H, Hagos H. Prevalence of Ixodid ticks infesting Raya cattle breeds in semi-arid areas of Raya Azebo district, Northern Ethiopia. Ethiop Vet J. 2018;22:53. https://doi.org/10.4314/evj.v22i2.5.
Hiluf G, Bsrat A, Kebede E, Hagos Y. Prevalence and identification of ectoparasites on indigenous chickens in Seharti-Samre district, Tigray, Northern Ethiopia. Ethiop Vet J. 2018;22:1. https://doi.org/10.4314/evj.v22i1.1.
Hornok S, Abichu G, Meli ML, Tánczos B, Sulyok KM, Gyuranecz M, et al. Influence of the biotope on the tick infestation of cattle and on the tick-borne pathogen repertoire of cattle ticks in Ethiopia. PLoS One. 2014;9:e106452. https://doi.org/10.1371/journal.pone.0106452.
Hornok S, Abichu G, Takács N, Gyuranecz M, Farkas R, De Fernndez MIG, et al. Molecular screening for Anaplasmataceae in ticks and Tsetse flies from Ethiopia. Acta Vet Hung. 2016;64:65–70. https://doi.org/10.1556/004.2016.007.
Hunde A, Assefa K, Mukarim A. Further Studies on bovine Ixodide ticks in and around Bedelle, Southwest Ethiopia. Afr J Agric Res. 2017;12:1922–9. https://doi.org/10.5897/AJAR2016.11380.
Kassa S, & Yalew A. Identification of Ixodide ticks of cattle in and around Hararamaya district, Eastern Ethiopia. Sci J Crop Sci. 2012; 1.
Kassaye E, Moser I, Woldemeskel M. Epidemiological study on clinical bovine dermatophilosis in Northern Ethiopia. Deut Tierarztl Woch. 2003;110:422–5.
Kebede N, Fetene T. Population dynamics of cattle ectoparasites in Western Amhara National Regional State, Ethiopia. J Vet Med Anim Hlth. 2012;4:22–6.
Kemal J, Abera T. Prevalence and infestation load of Ixodid ticks of cattle in Dassenech district, Southern Ethiopia. Ethiop Vet J. 2017;21:121. https://doi.org/10.4314/evj.v21i2.9.
Kemal J, Muktar Y, Alemu S. Distribution and prevalence of tick infestation in cattle in Babille district, Eastern Ethiopia. Livest Res Rural Dev. 2016;28:1.
Kemal J, Tamerat N, Tuluka T. Infestation and identification of Ixodid tick in cattle: the case of Arbegona district, Southern Ethiopia. J Vet Med. 2016. https://doi.org/10.1155/2016/9618291.
Kigaye MK, Jiffar T, Kigaye MK, Jiffar T. A survey of ectoparasites of cattle in Harar and Dire Dawa districts, Hararghe administrative region of Ethiopia. Bull Anim Hlth Prod Afr. 1991;39:15–24.
Kumsa B, Abiy Y, Abunna F. Ectoparasites infesting dogs and cats in Bishoftu, Central Oromia, Ethiopia. Vet Parasitol. 2019;15:100263. https://doi.org/10.1016/j.vprsr.2019.100263.
Kumsa B, Beyecha K, Geloye M. Ectoparasites of sheep in three agro-ecological zones in Central Oromia, Ethiopia. Onderstepoort J Vet Res. 2012;79:E1–7. https://doi.org/10.4102/ojvr.v79i1.442.
Kumsa BE, Mekonnen S. Ixodid ticks, fleas and lice infesting dogs and cats in Hawassa, Southern Ethiopia. Onderstepoort J Vet Res. 2011;78:326. https://doi.org/10.4102/ojvr.v78i1.326.
Kumsa B, Signorini M, Teshale S, Tessarin C, Duguma R, Ayana D, et al. Molecular detection of piroplasms in Ixodid ticks infesting cattle and sheep in Western Oromia, Ethiopia. Trop Anim Hlth Prod. 2013;46:27–31. https://doi.org/10.1007/s11250-013-0442-z.
Kumsa B, Socolovschi C, Raoult D, Parola P. Spotted fever group rickettsiae in Ixodid ticks in Oromia, Ethiopia. Ticks Tick-Borne Dis. 2015;6:8–15. https://doi.org/10.1016/j.ttbdis.2014.08.001.
Kumsa B, Tamrat H, Tadesse G, Aklilu N, Cassini R. Prevalence and species composition of Ixodid ticks infesting horses in three agroecologies in Central Oromia. Ethiopia Trop Anim Hlth Prod. 2012;44:119–24. https://doi.org/10.1007/s11250-011-9897-y.
Leul B, Berihun A, Etsay K. Epidemiological distribution of major ectoparasites species of small ruminant in the case of chemical control campaign in Welkait district, Tigray region, Ethiopia. J Trop Med. 2020. https://doi.org/10.1155/2020/4175842.
Mediannikov O, Abdissa A, Socolovschi C, Diatta G, Trape JF, Raoult D. Detection of a new Borrelia species in ticks taken from cattle in Southwest Ethiopia. Vector Borne Zoonot Dis. 2013;13:266–9. https://doi.org/10.1089/vbz.2011.0874.
Megersa B, Damena A, Bekele J, Adane B, Sheferaw D. Ticks and mange mites infesting camels of Boran pastoral areas and the associated risk factors. Southern Ethiopia. 2012;4:71–7.
Mekonnen S, Hussein I, Bedane B. The distribution of Ixodid ticks (Acari: ixodidae) in Central Ethiopia. Onderstepoort J Vet Res. 2001;68:243–51.
Mekuria S, Gezahegn E. Prevalence of external parasite of poultry in intensive and backyard chicken farm at Wolayta Soddo town. Southern Ethiopia Vet World. 2010;3:533–8.
Moges N, Bogale B, Fentahun T. Hard ticks (Ixodidae): species composition, seasonal dynamics and body site distribution on cattle in Chilga district. Northwest Ethiopia Asian J Agr Sci. 2012;4:341–5.
Mohamed B, Belay A, Hailu D. Species composition, prevalence and seasonal variations of Ixodid cattle ticks in and around Haramaya town. Ethiopia J Vet Med Anim Hlth. 2014;6:131–7. https://doi.org/10.5897/JVMAH2014.0275.
Mulugeta Y, Yacob HT, Ashenafi H. Ectoparasites of small ruminants in three selected agro-ecological sites of Tigray region. Ethiopia Trop Anim Hlth Prod. 2010;42:1219–24. https://doi.org/10.1007/s11250-010-9551-0.
Olkeba WG, Sarba EJ, Belay AD, Gebremedhin E. Prevalence of major skin diseases of cattle and associated risk factors around Ambo town, Ethiopia. Bull Anim Hlth Prod Afr. 2016;64:355.
Pegram RG, Hoogstraal H, Wassef HY. Ticks (Acari: Ixodoidea) of Ethiopia, distribution, ecology and host relationships of species infesting livestock. Bull Entomol Res. 1981;71:339–59. https://doi.org/10.1017/S0007485300008373.
Philip CB, Hoogstraal H, Reiss-gutfreund R, Clifford CM. Evidence of rickettsial disease agents in ticks from Ethiopian cattle. Bull World Hlth Organ. 1966;35:127–31.
Regassa A. Tick infestation of Borana cattle in the Borana province of Ethiopia. Onderstepoort J Vet. 2001;68:41–5.
Regassa A, de Castro JJ. Tick resistance to acaricides in Western Ethiopia. Trop Anim Hlth Prod. 1993;25:69–74. https://doi.org/10.1007/BF02236506.
Seid M, Zeryehun T, Kemal J, Tilahun B. Ectoparasites of small ruminants in and around Kombolcha, Northeastern Ethiopia. Ethiop Vet J. 2018;22:81. https://doi.org/10.4314/evj.v22i2.7.
Sertse T, Wossene A. A study on ectoparasites of sheep and goats in Eastern part of Amhara region. Northeast Ethiopia Small Rumin Res. 2007;69:62–7. https://doi.org/10.1016/j.smallrumres.2005.12.010.
Seyoum Z, Tadesse T, Addisu A. Ectoparasites prevalence in small ruminants in and around Sekela, Amhara regional state, Northwest Ethiopia. J Vet Med. 2015;2015:216085. https://doi.org/10.1155/2015/216085.
Shiferaw T, Onu S. Prevalence of ectoparasite infestations of cattle in Bench Maji zone, Southwest Ethiopia. Vet World. 2013;6:291. https://doi.org/10.5455/vetworld.2013.291-294.
Siyoum T, Kitaw G. Comparative milk production and prevalence study of parasites and sub clinical mastitis on indigenous lactating cows under different feeding regimes in Central highlands of Ethiopia. Ethiop Vet J. 2014;18:43–56.
Solomon G, Kaaya GP. Comparison of resistance in three breeds of cattle against African Ixodid ticks. Exp Appl Acarol. 1996;20:223–30. https://doi.org/10.1007/BF00054514.
Solomon G, Kaaya GP, Gebreab F, Gemetchu T, Tilahun G. Ticks and tick-borne parasites associated with indigenous cattle in Didtuyura ranch, Southern Ethiopia. Int J Trop Insect Sci. 1998;18:59–66. https://doi.org/10.1017/S1742758400007475.
Sulyok KM, Hornok S, Abichu G, Erdélyi K, Gyuranecz M. Identification of novel Coxiella burnetii genotypes from Ethiopian ticks. PLoS One. 2014;9:e113213. https://doi.org/10.1371/journal.pone.0113213.
Tadesse A, Fentaw E, Mekbib B, Abebe R, Mekuria S, Zewdu E. Study on the prevalence of ectoparasite infestation of ruminanats in and around Kombolcha and damage to fresh goat pelts and wet blue (pickled) skin at Kombolch tannary, Northestern Ethiopia. Ethiop Vet J. 2011. https://doi.org/10.4314/evj.v15i2.67697.
Tadesse B, Sultan A. Prevalence and distribution of tick infestation on cattle at Fitche Selale, North Shewa. Ethiopia Livest Res Rural Dev. 2014;28:1–8.
Tadesse F, Abadfaji G, Girma S, Kumsa B, Jibat T. Identification of tick species and their preferred site on cattle’s body in and around Mizan Teferi, Southwestern Ethiopia. J Vet Med Anim Hlth. 2012;4:1–5.
Tafesse B. Survey on the distribution of ticks of domestic animals in the Eastern zone of Ethiopia. Trop Anim Hlth Prod. 1996;28:145–6. https://doi.org/10.1007/BF02299564.
Taye DR, Assefa K, Hika W. Prevalence of major ectoparasites of calves and associated risk factors in and around Bishoftu town. Afr J Agric Res. 2015;10:1127–35. https://doi.org/10.5897/AJAR2014.9380.
Tesfaheywet Z, Simeon H. Major ectoparasites of small ruminants in Bench Maji zone, Southern Ethiopia. Livest Res Rural Dev. 2016;28:63.
Tesfaye A, Chanie M. Ectoparasites are major skin diseases of dogs in Gondar, Amhara national regional state. Ethiopia Int J Anim Vet Adv. 2011;3:392–6.
Tesfaye D. Ectoparasites of small ruminants presented at Bahir Dar veterinary clinic, Northwest Ethiopia. Afr J Agric Res. 2012. https://doi.org/10.5897/AJAR12.599.
Teshale S, Geysen D, Ameni G, Asfaw Y, Berkvens D. Improved molecular detection of Ehrlichia and Anaplasma species applied to Amblyomma ticks collected from cattle and sheep in Ethiopia. Ticks Tick-Borne Dis. 2015;6:1–7. https://doi.org/10.1016/j.ttbdis.2014.04.023.
Teshale S, Geysen D, Ameni G, Bogale K, Dorny P, Berkvens D. Molecular detection of Anaplasma species in questing ticks (Ixodids) in Ethiopia. Asian Pac J Trop Dis. 2016;6:449–52. https://doi.org/10.1016/S2222-1808(16)61066-6.
Teshale S, Kumsa B, Menandro ML, Cassini R, Martini M. Anaplasma, Ehrlichia and rickettsial pathogens in Ixodid ticks infesting cattle and sheep in Western Oromia. Ethiopia Exp Appl Acarol. 2016;70:231–7. https://doi.org/10.1007/s10493-016-0067-9.
Tessema T, Gashaw A. Prevalence of ticks on local and crossbred cattle in and around Asella town. Southeast Ethiopia Ethiop Vet J. 2011;14:79–89. https://doi.org/10.4314/evj.v14i2.63886.
Tiki B, Addis M. Distribution of Ixodid ticks on cattle in and around Holeta town, Ethiopia. Glob Vet. 2011;7:527–31.
Tilki T, Eshetu A, Waktola H. Major ectoparasites of cattle in Ada’a district, East Showa zone, Ethiopia. Livest Res Rural Dev. 2015;27:198.
Tomassone L, Grego E, Callà G, Rodighiero P, Pressi G, Gebre S, et al. Ticks and tick-borne pathogens in livestock from nomadic herds in the Somali region. Ethiopia Exp Appl Acarol. 2012;56:391–401. https://doi.org/10.1007/s10493-012-9528-y.
Wasihun P, Doda D. Study on prevalence and identification of ticks in Humbo district, Southern nations, nationalities, and people’s region (SNNPR). Ethiopia J Vet Med Anim Hlth. 2013;5:73–80.
Woldemeskel M, Mersha G. Study on caprine and ovine dermatophilosis in Wollo. Northeast Ethiopia Trop Anim Hlth Prod. 2010;42:41–4. https://doi.org/10.1007/s11250-009-9383-y.
Wood OL, Lee VH, Ash JS, Casals J. Crimean-Congo hemorrhagic fever, Thogoto, Dugbe, and Jos viruses isolated from Ixodid ticks in Ethiopia. Am J Trop Med Hyg. 1978;27:600–4. https://doi.org/10.4269/ajtmh.1978.27.600.
Yacob H, Yalew T, Dinka A. Part I: Ectoparasite prevalences in sheep and in goats in and around Wolaita Soddo. Southern Ethiopia Rev Med Vet. 2008;159:450–4.
Yacob H, Ataklty H, Kumsa B. Major ectoparasites of cattle in and around Mekelle. Northern Ethiopia Bull Entomol Res. 2008;38:126–30. https://doi.org/10.1111/j.1748-5967.2008.00148.x.
Yehualashet T, Gebreab F, Wakjira A, Tsega T. Preliminary observation on ticks: seasonal dynamics and resistance of three indigenous and three cross-bred cattle in Ethiopia. Bull Anim Hlth Prod Afr. 1995;43:105–14.
Yilma J, Adamu G, Zerbini E. Biossay of acaricide resistance on three common cattle tick species at Holotta. Central Ethiopia Rev Med Vet. 2001;152:385–90.
Yilma JM, Daniel WS, Dorchies P. Survey of ticks infesting domestic ruminants in South Wollo region of Ethiopia. Rev Med Vet. 1995;146:213–20.
Yishak I, Tsegalem A, Befekadu UW. Epidemiological study on ectoparasite infestation of small ruminants in Sodo Zuria district, Southern Ethiopia. J Vet Med Anim Hlth. 2015;7:140–4. https://doi.org/10.5897/JVMAH2014.0358.
Yonas M, Welegerima K, Laudisoit A, Bauer H, Gebrehiwot K, Deckers S, et al. Preliminary investigation on rodent-ectoparasite associations in the highlands of Tigray, Northern Ethiopia: implications for potential zoonoses. Integr Zool. 2011;6:366–74. https://doi.org/10.1111/j.1749-4877.2011.00265.x.
Zeleke M, Bekele T. Species of ticks on camels and their seasonal population dynamics in Eastern Ethiopia. Trop Anim Hlth Prod. 2004;36:225–31. https://doi.org/10.1023/B:TROP.0000016830.30194.2a.
Zeru F, Bedada H, Gebru M, Seid A, Gebregergious A. Epidemiology of major small ruminant ectoparasites and effectiveness of the control approaches employed in selected pastoral districts of Afar. Northeastern Ethiopia J Biol Agric Hlthc. 2015;5:63–72.
Zeryehun T, Atomsa M. Ectoparasite infestations of sheep and goats. Eurasian J Vet Sci. 2012;28:185–9.
Akinyi MY, Tung J, Jeneby M, Patel NB, Altmann J, Alberts SC. Role of grooming in reducing tick load in wild baboons (Papio Cynocephalus). Anim Behav. 2013;85:559–68. https://doi.org/10.1016/j.anbehav.2012.12.012.
Amoo AOJ, Dipeolu OO, Capstick PB, Munyinyi DM, Gichuru LN, Odhiambo TR. Ixodid ticks (Acari: Ixodidae) and livestock production: effect of varying acaricide treatments on ticks and productivity in East coast fever-immunized weaner and dairy cattle. J Med Entomol. 1993;30:503–12. https://doi.org/10.1093/jmedent/30.3.503.
Apanaskevich DA, Tomlinson JA. Description of four new species of Haemaphysalis Koch, 1844 (Acari: Ixodidae) from the H. (Rhipistoma) spinulosa subgroup, parasites of carnivores and rodents in Africa. Syst Parasitol. 2019;96:625–57. https://doi.org/10.1007/s11230-019-09875-7.
Bwangamoi O. A Report on generalized Equine ringworm (Trichophyton Equinum) complicated by tick infestation (Boophilus Decoloratus) and besnoitiosis. Bull Epizoot Dis Afr. 1972;20:211–20.
Campana MG, Hawkins MT, Henson LH, Stewardson K, Young HS, Card LR, et al. Simultaneous identification of host, ectoparasite and pathogen DNA via in-solution capture. Mol Ecol Resour. 2016;16:1224–39. https://doi.org/10.1111/1755-0998.12524.
Chiera JW, Newson RM, Karuhize GR. Adaptation of field strains of Rhipicephalus appendiculatus Neumann (Acarina: Ixodidae) to host resistance. Parasitol. 1989;99:149–55. https://doi.org/10.1017/S0031182000061138.
Clifford CM, Flux JE, Hoogstraal H. Seasonal and regional abundance of ticks (Ixodidae) on hares (Leporidae) in Kenya. J Med Entomol. 1976;13:40–7. https://doi.org/10.1093/jmedent/13.1.40.
Clifford CM, Kohls GM, Hoogstraal H. Ixodes Walkerae, n. sp., from a bird in Kenya (Agarina: Ixodidae). J Med Entomol. 1968;5:513–4. https://doi.org/10.1093/jmedent/5.4.513.
D’Amico G, Dumitrache MO, Široký P, Albrechtová K, Sloboda M, Domşa C, et al. Altitudinal and seasonal differences of tick communities in dogs from pastoralist tribes of Northern Kenya. Vet Parasitol. 2015;12:318–23. https://doi.org/10.1016/j.vetpar.2015.08.025.
Daubney R, Hudson JR. Nairobi sheep disease: natural and experimental transmission by ticks other than Rhipicephalus appendiculatus. Parasitol. 1934;26:496–509. https://doi.org/10.1017/S0031182000023817.
Davies FG. Karai virus, a probable arbovirus isolated from sheep and from the tick Rhipicephalus evertsi in Kenya. J Comp Pathol. 1982;92:9–14. https://doi.org/10.1016/0021-9975(82)90038-X.
Davies FG. Nairobi sheep disease in Kenya. The isolation of virus from sheep and goats, ticks and possible maintenance hosts. Epidemiol Infect. 1978;81:259–65. https://doi.org/10.1017/S0022172400025092.
de Castro JJ. Effects of artificial and natural tick infestations on cattle. Ticks Tick-Borne Dis. 1986;17:113–5.
de Castro JJ, Young AS, Dransfield RD, Cunningham MP, Dolan TT. Effects of tick infestation on Boran (Bos Indicus) cattle immunised against theileriosis in an endemic area of Kenya. Res Vet Sci. 1985;39:279–88. https://doi.org/10.1016/S0034-5288(18)31714-4.
Detray DE, Zaphiro D, Hay D. The incidence of African swine fever in wart hogs in Kenya-a preliminary report. J Am Vet Med A. 1961;138:78–80.
Dioli M, Jean-Baptiste S, Fox M. Ticks (Acari: Ixodidae) of the one-humped camel (Camelus Dromedarius) in Kenya and Southern Ethiopia: species composition, attachment sites, sex ratio and seasonal incidence. Trop Anim Hlth Prod. 2001;54:115–22.
Dolan R, Wilson AJ, Schwartz HJ, Newson RM, Field CR. Camel production in Kenya and its constraints. II. Tick infestation. Trop Anim Hlth Prod. 1983;15:179–85. https://doi.org/10.1007/bf02239930.
El Kammah KM, Hoogstraal H, Camicas JL. Notes on African Haemaphysalis ticks: XI. H. (Rhipistoma) paraleachi (Ixodoidea: Ixodidae) distribution & hosts of adults. Int J Acarol. 1992;18:205–12. https://doi.org/10.1080/01647959208683952.
Fotheringham W, Lewis EA. East coast fever; its transmission by ticks in Kenya colony: Hyalomma impressum near Planum P. Sch as a vector Parasitol. 1937;29:504–23. https://doi.org/10.1017/S0031182000025026.
Gitao CG. The Epidemiology and control of camel dermatophilosis. Rev Elev Med Vet Pay. 1993;46:309–11.
Gitau GK, McDermott JJ, Katende JM, O’callaghan CJ, Brown RN, Perry BD. Differences in the epidemiology of theileriosis on smallholder dairy farms in contrasting agro-ecological and grazing strata of highland Kenya. Epidemiol Infect. 2000;124:325–35. https://doi.org/10.1017/S0950268800003526.
Gregory MV. Diseases and parasites of the Central African hedgehog Erinaceus albiventris Wagner. Zool Beitr. 1981;27:205–13.
Grootenhuis JG, Morrison WI, Karstad L, Sayer PD, Young AS, Murray M, et al. Fatal theileriosis in eland (Taurotragus Oryx): pathology of natural and experimental cases. Res Vet Sci. 1980;29:219–29. https://doi.org/10.1016/S0034-5288(18)32667-5.
Guerra AS, Eckerlin RP, Dowling AP, Durden LA, Robbins RG, Dittmar K, et al. Host-parasite associations in small mammal communities in semiarid savanna ecosystems of East Africa. J Med Entomol. 2016;53:851–60. https://doi.org/10.1093/jme/tjw048.
Haig DA, Woodall JP, Danskin D. Thogoto virus: a hitherto undescribed agent isolated from ticks in Kenya. J Gen Microbiol. 1965;38:389–94.
Hassan SM, Dipeolu OO, Amoo AO, Odhiambo TR. Predation on livestock ticks by chickens. Vet Parasitol. 1991;38:199–204. https://doi.org/10.1016/0304-4017(91)90129-J.
Hassan SM, Dipeolu OO, Malonza MM. Natural attraction of livestock ticks by the leaves of a shrub. Trop Anim Hlth Prod. 1994;26:87–91. https://doi.org/10.1007/bf02239905.
Hassan SM, Dipeolu OO, Munyinyi DM. Influence of exposure period and management methods on the effectiveness of chickens as predators of ticks infesting cattle. Vet Parasitol. 1992;43:301–9. https://doi.org/10.1016/0304-4017(92)90171-5.
Hbisch EB, Mcpnee E, Eickman LE. The epidemiology of tick-typhus in Nairobi. East Afr Med J. 1995;34:459–77.
Heisch RB. Argas brumpti Neumann in the Kitui district of Kenya. East Afr Med J. 1954;31:483–4.
Heisch RB. Ornithodoros moubata (Murray) in a porcupine burrow near Kitui. East Afr Med J. 1954;31.
Heisch RB, Grainger WE, Harvey AEC, Lister G. Feral aspects of rickettsial infections in Kenya. T Roy Soc Trop Med H. 1962;56:272–82. https://doi.org/10.1016/0035-9203(62)90048-2.
Heisch RB, Guggisberg CAW. A description of Ornithodoros erraticus (Lucas) from Kenya. Ann Trop Med Parasitol. 1952;46:1–6. https://doi.org/10.1080/00034983.1952.11685499.
Heisch RB, Guggisberg CAW. On Ornithodoros graingeri n. sp., a tick from caves in Kenya. Parasitol. 1953;42:192–8. https://doi.org/10.1017/s0031182000084456.
Hoogstraal H, Kaiser MN, Walker JB, Ledger JA, Converse JD, Rica RC. Observations on the subgenus Argas (Ixodoidea: Argasidae: Argas) A. (A.) africolumbae, n. sp., a Pretoria virus-infected parasite of birds in Southern and Eastern Africa. J Med Entomol. 1975;12:194–201. https://doi.org/10.1093/jmedent/12.2.194.
Hoogstraal H, Clifford CM, Keirans JE. The Ornithodoros (Alectorobius) capensis group (Acarina: Ixodoidea: Argasidae) of the palearctic and oriental regions O. (A.) coniceps identity, bird and mammal hosts, virus infections, and distribution in Europe, Africa, and Asia. J Parasitol. 1979;65:395–407. https://doi.org/10.2307/3280282.
Hoogstraal H, El Kammah KM. Notes on African Haemaphysalis ticks. X. H. (Kaiseriana) aciculifer Warburton and H. (K.) rugosa Santos Dias, the African representatives of the Spinigera subgroup (Ixodoidea: Ixodidae). J Parasitol. 1972;58:960–78. https://doi.org/10.2307/3286594.
Ho Hoogstraal H, El Kammah KM, Camicas JL. Notes on African Haemaphysalis Ticks: XVI. H. (Rhipistoma) subterra sp. n., a new member of the Leachi group (Ixodoidea: Ixodidae). Int J Acarol. 1992;18:213–20. https://doi.org/10.1080/01647959208683953.
Hoogstraal H, Wassef HY, Easton ER, & Dixon JEW. Observations on the subgenus Argas (Ixodoidea: Argasidae: Argas) 12. Argas (A.) africolumbae: variation, bird hosts, and distribution in Kenya, Tanzania, and South and South-West Africa. J Med Entomol. 1977;13(4–5):441–45. https://doi.org/10.1093/jmedent/13.4-5.441.
Horak IG, Apanaskevich DA, Kariuki EK. A new species of Rhipicephalus (Acari: Ixodidae), a parasite of giraffes in Kenya. J Med Entomol. 2013;50:685–90.
Hornok S, Szőke K, Meli ML, Sándor AD, Görföl T, Estók P, et al. Molecular detection of vector-borne bacteria in bat ticks (Acari: Ixodidae, Argasidae) from eight countries of the old and new worlds. Parasit Vectors. 2019;12:50. https://doi.org/10.1186/s13071-019-3303-4.
Humke R. Spray race trails with the acaricide batestan in Kenya. Blue Book. 1973;23:7–16.
Irvin AD, Brown CG, Burridge MJ, Cunningham MP, Musoke AJ, Pierce MA, et al. A pathogenic theilerial syndrome of cattle in the Narok district of Kenya. Trop Anim Hlth Prod. 1972;4:220–9. https://doi.org/10.1007/BF02360114.
Irvin AD, Sale JB, Purnell RE. Babesia thomasi from rock hyraces in Kenya. J Parasitol. 1973;59:203–4.
Johnson BK, Chanas AC, Squires EJ, Shockley P, Simpson DI, Parsons J, et al. Arbovirus isolations from Ixodid ticks infesting livestock, Kano Plain, Kenya. T Roy Soc Trop Med H. 1980;74:732–7. https://doi.org/10.1016/0035-9203(80)90188-1.
Kagira JM, Kanyari PN, Maingi N, Githigia SM, Ng’ang’a C, Gachohi J. Relationship between the prevalence of ectoparasites and associated risk factors in free-range pigs in Kenya. ISRN Vet Sci. 2013. https://doi.org/10.1155/2013/650890.
Kanduma EG, Mwacharo JM, Sunter JD, Nzuki I, Mwaura S, Kinyanjui PW, et al. Micro- and minisatellite-expressed sequence tag (EST) markers discriminate between populations of Rhipicephalus appendiculatus. Ticks Tick-Borne Dis. Proceedings of the 7th International Ticks and Tick-borne Pathogens (TTP7) Conference Zaragosa, Spain, August 28th-September 2nd, 2011, 2012; 3: 128–36. https://doi.org/10.1016/j.ttbdis.2012.05.001.
Kariuki DP, Injairo R, Boyce WL, Wellde BT, Ngethe S. Parasite survey of eight wild animals in the Ruma national park. Ann Trop Med Parasitol. 1989;83:115–8. https://doi.org/10.1080/00034983.1989.11812415.
Kariuki DP, Young AS, Morzaria SP, Lesan AC, Mining SK, Omwoyo P, et al. Theileria parva carrier state in naturally infected and artificially immunised cattle. Trop Anim Hlth Prod. 1995;27:15–25. https://doi.org/10.1007/BF02236328.
Kariuki EK, Penzhorn BL, Horak IG. Ticks (Acari: Ixodidae) infesting cattle and African buffaloes in the Tsavo conservation area, Kenya. Onderstepoort J Vet Res. 2012;79:E1–4. https://doi.org/10.4102/ojvr.v79i1.437.
Keesing F, Allan BF, Young TP, Ostfeld RS. Effects of wildlife and cattle on tick abundance in Central Kenya. Ecol Appl. 2013;23:1410–8.
Keirans JE, Hoogstraal H, Clifford CM. Ornithodoros (Proknekalia) vansomereni, new subgenus and new species (Acarina: Ixodoidea: Argasidae), a swallow nest parasite in Kenya. Ann Entomol Soc Am. 1977;70:221–8. https://doi.org/10.1093/aesa/70.2.221.
Kimita G, Mutai B, Nyanjom SG, Wamunyokoli F, Waitumbi J. Phylogenetic variants of Rickettsia africae, and incidental identification of ‘Candidatus Rickettsia moyalensis’ in Kenya. PLoS Negl Trop Dis. 2016;10:e0004788. https://doi.org/10.1371/journal.pntd.0004788.
King’ori E, Obanda V, Chiyo PI, Soriguer RC, Morrondo P, Angelone S. Molecular identification of Ehrlichia, Anaplasma, Babesia and Theileria in African elephants and their ticks. PLoS One. 2019;14:e0226083.
Kipronoh KAI, Gathuma JM, Kitala PM, Kiara HK. Prevalence of tick-borne infections in extensive cattle management system in West Pokot district, Kenya. Bull Anim Hlth Prod Afr. 2011;59:43–52.
Knobel DL, Maina AN, Cutler SJ, Ogola E, Feikin DR, Junghae M, et al. Coxiella burnetii in humans, domestic ruminants, and ticks in rural Western Kenya. Am J Trop Med Hyg. 2013;88:513–8. https://doi.org/10.4269/ajtmh.12-0169.
Koka H, Sang R, Kutima HL, Musila L. Coxiella burnetii detected in tick samples from pastoral communities in Kenya. BioMed Res Int. 2018;2018:8158102. https://doi.org/10.1155/2018/8158102.
Koka H, Sang R, Kutima HL, Musila L. The detection of spotted fever group Rickettsia DNA in tick samples from pastoral communities in Kenya. J Med Entomol. 2017;54:774–80. https://doi.org/10.1093/jme/tjw238.
Kubasu SS, Makokkah GL, Kaaya G. Biological differences within Rhipicephalus appendiculatus Neumann (Acari: Ixodidae) populations in Kenya. J Egypt Soc Parasitol. 2007;37:411–8.
Kutima H, Horak IG, Kock M, Neves L, Jooste R, Kariuki E. Ixodid ticks (Acari: Ixodidae) collected from African savanna elephants (Loxodonta Africana) and african forest elephants (Loxodonta Cyclotis). Onderstepoort J Vet. 2019;86:1–5. https://doi.org/10.4102/ojvr.v86i1.1781.
Larkin PJ. Control of the blue tick (Boophilus decoloratus) on cattle with pyrethrum sprays. Vet Rec. 1961;73:298–300.
Latif AA, Punyua DK, Capstick PB, Newson RM. Tick infestations on Zebu cattle in Western Kenya: host resistance to Rhipicephalus appendiculatus (Acari: Ixodidae). J Med Entomol. 1991;28:127–32. https://doi.org/10.1093/jmedent/28.1.127.
Lewis EA. Rhipicephalus ayrei n. sp. (a tick) from Kenya colony. Parasitology. 1933. https://doi.org/10.1017/S0031182000019430.
Lutomiah J, Musila L, Makio A, Ochieng C, Koka H, Chepkorir E, et al. Ticks and tick-borne viruses from livestock hosts in arid and semiarid regions of the Eastern and Northeastern parts of Kenya. J Med Entomol. 2014;51:269–77. https://doi.org/10.1603/me13039.
Lwande OW, Lutomiah J, Obanda V, Gakuya F, Mutisya J, Mulwa F, et al. Isolation of tick and mosquito-borne arboviruses from ticks sampled from livestock and wild animal hosts in Ijara district, Kenya. Vector-Borne Zoonot Dis. 2013;13:637–42. https://doi.org/10.1089/vbz.2012.1190.
Maamun JM, Suleman MA, Akinyi M, Ozwara H, Kariuki T, Carlsson HE. Prevalence of Babesia microti in free-ranging baboons and African green monkeys. J Parasitol. 2011;97:63–7. https://doi.org/10.1645/GE-2391.1.
Macaluso KR, Davis J, Alam U, Korman A, Rutherford JS, Rosenberg R, et al. Spotted fever group rickettsiae in ticks from the Masai Mara region of Kenya. Am J Trop Med Hyg. 2003;68:551–3. https://doi.org/10.4269/ajtmh.2003.68.551.
Machado-Ferreira E, Vizzoni VF, Balsemão-Pires E, Moerbeck L, Gazeta GS, Piesman J, et al. Coxiella symbionts are widespread into hard ticks. Parasitol Res. 2016;115:4691–9. https://doi.org/10.1007/s00436-016-5230-z.
Maina AN, Jiang J, Omulo SA, Cutler SJ, Ade F, Ogola E, et al. High prevalence of Rickettsia africae variants in Amblyomma variegatum ticks from domestic mammals in rural Western Kenya: implications for human health. Vector-Borne Zoonot Dis. 2014;14:693–702. https://doi.org/10.1089/vbz.2014.1578.
Moll G, Lohding A, Young AS. Epidemiology of theilerioses in the Trans-Mara division, Kenya: husbandry and disease background and preliminary investigations on theilerioses in calves. Prev Vet Med. 1984;2:801–31. https://doi.org/10.1016/0167-5877(84)90035-7.
Moll G, Lohding A, Young AS, Leitch BL. Epidemiology of theileriosis in calves in an endemic area of Kenya. Vet Parasitol. 1986;19:255–73. https://doi.org/10.1016/0304-4017(86)90073-7.
Morzaria SP, Irvin AD, Taracha E, Spooner PR, Voigt WP, Fujinaga T, et al. Immunization against East coast fever: the use of selected stocks of Theileria parva for immunization of cattle exposed to field challenge. Vet Parasitol. 1987;23:23–41. https://doi.org/10.1016/0304-4017(87)90022-7.
Muenstermann S, Rinkanya FGR, Tome NR. Tick control in small ruminants with a cypermethrin ‘pour-on’ in Kenya. Trop Pest Manag. 1988;34:399–401. https://doi.org/10.1080/09670878809371286.
Mungube EO, Bauni SM, Tenhagen BA, Wamae LW, Nzioka SM, Muhammed L, et al. Prevalence of parasites of the local scavenging chickens in a selected semi-arid zone of Eastern Kenya. Trop Anim Hlth Prod. 2008;40:101–9. https://doi.org/10.1007/s11250-007-9068-3.
Mungube EO, Nzioka SM, Wamae LW. Effect of deltamethrin on Argas persicus within selected sites in Machakos and Kitui counties, semi-arid Eastern Kenya. Livest Res Rural Dev. 2014;26:12.
Muruthi C, Lwande O, Makumi J, Runo S, Otiende M, Arika W. Phenotypic and genotypic identification of ticks sampled from wildlife species in selected conservation sites of Kenya. J Vet Sci Technol. 2016. https://doi.org/10.4172/2157-7579.1000293.
Mutai BK, Wainaina JM, Magiri CG, Nganga JK, Ithondeka PM, Njagi ON, et al. Zoonotic surveillance for rickettsiae in domestic animals in Kenya. Vector Borne Zoonot Dis. 2013. https://doi.org/10.1089/vbz.2012.0977.
Mwamuye MM, Kariuki E, Omondi D, Kabii J, Odongo D, Masiga D, et al. Novel Rickettsia and emergent tick-borne pathogens: a molecular survey of ticks and tick-borne pathogens in Shimba Hills national reserve, Kenya. Ticks Tick-Borne Dis. 2017;8:208–18. https://doi.org/10.1016/j.ttbdis.2016.09.002.
Mwangi EN, Newson RM, Kaaya GP. A hymenopteran parasitoid of the bont tick Amblyomma variegatum Fabricius (Acarina: Ixodidae) in Kenya. Discov Innovat. 1993;5:331–5.
Mwangi EN, Sayer PD, Njanja JC, Bell JF. Tick survey on goats and sheep in Kenya. Trop Anim Hlth Prod. 1985;17:102–6. https://doi.org/10.1007/BF02360782.
Ndarathi CM, Waghela S, Semenye PP. Prevalence of ticks on rangeland domestic ruminants in Kajiado district, Kenya. Bull Anim Hlth Prod Afr. 1989;37:209.
Ndeereh D, Muchemi G, Thaiyah A, Otiende M, Angelone-Alasaad S, Jowers MJ. Molecular survey of Coxiella burnetii in wildlife and ticks at wildlife–livestock interfaces in Kenya. Exp Appl Acarol. 2017;72:277–89. https://doi.org/10.1007/s10493-017-0146-6.
Newson RM. The life cycle of Rhipicephalus appendiculatus on the Kenyan coast. Tick Borne Dis Vector. 1978;1978:46–50.
Njanja JC, Rinkanya FGF, Kiara HK. Ticks of camels, sheep and goats in Northwestern Kenya rangelands. Trop Pest Manag. 1991;37:166–8. https://doi.org/10.1080/09670879109371568.
Obanda V, Kagira JM, Chege S, Okita-Ouma B, Gakuya F. Trypanosomosis and other co-infections in translocated black (Diceros bicornis michaeli) and white (Ceratotherium simum simum) rhinoceroses in Kenya. Sci Parasitol. 2011;12:103–7.
Ogore PB, Baker RL, Kenyanjui M, Thorpe W. Assessment of natural Ixodid tick infestations in sheep. Small Rumin Res. 1999;33:103–7. https://doi.org/10.1016/S0921-4488(99)00009-7.
Oguge NO, Durden LA, Keirans JE, Balami HD, Schwan TG. Ectoparasites (sucking lice, fleas and ticks) of small mammals in Southeastern Kenya. Med Vet Entomol. 2009;23:387–92. https://doi.org/10.1111/j.1365-2915.2009.00820.x.
Oguge NO, Ondiaka P. A preliminary survey of the macroparasite communities of rodents of Kahawa, Central Kenya. Belgian J Zool. 1997;127:113–8.
Omondi D, Masiga DK, Fielding BC, Kariuki E, Ajamma YU, Mwamuye MM, et al. Molecular detection of tick-borne pathogen diversities in ticks from livestock and reptiles along the shores and adjacent islands of Lake Victoria and Lake Baringo, Kenya. Front Vet Sci. 2017. https://doi.org/10.3389/fvets.2017.00073.
Ong’are JO, Munyua WK, Wilson AJ, Rinkanya FGR. Survey of tick resistance in Kiambu district of Kenya, a comparison of two resistance test methods. Bull Anim Hlth Prod. 1985;1985:89–99.
Oswe M, Odhiambo R, Mutai B, Nyakoe N, Awinda G, Waitumbi JN. Zoonotic pathogens in ticks collected from livestock in Kenya. Open J Prev Med. 2018;8:248–59. https://doi.org/10.4236/ojpm.2018.88021.
Peirce MA. Rickettsia-like organisms in the blood of Turdus abyssinicus in Kenya. J Wildl Dis. 1972;8:273–4. https://doi.org/10.7589/0090-3558-8.3.273.
Pester FRN, Laurence BR. The parasite load of some African game animals. J Zool. 1974;174:397–406. https://doi.org/10.1111/j.1469-7998.1974.tb03167.x.
Price JE, Karstad LH. Free-living jackals (Canis mesomelas)-potential reservoir hosts for Ehrlichia canis in Kenya. J Wildl Dis. 1980;16:469–73. https://doi.org/10.7589/0090-3558-16.4.469.
Punyua DK, Newson RM. The brown ear tick Rhipicephalus appendiculatus Neumann (Acarina: Ixodidae) and associated tick species on wild and domestic hosts at Muguga, Kenya. J Parasitol. 1985;71:248–52. https://doi.org/10.2307/3281911.
Rinkanya FGR, Kinilya HSN. Evaluation of the efficacy of Ectopor Rhipicephalus appendiculatus ticks infesting cattle in Kenya. Bull Anim Hlth Prod Afr. 1992;40:197–200.
Roberts JI. The ticks of rodents and their nests, and the discovery that Rhipicephalus sanguineus Latr. is the vector of tropical Typhus in Kenya. Epidemiol Infect. 1935;35:1–22. https://doi.org/10.1017/S0022172400018933.
Rothen J, Githaka N, Kanduma EG, Olds C, Pflüger V, Mwaura S, et al. Matrix-assisted laser desorption/ionization time of flight mass spectrometry for comprehensive indexing of East African Ixodid tick species. Parasit Vectors. 2016;9:151. https://doi.org/10.1186/s13071-016-1424-6.
Rutagwenda T. A study of important camel diseases in Northern Kenya with special emphasis on their control. Camel Newsletter. 1984;1:12–6.
Sang R, Lutomiah J, Koka H, Makio A, Chepkorir E, Ochieng C, et al. Crimean-Congo hemorrhagic fever virus in Hyalommid ticks, Northeastern Kenya. Emerg Infect Dis. 2011;17:1502–5. https://doi.org/10.3201/eid1708.102064.
Sang R, Onyango C, Gachoya J, Mabinda E, Konongoi S, Ofula V, et al. Tickborne arbovirus surveillance in market livestock, Nairobi, Kenya. Emerg Infect Dis. 2006;12:1074–80. https://doi.org/10.3201/eid1207.060253.
Solberg IM, Aloo IA. Viral isolates from Ixodid ticks of wild animals in Kenya. Wildl Dis. 1976;1976:413–21. https://doi.org/10.1007/978-1-4757-1656-6_45.
Tatchell RJ, Chimwani D, Chirchir SJ, Ong’are JO, Mwangi E, Rinkanya F, et al. A study of the justification for intensive tick control in Kenyan rangelands. Vet Rec. 1986;119:401–3. https://doi.org/10.1136/vr.119.16.401.
Titcomb G, Allan BF, Ainsworth T, Henson L, Hedlund T, Pringle RM, et al. Interacting effects of wildlife loss and climate on ticks and tick-borne disease. P Biol Sci. 1862;2017:20170475. https://doi.org/10.1098/rspb.2017.0475.
Tomlinson JA, Horak IG, Apanaskevich DA. Identity of Haemaphysalis (Rhipistoma) muhsamae Santos Dias, 1954 (Acari: Ixodidae) and H. (R) subterra Hoogstraal, El Kammah & Camicas, 1992, parasites of carnivores and rodents in Eastern and Southern Africa. Syst Parasitol. 2018;95:673–91. https://doi.org/10.1007/s11230-018-9809-x.
Walker AR, Young AS, Leitch BL. Assessment of Theileria infections in Rhipicephalus appendiculatus ticks collected from the field. Z Parasitenkd. 1981;65:63–9. https://doi.org/10.1007/BF00926554.
Walton GA. Relapsing fever in the Digo district of Kenya colony. East Afr Med J. 1955;32:377.
Walton GA. Relapsing fever in the Meru district of Kenya. East Afr Med J. 1950;27:94–8.
Wanjohi JM, Ngeranwa JN, Rumberia RM, Muraguri GR, Mbogo SK. Immunization of cattle against East coast fever using Theileria parva (Marikebuni) and relaxation of tick control in North Rift, Kenya. Onderstepoort J Vet Res. 2001;68:217–23.
Wanzala W, Okanga S. Ticks (Acari: Ixodidae) associated with wildlife and vegetation of Haller park along the Kenyan coastline. J Med Entomol. 2006;43:789–94. https://doi.org/10.1603/0022-2585(2006)43[789:taiaww]2.0.co;2.
Wanzala W, Hassanali A, Mukabana WR, Takken W. Essential oils of indigenous plants protect livestock from infestations of Rhipicephalus appendiculatus and other tick species in herds grazing in natural pastures in Western Kenya. J Pest Sci. 2018;91:395–404. https://doi.org/10.1007/s10340-017-0853-0.
Wesonga FD, Kitala PM, Gathuma JM, Njenga MK, & Ngumi PN. An assessment of tick-borne diseases constraints to livestock production in a smallholder livestock production system in Machakos district, Kenya. Livest Res Rural Dev. 2010; 22.
Wesonga FD, Orinda GO, Ngae GN, Grootenhuis J. Comparative tick counts on game, cattle and sheep on a working game ranch in Kenya. Trop Anim Hlth Prod. 2006;38:35–42.
Wesonga FD, Wesongah JO, Chemuliti J, Wanjala K, Munga L, Gitau P. Seroprevalence of Ehrlichia ruminantium (heartwater) in small ruminants in a pastoral production system in Narok district, Kenya. Bull Anim Hlth Prod Afr. 2006;54:23–33. https://doi.org/10.4314/bahpa.v54i1.32727.
Wilson AJ, Schwartz HJ, Dolan R, Field CR, Rottcher D. Epidemiological aspects of important diseases of camels in selected areas of Kenya. Der Praktische tierarzt. 1982;63:974–85.
Young AS. The incidence of theilerial parasites in East African buffalo (Syncerus coffer). Trop Med Parasitol. 1978;29:281–8.
Young AS, de Castro JJ, Burns C, Murphy DL. Potential of ear tags impregnated with acaricides for control of the brown ear tick (Rhipicephalus appendiculatus) infesting cattle. Parasitol. 1985;90:391–9. https://doi.org/10.1017/S0031182000051088.
Young AS, Leitch BL, Dolan TT, Mbogo SK, Ndungu SG, Grootenhuis JG, et al. Evaluation of infection and treatment methods in immunization of improved cattle against theileriosis in an endemic area of Kenya. Vet Parasitol. 1990;35:239–57. https://doi.org/10.1016/0304-4017(90)90059-K.
Young AS, Leitch BL, Newson RM, Cunningham MP. Maintenance of Theileria parva parva infection in an endemic area of Kenya. Parasitol. 1986;93:9–16. https://doi.org/10.1017/s0031182000049787.
Young AS, Mutugi JJ, Kariuki DP, Lampard D, Maritim AC, Ngumi PN, et al. Immunisation of cattle against theileriosis in Nakuru district of Kenya by infection and treatment and the introduction of unconventional tick control. Vet Parasitol. 1992;42:225–40. https://doi.org/10.1016/0304-4017(92)90064-G.
Braae UC, Ngowi HA, Johansen MV. Smallholder pig production: prevalence and risk factors of ectoparasites. Vet Parasitol. 2013;196:241–4. https://doi.org/10.1016/j.vetpar.2012.12.058.
Culter SJ. Relapsing fever borreliae—clinics in laboratory medicine. 2015.
Cutler SJ, Browning P, Scott JC. Ornithodoros moubata, a soft tick vector for rickettsia in East Africa? Ann Ny Acad Sci. 2006;1078:373–7. https://doi.org/10.1196/annals.1374.074.
Easton ER, Tatchell RJ. Field studies involving ticks of cattle and wild animals in the Sukumaland area of Tanzania, 1973–1976. Proc Int Conf Tick Res Unit. 1981; 181–185.
Fukunaga M, Ushijima Y, Aoki Y, Talbert A. Detection of Borrelia duttonii, a tick-borne relapsing fever agent in Central Tanzania, within ticks by flagellin gene-based nested polymerase chain reaction. Vector-Borne Zoonot Dis. 2001;1:331–8. https://doi.org/10.1089/15303660160025949.
Fyumagwa RD, Hoare R, Simmler P, Meli ML, Hofmann-Lehmann R, Lutz H. Molecular detection of Anaplasma, Babesia and Theileria species in a diversity of tick species from Ngorongoro Crater, Tanzania. S Afr J Wildl Res. 2011;41:79–86. https://doi.org/10.10520/EJC117360.
Fyumagwa RD, Runyoro V, Horak IG, Hoare R. Ecology and control of ticks as disease vectors in wildlife of the Ngorongoro Crater, Tanzania. S Afr J Wildl Res. 2007;37:79–90. https://doi.org/10.3957/0379-4369-37.1.79.
Fyumagwa RD, Simmler P, Meli ML, Hoare R, Hofmann-Lehmann R, Lutz H. Prevalence of Anaplasma marginale in different tick species from Ngorongoro Crater, Tanzania. Vet Parasitol. 2009;162:154–7. https://doi.org/10.1016/j.vetpar.2008.12.018.
Geigy E, Mooser H. Studies on the epidemiology of African relapsing fever in Tanganyika. J Trop Med Hyg. 1955;58:199–201.
Hoogstraal H, Kaiser MN, Easton ER. Ornithodoros (Alectorobius) capensis Neumann (Ixodoidea: Argasidae) parasitizing a human and birds nesting on islands in East African lakes. J Med Entomol. 1976;12:703–4. https://doi.org/10.1093/jmedent/12.6.703.
Keirans J, Clifford CM, Hoogstraal H, Easton ER. Discovery of Nuttalliella namaqua Bedford (Acarina: Ixodoidea: Nuttalliellidae) in Tanzania and redescription of the female based on scanning electron microcopy. Ann Entomol Soc Am. 1976;69:926–32.
Kerario II, Muleya W, Chenyambuga S, Koski M, Hwang SG, Simuunza M. Abundance and distribution of Ixodid tick species infesting cattle reared under traditional farming systems in Tanzania. Afr J Agric Res. 2017;12:286–99.
Kimbita EN. First Report of Rhipicephalus appendiculatus, Echidnophaga gallinacea and Ctenocephalides felis on African pygmy hedgehogs (Atelerix albiventris) captured in Morogoro, Tanzania. Res Opinions in Anim Vet Sci. 2015;5:329–34.
Kusiluka LJM, Kambarage DM, Matthewman RW, Daborn CJ, Harrison LJS. Prevalence of ectoparasites of goats in Tanzania. J Appl Anim Res. 1995;7:69–74. https://doi.org/10.1080/09712119.1995.9706052.
Kwak YS, Kim TY, Nam SH, Lee IY, Kim HP, Mduma S, et al. Ixodid tick infestation in cattle and wild animals in Maswa and Iringa, Tanzania. Korean J Parasitol. 2014;52:565–8. https://doi.org/10.3347/kjp.2014.52.5.565.
Laisser EL, Chenyambuga SW, Karimuribo ED, Msalya G, Kipanyula MJ, Mwilawa AJ, et al. Tick burden and acquisition of immunity to Theileria parva by Tarime cattle in comparison to Sukuma cattle under different tick control regimes in the lake zone of Tanzania. J Vet Med Anim Hlth. 2016;8:21–8.
Laisser EL, Kipanyula MJ, Msalya G, Mdegela RH, Karimuribo ED, Mwilawa AJ, et al. Tick burden and prevalence of Theileria parva infection in Tarime Zebu cattle in the lake zone of Tanzania. Trop Anim Hlth Prod. 2014;46:1391–6. https://doi.org/10.1007/s11250-014-0651-0.
Lee S, Kim JY, Yi M, Lee IY, Fyumagwa R, Yong T. Comparative microbiomes of ticks collected from a black rhino and its surrounding environment. Inte J Parasitol Parasite Wildl. 2019;9:322–401. https://doi.org/10.1016/j.ijppaw.2019.05.008.
Lynen G, Zeman P, Bakuname C, Di Giulio G, Mtui P, Sanka P, et al. Cattle ticks of the genera Rhipicephalus and Amblyomma of economic importance in Tanzania: distribution assessed with GIS based on an extensive field survey. Exp Appl Acarol. 2007;43:303–19. https://doi.org/10.1007/s10493-007-9123-9.
Mamiro KA, Magwisha HB, Rukambile EJ, Ruheta MR, Kimboka EJ, Malulu DJ, et al. Occurrence of ticks in cattle in the new pastoral farming areas in Rufiji district, Tanzania. J Vet Med. 2016;2016:1–5. https://doi.org/10.1155/2016/3420245.
Mchinja SJ, Sirima EP. Field trial on the effects of the herbicide MCPA on Ixodid ticks. Trop Pest Manag. 1983;29:196–7. https://doi.org/10.1080/09670878309370800.
Msami HM. An epidemic of East coast fever on a dairy farm in Eastern Tanzania. Prev Vet Med. 2001;49:55–60. https://doi.org/10.1016/S0167-5877(01)00173-8.
Nagagi Y, Kimaro E, Temba V. Practical application and the possible emergence of tick resistance to commonly used acaricides in various districts of Tanzania. Livest Res Rural Dev. 2020;32:127.
Newson RM, Mella PNP, Franklin TE. Observations on the numbers of the tick Rhipicephalus appendiculatus on the ears of Zebu cattle in relation to hierarchical status in the herd. Trop Anim Hlth Prod. 1973;5:281–3. https://doi.org/10.1007/BF02240430.
Ogden NH, Gwakisa P, Swai E, French NP, Fitzpatrick J, Kambarage D, et al. Evaluation of PCR to detect Theileria parva in field-collected tick and bovine samples in Tanzania. Vet Parasitol. 2003;112:177–83. https://doi.org/10.1016/S0304-4017(02)00448-X.
Ogden NH, Swai E, Beauchamp G, Karimuribo E, Fitzpatrick JL, Bryant MJ, et al. Risk factors for tick attachment to smallholder dairy cattle in Tanzania. Prev Vet Med. 2005;67:157–70. https://doi.org/10.1016/j.prevetmed.2004.10.011.
Parker J, Plowright W, Pierce MA. The epizootiology of African swine fever in Africa. Vet Rec. 1969;85:668–74.
Phipps J. Ornithodoros moubata Murray in Tanganyika. East Afr Med J. 1950;27:475–82.
Scott JC, Wright DJM, Cutler SJ. Typing African relapsing fever spirochetes. Emerg Infect Dis. 2005;11:1722–9. https://doi.org/10.3201/eid1111.050483.
Senzota RBM. Rodent ectoparasites in the Serengeti National park, Tanzania. Trop Ecol. 1992;33:29–33.
Swai ES, Karimuribo ED, Kyakaisho P. Further evidence of occurrence of Argas persicus Oken 1881 in free-range village chickens in Tanzania. Livest Res Rural Dev. 2007;19:6.
Swai ES, Karimuribo ED, Rugaimukamu EA, Kambarage DM. Factors influencing the distribution of questing ticks and the prevalence estimation of T. parva infection in brown ear ticks in the Tanga region, Tanzania. J Vector Ecol. 2006;31:224–8. https://doi.org/10.3376/1081-1710(2006)31[224:FITDOQ]2.0.CO;2.
Tatchell RJ, Easton E. Tick (Acari: Ixodidae) ecological studies in Tanzania. Bull Entomol Res. 1986;76:229–46. https://doi.org/10.1017/S0007485300014711.
Walker JB. Rhipicephalus pulchellus Gerstäcker 1873: a description of the larva and nymph with notes on the adults and on its biology. Parasitol. 1955;45:95–8. https://doi.org/10.1017/S0031182000027463.
Walker JB. Rhipicephalus reichenowi Zumpt, 1943: a re-description of the male and female and descriptions of the nymph and larva, together with an account of its known hosts and distribution. Parasitol. 1966;56:457–69. https://doi.org/10.1017/S0031182000068943.
Walker JB, Wiley AJ. Rhipicephalus camelopardalis n. sp. (Ixodoidea, Ixodidae), a new species of tick from East African giraffes. Parasitol. 1959;49:448–53. https://doi.org/10.1017/S0031182000026974.
Walton GA. Ornithodorus moubata in wart-hog and porcupine burrows in Tanganyika territory. T Roy Soc Trop Med Hyg. 1953;47:410–1.
Wambura PN, Gwakisa PS, Silayo RS, Rugaimukamu EA. Breed-associated resistance to tick infestation in Bos indicus and their crosses with Bos taurus. Vet Parasitol. 1998;77:63–70. https://doi.org/10.1016/S0304-4017(97)00229-X.
Warwick BT, Bak E, Baldassarre J, Gregg E, Martinez R, Kioko J, et al. Abundance estimations of Ixodid ticks on Boran cattle and Somali sheep in Northern Tanzania. Int J Acarol. 2016;42:12–7. https://doi.org/10.1080/01647954.2015.1109708.
Yeoman, GH, Walker JB, Ross JPJ, Docker TM. The Ixodid ticks of Tanzania. A study of the zoogeography of the Ixodidae of an East African country. Commonwealth Inst Entomol. 1967: 215.
Balinandi S, Chitimia-Dobler L, Grandi G, Nakayiki T, Kabasa W, Bbira J, et al. Morphological and molecular identification of Ixodid tick species (Acari: Ixodidae) infesting cattle in Uganda. Parasitol Res. 2020;119:2411–20. https://doi.org/10.1007/s00436-020-06742-z.
Balinandi S, Mugisha L, Bbira J, Kabasa W, Nakayiki T, Bakkes DK, et al. General and local morphological anomalies in Amblyomma lepidum (Acari: Ixodidae) and Rhipicephalus decoloratus infesting cattle in Uganda. J Med Entomol. 2019;56:873–7. https://doi.org/10.1093/jme/tjy221.
Block W. Ticks from waterbuck and warthog in the Queen Elizabeth national park. Uganda East Afr J Ecol. 1968;6:140–1.
Byaruhanga C, Collins NE, Knobel D, Kabasa W, Oosthuizen MC. Endemic status of tick-borne infections and tick species diversity among Transhumant Zebu cattle in Karamoja region, Uganda: support for control approaches. Vet Parasitol. 2015;1–2:21–30. https://doi.org/10.1016/j.vprsr.2015.11.001.
Heisch RB. First record of Ornithodoros erraticus (Lucas) from Uganda, with some speculations on the origin of Spirochaeta duttoni Novy and Knapp. East Afr Med J. 1952;29:477–9.
Kaiser MN, Sutherst RW, Bourne AS. Relationship between ticks and Zebu cattle in Southern Uganda. Trop Anim Hlth Prod. 1982;14:63–74. https://doi.org/10.1007/bf02282583.
Kasaija PD, Contreras M, Kabi F, Mugerwa S, de la Fuente J. Vaccination with recombinant subolesin antigens provides cross-tick species protection in Bos indicus and crossbred cattle in Uganda. Vaccine. 2020;8:319. https://doi.org/10.3390/vaccines8020319.
Kitaka FX, Oteng AK, Kamya EP. Toxaphene-resistant ticks occurring on cattle in Uganda: Boophilus decoloratus, Rhipicephalus evertsi and Rhipicephalus appendiculatus. Bull Epizoot Dis Afr. 1970;18:137–42.
Magona JW, Walubengo J, Kabi F. Response of Nkedi Zebu and Ankole cattle to tick infestation and natural tick-borne, helminth and trypanosome infections in Uganda. Trop Anim Hlth Prod. 2011;43:1019–33. https://doi.org/10.1007/s11250-011-9801-9.
Matthysse JG, Colbo MH, Kamya EP. Acaricide trials against Rhipicephalus appendiculatus Neum., Amblyomma variegatum (F.) and Boophilus decoloratus (Koch) (Ixodidae) on cattle in Uganda. Bull Entomol Res. 1969;58:465–86. https://doi.org/10.1017/S0007485300057229.
Miyama T, Byaruhanga J, Okamura I, Uchida L, Muramatsu Y, Mwebembezi W, et al. Effect of chemical tick control practices on tick infestation and Theileria parva infection in an intensive dairy production region of Uganda. Ticks Tick-Borne Dis. 2020;11:101438. https://doi.org/10.1016/j.ttbdis.2020.101438.
Muhanguzi D, Byaruhanga J, Amanyire W, Ndekezi C, Ochwo S, Nkamwesiga J, et al. Invasive cattle ticks in East Africa: morphological and molecular confirmation of the presence of Rhipicephalus microplus in South-Eastern Uganda. Parasit Vectors. 2020;13:165. https://doi.org/10.1186/s13071-020-04043-z.
Nakao R, Qiu Y, Igarashi M, Magona JW, Zhou L, Ito K, et al. High prevalence of spotted fever group rickettsiae in Amblyomma variegatum from Uganda and their identification using sizes of intergenic spacers. Ticks Tick-Borne Dis. 2013;4:506–12. https://doi.org/10.1016/j.ttbdis.2013.07.001.
Nakao R, Stromdahl EY, Magona JW, Faburay B, Namangala B, Malele I, et al. Development of loop-mediated isothermal amplification (LAMP) Assays for rapid detection of Ehrlichia ruminantium. BMC Microbiol. 2010;10:296. https://doi.org/10.1186/1471-2180-10-296.
Ndekezi C, Nkamwesiga J, Ochwo S, Kimuda MP, Mwiine FN, Tweyongyere R, et al. Identification of Ixodid tick-specific aquaporin-1 potential anti-tick vaccine epitopes: an in-silico analysis. Front Bioeng Biotechnol. 2019;7:236. https://doi.org/10.3389/fbioe.2019.00236.
Obara I, Githaka N, Nijhof A, Krücken J, Nanteza A, Odongo D, et al. The Rhipicephalus appendiculatus tick vector of Theileria parva is absent from cape buffalo (Syncerus Caffer) populations and associated ecosystems in Northern Uganda. Parasitol Res. 2020;119:2363–7. https://doi.org/10.1007/s00436-020-06728-x.
Okello-Onen J, Tukahirwa EM, Perry BD, Rowlands GJ, Nagda SM, Musisi G, et al. Population dynamics of ticks on indigenous cattle in a pastoral dry to semi-arid rangeland zone of Uganda. Exp Appl Acarol. 1999;23:79–88. https://doi.org/10.1023/A:1006058317111.
Okello-Onen J, Tukahirwa EM, Perry BD, Rowlands GJ, Nagda SN, Musisi G, et al. The impact of tick control on the productivity of indigenous cattle under ranch conditions in Uganda. Trop Anim Hlth Prod. 2003;35:237–47. https://doi.org/10.1023/A:1023395413568.
Proboste T, Kalema-Zikusoka G, Altet L, Solano-Gallego L, Fernández de Mera IG, Chirife AD, et al. Infection and exposure to vector-borne pathogens in rural dogs and their ticks, Uganda. Parasit Vectors. 2015;8:306. https://doi.org/10.1186/s13071-015-0919-x.
Randolph SE, Rogers DJ. A generic population model for the African tick Rhipicephalus appendiculatus. Parasitol. 1997;115:265–79. https://doi.org/10.1017/S0031182097001315.
Rubaire-Akiiki CM, Okello-Onen J, Musunga D, Kabagambe EK, Vaarst M, Okello D, et al. Effect of agro-ecological zone and grazing system on incidence of East coast fever in calves in Mbale and Sironko districts of Eastern Uganda. Prev Vet Med. 2006;75:251–66. https://doi.org/10.1016/j.prevetmed.2006.04.015.
Schuh AJ, Amman BR, Apanaskevich DA, Sealy TK, Nichol ST, Towner JS. No evidence for the involvement of the Argasid tick Ornithodoros faini in the enzootic maintenance of Marburgvirus within Egyptian rousette bats Rousettus aegyptiacus. Parasit Vectors. 2016;9:128. https://doi.org/10.1186/s13071-016-1390-z.
Steyn JJ. A second locality record in Uganda for the tick parasite, Hunterellus hookeri Howard, and a few discussions on other tick parasites. East Afr Med J. 1955;32:357.
Tayebwa DS, Vudriko P, Tuvshintulga B, Guswanto A, Nugraha AB, Gantuya S, et al. Molecular epidemiology of Babesia species, Theileria parva, and Anaplasma marginale infecting cattle and the tick control malpractices in Central and Eastern Uganda. Ticks Tick-Borne Dis. 2018;9:1475–83. https://doi.org/10.1016/j.ttbdis.2018.06.012.
Vudriko P, Okwee-Acai J, Byaruhanga J, Tayebwa DS, Okech SG, Tweyongyere R, et al. Chemical tick control practices in Southwestern and Northwestern Uganda. Ticks Tick-Borne Dis. 2018;9:945–55. https://doi.org/10.1016/j.ttbdis.2018.03.009.
Vudriko P, Okwee-Acai J, Tayebwa DS, Byaruhanga J, Kakooza S, Wampande E, et al. Emergence of multi-acaricide resistant Rhipicephalus ticks and its implication on chemical tick control in Uganda. Parasit Vectors. 2016;9:4. https://doi.org/10.1186/s13071-015-1278-3.
Vudriko P, Umemiya-Shirafuji R, Okwee-Acai J, Tayebwa DS, Byaruhanga J, Jirapattharasate C, et al. Genetic mutations in sodium channel domain II and carboxylesterase genes associated with phenotypic resistance against synthetic pyrethroids by Rhipicephalus (Boophilus) decoloratus ticks in Uganda. Pestic Biochem Physiol. 2017;143:181–90. https://doi.org/10.1016/j.pestbp.2017.07.009.
Walker JB, Keirans JE, Pegram RG. Rhipicephalus aquatilis sp. nov. (Acari: Ixodidae), a new tick species parasitic mainly on the sitatunga, Tragelaphus spekei, in East and Central Africa. Onderstepoort J Vet Res. 1993;60:205–10.
Latif AA, Putterill JF, de Klerk DG, Pienaar R, Mans BJ. Nuttalliella amaqua (Ixodoidea: Nuttalliellidae): first description of the male, immature stages and re-description of the female. PLoS One. 2012;7:e41651.
Rodrigues R, Telles JN, Essere K, Ducournau C, Roqueplo C, Levieuge A, et al. Development of a one step real time RT-PCR assay to detect and quantify Dugbe virus. J Virol Methods. 2011;176:74–7.
Snellgrove AN, Krapiunaya I, Ford SL, Stanley HM, Wickson AG, Hartzer KL, et al. Vector competence of Rhipicephalus sanguineus sensu stricto for Anaplasma platys. Ticks Tick-Borne Dis. 2020;11:101517.
Alzahrani AG, Al Shaiban HM, Al Mazroa MA, Al-Hayani O, Macneil A, Rollin PE, et al. Alkhurma hemorrhagic fever in humans, Najran, Saudi Arabia. Emerg Infect Dis. 2010;16:1882.
Khumalo ZT, Catanese HN, Liesching N, Hove P, Collins NE, Chaisi ME, et al. Characterization of Anaplasma marginale subsp. centrale strains by use of Msp1aS genotyping reveals a wildlife reservoir. J Clin Microbiol. 2016;54:2503–12.
Bauer BU, Răileanu C, Tauchmann O, Fischer S, Ambros C, Silaghi C, et al. Anaplasma phagocytophilum and Anaplasma ovis–emerging pathogens in the German sheep population. Pathogens. 2021;10:1298.
Severo MS, Stephens KD, Kotsyfakis M, Pedra JHF. Anaplasma phagocytophilum: deceptively simple or simply deceptive? Future Microbiol. 2012;7:719–31.
Ouchene N, Nebbak A, Ouchene-Khelifi NA, Dahmani A, Zeroual F, Khelef D, et al. Molecular detection of avian spirochete Borrelia anserina in Argas persicus ticks in Algeria. Comp Immunol Microbiol Infect Dis. 2020;68:101408.
McCoy BN, Maïga O, Schwan TG. Detection of Borrelia theileri in Rhipicephalus geigyi from Mali. Ticks Tick-Borne Dis. 2014;5:401–3.
Blanda V, D’Agostino R, Giudice E, Randazzo K, La Russa F, Villari S, et al. New real-time PCRs to differentiate Rickettsia spp. and Rickettsia conorii. Molecules. 2020;25:4431.
Sarih M, Socolovschi C, Boudebouch N, Hassar M, Raoult D, Parola P. Spotted fever group rickettsiae in ticks. Morocco Emerg Infect Dis. 2008;14:1067–73.
Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129:S3-14.
Magaia V, Taviani E, Cangi N, Neves L. Molecular detection of Rickettsia africae in Amblyomma ticks collected in cattle from Southern and Central Mozambique. J Infect Dev Ctries. 2020;14:614–22.
Makenov MT, Toure AH, Bayandin RB, Gladysheva AV, Shipovalov AV, Boumbaly S, et al. Ngari virus (Orthobunyavirus, Peribunyaviridae) in Ixodid ticks collected from cattle in Guinea. Acta Trop. 2021;214:105790.
Fillâtre P, Revest M, Tattevin P. Crimean-Congo hemorrhagic fever: an update. Med Mal Infect. 2019;49:574–85.
Lledó L, Giménez-Pardo C, Gegúndez MI. Epidemiological study of Thogoto and Dhori virus infection in people bitten by ticks, and in sheep, in an area of Northern Spain. Int J Env Res Pu. 2020;17:2254.
Ogata S, Pereira JAC, Jhonny LVA, Carolina HPG, Matsuno K, Orba Y, Sawa H, Kawamori F, Nonaka N, Nakao R. Molecular survey of Babesia and Anaplasma infection in cattle in Bolivia. Vet Sci. 2021;8:188.
Puri A, Bajpai S, Meredith S, Aravind L, Krause PJ, Kumar S. Babesia microti: pathogen genomics, genetic variability, immunodominant antigens, and pathogenesis. Front Microbiol. 2021;12:697669.
Hubálek Z. Biogeography of tick-borne Bhanja virus (Bunyaviridae) in Europe. Interdiscip Perspect Infect Dis. 2009;2009:372691.
Boyd AS. Rickettsial pox. Dermatol Clin. 1997;15:313–8.
Krasteva S, Jara M, Frias-De-Diego A, Machado G. Nairobi sheep disease virus: a historical and epidemiological perspective. Front Vet Sci. 2020;7:419.
Rikihisa Y. The tribe ehrlichieae and ehrlichial diseases. Clin Microbiol. 1991;4:286–308.
Peng S, Yang S, Ho Y, Chen H, Shu P. Human case of Ehrlichia chaffeensis infection. Taiwan Emerg Infect Dis. 2019;25:2141–3.
Lescot M, Audic S, Robert C, Nguyen TT, Blanc G, Cutler SJ, et al. The genome of Borrelia recurrentis, the agent of deadly louse-borne relapsing fever, is a degraded subset of tick-borne Borrelia duttonii. PloS Genet. 2008;4:e1000185.
Peter SG, Aboge GO, Kariuki HW, Kanduma EG, Gakuya DW, Maingi N, et al. Molecular prevalence of emerging Anaplasma and Ehrlichia pathogens in apparently healthy dairy cattle in peri-urban Nairobi, Kenya. BMC Vet Res. 2020;16:1–12.
McCall PJ, Hume JC, Motshegwa K, Pignatelli P, Talbert A, Kisinza W. Does tick-borne relapsing fever have an animal reservoir in East Africa? Vector-Borne Zoonot. 2007;7:659–66.
Kirkbride M. Survival of the fittest: pastoralism and climate change in East Africa. Oxfam Int. 2008;116:1–47.
Acknowledgements
The authors thank David B. Pecor and Alex M. Potter for teaching and guiding students throughout the data collection process. The authors also thank Yvonne-Marie Linton and Michael E. von Fricken for their technical assistance in overlooking data and supporting the overall manuscript development and finalization.
Author’s note
The material in this manuscript has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting true views of the Department of the Army, the Department of Defense, or the U.S. Government. Title 17 U.S.C. 105 provides that “copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a U.S. Government work as work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
Funding
This work was funded by the Armed Forces Health Surveillance Division (AFHSD), Global Emerging Infections Surveillance (GEIS) Branch (ProMIS ID # P0031_21_WR to YML).
Author information
Authors and Affiliations
Contributions
AAL: data collection, data mining, writing—original draft. DBP: formal analysis, writing—review and editing, supervision, project administration. GM: data collection, data mining, data visualization, writing—original draft. AMP: formal analysis, writing—review and editing, supervision, project administration. RW, MFK, SM, FJ, AK, BK: data collection and data mining. DZ, JMH, MK: writing—review and editing, project administration. YML and MEV: conceptualization, formal analysis, funding acquisition, writing—review and editing. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
PRISMA checklist.
Additional file 2: Dataset S1.
Excel sheet of all articles and data.
Additional file 3: Table S1.
References listed by country
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lilak, A.A., Pecor, D.B., Matulis, G. et al. Data release: targeted systematic literature search for tick and tick-borne pathogen distributions in six countries in sub-Saharan Africa from 1901 to 2020. Parasites Vectors 17, 84 (2024). https://doi.org/10.1186/s13071-023-06086-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-06086-4