Abstract
Background
A significant decrease in malaria morbidity and mortality has been attained using long-lasting insecticide-treated nets and indoor residual spraying. Selective pressure from these control methods influences changes in vector bionomics and behavioural pattern. There is a need to understand how insecticide resistance drives behavioural changes within vector species. This study aimed to determine the spatio-temporal dynamics and biting behaviour of malaria vectors in different ecological zones in Ghana in an era of high insecticide use for public health vector control.
Methods
Adult mosquitoes were collected during the dry and rainy seasons in 2017 and 2018 from five study sites in Ghana in different ecological zones. Indoor- and outdoor-biting mosquitoes were collected per hour from 18:00 to 06:00 h employing the human landing catch (HLC) technique. Morphological and molecular species identifications of vectors were done using identification keys and PCR respectively. Genotyping of insecticide-resistant markers was done using the TaqMan SNP genotyping probe-based assays. Detection of Plasmodium falciparum sporozoites was determined using PCR.
Results
A total of 50,322 mosquitoes belonging to four different genera were collected from all the study sites during the sampling seasons in 2017 and 2018. Among the Anophelines were Anopheles gambiae s.l. 93.2%, (31,055/33,334), An. funestus 2.1%, (690/33,334), An. pharoensis 4.6%, (1545/33,334), and An. rufipes 0.1% (44/33,334). Overall, 76.4%, (25,468/33,334) of Anopheles mosquitoes were collected in the rainy season and 23.6%, (7866/33,334) in the dry season. There was a significant difference (Z = 2.410; P = 0.0160) between indoor-biting (51.1%; 15,866/31,055) and outdoor-biting An. gambiae s.l. (48.9%; 15,189/31,055). The frequency of the Vgsc-1014F mutation was slightly higher in indoor-biting mosquitoes (54.9%) than outdoors (45.1%). Overall, 44 pools of samples were positive for P. falciparum CSP giving an overall sporozoite rate of 0.1%.
Conclusion
Anopheles gambiae s.l. were more abundant indoors across all ecological zones of Ghana. The frequency of G119S was higher indoors than outdoors from all the study sites, but with higher sporozoite rates in outdoor mosquitoes in Dodowa and Kpalsogu. There is, therefore, an urgent need for a supplementary malaria control intervention to control outdoor-biting mosquitoes.
Graphical Abstract

Similar content being viewed by others
Background
The malaria burden in Africa is generally attributed to the relatively effective vector system that is made up of Anopheles gambiae s.l. and An. funestus [1, 2]. The transmission potential of these vectors varies between climatic seasons, ecological zones, and sometimes among areas in close proximity [3, 4]. In Ghana, studies have shown that An. gambiae s.l. and An. funestus are the predominant malaria vectors and they occur in sympatry over much of their range [5, 6]. Information on the various malaria vector species and their distribution under diverse ecological conditions are essential in their control strategies [7, 8].
There are three main ecological zones in Ghana: the coastal savannah zone in the south, the forest zone in the middle part, and the Sahel savannah zone in the northern part of Ghana. There are also transition areas between these zones. The coastal and forest zones have a bimodal rainfall pattern which allows for two peaks of malaria transmission, while the Sahel savannah zone has unimodal rainfall pattern which makes malaria transmission seasonal. The different climatic conditions experienced in the various ecological areas contribute to differences in malaria vector species composition, hence malaria transmission [9, 10].
Vector control is key in the malaria control strategy [11], and like many other African countries, long-lasting insecticide-treated nets (LLINs) and indoor residual spraying (IRS) are used in Ghana [12]. These methods, which target only indoor-biting mosquitoes, have achieved remarkable successes towards malaria elimination but the progress of these achievements has plateaued [12] because of the development and fast spread of insecticide resistance. The success of IRS and LLINs is largely based on the anthropophilic, endophagic, and endophilic behaviours of Anopheles vectors; however, there is a growing threat of both physiological and behavioural resistance to the insecticides used in these vector control strategies [13, 14]. The complexity of controlling malaria is attributed to changes in species and the behavioural pattern of the malaria vectors [13, 15,16,17,18] and these variations in mosquito bionomics are attributed to the possible influence of IRS and LLINs and the development of insecticide resistance [19, 20]. The development of resistance by these vectors may maintain transmission where control interventions have been successful [21]. The wide and prolonged use of LLINs and IRS causes certain behavioural changes in Anopheles vectors that help them circumvent or eschew insecticide-treated areas [13, 22]. The most principal of such adaptations includes a change in feeding behaviour.
Anopheles mosquitoes tend to change from their historically late night indoor-biting to early night and outdoor-biting times [13]. These mosquitoes avoid IRS and LLINs control by feeding and resting outdoors. They also feed in the early hours of the evening when people are outside and not in bed and/or early in the morning when people are out of their bed nets [13, 14, 23]. The local dynamics of insecticide resistance may be impacted by the spatio-temporal variation in insect vectors [24] albeit selection pressure may have resulted in the variations in mechanism of insecticide resistance in malaria vectors [24]. Studies have shown strong association between observed frequency of knock-down resistance (kdr) mutations and acetylcholine esterase (Ace-1) and resistance to pyrethroids and DDT in field mosquito populations [25, 26]; therefore, the presence of either kdr or Ace-1 gene in a field population of mosquitoes is a reliable indicator of both resistance prevalence and high individual resistance [27].
The variations in species distribution in different ecological zones could be influenced by some landscape barriers to gene flow and exposure of the vector population to different levels of insecticide pressures [28]. Mutations caused by these factors at the neurons might be having a pleiotropic effect on the mosquito behaviour. Therefore, this study was to determine the biting behaviour, spatio-temporal dynamics, and insecticide resistance status of malaria vectors in different ecological zones in Ghana in an era of high insecticide use for public health vector control. This information will provide a better understanding of how the ecology in Ghana affects vector seasonal dynamics and explain the interactions between increased insecticide resistance in the malaria vector population and the ensuing biting behaviour patterns.
Methods
Study sites
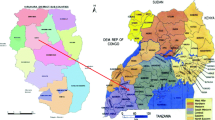
Adult mosquitoes were collected during the dry season (February–March) and the rainy season (May–July) in 2017 and 2018 from five study sites in Ghana. The study sites were selected from four ecological zones: Anyakpor (5° 46′ 51.96″ N 0° 35′ 12.84″ E) in the coastal savannah zone; Dodowa (5° 52′ 58.3212'' N 0° 5′ 52.9548″ W) in the coastal-forest transition zone; Dwease (6° 32′ 3.05 "N 1° 14′ 42.22″ W) in the forest zone; Pagaza (9° 22′ 33.34″ N 0° 42′ 29.67″ W) and Kpalsogu (9° 33′ 45.2″ N 1° 01′ 54.6″ W) both in the Sahel savannah zone. These sites are shown in Fig. 1.
Anyakpor is a rural coastal community in the Ada East District of Ghana. It has a dry equatorial climate with temperatures between 23°C – 33°C and a bimodal rainfall pattern with a long rainy season from April to June and a short rainy season from October to November. Farming activities occur all year round, supported by an irrigation scheme. This allows for uninterrupted farming activities throughout the year. There are dug-out wells and other water impoundments which collect water during the dry and rainy season, and these may serve as suitable breeding sites and eventually affect the densities of mosquitoes. A previous study by Hinne et al. [29], reported that the most dominant species present in this site was Anophelescoluzzii [29].
Dodowa is a town in the Shai Osudoku district with an average temperature of 27 ℃ and a bimodal rainfall pattern like Anyakpor. It has a secondary forest-type vegetation with little original virgin forest left because of deforestation. Dwease is also a rural community close to Dwease in the Asante-Akim Central municipality with a wet-semi equatorial climate characterized by bimodal rainfall just like Anyakpor. Dwease has a semi-deciduous forest vegetation of open and closed forests.
Kpalsogu and Pagaza are rural communities in the Kumbungu and Tamale municipalities respectively. They have a unimodal rainfall pattern from May to November and a long dry season from December to April. The mean annual temperature is around 28 °C but can get to a maximum of 42 °C. Kpalsogu is close to a dam linked to an irrigation scheme which allows uninterrupted farming activities throughout the year. There are other water impoundments which collect water during the rainy season for irrigation in the dry season. In the rainy season, these dams overflow, creating many swamps which are suitable breeding habitats for Anopheles mosquitoes. Water from the dam which is diverted through canals to farms also provides breeding sites for mosquitoes and may affect mosquito densities within the area. There is also an IRS campaign supported by the President’s Malaria Initiative (PMI) to prevent malaria ongoing within this community. Exposure of mosquitoes to sublethal doses of insecticide may facilitate resistance within the vector population. Moreover, this indoor vector control strategy may facilitate outdoor biting by the malaria vectors present there.
Mosquito collections
Indoor- and outdoor-biting mosquitoes were collected per hour from 18:00 to 06:00 h for four different nights in four houses per season by a two-person team of trained catchers in eight randomly selected houses employing the human landing catch (HLC) technique [30]. The study design ensured that randomly selected houses had household members sleeping under bednets or were covered under IRS for vector control. This was to ensure that there was no bias in selective factors such as IRS and presence of bednets. Collections were done in the five sentinel sites over four ecological zones of Ghana. Each study site was sectioned into four to ensure a fair representation of the mosquito population at the study site. Briefly, volunteers sat in the dark with their lower limbs exposed and, with the aid of a flashlight, located and collected the blood-seeking mosquitoes with a collection tube when they landed in search of a blood meal. Indoor and outdoor collectors were rotated hourly to avoid differences in individual attractiveness or repulsiveness to mosquitoes and as a precaution against dozing. Outdoor human biting catches were carried out at the same household 10 m away [31]. Independent staff supervised rotations and regularly walked between different groups for whole-night quality control of collectors placed inside and outside dwellings.
Hourly mosquitoes caught were killed by placing them in the − 20°C freezer for 15 min or chloroform for 1 min and then kept separately in individual tubes containing silica gel, pre-labelled with date, time, and location of capture, and taken to the laboratory for identification [32]. Mosquito biting pattern was classified as follows: early evening (EE) (18:00–22:00 h), late evening (LE) (22:00–4:00 h), and early morning (EM) (4:00–6:00 h).
Morphological and molecular identification of vector mosquitoes
Mosquitoes collected were identified morphologically using a simplified key adopted from Gillies and Coetzee [33]. A sub-sample from the total An. gambiae s.l. collected over the entire period was selected according to study site, season, and location (indoor or outdoor) in a proportion of 10%. This was used to further discriminate members of the An. gambiae complex by PCR and RFLP-PCR. The legs of each mosquito were used for DNA extraction as previously described by Scott et al. [34]. Four sets of primers (Anopheles gambiae, An. arabiensis, An. melas, and universal primer) were used in PCR for the identification of members of the An. gambiae s.l. species complex [34]. Anopheles gambiae s.s. and An. coluzzii were distinguished by PCR-RFLP using the method of Fanello et al. [35].
Genotyping for insecticide resistance mutations
Genomic DNA extracted from the legs of the indoor and outdoor mosquito samples were used to detect the presence of insecticide resistance mutations using a TaqMan SNP genotyping probe-based assay [36]. These markers include Vgsc-1014F and Vgsc-1014S. The same set of samples was also genotyped for Ace1-119S mutation [36].
Detection of sporozoite
The heads and thoraces of mosquito samples were used to detect the presence of Plasmodium falciparum sporozoite using polymerase chain reaction (PCR) as described by Echeverry et al. [37]. Twenty mosquitoes were pooled according to the site, species, and collection time for the detection of sporozoite; a total of 643 pools were constituted from 12,860 An. gambiae s.l. including those used for species identification. Pooling of mosquito samples was done for logistic reasons to minimize reagent consumption.
Data analysis
Descriptive analysis was performed to compare the abundance of malaria vectors in the different study sites (ecological zones), seasons, biting locations, and biting times. The chi-square and Fisher's exact tests were used to test the association between two categorical variables. The Mann-Whitney U and Kruskal-Wallis tests were used to test the associations between continuous and categorical variables. Generalized linear mixed model was used to model the effects of mosquito behaviour, season, and sampling period on Anopheles mosquito abundance. All statistical analyses were conducted in STATA version 15 software (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC). Alpha level was set at 0.05 and the proportions were estimated with confidence intervals in R (v 4.3.1). The sporozoite infection rate (IR) expressed as the proportion of mosquitoes positive for Plasmodium sporozoite was calculated according to the method previously described by Maia et al. [38]. The kdr L1014F and Ace 1 G119S mutation frequencies were calculated according to the following formula:
where RR is the number of homozygotes, RS is the number of heterozygotes, and n is the total number of specimens analysed.
Results
Abundance and seasonal distribution of malaria vectors
Overall, a total of 50,322 mosquitoes belonging to three different genera were collected from all the study sites during the two sampling seasons. In general, more mosquitoes were collected in 2017 (n = 26,415; 95% CI = 0.52–0.53) than in 2018 (n = 23,907, 95% CI = 0.47–0.48). The mosquitoes collected belonged to the Anopheline, Culicine, and Mansonia genera. Regarding the mosquito genera, only Culicine mosquitoes were more abundant in the 2018 sampling year (6614/23,907, 95% CI = 0.27–0.28) compared to 2017 (5856/26,415, 95% CI = 0.22–0.23). Among the Anophelines were An. gambiae s.l. (93.2%), An. pharoensis (4.6%), An. funestus (2.1%), and An. rufipes (0.1%) (Table 1).
Throughout the combined period of the study, abundance of An. gambiae s.l. varied significantly amongst the study sites (χ2 = 213.404; df = 4; P = 0.0001). The mean abundance of An. gambiae s.l. was highest in Pagaza (5.82, 95% CI = 5.43–6.24) and Dwease had the least (2.77, 95% CI = 2.65–2.90) (Additional file 1: Table S1). Overall, An. gambiae s.l. were more abundant in the rainy season (75.5%) than in the dry season (24.5%) during both sampling years (Z = − 36.037; P < 0.0001) (Additional file 1: Table S1). However, in Anyakpor and Dwease, more An. gambiae s.l. were collected in the dry season. Compared to the dry season, the mean abundance of An. gambiae s.l. was four-fold higher during the rainy season (B = 4.15, 95% CI = 3.916–4.381, P = 0.0001). Relatively fewer An. gambiae s.l. mosquitoes were collected in 2018 compared to the 2017 sampling year (B = − 0.871, 95% CI = − 1.103 to − 0.0639, P = 0.0001) (Additional file 1: Table S3).
During both sampling years, almost all An. funestus were collected in the rainy season (B = 0.117, 95% CI = 0.15–0.20, P < 0.001) compared to the dry season (Additional file 1: Table S4). Unlike An. gambiae s.l., more An. funestus were collected during the 2018 sampling period (B = 0.136, 95% CI = 0.11–0.16, P < 0.001) than in the 2017 sampling period (Additional file 1: Table S4).
The most predominant species sampled were An. pharoensis [2017 n = (815/908); 2018 n = (730/1,371)], followed by An. funestus [2017 n = (80/908), 2018 n = (610/1371)], and then An. rufipes [2017 n = (13/908), 2018 n = (31/1371)]. More other Anopheline species were collected during the rainy season [2017 (An. pharoensis = 681/908, An. funestus = 80/908; An. rufipes = 10/908); 2018 (An. pharoensis = 635/1371, An. funestus = 609/1371, An. rufipes = 4/1371] compared to the dry season [2017 (An. pharoensis = 134/908; An. funestus = 0/908; An. rufipes = 3/908); 2018 (An. pharoensis = 95/1371, An. funestus = 1/1371, An. rufipes = 27/1371)].
Indoor and outdoor distribution of vectors
Overall, more An. gambiae s.l. were collected indoors (51.1%; 15,866/31,055) than outdoors (48.9%; 15,189/31,055) (Z = 2.410; P = 0.0160) (Table 2, Additional file 1: Table S1). Contrarily, a total of 59.3% (409/690) An. funestus mosquitoes were collected outdoors while 40.7% (281/690) were collected indoors. There was a non-significant decrease in outdoor biting in An. gambiae s.l. (B = − 0.175, 95% CI = − 0.41 to 0.06 P = 0.140); however, the abundance of An. funestus was slightly increased outdoors (B = 0.033, 95% CI = 0.01–0.06, P = 0.005) compared to indoors (Additional file 1: Table S4).
In 2017, 48.2% (8,311/17,245; 95% CI = 0.47–0.49) An. gambiae s.l. were collected indoors, and 54.7% (7555/13,810, 95% CI = 0.54–0.56) in 2018 as shown in Fig. 2, at all the study sites. The abundance of indoor-biting An. gambiae s.l. increased in the 2018 sampling period except in Dwease in the forest area where the abundance of indoor An. gambiae s.l. reduced from 53.7% (1245/2317, 95% CI = 0.52–0.56) in 2017 to 50.6% (623/1232, 95% CI = 0.48–0.53) 2018. During the 2017 sampling period, more An. gambiae s.l. were collected indoors in all sites, except in Dodowa where more were collected outdoors (59.6%; 3139/5263, 95% CI = 0.58–0.61) than indoors (40.4%; 2124/5263, 95% CI = 0.39–0.42) (Table 2, Fig. 2). During the 2017 sampling period, an equal number of An. funestus were collected both indoors (5%, 40/80) and outdoors (5%, 40/80) collections. However, in the 2018 sampling period, more An. funestus were collected outdoors (60.5%, 369/610) than indoors (39.5%, 241/610). Moreover, more An. funestus were collected (57.1%, 20/35) in indoor collection in Pagaza during the 2017 sampling period but during the 2018 sampling period more were collected outdoors (61.6%, 331/537). However, in Kpalsogu 43.2% (19/44) of An. funestus were collected during the 2017 sampling year and 53.3% (8/15) during the 2018 sampling year (Table 2).
Species discrimination in the An. gambiae complex
A subsample of 1670 An. gambiae s.l. from all the study sites was randomly selected and used to discriminate the sibling species: An. coluzzii 55.9% (935/1670), An. gambiae s.s. 39.5% (659/1670), An. arabiensis 2.3% (39/1670), and An. melas 2.2% (37/1670). Overall, more An. coluzzii were collected in all the ecological zones, except in the Sahel savannah zone, where the species were dominated by the An. gambiae s.s. [Sahel (An. gambiae s.s. = 323/1670; An. coluzzii = 298/1670; An Arabiensis = 39/1670; An. melas = 0/1670); coastal (An. gambiae s.s. = 288/1670; An. coluzzii = 432/1670; An Arabiensis = 0/1670; An. melas = 37/1670); forest (An. gambiae s.s. = 48/1670; An. coluzzii = 205/1670; An. Arabiensis = 0/1670; An. melas = 0/1670)] (Table 3, Fig. 3).
The composition and distribution of these species differed significantly by study sites (χ2 = 967.48, df = 12, P < 0.001) and year (χ2 = 256.67, df = 3, P < 0.001). Anopheles coluzzii was the most abundant in Anyakpor (83.7%, 394/471), Dwease (81.0%, 205/253), and Kpalsogu (80.2%, 239/298) while An. gambiae s.s. was the most abundant in Dodowa (86.7%, 248/286) and Pagaza (74.0%, 268/362) respectively. All the An. melas collected during the study were only from the coastal savannah site of Anyakpor (7.9%, 37/471). All Anopheles arabiensis were from Sahel savannah sites of Kpalsogu (1.3%, 4/298) and Pagaza (9.7%, 35/362) (Table 3, Fig. 4).
Biting times of An. gambiae s.l. and An. funestus in the study sites
Anopheles gambiae s.l. were found to bite the most during the late evening (LE) (66.6%; 20,685/31,055), followed by the early morning (EM) (20.1%; 6228/31,055), and less during the early evening (EE) (13.3%; 4142/31,055). This was the same pattern for both indoor [LE: (66.1%, 10,490/15,866); EM: (20.5%, 3255/15,866); EE: (11.4%, 2121/18,566)] and outdoor biting [LE: (67.1%, 10,195/15,189); EM: (19.6%, 2973/15,189), EE: (14.0%, 2021/15189)] (Fig. 4).
Anopheles funestus were found to bite mostly during the late evening (64.78%; 447/690) followed by the early evening (24.5%, 169/690) and early morning (10.7%; 74/690). This observed biting behaviour was similar for both indoor- [LE: (66.9%, 188/281); EE: (23.8%, 67/281); EM: (9.3%, 26/281)] and outdoor-biting An. funestus mosquitoes [LE: (63.3%, 259/409); EE: (24.9%, 102/409); EM: (11.7%, 48/409)] (Fig. 5). Compared to early evening biting activity, An. gambiae s.l. preferred to bite more in the late evenings (B = 3.723, 95% CI = 3.46–3.98, P = 0.000) and the early mornings (B = 3.209, 95% CI = 2.86–3.56, P = 0.000) (Additional file 1: Table S3). However, An. funestus biting activity increased significantly only in the late evenings compared to the early evenings (B = 0.050, 95% CI = 0.02–0.08, P < 0.0001) (Additional file 1: Table S4).
Regarding the species of An. gambiae s.l., An. coluzzii and An. melas had a different biting pattern from An. gambiae s.s. and An. arabiensis. Anopheles coluzzii preferred late evening feeding (48.0%, 449/935) followed by early morning (26.5%, 248/935) and early evening (25.5%, 238/935) feeding. Anopheles melas on the other hand preferred to bite in the early evening (43.2%, 16/37) followed by the late evening (40.5%, 15/37) and early morning (16.2%, 6/37). Anopheles gambiae s.s. preferred to bite in the late evening (54.5%, 359/659) followed by the early evening (25.0%, 165/659) and the early morning (20.5%, 135/659). Anopheles arabiensis preferred late evening (41.0%, 16/36) biting followed by early evening (33.3%, 13/36) and early morning (25.6%, 10/36) biting.
Insecticide resistance genotypes in An. gambiae s.l.
Anopheles gambiae s.l. samples were genotyped for the presence of Vgsc-1014S and 1014F mutations as well as the G119S mutation. The Vgsc-1014S mutation was not detected in any mosquito for this study; however, the frequency of the Vgsc-1014F mutation was slightly varied in indoor-biting mosquitoes (54.9%) compared with those biting outdoors (45.1%). Overall, Vgsc-1014S mutation frequency in An. melas was 87.1%, whereas that of An. arabiensis was 50% (Table 4).
Similarly, the frequency of the G119S mutation in An. gambiae s.l. varied in indoor host-seeking mosquitoes (52.9%) compared with outdoor-biting mosquitoes (47.1%). Resistance mutation genotypes in the other Anopheles mosquitoes were An. melas 0.63 (30.1%) and An. arabiensis 0.44 (21.0%) (Table 4).
Sporozoite infection rates in the sampled vectors
A total of 12,860 An. gambiae s.l. were pooled in groups of 20 into 643 pools and tested for Plasmodium falciparum circumsporozoite (CSP). Overall, 44 pools were positive for P. falciparum CSP—Anyakpor (n = 8), Dodowa (n = 7), Dwease (n = 10), Kpalsogu (n = 8), and Pagaza (n = 11)—giving an overall sporozoite rate of 0.1% (Table 5).
Regarding the individual study sites, sporozoite rate varied in indoor-collected mosquitoes compared to those collected outdoors except in Dodowa [(indoor (0.1%); outdoor (0.2%)] and Kpalsogu [(indoor (0.1%); outdoor (0.18%)] where outdoor-biting An. gambiae s.l. had a higher sporozoite rate.
Furthermore, all the study sites had similar sporozoite rates in both seasons as represented in Table 5 except in Dodowa where the sporozoite rate varied in the dry season (0.2%) compared to the rainy season (0.1%) and in Pagaza [rainy season (0.3%); dry season (0.2%)].
Discussion
Evidence has shown that successful malaria elimination strategies require vector control intervention that target the changing vector behaviour [39]. It is, therefore, essential to monitor the changing vector behaviour and ecology in the era of increasing malaria intervention to reduce the high disease burden. This study investigated the biting behaviour, resistant gene genotyping, and spatiotemporal dynamics of malaria vectors in Ghana.
Findings from this study indicated that many malaria vectors were sampled during the 2017 sampling year, with a decline in vectors in the subsequent year. This may be because of the effectiveness of the vector control tools deployed in those areas or probably a reduction in breeding sites. Moreover, most vectors were collected during the rainy season for both sampling years, likely due to the availability of breeding habitats that facilitate oviposition by gravid females. However, the presence of mosquitoes in high abundance during the dry season in Anyakpor and Kpalsogu was likely due to the irrigated farming, which supports the breeding of vectors during the dry season [5, 29]. During the rainy season, these low-lying areas get flooded, therefore disrupting malaria vector breeding; however, during the dry season, the irrigation areas provide breeding habitats for continuous vector breeding. This implies that malaria transmission may be occurring in these areas year round [40, 41].
Malaria vectors in Africa have been efficient in malaria transmission largely because of their anthropophilic and endophilic nature [6, 42]. Therefore, knowledge of the biting behaviour in disease vectors is important to understand the role of the vectors in disease transmission and hence the deployment of effective control tools. In this study, the abundance of Anopheles mosquitoes biting indoors was relatively similar to the outdoor biting. This behavioural trait by the vectors was consistent for both sampling years. Compared to a study done in Ghana by Tuno et al. [43] in which the abundances of outdoor An. gambiae were 15% and 23% during the dry and rainy season, respectively, this study has shown a drastic increase in the outdoor-biting activity in An. gambiae s.l. According to Sherrard-Smith et al. (2019), mathematical models suggest that even the smallest changes in outdoor host-seeking activity of malaria vectors can have a substantial public health impact.
Larger increases in outdoor biting behaviour lead to reduced effectiveness of LLINs [44]. The shift in biting behaviour may be due to selective pressure mounted by the use of LLINs and IRS. Because LLINs and IRS are indoor based, increase in outdoor-biting mosquitoes may indicate possible outdoor malaria transmission, showing the need for outdoor vector interventions [45, 46]. Historically, the large-scale use of LLINs and IRS as led to an increase in the abundance of outdoor-biting vectors [23]. The high densities of outdoor-biting An. gambiae contribute to the persistence of malaria in the Sahel savannah area despite LLIN and IRS interventions [23]. The presence of outdoor-feeding mosquitoes limits the effectiveness of these interventions [23, 47] and may be of major public health concern. It will be important for vector control strategies targeting both indoor and outdoor malaria vectors to be introduced in these areas.
Overall, An. coluzzii was the most abundant vector; however, An. gambiae s.s. was the predominant species present in the costal savannah and forest zone. This is indicative that the primary malaria vectors are well established across all ecological zones; hence, constant surveillance and strengthening of control strategies are essential. These findings corroborate those of Hinne et al. [29] whose study was done in similar study sites and reported high abundance of An. coluzzii compared to An. gambiae s.s. in these areas [29].
Findings from this study showed that both indoor and outdoor An. gambiae s.l. preferred to bite late in the night when people were asleep. Peak biting activity in the late night occurs because household members begin to rise as early as 03:00 h to begin morning chores including fetching water and firewood, feeding animals, cooking, bathing, and preparing for market days. Outdoor sleeping is also a major factor contributing to peak outdoor biting in the late evenings. People sleep outside during the early nighttime because of high temperatures in the rooms and wait till about 02:00 h [47] when their rooms are cool enough to sleep indoors. During the dry season in the Sahel savannah areas, some people spend the entire night sleeping outdoors because their rooms become extremely warm. Other outdoor activities such as funerals, church activities, and trading are reasons for people to stay outside in the late evening [47]. This finding corroborates a study in Uganda that reported that the peak biting time for An. gambiae s.l. was between 23:00 and 5:00 h [14]. The biting behavioural activity observed in An. melas and An. arabiensis in correlation to the vector densities observed for both indoor and outdoor settings could have major public health implications, because with the level of resistance and sporozoite rate observed in the malaria vectors, there could be possible transmission of malaria outdoors (residual malaria). Malaria vector feeding and resting behaviours are likely to change to maximize available feeding opportunities. Anopheles melas and An. arabiensis preferred to bite their host outdoors compared to indoors, whereas An. coluzzii and An. gambiae s.s. preferred indoor biting.
The frequency of kdr mutations was very high but similar in outdoor- and indoor-biting mosquitoes. This could be because of frequent exposure to sub-lethal doses of insecticides for public health use, i.e. IRS, aerosol sprays, and LLINs used in houses, use of pesticides in agriculture [48], other volatiles in outdoor settings, and other factors that may be associated with insecticide resistance. Furthermore, this finding may also imply that the mosquito population from the study sites can resist the presence of insecticides employed for vector control and may lead to increased human-vector contact and malaria transmission in the region despite the high LLIN coverage. The Vgsc-1014F mutation has been found to be strongly associated with pyrethroid resistance in West Africa; consequently, their presence in indoor-biting mosquitoes may be of particular concern [49]. The presence of Vgsc-1014F mutation in a mosquito population is a reliable marker of both high individual target-site resistance and pyrethroid-resistance prevalence [50, 51]. Studies have shown a relationship between the spread of Vgsc-1014F alleles with the use of LLINs [52, 53]. The lowest frequency of Vgsc-1014F was found in An. arabiensis, a gene that confers target site resistance, which could be explained by the biting behaviour of this mosquito species. Anopheles arabiensis prefers to bite and rest outdoors, and this could have limited their exposure to the insecticides used in vector control.
The results from the study showed that the frequency of G119S from the mosquito population was higher in the indoor than outdoor mosquito population from all the study sites. The highest frequency of G119S was observed in An. melas and the lowest in An. arabiensis. That high frequency of Ace-1 mutation observed in Anyarkpor may be due to the frequent use of pesticides in agricultural activities in the area and exposure to malaria vectors since most of these pesticides contain the same active ingredients as insecticides used for public health control of malaria vectors [54,55,56]. This may imply that vector control management tools may fail in such areas and requires careful monitoring. A similar observation was made in southern Ghana by Essandoh et al. [25], who reported that high prevalence of resistance in malaria vectors was consistent with agriculture-driven selection.
Sporozoite rates determined during the study were relatively similar for both sampling seasons (dry and rainy) whereas relatively similar for indoor and outdoor sampling. This finding suggests that malaria transmission did not change between the seasons. However, sporozoite rate was not determined according to species and year of sampling from the various study sites. This was because blood-fed mosquito samples were pooled according to study sites for the determination of sporozoite rate, and this was a limitation to our study. Higher sporozoite rates were recorded in indoor mosquitoes compared to outdoor-biting mosquitoes from all the study sites except in Dodowa and Kpalsogu where the sporozoite rates were higher in mosquitoes collected outdoors than those from indoors. This observation was suggestive of outdoor malaria transmission (residual malaria), and it is important for vector control tools to be implemented to target outdoor-biting mosquitoes as well. The infection rates found in the indoor-biting mosquitoes could suggest ongoing malaria transmission regardless of vector control tools employed in the study sites.
Conclusions
This study revealed that An. gambiae s.l. were more abundant indoors across all ecological zones of Ghana. Furthermore, the abundance of Anopheles mosquitoes and frequency of kdr mutations were similar in both indoor- and outdoor-biting mosquitoes. However, the frequency of G119S from the mosquito population was higher in the indoor than outdoor mosquito population from all the study sites. Higher sporozoite rates were recorded in outdoor mosquitoes in Dodowa and Kpalsogu. There is thus an urgent need for a supplementary malaria control intervention to control outdoor-resting and -biting mosquitoes as the current tools only target indoor-resting and -biting mosquitoes. Continued surveillance of vector behaviours is recommended to help in the control of malaria.
Availability of data and materials
All datasets generated and/or analysed during this study are included in the manuscript.
Abbreviations
- WHO:
-
World Health Organisation
- LLINs:
-
Long lasting insecticide-treated nets
- IRS:
-
Indoor residual sprays
- PMI:
-
President Malaria Initiative
- Ace-1:
-
Acetylcholinesterase 1
- kdr:
-
Knockdown resistance
- HLC:
-
Human landing catch
- EE:
-
Early evening
- LE:
-
Late evening
- EM:
-
Early morning
- PCR:
-
Polymerase chain reaction
- DNA:
-
Deoxyribonucleic acid
- PCR-RFLP:
-
Polymerase chain reaction-restriction fragment length polymorphism
- Vgsc:
-
Voltage-gated sodium channel
- SNP:
-
Single nucleotide polymorphism
- G:
-
Glycine
- L:
-
Leucine
- F:
-
Phenylalanine
- S:
-
Serine
- RR:
-
Homozygote resistant
- RS:
-
Heterozygote resistant
- SS:
-
Homozygote susceptible
- N:
-
Number of samples tested
- n:
-
Total number of positive samples
- NMIMR:
-
Noguchi Memorial Institute for Medical Research
- CSP:
-
Circumsporozoite protein
References
Cohuet A, Simard F, Wondji CS, Antonio-Nkondjio C, Awono-Ambene P, Fontenille D. High malaria transmission intensity due to Anopheles funestus (Diptera: Culicidae) in a village of savannah-forest transition area in Cameroon. J Med Entomol. 2004;41:901–5.
Coetzee M, van Wyk P, Booman M, Koekemoer LL, Hunt RH. Insecticide resistance in malaria vector mosquitoes in a gold mining town in Ghana and implications for malaria control. Bull Soc Pathol Exot. 2006;99:400–3.
Drakeley C, Schellenberg D, Kihonda J, Sousa CA, Arez AP, Lopes D, et al. An estimation of the entomological inoculation rate for Ifakara: a semi-urban area in a region of intense malaria transmission in Tanzania. Trop Med Int Health. 2003;8:767–74.
Shililu J, Ghebremeskel T, Seulu F, Mengistu S, Fekadu H, Zerom M, et al. Seasonal abundance, vector behavior, and malaria parasite transmission in Eritrea. J Am Mosq Control Assoc. 2004;20:155–64.
Appawu M, Owusu-Agyei S, Dadzie S, Asoala V, Anto F, Koram K, et al. Malaria transmission dynamics at a site in northern Ghana proposed for testing malaria vaccines. Trop Med Int Health. 2004;9:164–70.
Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2010;3:117.
Coulibaly B, Kone R, Barry MS, Emerson B, Coulibaly MB, Niare O, et al. Malaria vector populations across ecological zones in Guinea Conakry and Mali, West Africa. Malar J. 2016;15:191.
Yokoly FN, Zahouli JBZ, Small G, Ouattara AF, Opoku M, de Souza DK, et al. Assessing Anopheles vector species diversity and transmission of malaria in four health districts along the borders of Côte d’Ivoire. Malar J. 2021;20:409.
Appawu MA, Baffoe-Wilmort A, Afari EA, Dunyo S, Koram KA, Nkrumah FK. Malaria vector studies in two ecological zones in southern Ghana. Afr Entomol. 2001;9:59–65.
Kasasa S, Asoala V, Gosoniu L, Anto F, Adjuik M, Tindana C, et al. Spatio-temporal malaria transmission patterns in Navrongo demographic surveillance site, northern Ghana. Malar J. 2013;12:63.
WHO. A Framework for Malaria Elimination. World Health Organisation. 2017. https://www.who.int/publications/i/item/9789241511988. Assessed 20 May 2020
PMI-Ghana. President’s Malaria Initiative: Ghana - Malaria Operational Plan FY 2018. 2018. http://www.pmi.gov/docs/default-source/default-document-library/malaria-operational-plans/fy14/ghana_mop_fy14.pdf?sfvrsn=20. Accessed 7 Mar 2021.
Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–11.
Gatton ML, Chitnis N, Churcher T, Donnelly MJ, Ghani AC, Godfray HC, et al. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution. 2013;67:1218–30.
Kabbale FG, Akol AM, Kaddu JB, Onapa AW. Biting patterns and seasonality of Anopheles gambiae sensu lato and Anopheles funestus mosquitoes in Kamuli District, Uganda. Parasit Vectors. 2013;6:340.
Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province. Kenya Malar J. 2010;9:62. https://doi.org/10.1186/1475-2875-9-62.
Russell TL, Lwetoijera DW, Maliti D, Chipwaza B, Kihonda J, Charlwood JD, et al. Impact of promoting longer-lasting insecticide treatment of bed nets upon malaria transmission in a rural Tanzanian setting with pre-existing high coverage of untreated nets. Malar J. 2010;9:187.
Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–8.
Derua YA, Alifrangis M, Hosea KM, Meyrowitsch DW, Magesa SM, Pedersen EM, et al. Change in composition of the Anopheles gambiae complex and its possible implications for the transmission of malaria and lymphatic filariasis in north-eastern Tanzania. Malar J. 2012;11:188.
Mwangangi JM, Mbogo CM, Orindi BO, Muturi EJ, Midega JT, Nzovu J, et al. Shifts in malaria vector species composition and transmission dynamics along the Kenyan coast over the past 20 years. Malar J. 2013;12:13.
WHO. Insecticides resistance. World Health Organisation. 2015. https://www.who.int/teams/global-malaria-programme/prevention/vector-control/insecticide-resistance. Accessed 20 Jul 2022.
Hemingway J. The role of vector control in stopping the transmission of malaria: threats and opportunities. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130431.
Chareonviriyaphap T, Bangs MJ, Suwonkerd W, Kongmee M, Corbel V, Ngoen-Klan R. Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasit Vectors. 2013;6:280.
Musiime AK, Smith DL, Kilama M, Rek J, Arinaitwe E, Nankabirwa JI, et al. Impact of vector control interventions on malaria transmission intensity, outdoor vector biting rates and Anopheles mosquito species composition in Tororo, Uganda. Malar J. 2019;18:445.
Sanou A, Nelli L, Guelbéogo WM, Cissé F, Tapsoba M, Ouédraogo P, et al. Insecticide resistance and behavioural adaptation as a response to long-lasting insecticidal net deployment in malaria vectors in the Cascades region of Burkina Faso. Sci Rep. 2021;11:17569.
Essandoh J, Yawson AE, Weetman D. Acetylcholinesterase (Ace-1) target site mutation 119S is strongly diagnostic of carbamate and organophosphate resistance in Anopheles gambiae s.s. and Anopheles coluzzii across southern Ghana. Malar J. 2013;12:404.
Edi CV, Djogbénou L, Jenkins AM, Regna K, Muskavitch MAT, Poupardin R, et al. CYP6 P450 Enzymes and ACE-1 duplication produce extreme and multiple insecticide resistance in the malaria mosquito Anopheles gambiae. PLoS Genet. 2014;10:e1004236.
Feng X, Yang C, Yang Y, Li J, Lin K, Li M, et al. Distribution and frequency of G119S mutation in ace-1 gene within Anopheles sinensis populations from Guangxi, China. Malar J. 2015;14:470.
Amvongo-Adjia N, Riveron JM, Njiokou F, Wanji S, Wondji CS. Influence of a Major Mountainous Landscape Barrier (Mount Cameroon) on the Spread of Metabolic (GSTe2) and Target-Site (Rdl) Resistance Alleles in the African Malaria Vector Anopheles funestus. Genes. 2020;11:1492.
Hinne IA, Attah SK, Mensah BA, Forson AO, Afrane YA. Larval habitat diversity and Anopheles mosquito species distribution in different ecological zones in Ghana. Parasit Vectors. 2021;14:193.
Kenea O, Balkew M, Tekie H, Gebre-Michael T, Deressa W, Loha E, et al. Human-biting activities of Anopheles species in south-central Ethiopia. Parasit Vectors. 2016;9:527.
Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, et al. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–25.
Mathenge EM, Killeen GF, Oulo DO, Irungu LW, Ndegwa PN, Knols BGJ. Development of an exposure-free bednet trap for sampling Afrotropical malaria vectors. Med Vet Entomol. 2002;16:67–74.
Coetzee M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar J. 2020;19:70.
Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–9.
Fanello C, Santolamazza F, della Torre A. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med Vet Entomol. 2002;16:461–4.
Bass C, Nikou D, Donnelly MJ, Williamson MS, Ranson H, Ball A, et al. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar J. 2007;6:111.
Echeverry DF, Deason NA, Makuru V, Davidson J, Xiao H, Niedbalski J, et al. Fast and robust single PCR for Plasmodium sporozoite detection in mosquitoes using the cytochrome oxidase I gene. Malar J. 2017;16:230.
Maia MF, Kapulu M, Muthui M, Wagah MG, Ferguson HM, Dowell FE, et al. Detection of Plasmodium falciparum infected Anopheles gambiae using near-infrared spectroscopy. Malar J. 2019;18:85.
Russell TL, Beebe NW, Cooper RD, Lobo NF, Burkot TR. Successful malaria elimination strategies require interventions that target changing vector behaviours. Malar J. 2013;12:56.
Minakawa N, Dida GO, Sonye GO, Futami K, Njenga SM. Malaria Vectors in Lake Victoria and Adjacent Habitats in Western Kenya. PLoS ONE. 2012;7:e32725.
De Silva PM, Marshall JM. Factors Contributing to Urban Malaria Transmission in Sub-Saharan Africa: A Systematic Review. J Trop Med. 2012;89:819563.
Antonio-Nkondjio C., Simard F. Highlights on Anopheles nili and Anopheles moucheti, Malaria Vectors in Africa. Anopheles Mosquitoes : New Insights into Malaria Vectors. Tech.2013;89:7
Tuno N, Kjaerandsen J, Badu K, Kruppa T. Blood-feeding behavior of Anopheles gambiae and Anopheles melas in Ghana, western Africa. J Med Entomol. 2010;47:28–31.
Sherrard-Smith E, Skarp JE, Beale AD, Fornadel C, Norris LC, Moore SJ, et al. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc Natl Acad Sci U S A. 2019;116:15086–95.
Sougoufara S, Ottih EC, Tripet F. The need for new vector control approaches targeting outdoor biting anopheline malaria vector communities. Parasit Vectors. 2020;13:295.
Nalinya S, Musoke D, Deane K. Malaria prevention interventions beyond long-lasting insecticidal nets and indoor residual spraying in low- and middle-income countries: a scoping review. Malar J. 2022;21:31.
Monroe A, Asamoah O, Lam Y, Koenker H, Psychas P, Lynch M, et al. Outdoor-sleeping and other night-time activities in northern Ghana: implications for residual transmission and malaria prevention. Malar J. 2015;14:35.
Lynd A, Gonahasa S, Staedke SG, Oruni A, Maiteki-Sebuguzi C, Dorsey G, et al. LLIN Evaluation in Uganda Project (LLINEUP): a cross-sectional survey of species diversity and insecticide resistance in 48 districts of Uganda. Parasit Vectors. 2019;12:94.
Weetman D, Mitchell SN, Wilding CS, Birks DP, Yawson AE, Essandoh J, et al. Contemporary evolution of resistance at the major insecticide target site gene Ace-1 by mutation and copy number variation in the malaria mosquito Anopheles gambiae. Mol Ecol. 2015;24:2656–72.
Lucas ER, Miles A, Harding NJ, Clarkson CS, Lawniczak MKN, Kwiatkowski DP, et al. Whole-genome sequencing reveals high complexity of copy number variation at insecticide resistance loci in malaria mosquitoes. Genome Res. 2019;29:1250–61.
Ochomo EO, Bayoh NM, Walker ED, Abongo BO, Ombok MO, Ouma C, et al. The efficacy of long-lasting nets with declining physical integrity may be compromised in areas with high levels of pyrethroid resistance. Malar J. 2013;12:368.
Trape JF, Tall A, Diagne N, Ndiath O, Ly AB, Faye J, et al. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. Lancet Infect Dis. 2011;11:925–32.
Klinkenberg E, McCall PJ, Wilson MD, Amerasinghe FP, Donnelly MJ. Impact of urban agriculture on malaria vectors in Accra, Ghana. Malar J. 2008;7:151.
Chabi Joseph, Eziefule Miracle C., Pwalia Rebecca, Joannides Joannitta, Obuobi Dorothy, Amlalo Godwin, et al. Impact of Urban Agriculture on the Species Distribution and Insecticide Resistance Profile of s gambiae s.s. and Anopheles coluzzii in Accra Metropolis, Ghana. Advances in Entomology. 2018;6 (3):198–211.
Hien AS, Soma DD, Hema O, Bayili B, Namountougou M, Gnankiné O, et al. Evidence that agricultural use of pesticides selects pyrethroid resistance within Anopheles gambiae s.l. populations from cotton growing areas in Burkina Faso, West Africa. PLoS ONE. 2017;12:e0173098.
Acknowledgements
We thank the residents at our study sites for their support during the study. Our sincere gratitude goes to Michelle Asigbaase, Andy Asafu-Adjaye, Dr. Joseph Harold Nyarko Osei, Sakyi Kojo Yirenkyi, Dr. Sellase Pi-Bansa, and Dr. Kojo Frimpong, who are staff in the Parasitology Unit of the Noguchi Memorial Institute for Medical Research (NMIMR), for their support during laboratory work and to Isaac Sraku, Anisa Abdulai, and Prince Ashong of the IMAT research team of the Medical Microbiology Department, University of Ghana, for their help during the laboratory activities. We also thank Thomas Peprah Agyekum for his help during data analysis.
Funding
This study was supported by grants from the National Institute of Health (RO1A1123074 and D43TW011513), and it is also part of the research undertaken towards the award of Doctor of Philosophy (PhD) Degree in Entomology under the sponsorship of the Deutscher Akademischer Austauschdienst (DAAD).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: OKA, SKD, DAB, RK, MDW, YAA. Supervised data collection: OKA, SBD, ARM, CMO-A, SC, YAA. Performed the experiments: OKA, SBD, ARM, CMO-A. Analysed the data: OKA, SBD, IAH, CMO-A, YAA. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Scientific and ethical approval for the study was obtained from the Institutional Review Board of the Noguchi Memorial Institute for Medical Research (NMIMR). Verbal and written informed consents of the village elders and compound/household heads and the mosquito collectors were taken before mosquitoes were collected from the study sites. The mosquito catchers were recruited from the various sampling sites to foster acceptance from residents. The catchers were trained to collect landing mosquitoes prior to blood feeding to minimize the risk of malaria transmission.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.Table S1.
Univariate analysis of sampling parameters on An. gambiae s.l. abundance.Univariate analysis of sampling parameters on An. funestus abundanceTable S2. Univariate analysis of sampling parameters on An. funestus abundance. Table S3. Generalized linear mixed model of the effect of sampling parameters on An. gambiae s.l. abundance. Table S3. Generalized linear mixed model of the effect of sampling parameters on An. funestus abundance.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Akuoko, O.K., Dhikrullahi, S.B., Hinne, I.A. et al. Biting behaviour, spatio-temporal dynamics, and the insecticide resistance status of malaria vectors in different ecological zones in Ghana. Parasites Vectors 17, 16 (2024). https://doi.org/10.1186/s13071-023-06065-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-06065-9