Abstract
Background
Insecticide-treated nets (ITNs) using pyrethroids have been the main vector control tools deployed in malaria endemic countries and are responsible for the dramatic reduction in African malaria cases in the early 2000s. The World Health Organization (WHO) cone test was designed to assess the rapid toxicity effects of pyrethroid exposure on mosquito vectors but has yielded no insights beyond 60-min knockdown and 24-h mortality. As dual-active-ingredient (AI) ITNs become more widespread, bioassays that can provide realistic assessment of single- and dual-treated ITNs (i.e. nets with more than one active ingredient) are urgently needed.
Methods
We present an augmentation of the cone test that enables accurate quantification of vector behavioural responses (specifically movement, spatial and temporal occupancy) to ITNs using video recording and bespoke software that uses background segmentation methods to detect spatial changes in the movement of mosquitoes within the cone. Four strains of Anopheles gambiae sensu lato (s.l.) were exposed to four ITNs (PermaNet 2.0, PermaNet 3.0, Olyset Net, Interceptor G2) and untreated nets in these modified cone tests. Life history data (post-exposure blood-feeding, blood meal weight, longevity) for individual mosquitoes were recorded.
Results
All mosquitoes responded to the presence of ITNs, spending from 1.48 to 3.67 times more time in the upper region of the cone, depending on the ITN type. Of all ITNs, PermaNet 2.0 provoked the smallest change in behavioural response. Activity in the cone influenced observed post-exposure longevity, and in resistant strains exposed to Interceptor G2, the higher the activity, the greater the risk of dying, as long as the proportion of activity at the net surface was less than 50%. All ITNs inhibited blood-feeding, and smaller blood meals were taken when mosquitoes fed.
Conclusions
The additional mosquito behaviour data obtained by using this modification to the WHO cone test provides unique insight into the innate responses of different mosquito strains on untreated nets and the entomological mode of action of ITNs, important evidence when evaluating ITN characteristics.
Graphical Abstract

Similar content being viewed by others
Background
Insecticide-treated nets (ITNs) have contributed substantially to a decline in the burden of malaria caused by the principal African malaria vectors Anopheles gambiae sensu lato (s.l.) and Anopheles funestus in at-risk communities across Africa [1, 2]. Seemingly simple tools, ITNs achieve an impact through a multifunctional mechanism that reduces the contact rate between humans and malaria vectors, alters blood-feeding and host-seeking behaviours, and causes mosquito death [3,4,5,6,7]. Increased insecticide resistance has reduced the instantaneous killing capacity of ITNs, but the effects of ITNs on host-seeking behaviours and blood-feeding remain, regardless of the insecticide resistance status of local vector populations [8,9,10,11,12,13,14].
Assessments of ITN impact are principally conducted using the World Health Organization (WHO) cone test and tunnel tests during laboratory testing and experimental hut trials during semi-field testing [15]. The most widely used laboratory evaluation method is the WHO cone test, where five mosquitoes are exposed to a net surface for 3 min, and knockdown at 60 min and mortality at 24 h are collected as parameters by which to adjudge ITN efficacy [15]. The test is designed to capture the rapid toxicity effects induced by fast-acting pyrethroid insecticides, and yields no insight into mosquito behaviours that might influence ITN performance [16] or the effects of compounds that target different stages of the mosquito life cycle or which induce delayed mortality [17,18,19,20]. With multiple classes of insecticides now in use as ITN treatments, there is an urgent need for ITN evaluation methods to accurately measure a broad range of insecticidal effects [21].
Recognising the range of immediate and delayed impacts of ITNs requires a thorough understanding of the effects of insecticide exposure. For example, if a mosquito successfully takes a blood meal through an ITN, its chances of survival are higher than a mosquito that is exposed but remains unfed [22]. ITNs reduce overall post-exposure (PE) blood-feeding success, blood-feeding duration and the time spent on the net, relative to the untreated (UT) net [23]. Pyrethroid-resistant mosquitoes that are not immediately killed by an ITN may nonetheless exhibit a reduced life span [24], and the effects of pro-insecticides that require metabolization into active forms may be enhanced by assays that allow mosquitoes to have a free range of movement [25].
Advances in video recording and machine vision techniques have enabled fine-grained studies of mosquito behaviours ranging in scale from house entry dynamics [26] to flight distribution within a small wind tunnel [27]. Measuring key behavioural parameters during and after ITN exposure provides additional insights about the likely effect of a given ITN on a particular vector population. In an experimental field hut study, mosquitoes reduced the proportion of time spent in a ‘bouncing’ flight mode when an ITN was present [28]. Tracking of mosquito positions in smaller-scale assays can quantify net contact and excito-repellency effects, and investigate the relationship between these parameters and observed mortality [14].
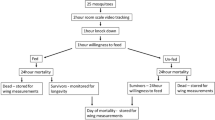
To address this goal, we present an automated method, video cone test analysis (ViCTA), designed to use the video output of mosquito activity inside the cone recorded during the video-modified WHO cone assay, as recently reported by Hughes et al. [14], where mosquito behavioural states were manually assessed at 5-s intervals. In this new study, video recordings of standard WHO cone assays are automatically analysed using background subtraction methods to detect and segment moving mosquitoes at 0.1-s intervals. The detected movements are aggregated to report total movement activity and total and proportional distribution of mosquitoes within the cone, before screening for correlation with a range of subsequent life history outcomes to evaluate for indications of the ‘whole life’ impact of ITNs on mosquitoes (Additional file 1: Fig. S1).
Methods
Physical apparatus
The WHO cone and smartphone (Apple iPhone SE, Apple Inc., Cupertino, CA, USA) were mounted on a bespoke frame as per Hughes et al. [14], shown in Fig. 1.
Experimental rig for video cone test analysis. The test netting is sandwiched between two pieces of 3-mm acrylic mounted at 45° and containing a 9-cm hole to allow exposure to the host stimulus scent. The host rests its arm below the scent stimulus hole, and mosquitoes are not able to contact the host arm. A smartphone is placed above the cone and immobilised at a fixed distance and angle using a z-clamp
Insecticidal netting
A UT net (Bayer, Germany) was used in control tests. ITNs tested were the PermaNet 2.0 (Vestergaard Frandsen, Switzerland, deltamethrin 1.4/1.8 g/kg, P2), PermaNet 3.0 [Vestergaard Frandsen, Switzerland, deltamethrin 4.0 g/kg (roof), 2.8/1 g/kg (sides), piperonyl butoxide (PBO) 4.0 g/kg (roof), P3], Olyset Net [Sumitomo Chemical Co., Ltd., Japan, 2% (w/w) permethrin 20 g/kg, OS], and Interceptor G2 (BASF, Germany, alpha-cypermethrin 100 mg/m2, chlorfenapyr 200 mg/m2, IG2). ITNs were aired at ambient temperature for 1 week, then stored at 4 °C until acclimatisation at laboratory temperature 24 h prior to testing.
Mosquito strains
Experiments were performed at the Liverpool School of Tropical Medicine (LSTM) using four colonised strains: the insecticide-susceptible Kisumu (KS) (An. gambiae sensu stricto) and N’gousso (NG) (Anopheles coluzzii) strains and pyrethroid-resistant Banfora (BF) and VK7 strains (both An. coluzzii). Both insecticide-resistant strains are maintained under 6-monthly selection pressure with deltamethrin and have been previously described [29, 30].
All experiments were performed in a climate-controlled laboratory (27 ± 2 °C, 80 ± 8% RH) using non-blood-fed 3–5-day-old female mosquitoes. Mosquitoes were sugar-starved for at least 5 h prior to testing.
ViCTA video cone tests
A mean of 43 cone test replicates each were performed for KS, NG and VK7 strains, respectively; fewer BF replicates (mean = 19) were conducted due to colony issues. All cone tests were conducted using the modifications to the WHO guidelines described in Hughes et al. with a host attractant. Mortality at 24 h and life history data were recorded.
Life history data
The life history data collected were blood-feeding at 1 and 24 h PE, blood meal weight (BMW) and longevity as described in Hughes et al. A human arm was offered as a blood meal source at 1 h PE; mosquitoes that did not feed at 1 h were offered a second opportunity to blood-feed at 24 h PE. Mosquitoes were monitored individually in individual 50-ml Falcon tubes with a lid of UT netting. Seventy-two hours after blood-feeding, individual BMWs were measured using excreted haematin [31]. One millilitre of 1% lithium carbonate was used to dissolve all excreted haematin and the absorbance at 397 nm was measured in triplicate. A standard curve comprising standards from 1.76 to 30 µg/ml was used to calculate the total weight of haematin (µg/ml) in each sample. Mosquitoes were maintained in individual housing with access to 10% sugar solution until death. Mortality was recorded every 24 h. Wing length was measured after death and was used as an index of mosquito mass [32].
Video recording and analysis
Video files were converted to 540 × 960 pixels, 30 frames per second, H264 encoding at 1500 Kb bitrate, inside an MPEG-4 container prior to analysis. Background segmentation methods detect actively moving mosquitoes within the cone and log the location of moving mosquitoes at 0.1-s intervals. Preprocessing morphological operators are used for each video frame and interpreted by a Gaussian mixture model for background segmentation [33] to generate regularised contour objects representing moving mosquitoes whilst removing noise artefacts (Supplementary methods 1).
Mosquito movement counts are calculated by the software and aggregated at 5-s intervals for 35 epochs of spatio-temporal activity per test. The first 5 s of the test is excluded to allow for acclimatisation of the background segmentation model. Metrics produced for data analysis include (i) total mosquito activity (movements of all five mosquitoes in the cone over 180 s) and (ii) regional activity (movements of all five mosquitoes in the cone stratified into the upper half (UHC) and lower half (LHC) of the cone volume. Frames with no moving mosquitoes constitute mosquito inactivity. Output result files were parsed and combined with post-test monitoring data using R.
Statistical analysis
Statistical analysis was performed using R statistical software version 4.1.2 and R Studio [34, 35]. Descriptive statistics were generated using the number of observations, mean and standard deviation (SD) for continuous variables, the number and percentage of observations for categorical variables, and the number and percentage of observations, mean, SD, minimum, maximum, median survival and 95% confidence interval (CI) for time-to-event variables.
Behavioural data were analysed using linear mixed models (LMMs) and generalised linear mixed models (GLMMs) in the R lme4 and glmmTMB packages, respectively [36, 37]. The normal, negative binomial, and beta-binomial distributions were used to evaluate the total, regional and proportion of activity in the LHC, respectively. The mean difference, incidence risk ratio (IRR), odds ratio (OR) and 95% CIs were generated. Mosquito strain, treatment, number of inactive frames, valid frames proportion, and two-way interaction (treatment and strain) were included for all the models, and three-way interaction (treatment, strain, and region) and two-way interactions (region and inactive frames, and strain and region) for the regional activity model only. The test date and the operator within the test date were fitted as random effects for the regional and proportion of activity at LHC outcomes, respectively. The test date was too variable for total activity (model without test date fitted the data better) and hence was not retained in the final model.
For the life history data, GLMMs were employed to analyse PE blood-feeding success and 24-h mortality using the binomial distribution, and LMMs for the BMW, using the normal distribution, respectively. Longevity was analysed using the mixed-effects Cox regression model using the R ‘coxme’ package [38]. Treatment, wing-length measurement (BMV and longevity only), PE feeding time, BMW, the predicted proportion of activity in LHC (PLHC), two-way interactions (treatment and strain, treatment and PLHC, treatment and wing-length measurement) and three-interaction (strain, treatment and PE feeding time) for the PE blood-feeding success model only were included as fixed effects while replicate and test date were included as random effects.
The likelihood ratio test was used under the restricted maximum likelihood estimation to determine the statistical significance of the random effects. The fixed effects for the ‘best’ model were determined using the Akaike information criterion and the residuals based on the DHARMa package [39]. All comparisons were conducted within a strain comparing each ITN to UT using the ‘emmeans’ package [40]. Dunnett’s method was employed to control for the probability of making false-positive findings. Statistical significance was set at 5%.
Ethical approval
Ethical approval for this laboratory study was obtained from the LSTM Research Ethics Committee as part of the project: Developing entomological indicators to assess the public health value of next-generation LLINs (protocol number 19-038, approval 06.08.2019). Volunteers acting as arm feeders were registered with LSTM as mosquito colony arm feeders and had previously signed consent forms which were kept on file.
Results
Total mosquito activity at untreated and treated nets
A total of 3725 mosquitoes were assessed over 745 WHO cone tests using strains of KS, NG, BF and VK7 exposed to P2, OS, P3, IG2 and UT nets. Mosquitoes exposed to UT nets had a propensity to crawl along the net surface (Fig. 2a). The mean total movement events observed during cone tests on UT nets was 4175 (SD 2154) in KS, 4671 (SD 1655) in NG, 3636 (SD 1619) in BF and 1975 (SD 1769) in VK7 (Fig. 3). Susceptible strains typically had higher total activity than resistant strains. During exposure to treated nets, mosquito activity became dispersed throughout the cone and crawling on the net surface was reduced (Fig. 2b). The mean total movement events (Fig. 3) during ITN tests by strain was 4905 (SD 1120) in KS, 5326 (SD 904) in NG, 4022 (SD 1470) in BF, and 2737 (SD 1582) in VK7. Significant differences between the total movement comparing UT nets and ITNs by strain are indicated in Additional file 2: Table S1.
Composite outputs from individual ViCTA analyses demonstrate behavioural differences between untreated and insecticide-treated bednets. a composite summary image using the Kisumu strain of An. gambiae (KS) on an untreated net constructed by sampling video every 0.1 s and merging darkest components of each frame, b Composite summary output of KS on net treated with Interceptor G2 (IG2), c composite image of N’gousso strain of An. coluzzii (NG) on untreated net, d Composite summary output of NG exposed to PermaNet 3
Regional mosquito activity at ITNs
The regional occupancy of mosquitoes in cones revealed variation in the responses of Anopheles spp. to different ITNs in terms of absolute (Fig. 4a) and relative (Fig. 4b) occupancy. During exposure to P2, mosquitoes were 1.48–2.11 times more active in the upper half of the cone (UHC) than in the UT net (Table 1, P2 vs UT upper region: KS OR 2.11; 95% CI 1.64, 2.7; P ≤0.0010; NG OR 1.78; 95% CI 1.39, 2.27; P ≤ 0.0010; BF OR 1.48; 95% CI 1.14, 1.92; P ≤ 0.0010; VK7 OR 1.67; 95% CI 1.29, 2.15, P ≤ 0.0010). Although activity was increased, there were no significant differences in the proportion of time spent in the upper and lower parts of the cone compared to the UT net (borderline result for KS Table 2 P2 vs UT: KS OR 0.51; 95% CI 0.25, 1.02; P = 0.0582).
In OS tests, all strains spent 1.55–2.67 times more time in the UHC than in the UT net, and the proportional LHC occupancy was 66–75% lower (Table 1, OS vs UT upper region: KS OR 2.67; 95% CI 2.1, 3.41; P ≤ 0.0010; NG OR 2.54; 95% CI 2.01, 3.2; P ≤ 0.0010; BF OR 1.75; 95% CI 1.31, 2.34; P ≤ 0.0010; VK7 OR 1.55; 95% CI 1.21, 1.98, P ≤ 0.0010; Table 2 OS vs UT KS OR 0.34; 95% CI 0.25, 1.02; P = 0.0582; NG OR 0.59; 95% CI 0.29, 1.18; P = 0.1922; BF OR 0.56; 95% CI 0.26, 1.22; P = 0.2117; VK7 OR 0.72; 95% CI 0.36, 1.42, P = 0.5579); in P3 tests, mosquitoes spent 1.48–3.6 times more time in the UHC and were 65% less likely to move in the LHC (Table 1, P3 vs UT upper region: KS OR 2.79; 95% CI 2.16, 3.60; P ≤ 0.0010; NG OR 3.38; 95% CI 2.67, 4.27; P ≤ 0.0010; BF OR 1.48; 95% CI 1.13, 1.94; P = 0.0015; VK7 OR 2.43; 95% CI 1.91, 3.08, P ≤ 0.0010; Table 2, P3 vs UT: KS OR 0.35; 95% CI 0.18, 0.71; P ≤ 0.0010; NG OR 0.23; 95% CI 0.11, 0.46; P ≤ 0.0010; BF OR 0.55; 95% CI 0.25, 1.21; P = 0.1930; VK7 OR 0.23; 95% CI 0.11, 0.45, P ≤ 0.0010). Insecticide-susceptible and insecticide-resistant strains exhibited different behaviours during exposure to IG2: in the susceptible strains, between 50 and 74% less activity was observed in the LHC during IG2 tests compared to the UT net (Table 2 IG2 vs UT: KS OR 0.50; 95% CI 0.25, 0.99; P = 0.0470; NG OR 0.26; 95% CI 0.13, 0.52; P ≤ 0.0010), which was not observed in the two resistant strains (Table 2 IG2 vs UT: BF OR 1.74; 95% CI 0.8, 3.75; P = 0.2372; VK7 OR 0.55; 95% CI 0.28, 1.08; P = 0.1025).
Resting behaviour
Although mosquito resting could be indirectly inferred as the reciprocal of mosquito movement activity counts per frame (5 − n, where n is the number of moving mosquitoes detected per frame), a stricter measure was used. A resting frame was defined as a video frame where none of the five mosquitoes moved, compared to the previous frame. The total number of resting frames was calculated for each assay. Resting behaviour showed stark differences between susceptible and resistant strains (Fig. 5) with VK7 mosquitoes found to be resting for a large proportion of the cone assays.
Mean mosquito resting period by strain and treatment. Seconds count based on number of measured frames where none of the five mosquitoes moved compared to the previous frame, out of a total of 1800 frames over the 3 min of the cone test. Numbers adjacent to each bar indicate mean number of seconds resting
Blood-feeding success
Over 92% of mosquitoes successfully blood-fed after tests with UT nets, with most mosquitoes feeding at 1 h [KS 91.7% (176/192); NG 96% (192/200); BF 95.88% (93/97) and VK7 93.43% (199/213)]. After ITN tests, none of the mosquitoes exposed to P3, or the susceptible strains exposed to P2 and OS, survived long enough to feed. Of the few KS and NG mosquitoes that lived long enough to take a blood meal after IG2 tests, approximately half fed at 1 h PE (PE) [KS 53.85% (7/13), NG 53.33% (16/30)]. In the resistant strains, compared to UT nets, BF mosquitoes were at least 90% less likely to feed at 1 h PE when exposed to ITNs (Additional file 2: Table S2 BF: IG2 OR 0.10; 95% CI 0.00, 2.56; P = 0.2325; OS OR 0.01; 95% CI 0.00, 0.25; P = 0.0028; P2 OR 0.00; 95% CI 0.00, 0.02; P ≤ 0.0010). P2 had the largest immediate inhibitory effect [blood-feeding success = 9.8% (9/92)], followed by OS [48.57% (17/35)] and IG2 [61.86% (73/118)]. At 24 h PE, approximately one third of BF mosquitoes (31.11%, 14/45) that were unfed at 1 h after IG2 exposure, and all mosquitoes that were unfed at 1 h after exposure to P2 and OS tests, blood-fed (P2 n = 83; OS n = 18).
ITN exposure did not have a significant effect on the blood-feeding behaviour of VK7 mosquitoes at either 1 h or 24 h PE. Most mosquitoes successfully fed at 1 h [P2: 93.90% (77/82); OS: 87.50% (42/48); IG2 62.07% (18/29)].
Blood meal weight
The mean total weight of blood meals taken after tests with UT netting was 12.47 µg (SD = 7.64). BMW per strain on UT netting was as follows: KS 12.26 µg (SD = 7.89), NG 11.74 µg (SD = 6.13), BF 10.48 µg (SD = 6.42) and VK7 13.10 µg (SD = 8.93). After ITN exposure, blood meal weights decreased (range 6.86 µg to 12.20 µg Fig. 6 and Additional file 2: Table S3), significantly so in NG and VK7 strains (NG χ2 = 5.47; df = 1; P = 0.0193; VK7 χ2 = 13.94; df = 3; P = 0.0030), where, compared to UT, at least 4.0 µg less blood was ingested (Additional file 2: Table S3 NG IG2 = −4.44 µg; 95% CI −8.23, −0.65; P = 0.0225, VK7: OS = −4.06 µg; 95% CI −6.84, −1.27; P = 0.0045 and P2 = −4.19 µg; 95% CI −7.12, −1.36; P = 0.0042). Activity in the LHC during tests was significantly associated with smaller blood meal weights in VK7 mosquitoes, and blood meals became 9.3 times smaller as activity increased (estimate = −9.35 µg; 95% CI −17.76, −0.93; P = 0.0298) regardless of treatment.
In VK7 mosquitoes that could not feed at 1 h PE but which recovered to feed at 24 h PE, blood meal weights were 1.45 times smaller blood meals compared to those that fed at 1 h PE (estimate = −1.45 µg; 95% CI −2.93, −0.03; P = 0.0551). The sizes of individual mosquitoes had a significant effect on blood meal weights in the NG, BF and VK7 strains (NG χ2 = 5.94; df = 1; P = 0.0148; BF χ2 = 17.55; df = 1; P < 0.0001; and VK7 χ2 = 11.18; df = 1; P = 0.0008) and as size increased, so did the size of the blood meal (NG estimate = 5.49; 95% CI 0.91, 10.03; P = 0.0201, BF estimate = 8.28; 95% CI 4.09, 12.47; P ≤ 0.0010 and VK7 estimate = 4.19; 95% CI 1.73, 6.65; P ≤ 0.0010).
Longevity
After UT net tests, the 24-h mortality was 0.48–5.45% and the median longevity was 12–14 days (Additional file 2: Table S4, Additional file 2: S5). For all ITNs, 24-h mortality for KS and NG was 85.37–100% and 3.46–20.83% for resistant BF and VK7 (Additional file 2: Table S4). No mosquitoes survived P3 exposure and no susceptible mosquitoes survived P2. The median longevity for resistant strains after ITN tests was 9–14 days (Additional file 2: Table S5). KS and NG mosquitoes were at least 47.55 times more likely to die when exposed to IG2 or OS compared to a UT net (Additional file 2: Table S4).
The longevity of KS mosquitoes was influenced by activity during tests and mosquitoes were 11.93 times more likely to die as the LHC activity increased regardless of treatment (including UT). In VK7 mosquitoes, observed longevity also was influenced by the activity in the cone during tests (treatment*activity interaction: χ2 = 9.11; df = 3; P = 0.0278) and as the proportion of activity in the LHC increased, the likelihood of dying increased for UT and P2 (Additional file 2: Fig. S3a). In IG2 tests, mosquitoes were more likely to die when greater activity was recorded in the UHC, and, compared with UT, mosquitoes were significantly more likely to die when the proportion of activity close to the net was less than 50% (Additional file 2: Fig. S3b).
Mosquitoes that did not blood-feed at 1 h PE were at least 17 times more likely to die at 24 h PE (Additional file 2: Table S4 KS χ2 = 0.00; df = 1; P = 0.9987, NG χ2 = 25.80; df = 1; P < 0.0001, BF χ2 = 19.40; df = 1; P < 0.0001 and VK7 χ2 = 24.54; df = 1; P < 0.0001). The amount of blood ingested had a slight impact on longevity: BF and VK7 mosquitoes were 7% and 2% less likely to die as the BMW increased, respectively (Additional file 2: Table S5 BF HR 0.93; 95% CI 0.90, 0.93; P < 0.0001 and VK7 HR 0.98; 95% CI 0.97, 0.99; P = 0.0012). The effect of treatment on longevity varied with wing length for all strains, for example, smaller mosquitoes lived longer PE to UT netting (all strains) and were more likely to die if they were exposed to IG2 for all the strains except BF (Additional file 2: Fig. S2 a, b).
Discussion
The laboratory methods that are currently used to evaluate ITN performance suffer from a lack of correlation between laboratory and semi-field findings, and the WHO cone test, the most widely implemented laboratory test, does not incorporate mosquito–host responses, mosquito–ITN interactions or post-exposure life history monitoring into its outcome measures [41]. Incorporating video capture and static scan-sampling analysis into the WHO cone test method in a previous study revealed differences in the behaviours of insecticide-susceptible and resistant mosquitoes in response to pyrethroid ITNs, which were modified depending on whether a human host attractant was present [14]. In this study the potentially subjective and time-consuming (approximately 10 min per video) manual scan sampling analysis was replaced by an automated movement quantification method that detected the mosquitoes’ spatial location and movements at 0.1 s intervals, providing an increase from 36 data sample points to 1800 in a typical run time of less than 2 min. In addition to confirming the irritant responses to ITNs previously shown [14, 42], in this study we demonstrate that the amount that a mosquito moves when exposed to an ITN directly impacts subsequent life history such as blood meal sizes and post-exposure life spans.
Within the cone, all mosquitoes responded to ITNs, reducing the amount of time spent crawling on the net surface. Mosquitoes responded the least strongly to PermaNet 2.0, confirming observations in previous wind tunnel experiments that showed a lack of close-range excito-repellent effects during exposure to deltamethrin-treated ITNs [27] and demonstrating that activity tracking in a small-scale cone can be used instead of additional experimental set-ups to characterise the excito-repellent and irritant properties of ITNs. Olyset Net and P3 nets provoked the strongest irritant responses; such responses to OS have been observed previously [42], whilst the increased irritant responses that were observed in PermaNet 3.0 compared to PermaNet 2.0 could be due to the doubled concentration of deltamethrin in the combination net [43, 44].
The amount and location of mosquito activity within the cone had a direct influence on the subsequent observed longevity. Kisumu strain mosquitoes were much more likely to die if they spent more time active at the net surface, even during UT net tests. Notably, in Interceptor G2 tests, the more time in which resistant mosquitoes spent flying away from the net surface, the higher the risk of dying. It has been previously found that the testing modality used for Interceptor G2 bioassays influences observed mortality [25, 45]. Our results suggest that mosquito activity during bioassays forms a key component of subsequent mortality, and that capturing such activity can be useful in understanding the impact of chemistries with delayed mortality effects.
All ITNs tested inhibited blood-feeding at 1 h PE in the resistant BF strain and in the few insecticide-susceptible mosquitoes that survived. By 24 h, the response to host cues was restored in mosquitoes exposed to P2 and OS nets. This waning of inhibition, combined with previous studies demonstrating that mosquitoes that feed successfully through a permethrin-treated net both recover from and lose permethrin-associated feeding inhibition [42, 46], suggests that the blood-feeding inhibition observed at 1 h would not be repeated if those mosquitoes were offered subsequent blood meals. As mosquitoes may encounter ITNs multiple times during their life span, incorporating multiple exposures and blood-feeding opportunities into post-test monitoring may enable this test system to record a more realistic ‘whole life’ impact on mosquitoes [24, 42, 46, 47]. Although no significant effects on blood-feeding success in VK7 mosquitoes were observed in post-ITN exposure, as the amount of movement in the lower half of the cone increased, the size of the blood meal ingested was reduced (regardless of whether the meal was at 1 or 24 h PE). This reduced blood meal weight had significant effects on longevity in resistant strains: BF mosquitoes were 7.1% less likely to die per unit of blood meal weight increase, and VK7 mosquitoes were 2.2% less likely to die per unit of blood meal weight increase.
The changes observed in the character of mosquito–ITN interactions within an overall lack of significant changes in total mosquito activity suggest that the observed irritancy with the four tested ITNs is unlikely to reduce mosquito contact time below that required to deliver a lethal dose of insecticide [48]. Such profiles could be used by bednet developers to assess the likely impact of new chemistries on mosquito behaviour and optimise the net profile for maximum mortality or sub-lethal impairment [42]. Furthermore, the ability to identify strain-specific differences, such as the different responses shown by insecticide-susceptible and insecticide-resistant mosquito strains to IG2 exposure, could be useful to both manufacturers who wish to design a bednet to target a specific mosquito population and to control programmes aimed at assessing the likely efficacy of an ITN against local vector populations.
As the ViCTA method uses an augmentation of the WHO cone assay, it has some limitations imposed by the assay design. The shape of the cone is not an ideal ‘window’ through which to observe flight behaviour; the curvature of the cone affects the light transmission, and focusing at the cone sides and the lip at the top of the cone can temporarily occlude moving mosquitoes. The placement angle of the camera (designed to capture the net surface in addition to the upper parts of the cone) and the steeply inward tapering of the cone impede the absolute determination of vertical mosquito flight, and thus changes in cone region occupancy are best evaluated as relative occupancy proportions. A more bespoke equipment setup such as that used in the baited box study [49] would resolve some of these issues, but an advantage of the ViCTA test is the use of a standard cone assay configuration. The background subtraction mechanism used in the ViCTA algorithm detects only moving mosquitoes, and the resting period (inactivity) of individual mosquitoes may only be inferred (as 5 − n, where n is the number of moving mosquitoes per frame). The stricter measure of resting used in this report (consecutive frames where none of the five mosquitoes moved) demonstrated large differences in resting between susceptible and resistant strains (Fig. 5) on UT and treated nets. It is interesting to speculate as to how the genetic changes which confer resistance to these strains may also impact mosquito behaviour, resulting in the relative lack of activity during the assays. A more complex time series analysis of resting behaviour and spatio-temporal patterns of activity during the course of the test is underway and may yet yield more subtle behavioural features.
Conclusions
By using a simple augmentation of the WHO cone bioassay combined with automated video analysis we were able to demonstrate a wide range of complex behavioural responses of mosquitoes in response to insecticide-treated nets during the bioassay. Such behavioural responses (for example, the strong mosquito preference for the top surface of a bednet) have already been proven invaluable in the translation of this information to bednet designs [48, 50], with some dual-active-ingredient (AI) ITNs exploiting these preferences by specifically adding higher concentration or combination AIs to these regions [51]. Detailed behavioural insights can speed up the development process whilst reducing development costs and minimising the public health and financial risks of deploying ineffective vector control tools. Furthermore, these novel assays exploit complex video recording and information processing techniques that are now found in inexpensive and widely available technologies, helping to democratise their widespread adoption.
Availability of data and materials
ViCTA software and designs for cone test rig equipment are freely available on request to authors.
References
Bhatt S, Weiss D, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207.
Pryce J, Richardson M, Lengeler C. Insecticide-treated nets for preventing malaria. Cochrane Database Syst Rev. 2018;11:CD000363.
Birget PLG, Koella JC. An epidemiological model of the effects of insecticide-treated bed nets on malaria transmission. PLoS ONE. 2015;10:e0144173.
Sherrard-Smith E, Skarp JE, Beale AD, Fornadel C, Norris LC, Moore SJ, et al. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc Natl Acad Sci. 2019;116:15086–95.
Strode C, Donegan S, Garner P, Enayati AA, Hemingway J. The impact of pyrethroid resistance on the efficacy of insecticide-treated bed nets against African anopheline mosquitoes: systematic review and meta-analysis. PLoS Med. 2014;11:e1001619.
Tainchum K, Nararak J, Boonyuan W, Bangs MJ, Chareonviriyaphap T. Behavioral responses of Anopheles species (Culicidae: Diptera) with varying surface exposure to pyrethroid-treated netting in an excito-repellency test system. J Vector Ecol. 2016;41:254–64.
Yamada H, Soliban SM, Vreysen MJB, Chadee DD, Gilles JRL. Eliminating female Anopheles arabiensis by spiking blood meals with toxicants as a sex separation method in the context of the sterile insect technique. Parasit Vectors. 2013;6:1–10.
Glunt KD, Abı́lio AP, Bassat Q, Bulo H, Gilbert AE, Huijben S. Long-lasting insecticidal nets no longer effectively kill the highly resistant Anopheles funestus of southern Mozambique. Malaria J. 2015;14:1–7.
Hancock PA, Hendriks CJM, Tangena J-A, Gibson H, Hemingway J, Coleman M, et al. Mapping trends in insecticide resistance phenotypes in African malaria vectors. PLoS Biol. 2020;18:e3000633.
Kleinschmidt I, Bradley J, Knox TB, Mnzava AP, Kafy HT, Mbogo C, et al. Implications of insecticide resistance for malaria vector control with long-lasting insecticidal nets: a WHO-coordinated, prospective, international, observational cohort study. Lancet Infect Dis. 2018;18:640–9.
Kleinschmidt I, Mnzava AP, Kafy HT, Mbogo C, Bashir AI, Bigoga J, et al. Design of a study to determine the impact of insecticide resistance on malaria vector control: a multi-country investigation. Malar J. 2015;14:1–13.
Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32:187–96.
Riveron JM, Huijben S, Tchapga W, Tchouakui M, Wondji MJ, Tchoupo M, et al. Escalation of pyrethroid resistance in the malaria vector Anopheles funestus induces a loss of efficacy of piperonyl butoxide–based insecticide-treated nets in Mozambique. J Infect Dis. 2019;220:467–75.
Hughes A, Matope A, Emery M, Steen K, Murray G, Ranson H, et al. A closer look at the WHO cone bioassay: video analysis of the hidden effects of a human host on mosquito behaviour and insecticide contact. Malar J. 2022;21:1–11.
World Health Organization. WHO guidance note for estimating the longevity of long-lasting insecticidal nets in malaria control. Geneva: World Health Organization; 2013.
Lindsay SW, Thomas MB, Kleinschmidt I. Threats to the effectiveness of insecticide-treated bednets for malaria control: thinking beyond insecticide resistance. Lancet Glob Health. 2021;9:e1325–31.
Mulligan F, Schaefer C. Efficacy of a juvenile hormone mimic, pyriproxyfen (S-31183), for mosquito control in dairy wastewater lagoons. J Am Mosq Control Assoc. 1990;6:89–92.
Yapabandara A, Curtis C, Wickramasinghe M, Fernando W. Control of malaria vectors with the insect growth regulator pyriproxyfen in a gem-mining area in Sri Lanka. Acta Trop. 2001;80:265–76.
Oxborough RM, N’Guessan R, Jones R, Kitau J, Ngufor C, Malone D, et al. The activity of the pyrrole insecticide chlorfenapyr in mosquito bioassay: towards a more rational testing and screening of non-neurotoxic insecticides for malaria vector control. Malar J. 2015;14:1–11.
Raghavendra K, Barik TK, Sharma P, Bhatt RM, Srivastava HC, Sreehari U, et al. Chlorfenapyr: a new insecticide with novel mode of action can control pyrethroid resistant malaria vectors. Malar J. 2011;10:1–7.
MacDonald M. Landscape of new vector control products. Baltimore: John Hopkins Center for Communication Programs; 2015.
Hauser G, Thiévent K, Koella JC. The ability of Anopheles gambiae mosquitoes to bite through a permethrin-treated net and the consequences for their fitness. Sci Rep. 2019;9:1–8.
Barreaux P, Koella JC, N’Guessan R, Thomas MB. Use of novel lab assays to examine the effect of pyrethroid-treated bed nets on blood-feeding success and longevity of highly insecticide-resistant Anopheles gambiae s.l. mosquitoes. Parasit Vectors. 2022;15:1–9.
Viana M, Hughes A, Matthiopoulos J, Ranson H, Ferguson HM. Delayed mortality effects cut the malaria transmission potential of insecticide-resistant mosquitoes. Proc Natl Acad Sci. 2016;113:8975–80.
Kibondo UA, Odufuwa OG, Ngonyani SH, Mpelepele AB, Matanilla I, Ngonyani H, et al. Influence of testing modality on bioefficacy for the evaluation of Interceptor® G2 mosquito nets to combat malaria mosquitoes in Tanzania. Parasit Vectors. 2022;15:1–18.
Spitzen J, Koelewijn T, Mukabana WR, Takken W. Visualization of house-entry behaviour of malaria mosquitoes. Malar J. 2016;15:1–10.
Spitzen J, Ponzio C, Koenraadt CJ, Jamet HVP, Takken W. Absence of close-range excitorepellent effects in malaria mosquitoes exposed to deltamethrin-treated bed nets. Am J Trop Med Hyg. 2014;90:1124–32.
Parker JE, Jaimes NCA, Gleave K, Mashauri F, Abe M, Martine J, et al. Host-seeking activity of a Tanzanian population of Anopheles arabiensis at an insecticide treated bed net. Malar J. 2017;16:1–14.
Toé KH, N’Falé S, Dabiré RK, Ranson H, Jones CM. The recent escalation in strength of pyrethroid resistance in Anopheles coluzzi in West Africa is linked to increased expression of multiple gene families. BMC Genomics. 2015;16:1–11.
Williams J, Ingham VA, Morris M, Toé KH, Hien AS, Morgan JC, et al. Sympatric populations of the Anopheles gambiae complex in Southwest Burkina Faso evolve multiple diverse resistance mechanisms in response to intense selection pressure with pyrethroids. Insects. 2022;13:247.
Briegel H. Determination of uric acid and hematin in a single sample of excreta from blood-fed insects. Experientia. 1980;36:1428–528.
Lyimo EO, Takken W. Effects of adult body size on fecundity and the pre-gravid rate of Anopheles gambiae females in Tanzania. Med Vet Entomol. 1993;7:328–32.
Power PW, Schoonees JA. Understanding background mixture models for foreground segmentation. Auckland: Industrial Research Limited; 2002. p. 10–1.
Allaire J. RStudio: integrated development environment for R. Boston MA. 2012;770:165–71.
R Core Team. R: a language and environment for statistical computing, R Foundation for Statistical Computing. 2022
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015; 67(1):1–48
Brooks ME, Kristensen K, Van Benthem KJ, Magnusson A, Berg CW, Nielsen A, et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017;9:378–400.
Therneau TM, Therneau MTM. Package ‘coxme.’ R Package Version. 2015;2:5.
Hartig F. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.3. 3.0. CRAN 2020.
Russell L. emmeans: Estimated marginal means, aka least-squares means R package version 1.4.3.01. Iowa: The University of Iowa; 2019.
Sokhna C, Ndiath M, Rogier C. The changes in mosquito vector behaviour and the emerging resistance to insecticides will challenge the decline of malaria. Clin Microbiol Infect. 2013;19:902–7.
Siegert PY, Walker E, Miller JR. Differential behavioral responses of Anopheles gambiae (Diptera: Culicidae) modulate mortality caused by pyrethroid-treated bednets. J Econ Entomol. 2009;102:2061–71.
World Health Organization. Vector Control Products: PermaNet 2.0. Geneva: World Health Organization; 2017.
World Health Organization. Vector Control Products: PermaNet 3.0. Geneva: World Health Organization; 2018.
Tungu PK, Michael E, Sudi W, Kisinza WW, Rowland M. Efficacy of interceptor® G2, a long-lasting insecticide mixture net treated with chlorfenapyr and alpha-cypermethrin against Anopheles funestus: experimental hut trials in north-eastern Tanzania. Malar J. 2021;20:1–15.
Mulatier M, Pennetier C, Porciani A, Chandre F, Dormont L, Cohuet A. Prior contact with permethrin decreases its irritancy at the following exposure among a pyrethroid-resistant malaria vector Anopheles gambiae. Sci Rep. 2019;9:1–11.
Iacovidou MA, Barreaux P, Spencer SE, Thomas MB, Gorsich EE, Rock KS. Omitting age-dependent mosquito mortality in malaria models underestimates the effectiveness of insecticide-treated nets. PLoS Comput Biol. 2022;18:e1009540.
Parker JE, Angarita-Jaimes N, Abe M, Towers CE, Towers D, McCall PJ. Infrared video tracking of Anopheles gambiae at insecticide-treated bed nets reveals rapid decisive impact after brief localised net contact. Sci Rep. 2015;5:13392.
Hughes A, Foster GM, Guy A, Matope A, Abe M, Towers D, et al. Quantifying late-stage host-seeking behaviour of Anopheles gambiae at the insecticidal net interface using a baited-box bioassay. Malar J. 2020;19:1–11.
Lynd A, McCall PJ. Clustering of host-seeking activity of Anopheles gambiae mosquitoes at the top surface of a human-baited bed net. Malar J. 2013;12:1–8.
Vestergaard. Vestergaard: PermaNet 3.0. https://vestergaard.com/products/public-health/permanet-3-0/. Accessed 15 April 2023
Acknowledgements
ITNs used in this study were kindly provided by Vestergaard Frandsen, Sumitomo Chemical Co., Ltd. and BASF. The net manufacturers had no role in the design of the study or interpretation of results.
Funding
This research was funded by the Bill & Melinda Gates Foundation [under Grant Agreement No. OPP1200155]. The findings and conclusions contained within are those of the authors and do not necessarily reflect the positions or policies of the Bill & Melinda Gates Foundation.
Author information
Authors and Affiliations
Contributions
JJ conceived and developed the ViCTA software and conducted data management and analysis. AM developed and implemented data conversion and statistical analysis methods. PB performed data management, wing measurements and data analysis. KG and KS performed the cone assays and post-exposure follow-up studies. HR, PJM and GF secured project funding and project support. All authors contributed to the writing and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
13071_2023_6029_MOESM1_ESM.docx
Additional file 1: Figure S1. Flowchart of image analysis pipeline during a ViCTA experiment (top) and output files exported at the end of an experiment (bottom).
13071_2023_6029_MOESM2_ESM.docx
Additional file 2: Figure S2. Treatment effect on longevity as the wing length measurement increases by An. gambiae strain based on the fitted mixed-effects Cox regression model. a Effect by treatment, b contrast (insecticide-treated net [ITN] vs untreated netting) effect, KS Kisumu, NG N’Gousso, BF Banfora, UT untreated net, IG2 Interceptor® G2 net, OS Olyset net, P2 PermaNet 2.0 net, and the solid black horizontal line is the reference (panel b, HR 1) with values above 1 showing more likelihood of dying when exposed to an ITN compared to UT and vice versa. Figure S3. Treatment effect on longevity as the activity at the lower half of the cone increases based on the fitted mixed-effects Cox regression model (VK7 strain only). a Effect by treatment, b contrast (insecticide-treated net [ITN] vs untreated netting) effect, UT untreated net, IG2 Interceptor® G2 net, OS Olyset net, P2 PermaNet 2.0 net, and the solid black horizontal line is the reference (panel b, HR 1) with values above one showing more likelihood of dying when exposed to an ITN compared to UT and vice versa. Table S1. Total movement activity, insecticide treated net versus untreated netting comparisons within An. gambiae strain from a linear regression model. Multiple pairwise comparisons 95% confidence intervals and P-values corrected using the Dunnett adjustment. UT untreated net, IG2 Interceptor® G2 net, OS Olyset net, P2 PermaNet 2.0 net, P3 PermaNet 3.0 net, KS Kisumu, NG N’Gousso and BF Banfora. Table S2. Treatment comparisons for blood-feeding success post-exposure based on generalised linear mixed models (GLMMs) with a binomial distribution fitted for each An. gambiae strain separately within the time the blood meal was offered (1 or 24 h). Only the predictor variables in the final model for each strain are provided. KS Kisumu, NG N’Gousso, BF Banfora, UT untreated net, IG2 Interceptor® G2 net, P2 PermaNet 2.0 net, OS Olyset net, Ref reference group, OR odds ratio, CI confidence interval, *significant at 5% significance level. Random effects include testing day and replicate. Table S3. Blood meal size results based on linear mixed effects models fitted for each An. gambiae strain separately. Only the predictor variables in the final model for each strain are provided. KS Kisumu, NG N’Gousso, BF Banfora, UT untreated net, IG2 Interceptor® G2 net, P2 PermaNet 2.0 net, OS Olyset net, Ref reference group, CI confidence interval, SD standard deviation, cone activity = predicted proportion of activity at the lower half of the cone during exposure (behaviour data), DE during exposure, *significant at 5% significance level. Random effects include testing day and replicate. Table S4. Mortality at 24-h results based on generalised linear mixed models (GLMMs) with a binomial distribution fitted for each An. gambiae strain separately within the time the blood meal was offered (1 or 24 h). Only the predictor variables in the final model for each strain are provided. KS Kisumu, NG N’Gousso, BF Banfora, UT untreated net, IG2 Interceptor® G2 net, P2 PermaNet 2.0 net, OS Olyset net, Ref reference group, OR odds ratio, CI confidence interval, *significant at 5% significance level. Random effects include testing day and replicate. Table S5. Longevity results based on the final model mixed-effects Cox regression models fitted for each An. gambiae strain separately. Only the predictor variables in the final model for each strain are provided. KS Kisumu, NG N’Gousso, BF Banfora, UT untreated net, IG2 Interceptor® G2 net, P2 PermaNet 2.0 net, OS Olyset net, Ref reference group, CI confidence interval, SD standard deviation, min minimum, max maximum, HR hazard ratio, cone activity = predicted proportion of activity at the lower half of the cone during exposure (behaviour data), DE during exposure, *significant at 5% significance level and +overall descriptive results. Random effects include testing day and replicate.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jones, J., Matope, A., Barreaux, P. et al. Video augmentation of the WHO cone assay to quantify mosquito behavioural responses to insecticide-treated nets. Parasites Vectors 16, 420 (2023). https://doi.org/10.1186/s13071-023-06029-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-06029-z