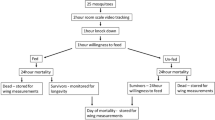
Abstract
Background
The WHO cone test is one of three tests currently used to evaluate the efficacy of insecticide-treated bed nets (ITNs). It generates two test outputs, knockdown and 24-h mortality, both indicative of immediate toxicity but that reveal little about the nature of mosquito and ITN interaction or how results translate to real-world settings.
Methods
A human arm held 5 mm behind the net surface acted as a host attractant during cone tests and a smartphone was used to capture mosquito behaviour in the cone. Post-exposure blood feeding and survival for nine days were recorded; ingested blood meal size was determined by measuring excreted haematin. Four strains of Anopheles gambiae (insecticide susceptible: Kisumu and N’gousso; insecticide resistant: Banfora and VK7) were tested with and without the host attractant using untreated, Permanet 2.0 and Olyset nets. Video recordings were scan sampled every five seconds to record mosquito positions on either the net, in flight or in contact with the cone. Generalized estimating equations were used to analyse all data except survival within nine days which was analysed using Weighted Cox Regression.
Results
Net contact was the most frequently recorded behaviour in all Anopheles spp. strains on all nets. Adding the human host as attractant triggered excitatory behaviours: in all strains, the magnitude of net contact was significantly decreased compared to tests without a host. ITN exposure altered the observed behaviour of the two susceptible strains, which exhibited a decreased response to the host during ITN tests. The resistant strains did not alter their behaviour during ITN tests. Significantly less net contact was observed during Olyset Net tests compared to Permanet 2.0. The host presence affected survival after exposure: Banfora and VK7 mosquitoes exposed to Permanet 2.0 with a host lived longer compared to tests performed without a host. However, mosquitoes that blood-fed and survived long enough to digest the blood meal did not exhibit significantly reduced longevity regardless of the presence of the host attractant.
Conclusions
Simple modifications to the WHO cone test and extension of post-test monitoring beyond the current 24 h enable detailed behavioural characterizations of individual ITNs to be compiled. The effects observed from testing with a host and including blood feeding suggest that more representative estimates of true of ITN efficacy are gained with these modifications than when using the current testing protocol.
Similar content being viewed by others
Background
Insecticide-treated nets (ITNs) are a fundamental tool in the continuing drive for sustainable malaria reduction and elimination in at-risk communities in Africa [1,2,3]. Widespread insecticide resistance in the major African malaria vectors Anopheles gambiae sensu lato (s.l.) and Anopheles funestus s.l. has reduced the entomological efficacy of ITNs; by reducing mosquito mortality, the critical community effect achieved through widespread ITN usage is diminished [4,5,6,7,8,9]. Although in some resistant mosquito populations sub-lethal insecticide doses can deliver reductions in vector longevity below that required to transmit the malaria parasite, studies conducted in highly-resistant vector populations in Burkina Faso indicate that as resistance intensity increases, such sub-lethal impacts disappear [10, 11]. In the current landscape where a plurality of resistance states exists across African Anopheline vector populations, a nuanced approach to ITN efficacy evaluations that characterizes mosquito-ITN interactions and incorporates measures of insecticide-induced impairment has the potential to deliver additional insights, especially in the advent of combination ITNs containing two or more active ingredients [12, 13].
Laboratory measurements of ITN entomological efficacy predominantly depend on results from the WHO cone test, which was introduced in the 1980s and is designed to capture the rapid toxicity effects of pyrethroid insecticides using two post-exposure endpoints: knock down (KD) at 60 min and 24 h mortality [14, 15]. The WHO cone test does not incorporate a host to which the mosquito can respond, unlike the other two procedures described in the current World Health Organization (WHO) ITN testing guidelines, the tunnel test and experimental hut trials [16]. However, the secondary tunnel test incorporates a non-human host, typically a rabbit or guinea pig, and experimental hut trials incorporate a human host but are performed after the laboratory stages of efficacy evaluations are complete [16]. Thus, the laboratory evaluations of ITN efficacy are primarily performed using a test that does not assess the efficacy of insecticide-treated materials in the presence of human prey, and for which the limited applicability of results to ‘real life’ situations has previously been noted [17].
To understand how effective an ITN might be once deployed into communities, there is an urgent need for bioassays that are able to characterize vector behavioural responses to, and downstream insecticidal effects of, ITN exposure. Such bioassays should be reasonably high-throughput, produce test outputs that capture a range of ITN modes of action, and able to be readily implemented into existing laboratory and field station set ups [18, 19]. Systems using infra-red video capture and tracking to characterize the entomological mode of action of ITNs have been previously described, but these tests are large-scale, difficult to transport and expensive, rendering them unsuitable for mass implementation into evaluation laboratories and stations [13, 20].
This report describes investigations of adaptations to the WHO cone test that allow the characterization of behavioural responses to ITNs through the incorporation of a host attractant and the addition of video capture. An extended post-exposure monitoring pipeline is implemented to assess the contribution of insecticide resistance to the lifetime impact of active ingredient (AI) exposure.
Methods
Mosquito colonies
All experiments were performed at the Liverpool School of Tropical Medicine (LSTM) using unfed 3–5 day old female Anopheles gambiae s.l. adults. Four strains of An. gambiae s.l. mosquitoes were used in the experiments, two of which were insecticide susceptible (Kisumu, N’gousso) and two which were insecticide resistant (Banfora, VK7).
The An. gambiae sensu stricto (s.s.) Kisumu (KS) colony originated in Kenya [21] and has been maintained at LSTM since 1975. Anopheles coluzzii N’guosso (NG) was colonized from Cameroon in 2006 [22] and is susceptible to most classes of insecticide with low level of resistance to organochlorides, DDT and Dieldrin (LSTM profiling results). The An. coluzzii VK7 (Vallee du Kou, village no. 7) and Banfora (BF) strains from Burkina Faso were colonized in 2014, are resistant to pyrethroids and DDT [23,24,25] and are maintained under six-monthly selection pressure with deltamethrin. Both insecticide resistant strains have a high frequency of 1014F kdr and express elevated levels of P450s known to metabolize pyrethroids [25].
Insecticidal netting
Untreated polyester net (Bayer, Germany) was used as a baseline control. Two commercial ITNs were tested: PermaNet®2.0 (Vestergaard Frandsen, Switzerland, deltamethrin 1.4–1.8 g/kg, P2) and Olyset® Net (Sumitomo Chemical Ltd, Japan 2% (w/w) permethrin 20 g/kg, OS). ITNs were donated by the manufacturer, aired at ambient temperature for one week, then stored at 4 °C until acclimatization at laboratory temperature 24 h prior to testing.
Video Cone Test (VCT) apparatus and design
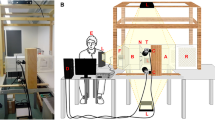
Two modifications were introduced to the WHO cone test [16]: (a) a basic smartphone (iPhone SE, release date Mar 2016) with video capture (60 frames per second (fps), 1080p HD, Apple Inc, USA); (b) a human attractant (host arm), positioned approximately 5 mm from the test netting. The smartphone was clamped to a 420 mm × 297 mm (H × W) cone board held at 45° by a bespoke fused PLA plastic filament base (237 mm × 280 mm × 266 mm (H × W × D) (Fig. 1A) and positioned such that the entire cone and net were visible in the recording field (Fig. 1B).
VCT apparatus and design. A bespoke stand made from fused PLA plastic filament (A) supports the Perspex cone board at 45°. Openings in the side of the stand allows the host arm to be placed horizontally behind the net. The smartphone is clamped to the board and positioned in front of the cone. The recording field (B) comprises of the entire volume of the cone and net. The presence of a gap between the lower rim and upper opening of the cone (red arrow) ensures the optimal camera angle and position
Experimental protocol
All experiments were performed in a climate-controlled laboratory (27 ± 2 °C, 80 ± 8% RH) after the first hour of the scotophase using mosquitoes acclimatized to laboratory conditions. Prior to testing, mosquitoes were starved of 10% sugar solution for at least five hours.
VCT
A minimum of 200 mosquitoes each of Kisumu, N’gousso, VK7 and Banfora strains were exposed to P2, OS and untreated nets. A total of 100 mosquitoes were exposed in the presence of the host attractant, (host present tests, HP) and 100 without (host absent tests, HA). Observed differences in mosquito behaviour between: (a) the presence and absence of the host attractant, and (b) untreated and insecticide-treated netting materials, were evaluated.
After exposure, 60-min KD, 24-h mortality and life history traits were recorded.
The number of replicates performed per day were allowed to fluctuate according to mosquito availability, to mimic routine availability in field conditions, with a minimum number of three test days per strain using untreated net and two days using each treated net to capture routine day-to-day test variation. To reduce inter-person variation, a single host was used for all host present tests.
Video analyses
Videos were converted to Windows Media Video (WMF) format using Windows Movie Maker (©2012 Microsoft Corporation, USA) and analysed using event-logging software BORIS [26]. The positions of all five mosquitoes were categorized as either ‘net’ (in contact with the net),’cone’ (in contact with the cone) or ‘in flight’ at five-second intervals for the duration of the 180-s assay, producing 36 data points per three-minute test. These data points were used for the statistical comparisons of observed mosquito behaviours in the cone.
Life history traits
Blood feeding at 1- and 24-h post-exposure, blood meal size and longevity were recorded. Briefly, after recording KD at 60 min, mosquitoes were given the opportunity to blood feed for 20 min on a human host arm. Mosquitoes that blood fed were transferred into individual 50 ml Falcon tubes with a lid of untreated netting to enter the monitoring pipeline. Unfed mosquitoes were offered a second blood meal 24 h post-exposure, and then transferred to individual Falcon tubes to begin monitoring. Seventy-two hours after mosquitoes were transferred to the Falcon tubes, mosquitoes were transferred into a fresh Falcon tube and the original housing was used to measure individual blood meal sizes using excreted haematin [27]. Briefly, the excreted haematin collected in individual falcon tubes was dissolved in 1 ml 1% Lithium carbonate and the absorbance at 397 nm measured in triplicate. The total concentration of haematin (µg/ml) in each sample was determined using a seven point standard curve comprising standards from 1.76 to 20 µg/ml. As the concentration of haematin is directly stoichiometrically related to haemoglobin input, which is indicative of the total volume of blood ingested, the haematin measurements were used as a measurement of ingested blood meal size [28].
Mosquitoes were maintained in individual housing with access to 10% sugar solution and mortality was recorded every 24 h until natural death, after which they were stored at 20 °C until measuring wing‐length as an index of mosquito mass [29]. The right wing of each mosquito was removed and mounted onto glass slides, and a GXCAM ECLIPSE Wi-Fi camera attached to a GX Stereo microscope (GT Vision Ltd) was used to capture an image. The length of the wing was measured from the axial vein to the distal end of the R1 vein using GXCAM software (GXCAM Ver.6.7).
Statistical analysis
Descriptive statistics were generated using number of observations, mean, standard deviation (SD), minimum, maximum, and coefficient of variation for continuous variables results, the number and percentage of observations for categorical variables, and number and percentage of events, mean, SD, minimum, maximum, median survival and its corresponding 95% confidence interval (CI) for time-to-event variables.
Within- and between-day test variability was used to evaluate VCT robustness. Variability of less than 30% within each An. gambiae strain in host absent tests using untreated net were considered to comply with previous measurements of variability within cone tests [30]. As the addition of a host attractant to the test was assumed to prompt behavioural changes in the test mosquitoes, and as no published precedent regarding small-scale responses to ITNs exists, an additional 10% variability in within- and between-day imprecision in host present untreated net tests were set as putative acceptability criteria for test acceptance. Any additional variability in behaviour during ITN tests was therefore assumed to be driven by the insecticidal net treatment.
A marginal logistic regression model using generalized estimating equations (GEE) was employed to analyse mosquito behaviour in the cone. The variance component covariance structure was used due to non-convergence issues of other complex covariance structures. The beta-binomial distribution and a logit link function were considered for the analysis, with the mean proportion of mosquitoes in contact with the net and flight or cone for the entire assay as an outcome. Host (absent or present), strain, treatment, location (net, flight or cone), and all the possible interactions among these variables were fitted as fixed effects and replicate as a random effect. The odds ratio (OR) together with its corresponding 95% CI of the proportion of contact between net and flight/cone were generated. Cone contact in all assays was minimal and unfluctuating; these data were combined with flight data for the statistical analyses. Comparisons were conducted comparing the tests where the host was present or absent within a net treatment (untreated, P2, OS) and within a test comparing the net treatments. Due to the multiple comparisons that were performed, the Bonferroni adjustment procedure was employed to control for the probability of making false positive findings.
For the life history traits, GEE was used to analyse the blood feeding (at 1 h or 24 h) post-exposure and blood meal volume, using the binomial distribution with a logit link function, and a normal distribution with an identity link function, respectively. Longevity within 9 days was analysed using Weighted Cox Regression to generate unbiased averaged hazard ratios (HR) together with their corresponding 95% CI since the proportional hazard assumption was violated [31]. This analysis was performed using the R Package coxphw with replicate as a cluster [32]. Treatment, wing-length measurement, time the mosquito fed post-exposure (1 h, 24 h, no feeding or dead), blood meal volume and the predicted mean proportion of mosquitoes in contact with the net were considered as fixed effects and replicate as a cluster. All comparisons were performed within strain and host (net treatment vs untreated) or treatment (host present vs absent).
All statistical tests were conducted at 5% significance level. Statistical analysis was performed using SAS software, Version 9.4 (© 2002–2012 SAS Institute Inc., Cary, NC, USA) and R 4.0.1 [33].
Ethical approval
Ethical approval was not required for this study as all activities were considered to fall under daily routine laboratory and colony maintenance tasks. Members of staff who performed post-exposure arm feeding were registered with LSTM as mosquito colony arm feeders and had previously signed consent forms which were kept on file. Arm feeding was considered part of routine daily colony maintenance activities.
Results
Evaluation of VCT robustness
Between 105 and 125 (host absent) and 100 and 130 (host present) An. gambiae mosquitoes per strain were used in untreated net tests (Additional file 1: Table S1), with a minimum of three test days per strain (maximum seven days, Additional file 1: Table S2). Net contact passed the robustness criteria for both within- and between-day imprecision, regardless of the number of replicates performed per day (Additional file 1: Tables S1 and S2). The total test imprecision for net contact was < 30% for each strain in host absent tests (KS 24.19%; NG 17.11%; BF 14.69%; VK7 13.56%, Additional file 1: Table S1) and < 40% for each strain in host present tests (KS 22.04%; NG 30.04%; BF 29.90%; VK7 26.19%, Additional file 1: Table S1); within each test day the maximum imprecision observed was 29.43%, 23.55%, 18.37% and 15.35% for Kisumu, N’gousso, Banfora and VK7 strains in host absent tests and 26.14%, 39.64%, 32.99%, 27.08% for Kisumu, N’gousso, Banfora and VK7 strains, respectively, in host present tests (Additional file 1: Table S2). No trends in imprecision were noted when net contact results were binned by timepoint (Additional file 1: Table S3), indicating the stability of using net contact as a measure of response.
Both flight and cone contact were too variable to be used as a robust measure of mosquito response (Additional file 1: Tables S1 and S2); results and interpretations presented hereafter use net contact as the basis for evaluation.
Host and ITN effects on An. gambiae strains
The most frequently occurring observed behaviour in all An. gambiae strains during baseline, untreated net, host absent tests was net contact (Table 1, Host absent net vs Host absent flight KS OR 18.07; 95% CI 17.16, 19.03; P ≤ 0.0001; NG OR 40.79; 95% CI 39.18, 42.46; P ≤ 0.0001; BF OR 56.43; 95% CI 54.12, 58.85; P ≤ 0.0001; VK7 OR 86.26; 95% CI 83.11, 89.53, P ≤ 0.0001). The addition of a host attractant triggered excitatory behaviours: in all four strains the magnitude of net contact decreased significantly (Table 1, Host present net vs Host present flight KS OR 16.41; 95% CI 15.83, 17.01; P ≤ 0.0001; NG OR 17.44; 95% CI 15.78, 19.27; P ≤ 0.0001; BF OR 6.25; 95% CI 5.96, 6.56; P ≤ 0.0001; VK7 OR 7.25; 95% CI 6.98, 7.53; P ≤ 0.0001; full multivariable analysis in Additional file 1: Table S4). Notably, the two resistant strains spent approximately three times longer in contact with the net when the host was absent compared to present, indicating a higher excitatory response to the host presence than shown by the two susceptible strains (Table 1, Host absent net vs host present net KS OR 1.05; 95% CI 1.00, 1.10; P = 0.0256; NG OR 1.53; 95% CI 1.42, 1.65; P ≤ 0.0001; BF OR 3.01; 95% CI 2.87, 3.14; P ≤ 0.0001; VK7 OR 3.45; 95% CI 3.32, 3.58; P ≤ 0.0001).
The presence of insecticide-treated material altered the observed behaviour of the two susceptible strains and, with the exception of Kisumu during exposure to P2, both strains had significantly higher net contact when the host was present (Table 1, Host absent net vs host present net P2: KS OR 0.99; 95% CI 0.94, 1.03; P = 1.0; NG OR 0.85; 95% CI 0.81, 0.88; P ≤ 0.0001; OS: KS OR 0.39; 95% CI 0.37, 0.41; P ≤ 0.0001; NG OR 0.70; 95% CI 0.66, 0.73; P ≤ 0.0001). This was not observed in resistant strains, which largely retained the host excitatory effects observed during untreated net tests (Table 1, Host absent net vs host present net P2: BF OR 2.14; 95% CI 2.06, 2.23; P ≤ 0.0001; VK7 OR 1.75; 95% CI 1.60, 1.90; P ≤ 0.0001; OS: BF OR 1.02; 95% CI 0.96, 1.09; P = 1.0; VK7 OR 5.29; 95% CI 4.98, 5.63; P ≤ 0.0001).
During P2 exposure there was a trend for Anopheles spp. mosquitoes to make less net contact compared to untreated net, indicative of a mild irritant effect. This effect was reduced in host tests (Table 2, Host absent P2 net vs UT net KS OR 1.00; 95% CI 0.96, 1.05; P = 1.0; NG OR 0.51; 95% CI 0.48, 0.53; P ≤ 0.0001; BF OR 0.92; 95% CI 0.88, 0.96; P ≤ 0.0001; VK7 OR 0.34; 95% CI 0.31, 0.38; P ≤ 0.0001 and Host present P2 net vs UT net KS OR 1.07; 95% CI 1.01, 1.13; P = 0.0061; NG OR 0.91; 95% CI 0.84, 0.99; P = 0.022; BF OR 1.29; 95% CI 1.23, 1.36; P ≤ 0.0001; VK7 OR 0.67; 95% CI 0.65, 0.70; P ≤ 0.0001; full multivariable analysis in Additional file 1: Table S5). In OS tests, mosquitoes of all strains made significantly less net contact compared to untreated net, regardless of the host presence (Table 2, Host absent OS net vs UT net KS OR 0.33; 95% CI 0.31, 0.35; P ≤ 0.0001; NG OR 0.14; 95% CI 0.13, 0.14; P ≤ 0.0001; BF OR 0.32; 95% CI 0.29, 0.34; P ≤ 0.0001; VK7 OR 0.58; 95% CI 0.55, 0.62; P ≤ 0.0001; Host present OS net vs UT net KS OR 0.88; 95% CI 0.84, 0.92; P ≤ 0.0001; NG OR 0.30; 95% CI 0.27, 0.33; P ≤ 0.0001; BF OR 0.92; 95% CI 0.88, 0.97; P ≤ 0.0001; VK7 OR 0.38; 95% CI 0.36, 0.40; P ≤ 0.0001).
Comparing net contact at OS with P2 revealed differences in responses between the two pyrethroid-treated nets: significantly less net contact was observed during exposure to OS than P2 regardless of the host presence with the exception of VK7 in host absent tests (Table 2, Host absent OS net vs P2 net KS OR 0.33; 95% CI 0.31, 0.34; P ≤ 0.0001; NG OR 0.27; 95% CI 0.26, 0.28; P ≤ 0.0001; BF OR 0.34; 95% CI 0.32, 0.37; P ≤ 0.0001; VK7 OR 1.71; 95% CI 1.53, 1.90; P ≤ 0.0001; Host present OS net vs P2 net KS OR 0.82; 95% CI 0.78, 0.87; P ≤ 0.0001; NG OR 0.33; 95% CI 0.31, 0.35; P ≤ 0.0001; BF OR 0.72; 95% CI 0.68, 0.75; P ≤ 0.0001; VK7 OR 0.56; 95% CI 0.54, 0.59; P ≤ 0.0001).
Impacts of exposure on life history traits
KD and 24 h mortality
VCT mortality was low (< 5%) in untreated net tests with the exception of the VK7 in host absent tests, where it reached 9%. Knockdown and 24 h mortality exceeded 90% in both susceptible strains following exposure to P2 and OS (Additional file 1: Table S6); mortality in resistant strains was 19% and 3% for Banfora and VK7, respectively, after P2 tests and 5% for both strains after OS tests (Additional file 1: Table S6).
Blood feeding and blood meal size
Due to mortality in the susceptible strains, analyses of recovery of blood feeding ability and blood meal size were only performed on resistant strains. After baseline untreated net tests, most Banfora and VK7 mosquitoes fed at 1-h post-exposure (Banfora: 85.7% (84/98), 93.8% (106/113); VK7: 86.7% (104/120), 93.5% (115/123) host absent and host present respectively, Additional file 1: Table S7); at 24 h, 78.6% (11/14) and 71% (5/7) of Banfora mosquitoes that did not feed at 1 h fed successfully in host absent and host present tests, respectively, and 12.5% (2/16) and 87.5% (7/8) of VK7 mosquitoes fed in host absent and host present tests (Additional file 1: Table S7).
Results from treated nets revealed the effects of treatment and host presence on blood feeding recovery. One hour after exposure, Banfora mosquitoes were significantly more likely to feed if the exposure was to untreated net compared to P2 and OS (Additional file 1: Table S7, Banfora 1 h Host absent OS vs UT OR 0.0349; 95% CI 0.0121, 0.1001, P ≤ 0.0001; Host present P2 vs UT OR 0.0077, 95% CI 0.0023, 0.0258, P ≤ 0.0001; OS vs UT OR 0.0533, 95% CI 0.0207, 0.1374; P ≤ 0.0001); after exposure to OS the presence of the host significantly increased chances of feeding at 1 h (Additional file 1: Table S8, Banfora 1 h OS Present vs Absent OR 7.1950, 95% CI 2.8809, 17.9689, P ≤ 0.0001). By 24 h the presence of the host during exposure no longer had an effect on blood feeding (Additional file 1: Table S8, Banfora 24 h P2 Present vs Absent OR 0.7062, 95% CI 0.2436, 2.0469, P = 0.5217; OS Present vs Absent OR 1.8564, 95% CI 0.6230, 5.5318, P = 0.2668).
At one hour post-exposure VK7 mosquitoes were also significantly more likely to feed after exposure to untreated net compared to P2, regardless of the presence of the host (Additional file 1: Table S7, VK7 1 h Host absent P2 vs UT OR 0.0177; 95% CI 0.0068, 0.0460, P ≤ 0.0001; Host present P2 vs UT OR 0.0110, 95% CI 0.0032, 0.0384, P ≤ 0.0001). However, a significant difference between feeding after untreated and OS exposure only occurred after host present tests (Additional file 1: Table S7, VK7 1 h Host absent OS vs UT OR 0.5464; 95% CI 0.2782, 1.0734, P = 0.0794; Host present OS vs UT OR 0.1115, 95% CI 0.0317, 0.3930, P = 0.0006). Unlike Banfora mosquitoes, the presence of the host did not have a significant effect on willingness to blood feed (Additional file 1: Table S8).
The mean blood meal size after untreated net tests was 12.67 µg/ml (SD = 6.65) and 13.25 µg/ml (SD = 6.39) for Banfora and VK7, respectively. No consistent effect of the host was observed in Banfora mosquitoes but in VK7 the host presence had a significant effect on the blood meal size, resulting in larger blood meals regardless of whether treated or untreated net was used (Additional file 1: Table S9, Host present vs Host Absent VK7 untreated mean haematin difference 2.86; 95% CI 1.25, 4.47; P = 0.0005; P2 mean haematin difference 2.26; 95% CI 0.06, 4.46; P = 0.0437; OS mean haematin difference 4.23; 95% CI 2.22, 6.24; P ≤ 0.0001). Blood meal sizes were consistently smaller after treated net exposures, although significantly so only in host absent tests for OS (Additional file 1: Table S10, Host absent OS vs Untreated Banfora mean difference − 5.29, 95% CI − 8.40, − 2.18; P = 0.0009; VK7 mean difference − 2.05, 95% CI − 3.48, − 0.62; P = 0.0049).
Longevity
The median longevity after VCT tests with untreated net was a minimum of 14 days for each strain, except for VK7 in host absent tests (Additional file 1: Table S11); accordingly, results for VK7 were only evaluated within each net treatment.
The median longevity of Banfora mosquitoes exposed to P2 was one and five days for host absent and host present tests, respectively (Additional file 1: Table S11, host absent Range 1–33; host present range 1–37). P2 had a relatively weak effect on the survival of Banfora mosquitoes: compared to those exposed to untreated nets, mosquitoes exposed to P2 were twice as likely to be dead by nine days (Additional file 1: Table S12, Banfora host absent P2 Hazard Ratio (HR) 2.03, 95% CI 1.40, 2.93, P = 0.0002; host present P2 HR 2.01, 95% CI 1.00, 4.06, P = 0.0510). Host effects on longevity were observed: Banfora and VK7 mosquitoes exposed to P2 with a host lived longer compared to tests without a host (Additional file 1: Table S13, P2 Banfora survival HR 0.64, 95% CI 0.49, 0.83, P = 0.0010; P2 VK7 host present HR 0.65, 95% CI 0.40, 1.06, P = 0.0836). Mosquitoes that blood fed and survived long enough to digest the blood meal did not exhibit significantly reduced longevity regardless of the presence of the host attractant (Additional file 1: Table S13, haematin Banfora HR 1.12, 95% CI 0.32, 3.87, P = 0.8587; VK7 HR 0.34, 95% CI 0.14, 0.86, P = 0.0231).
On average, Banfora mosquitoes exposed to OS died one day after exposure (Median = 1 day, Range 1–32 days). Compared to untreated net, Banfora mosquitoes exposed to OS were significantly more likely to die within 9 days, unless mosquitoes survived to take and digest a blood meal (Additional file 1: Table S12, Banfora host absent OS HR 1.66, 95% CI 1.09, 2.52, P = 0.0189; haematin HR 0.71, 95% CI 0.01, 39.39, P = 0.8667; host present OS HR 2.83, 95% CI 1.36, 5.87, P = 0.0053; haematin HR 1.45, 95% CI 0.53, 3.99, P = 0.4745).
VK7 mosquitoes exhibited reduced longevity after tests with a host compared to tests without a host, corresponding with the observed magnitude of net contact (Additional file 1: Table S13, VK7 OS host present HR 0.12; 95% CI 0.07, 0.20; P ≤ 0.0001; survival HR 0.09; 95% CI 0.04, 0.22; P ≤ 0.0001; Additional file 1: Table S4, VK7 host absent OS net vs OS flight OR 29.21; 95% CI 26.85, 31.77; P ≤ 0.0001; host present OS net vs OS flight OR 1.05; 95% CI 0.99, 1.09; P = 0.1436).
Discussion
In the current malaria control landscape, where combating increases in insecticide resistance is an urgent priority, understanding the impacts of ITNs on local vector populations, especially in regions with different endemicities and varying uptakes of vector control tools, is of paramount importance [20, 34]. Previously reported characterizations of An. gambiae s.l. strains using free-flying mosquitoes revealed significant differences in mosquito behavioural responses at unbaited, human-baited untreated and ITNs [35]. These dissimilar responses, in addition to earlier studies showing that laboratory and field data do not always align [35, 36], suggest that evaluations of ITN efficacy would more accurately predict ‘real world’ performance if measures of vector behaviour were incorporated [12, 34].
The cone bioassay is one of WHO’s standard procedures for measuring the efficacy of ITNs and is widely implemented as part of routine malaria control practices across sub-Saharan Africa. However, the two endpoints, KD and 24-h mortality, are not designed to capture the effects of active ingredients beyond the rapid toxicity characteristic of pyrethroid exposure and are therefore unsuitable for characterizing the effects of nets co-formulated with active ingredients other than pyrethroids. The insights to be gained from extracting mosquito behaviour data with and without a host attractant during the cone test, with extended monitoring to capture life history traits were investigated.
Distinct differences were observed in the responses of An. gambiae to the host attractant and to ITN exposure. The host attractant elicited an excitatory effect, expressed as decreased net contact in the presence of the host, in all strains which was more pronounced in the two insecticide resistant strains compared to the two susceptible strains. This stimulative effect was preserved during the exposure of resistant strains to ITNs but lessened in the susceptible strains, suggesting a complex interplay between resistance status, host-seeking behaviours and ITN efficacy. This was further developed in our comparisons between two ITNs in widespread use treated with the same class of insecticide, Permanet 2.0 and Olyset. The behavioural profiles generated from the VCT were sensitive enough to clearly differentiate between the two ITNs and revealed that Permanet 2.0 elicited responses largely unaltered from those observed during testing with untreated net, whilst more pronounced irritative effects, i.e., significantly more time spent in flight, were observed with Olyset. Comparable profiles of both of these ITNs have been previously described using arm-in-cage (or similar) assays [39, 40], but until now it has not been possible to capture these data contemporaneously during routine efficacy testing.
ITN exposure reduced the likelihood of post-exposure blood feeding in both insecticide resistant strains, with an observed decrease in host absent tests from 86% blood feeding success after untreated netting tests to 0% and 11% in the Banfora strain after Permanet 2.0 and Olyset exposure, respectively, and in the VK7 strain from 87 to 12% after Permenet 2.0 exposure. In some cases, this reduction was ameliorated by exposure in the presence of a host, which significantly increased the chances of a mosquito taking a blood meal at one-hour post-exposure. This effect had disappeared by 24 h post-exposure; nevertheless, the magnitude of this effect suggests that post-exposure feeding should be incorporated into efficacy studies, especially for ITNs treated with AIs that might be expected to have a delayed effect on mortality.
The observed longevity following ITN exposure was also affected by the presence of a host during exposure: in the absence of blood feeding mosquitoes exposed to P2 with a host lived longer than those exposed without the host present. For those highly resistant mosquitoes that survived insecticide exposure long enough to feed and digest a blood meal, no delayed effects of longevity were observed, in keeping with previous studies and further suggesting the importance of measurements of blood feeding success when ITN efficacy is evaluated [11].
Although several behavioural trends that could be broadly classified into ‘susceptible’ or ‘resistant’ patterns were observed, strain-specific differences were apparent in some responses. Notably, the responses of the VK7 strain differed during exposure to OS compared to the other strains. Both resistant strains tested in this study originate from Southwest Burkina Faso and were colonized during the same year. The high pyrethroid resistance phenotype in each strain is partially conferred by both target site and metabolic resistance but each strain has additional resistance mechanisms; notably, resistance in the Banfora strain is partially conferred by elevated levels of sensory appendage protein, SAP2 and is also associated with elevated rates of respiration whereas elevated levels of gene families putatively involved in pyrethroid sequestration are found in the VK7 strain [23, 25, 37, 38]. It would be instructive to repeat this study using Anopheles strains carrying moderate pyrethroid resistance and/or single mechanisms of resistance.
As has been previously noted, the smaller scale of test arenas and standardized environmental conditions in most laboratories contributes to discrepancies found between laboratory and field studies [35, 41,42,43,44,45,46,47]. Whilst the shape and volume of the WHO cone undoubtedly create artificial conditions of near-forced net contact, it is encouraging to note that the excitatory effect provoked by the addition of a host captures at a small scale the finding from Parker et al. that An. gambiae activity at an untreated net was significantly lower in the absence of human bait. This indicates that data more representative of those typically obtained from field-scale experiments can be collected in the laboratory using appropriately modified assays.
Using scan sampling to translate mosquito activity in the cone into quantified behavioural composites has proved very informative but has its limitations. For example, the static nature of scan sampling does not allow determination of whether time spent in contact with the net is spent resting or in active host seeking, information that would be useful in understanding the apparent lessening of the host effect on susceptible mosquitoes. Furthermore, although the addition of the video recording does not appreciably increase the time required to perform laboratory experiments, subsequent scan sampling adds additional analysis time. It is possible that this could be mitigated by applying an automated video analysis method to record mosquito positions.
Conclusions
The results demonstrate that the WHO cone test, with appropriate adaptations and life history trait monitoring, can be used to construct behavioural composites for standard ITNs that are more informative for routine efficacy testing than the standard cone test method. Adding a host to the test reproduces at a small scale behavioural modes that can usually only be observed in larger scale tests; even in the three-minute test window these alterations in mosquito-host-ITN interactions yield knock-on effects to post-exposure blood feeding and subsequent survival that are directly applicable to efficacy evaluations in local vector populations. Such data could prove invaluable in evaluating the success of combination nets currently being rolled out across Africa.
Availability of data and materials
The data generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–11.
Pryce J, Richardson M, Lengeler C. Insecticide-treated nets for preventing malaria. Cochrane Database Syst R. 2018;11:CD000363.
Roll Back Malaria Partnership. The global malaria action plan: for a malaria free world. Geneva; World Health Organization. 2008. https://reliefweb.int/report/world/global-malaria-action-plan. Accessed 30 May 2022.
Bagi J, Grisales N, Corkill R, Morgan JC, N’Falé S, Brogdon WG, et al. When a discriminating dose assay is not enough: measuring the intensity of insecticide resistance in malaria vectors. Malar J. 2015;14:210.
Churcher TS, Lissenden N, Griffin JT, Worrall E, Ranson H. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. Elife. 2016;5: e16090.
Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends parasitology. 2016;32:187–96.
WHO. World Malaria Report 2018. Geneva, World Health Organization. 2018. https://apps.who.int/iris/handle/10665/275867. Accessed 25 May 2022.
WHO. Global report on insecticide resistance in malaria vectors: 2010–2016. Geneva, World Health Organization. 2018. https://apps.who.int/iris/handle/10665/272533. Accessed 25 May 2022.
Kleinschmidt I, Bradley J, Knox TB, Mnzava AP, Kafy HT, Mbogo C, et al. Implications of insecticide resistance for malaria vector control with long-lasting insecticidal nets: a WHO-coordinated, prospective, international, observational cohort study. Lancet Infect Dis. 2018;18:640–9.
Hughes A, Lissenden N, Viana M, Toé KH, Ranson H. Anopheles gambiae populations from Burkina Faso show minimal delayed mortality after exposure to insecticide-treated nets. Parasit Vectors. 2020;13:17.
Viana M, Hughes A, Matthiopoulos J, Ranson H, Ferguson HM. Delayed mortality effects cut the malaria transmission potential of insecticide-resistant mosquitoes. Proc Natl Acad Sci USA. 2016;113:8975–80.
Gatton ML, Chitnis N, Churcher T, Donnelly MJ, Ghani AC, Godfray HC, et al. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution. 2013;67:1218–30.
Hughes A, Foster GM, Guy A, Matope A, Abe M, Towers D, et al. Quantifying late-stage host-seeking behaviour of Anopheles gambiae at the insecticidal net interface using a baited-box bioassay. Malar J. 2020;19:140.
Okumu F. The fabric of life: what if mosquito nets were durable and widely available but insecticide-free? Malar J. 2020;19:260.
WHO. Test procedures for insecticide resistance monitoring in malaria vectors, bio-efficacy and persistence of insecticides on treated surfaces: report of the WHO informal consultation. Geneva: World Health Organization; 1998.
WHO. Guidelines for laboratory and field testing of long-lasting insecticidal nets. Geneva, World Health Organization. 2013. https://apps.who.int/iris/handle/10665/80270. Accessed 25 May 2022.
Sokhna C, Ndiath MO, Rogier C. The changes in mosquito vector behaviour and the emerging resistance to insecticides will challenge the decline of malaria. Clinl Microbiol Infect. 2013;19:902–7.
Grieco JP, Achee NL, Chareonviriyaphap T, Suwonkerd W, Chauhan K, Sardelis MR, et al. A new classification system for the actions of IRS chemicals traditionally used for malaria control. PLoS ONE. 2007;2: e716.
N’Guessan R, Asidi A, Boko P, Odjo A, Akogbeto M, Pigeon O, Rowland M. An experimental hut evaluation of PermaNet® 3.0, a deltamethrin—piperonyl butoxide combination net, against pyrethroid-resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes in southern Benin. Trans R Soc Trop Md Hyg. 2010;104:758–65.
Parker JE, Angarita-Jaimes N, Abe M, Towers CE, Towers D, McCall PJ. Infrared video tracking of Anopheles gambiae at insecticide-treated bed nets reveals rapid decisive impact after brief localised net contact. Sci Rep. 2015;5: 113392.
Shute GT. A method of maintaining colonies of East African strains of Anopheles gambiae. Ann Trop Med Parasitol. 1956;50:92–4.
Harris C, Lambrechts L, Rousset F, Abate L, Nsango SE, Fontenille D, Morlais I, Cohuet A. Polymorphisms in Anopheles gambiae immune genes associated with natural resistance to Plasmodium falciparum. PLoS Pathog. 2010;6: e1001112.
Toé KH, Jones CM, N’Fale S, Ismail HM, Dabiré RK, Ranson H. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerg Infect Dis. 2014;20:1691.
Toé KH, N’Falé S, Dabiré RK, Ranson H, Jones CM. The recent escalation in strength of pyrethroid resistance in Anopheles coluzzi in West Africa is linked to increased expression of multiple gene families. BMC Genomics. 2015;16:146.
Williams J, Ingham VA, Morris M, Toé KH, Hien AS, Morgan JC, et al. Sympatric populations of the Anopheles gambiae complex in Southwest Burkina Faso evolve multiple diverse resistance mechanisms in response to intense selection pressure with pyrethroids. Insects. 2022;13:247.
Friard O, Gamba M. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol. 2016;7:1325–30.
Briegel H. Determination of uric acid and hematin in a single sample of excreta from blood-fed insects. Experientia. 1980;36:1428.
Briegel H. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J Insect Physiol. 1990;36:165–72.
Lyimo EO, Takken W. Effects of adult body size on fecundity and the pre-gravid rate of Anopheles gambiae females in Tanzania. Med Vet Entomol. 1993;7:328–32.
Lissenden N, Kont MD, Essandoh J, Ismail HM, Churcher TS, Lambert B, et al. Review and meta-analysis of the evidence for choosing between specific pyrethroids for programmatic purposes. Insects. 2021;12:826.
Schemper M, Wakounig S, Heinze G. The estimation of average hazard ratios by weighted Cox regression. Stat Med. 2009;28:2473–89.
Dunkler D, Ploner M, Schemper M, Heinze G. Weighted Cox regression using the R package coxphw. J Stat Softw. 2018;84:1–26.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2020 Jun. https://www.R-project.org/. Accessed 30 June 2021.
Seyoum A, Sikaala CH, Chanda J, Chinula D, Ntamatungiro AJ, Hawela M, et al. Human exposure to anopheline mosquitoes occurs primarily indoors, even for users of insecticide-treated nets in Luangwa Valley, South-east Zambia. Parasit Vectors. 2012;5:101.
Parker JE, Jaimes NC, Gleave K, Mashauri F, Abe M, Martine J, et al. Host-seeking activity of a Tanzanian population of Anopheles arabiensis at an insecticide treated bed net. Malar J. 2017;16:270.
Grossman MK, Oliver SV, Brooke BD, Thomas MB. Use of alternative bioassays to explore the impact of pyrethroid resistance on LLIN efficacy. Parasit Vectors. 2020;13:179.
Ingham VA, Anthousi A, Douris V, Harding NJ, Lycett G, Morris M, Vontas J, Ranson H. A sensory appendage protein protects malaria vectors from pyrethroids. Nature. 2020;577:376–80.
Ingham VA, Tennessen JA, Lucas ER, Elg S, Carrington-Yates H, Carson J, Guelbeogo WM, Sagnon N, Hughes G, Heinz E, Neafsey DE. Integration of whole genome sequencing and transcriptomics reveals a complex picture of insecticide resistance in the major malaria vector Anopheles coluzzii. bioRxiv. 2021. https://doi.org/10.1101/2021.08.21.457189.
Siegert PY, Walker E, Miller JR. Differential behavioral responses of Anopheles gambiae (Diptera: Culicidae) modulate mortality caused by pyrethroid-treated bednets. J Econom Entomol. 2009;102:2061–71.
Hauser G, Thiévent K, Koella JC. The ability of Anopheles gambiae mosquitoes to bite through a permethrin-treated net and the consequences for their fitness. Sci Rep. 2019;9:8141.
Hossain MI, Curtis CF. Permethrin-impregnated bednets: behavioural and killing effects on mosquitoes. Med Vet Entomol. 1989;3:367–76.
Barnard DR, Posey KH, Smith D, Schreck CE. Mosquito density, biting rate and cage size effects on repellent tests. Med Vet Entomol. 1998;12:39–45.
McMeniman CJ, Corfas RA, Matthews BJ, Ritchie SA, Vosshall LB. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell. 2014;156:1060–71.
Murlis J, Elkinton JS, Cardé RT. Odor plumes and how insects use them. Annu Rev Entomol. 1992;37:505–32.
Geier M, Bosch O, Boeckh J. Influence of odour plume structure on upwind flight of mosquitoes towards hosts. J Exp Biol. 1999;202:1639–48.
Ferguson HM, Ng’habi K, Walder T, Kadungula D, Moore SJ, Lyimo IN, Russell TL, Urassa H, Mshinda H, Killeen GF, Knols BGJ. Establishment of a large semi-field system for experimental study of African malaria vector ecology and control in Tanzania. Malar J. 2008;7:158.
Lacey ES, Cardé RT. Location of and landing on a source of human body odour by female Culex quinquefasciatus in still and moving air. Physiol Entomol. 2012;37:153–9.
Acknowledgements
Thanks are extended to CNRFP and IRSS institutions for resistant mosquito populations.
Funding
The work was funded by the Bill and Melinda Gates Foundation, as part of the project entitled, Accelerating time to market of new vector control tools by strengthening the Phase I evaluation (OPP1159078).
Author information
Authors and Affiliations
Contributions
The VCT was conceived by PJM and realised by GM (software) and AH (initial testing). GMF, HR, AH and PJM designed the experiments and AH, ME and KS performed the tests. AH, GMF and AM analysed the data. AH, GMF, AM and PJM wrote the manuscript the final draft of which was approved by all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. Total imprecision assessment of VCT on untreated net by event type (net contact, flight, cone contact) for each mosquito strain (Kisumu, N’gousso, Banfora and VK7) of An. gambiae s.l. in the absence and presence of a host. Where n (mos) = number of mosquitoes per group, n (reps) = number of replicates performed per group, mean = mean number of mosquitoes per event, SD = standard deviation of the mean, LCLM = lower confidence level mean, UCLM = upper confidence level mean, %CV = percentage coefficient of variation, IS = insecticide susceptible, and IR = insecticide resistant. Table S2. Within-day imprecision assessment of VCT on untreated net by event type (net contact, flight, cone contact) for each strain (Kisumu, N’gousso, Banfora and VK7) of An. gambiae s.l. in the absence and presence of a host. Where n (test days) = number of test days per group, n (reps per test day) = number of replicates performed per test day per group, mean = mean number of mosquitoes per event, SD = standard deviation of the mean, %CV = percentage coefficient of variation, IS = insecticide susceptible, and IR = insecticide resistant. Table S3. Variation in test stages. Assessment of VCT on untreated net by event type (net contact only) for each strain (Kisumu, N’gousso, Banfora and VK7) of An. gambiae s.l. in the absence and presence of a host. Where n (reps) = number of replicates performed per group, %CV = percentage coefficient of variation by time (s = seconds), IS = insecticide susceptible, and IR = insecticide resistant. Table S4. Host-Location comparisons within An. gambiae s.l. strain and net treatment. A Beta-binomial Distribution model fitted. Multiple pairwise comparisons 95% Confidence Intervals and P-values corrected using the Bonferroni adjustment. Where IS = insecticide susceptible, IR = insecticide resistant, UT = Untreated net, OS = Olyset net, P2 = PermaNet 2.0 net and * = Significant at 5% significance level. Table S5. Treatment-Location comparisons within An. gambiae strain (Kisumu, N’gousso, Banfora and VK7) and host (present or absent). A Beta-binomial Distribution model Results. Multiple pairwise comparisons 95% Confidence Intervals and P-values corrected using the Bonferroni adjustment. Where IS = insecticide susceptible, IR = insecticide resistant, UT = Untreated net, OS = Olyset net, P2 = PermaNet 2.0 net and * = Significant at 5% significance level. Table S6. Knock-down (KD) (1 h) and mortality (24 h) after net exposure for insecticide susceptible (Kisumu and N’gousso) and insecticide resistant (VK7 and Banfora) strains of An. gambiae s.l. against PermaNet 2.0 (P2) and Olyset (OS) ITNs, in VCT validation experiments. Table S7. Treatment comparisons—Willingness to refeed at 1 or 24 h within An. gambiae strain and host (present or absent). A Binary Logistic Regression model fitted using Generalised Estimating Equation. Where IR = insecticide resistant, UT = Untreated net, OS = Olyset net, P2 = PermaNet 2.0 net and * = Significant at 5% significance level. Table S8. Host comparisons—Willingness to refeed at 1 or 24 h within An. gambiae strain and treatment. A Binary Logistic Regression model fitted using Generalised Estimating Equation. Where IR = insecticide resistant, UT = Untreated net, OS = Olyset net, P2 = PermaNet 2.0 net and * = Significant at 5% significance level. Table S9. Treatment comparisons – Blood meal size within An. gambiae strain and host (present or absent). A Linear Regression model fitted using Generalised Estimating Equation. Where IS = insecticide susceptible, IR = insecticide resistant, UT = Untreated net, OS = Olyset net, P2 = PermaNet 2.0, CI = Confidence Interval net and * = Significant at 5% significance level. Table S10. Host comparisons – Blood meal size within An. gambiae strain and treatment. A Linear Regression model fitted using Generalised Estimating Equation. Where IS = insecticide susceptible, IR = insecticide resistant, UT = Untreated net, OS = Olyset net, P2 = PermaNet 2.0 net and * = Significant at 5% significance level. Table S11. Descriptive statistics for longevity data within host, An. gambiae strain and net treatment. Where BF = Banfora, VK = VK7, UT = Untreated net, P2 = PermaNet 2.0 net, OS = Olyset net, IR = insecticide resistant, n = sample size, % = percentage, SD = Standard Deviation, Min = Minimum, Max = Maximum, CI = Confidence Interval, Yes n = number of mosquitoes that died within 9 days and * results for mosquitoes that died within 9 days post-exposure only. Table S12. Net treatment comparisons—Mortality within 9 days within An. gambiae strain and host. A Weighted Cox Regression Model fitted adjusted for other predictors (time fed, wingspan, haematin and net proportion). Where UT = Untreated net, P2 = PermaNet 2.0 net, OS = Olyset net, IR = insecticide resistant, Ref = Reference group, Net proportion = Mean proportion of mosquitoes on the net during exposure (behaviour data) and * = Significant at 5% significance level. Censoring at 9 days. Table S13. Host comparisons—Mortality within 9 days within An. gambiae strain and net treatment. A Weighted Cox Regression Model fitted adjusted for other predictors (time fed, wingspan, haematin and net proportion). Where UT = Untreated net, P2 = PermaNet 2.0 net, OS = Olyset net, IR = insecticide resistant, Ref = Reference group, Net proportion = Mean proportion of mosquitoes on the net during exposure (behaviour data) and * = Significant at 5% significance level. Censoring at 9 days.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hughes, A., Matope, A., Emery, M. et al. A closer look at the WHO cone bioassay: video analysis of the hidden effects of a human host on mosquito behaviour and insecticide contact. Malar J 21, 208 (2022). https://doi.org/10.1186/s12936-022-04232-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-022-04232-4