Abstract
The spatial distribution of mosquito species in the course of globalization and climate warming is highly dynamic. Different studies have demonstrated the spread and establishment of thermophilic mosquito species, potentially increasing the prevalence of ‘nuisance’ mosquitoes and the local transmission of pathogens. Here we report the first recorded sampling of Anopheles hyrcanus in Wrocław, southwest Poland. This is the most northern detection of this species to date in Europe. Future spread and population development of this potential vector of malaria parasites, viruses or zoonotic helminths, such as Dirofilaria spp., must be monitored carefully. Potential factors underlying the spread of this species are discussed.
Graphical Abstract

Similar content being viewed by others
Mosquitoes (Diptera: Culicidae) are a main focus of medical entomological research. While several mosquito species are considered to be ‘nuisance’ mosquitoes only, others are vectors of various pathogens, such as malaria parasites or arboviruses [1]. The spread of exotic vector mosquitoes increases the threats to global health, not only in the tropics but also in temperate climate zones [2, 3]. Several Aedes mosquito species are particularly known for their high potential to colonize new regions far beyond their native geographical distribution. The most important trait enabling such long-distance dispersal is the high resistance of the eggs of many Aedes species to desiccation, i.e. the eggs can survive dryness for months or sometimes even years [4]. In addition, successful invasive mosquito species are well adapted to modern human environments in that they are able to colonize artificial breeding sites, such as used tires, and feed on humans [3]. The intercontinental spread of Aedes species is predominantly facilitated through the global transport of eggs with different commercial goods (e.g. used tires and plants such as lucky bamboo). Once locally established, medium-scale dispersal is mediated by the transport of adults as blind passengers in vehicles and boats [5]. In Europe, the best known example of an invasive mosquito species is the Asian tiger mosquito (Aedes albopictus) [3]. In the early 1990s, this species was introduced from the USA to Italy and then rapidly spread within Italy and to areas around the Mediterranean basin. Current observations have revealed the spread of this mosquito species towards Central Europe, with several populations reported to be established in areas north of the Alps [6].
For mosquito species which do not lay eggs that can remain dormant for an extended period of time (e.g. Culex, Culiseta or Anopheles), long-distance dispersal is less likely, but not impossible. For example, the Culex coronator complex, which was first described in Trinidad and Tobago, has shown a rapid range expansion throughout the southern US states [7]. The regular import of exotic taxa of the above-mentioned genera into Europe has also been reported [8]. At the same time, there are indications of changes in the distribution of mosquito species native to southern Europe, with a trend towards Central Europe, such as the spread of Culiseta longiareolata to Germany [9].
Monitoring programs aimed at assessing exotic mosquito populations can provide information on the local risk of nuisance mosquitoes or pathogen transmission. In addition, such programs allow the implementation of a quick response, such as specific control measures, to the spread of exotic mosquito species.
In the study reported here, we describe the first detection of Anopheles hyrcanus in Poland. Mosquito sampling was carried out in fields designated for water infiltration (51°04′58.16″ N, 17°06′42.20″ E; Fig. 1) that are located on the outskirts of the southeastern part of the Polish city of Wrocław, along the left bank of the Odra River. Wrocław is one of the warmest cities in Poland and is characterized by a humid, temperate continental climate. The mean annual temperature in the region is approximately 9.0 °C, and the average annual precipitation is approximately 600 mm [10]. The entire region belongs to the Odra catchment area, where the Bystrzyca, Ślęza, Oława and Widawa rivers form an extensive system of ditches and tributaries. This aquatic ecosystem was constructed in 1896 to provide surface and underground water filtration of 160 m3 water/day for drinking water production. It was selected for this study because it is characterized by a high diversity of different aquatic and semi-aquatic habitats that provide ideal breeding sites for mosquitoes [11].
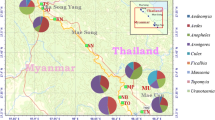
Occurrence map of Anopheles hycranus sensu lato at three different geographical levels. Countries are given in yellow, and provinces/districts are shown in orange. The filled black circles are global positioning system (GPS) coordinates derived by Bertola et al. [15]. The red dot indicates the new occurrence site reported in this study
The area is mostly marshy and dominated by water reservoirs, such as floodplains, wells and ponds, and by 12 km of canals and ditches. Willow-poplar alluvial forests dominated by white willow (Salix alba) and white poplar (Populus alba) are the predominant vegetation cover along the river banks. Swamp vegetation, including high rushes and large sedge communities with common reed (Phragmites australis), sweet flag (Acorus calamus), acute sedge (Carex gracilis), yellow iris (Iris pseudacorus) and common sedge (Carex fusca), are found near both stagnant and flowing waters. The high diversity of aquatic biotopes results in a high species diversity [12]. The area is contained within the sanitary protection zone and is protected under the Natura 2000 sites, a European network of protected nature areas, as hornbeam forest in the Odra River Valley; it is one of the 118 important areas in Poland that meet the criteria for Important Bird Areas. Thus, the area plays important roles in both nature conservation and the water supply of Wrocław, and also hosts a large mosquito population that shows almost yearly massive emergence [11].
Adult mosquito collections were conducted with CO2-baited Encephalitis Vector Survey (EVS) traps (BioQuip Products Inc., Rancho Dominiquez, CA, USA) at two locations along the Odra river: Starodworska (51°4′21.48″ N, 17°6′49.55″ E) and Świątnicka (51°4′54.33″ N, 17°5′46.54″ E). The traps were installed 1 m above the ground and run overnight approximately from 1600 hours to 800 hours the following day. In Starodwoska, four collections were performed in July and September 2019 and four collections in July, August and September 2020. In Świątnicka, two collections were conducted in July and August 2019 and 2020. Mosquitoes were transported in dry ice containers to the laboratory where they were sorted under a stereo microscope and identified to species level using the dichotomous keys described by Becker et al. [1].
In 2019 and 2020, a total of 11,194 female mosquitoes were collected in Starodworska and Świątnicka (Table 1). The most abundant species was Aedes vexans (86.8% of all collected specimens), followed by Aedes sticticus (7.5%) and Anopheles maculipennis sensu lato (1.2%). Less frequent species were Culex pipiens sensu stricto/Culex torrentium (0.7%), Aedes cinereus/geminus (0.2%), Anopheles claviger/Anopheles petragnani (0.2%), Aedes annulipes group (0.2%), Aedes rossicus (0.1%), Aedes geniculatus (0.1%), Culiseta annulata (0.1%) and Anopheles plumbeus (< 0.1%). A remaining 136 specimens were damaged and their identity could not be determined.
A total of 42 (0.4%) An. hyrcanus specimens were collected over the 2-year collection period (Table 1). This species was identified based on different morphological features: (i) two pale spots on the apical half of the costal wing margin; (ii) distinctly swollen base of the fore femora; and (iii) a mostly dark tarsomere 4 of the hind leg that was pale at the apex. The wing veins were covered with dark and pale scales, forming contrasting spots. The antennae were dark brown, and the basal five to seven flagellomeres showed only a few white scales.
To confirm the morphological identification of An. hyrcanus, we performed molecular barcoding of the COI (cytochrome oxidase subunit I) gene region. Six specimens were individually deposited in 2-ml safe-lock tubes (Eppendorf, Hamburg, Germany), followed by the addition of approximately 20 pieces of 2.0-mm zirconia beads (BioSpecProducts, Bartlesville, OK, USA) and 500 µl of cell culture medium (high-glucose Dulbecco’s modified Eagle’s medium; Sigma-Aldrich, St. Louis, MO, USA) to each tube. The specimens were then homogenized in the Qiagen TissueLyser (Qiagen, Hilden, Germany) for 2 min at 30–50 Hz. DNA extraction was conducted using the QIAamp viral RNA mini kit according to the manufacturer's instructions (Qiagen). DNA elutions were used to amplify the COI gene region [13]. A comparison of the sequences of the six An. hyrcanus specimens with sequences deposited in GenBank showed a homology of 99.8–100% with available sequences of An. hyrcanus respectively Anopheles pseudopictus. All sequences were identical. In this study, we followed Ponçon et al. [14] in accepting that An. hyrcanus and An. pseudopictus probably belong to the same species, based on genetic analyses. One representative sequence has been submitted to GenBank (accession no. MZ093049).
This is the first record of An. hyrcanus in Poland. The detection of a total of 42 specimens during different sampling sessions conducted in 2019 and 2020 at two different sites (Table 1) indicates that the species is well-established at these locations. These newly described sites also represent the most northern occurrence of An. hyrcanus in Europe reported to date (Fig. 1). Anopheles hyrcanus has been previously documented in the Ukraine and Czech Republic, but no sightings have been reported in Germany, Belarus, Slovakia, Lithuania and Kaliningrad (Russia) [15]. The environmental conditions at both of our sampling sites resemble those of breeding sites reported for An. hyrcanus, which has a preference for large, stagnant water bodies with a rich aquatic vegetation, including reeds [1].
The interpretation of data on new and individual foci of a species at selected sites is difficult as the presence of that species could be driven by various factors, such as environmental change, including climate warming or intensified sampling. However, different studies indicate that An. hyrcanus is spreading in Europe, with new foci detected in Serbia, Slovakia, Czech Republic and Austria [16]. This mosquito species is expected to continue to spread further across Europe as the trend towards increasing annual temperatures continue.
Anopheles hyrcanus is a highly mammalophilic mosquito species, predominantly feeding on cattle and horses, but also on humans [17]. The species is considered an important vector of malaria in France [18]. In addition, a potential role as vector for Dirofilaria immitis or D. repens can be expected [19]. This is especially important in the light of the ongoing import of human malaria [20] and the ongoing circulation of D. repens in Poland [21]. Therefore, the ongoing spread of the species in Europe must be further monitored carefully.
Availability of data and materials
All data are available in the manuscript or open databases.
References
Becker N, Petric D, Zgomba M, Boase C, Madon M, Dahl C, et al. Mosquitoes and their control. Berlin: Springer; 2010.
European Centre for Disease Prevention and Control. Autochthonous transmission of chikungunya virus in mainland EU/EEA, 2007–present. https://www.ecdc.europa.eu/en/all-topics-z/chikungunya-virus-disease/surveillance-threats-and-outbreaks/autochthonous. Accessed 28 Sept 2023.
Medlock JM, Hansford KM, Versteirt V, Cull B, Kampen H, Fontenille D, et al. An entomological review of invasive mosquitoes in Europe. Bull Entomol Res. 2015;105:637–63.
Sota T, Mogi M. Interspecific variation in desiccation survival time of Aedes (Stegomyia) mosquito eggs is correlated with habitat and egg size. Oecologia. 1992;90:353–8.
Becker N, Geier M, Balczun C, Bradersen U, Huber K, Kiel E, et al. Repeated introduction of Aedes albopictus into Germany, July to October 2012. Parasitol Res. 2013;112:1787–90.
Lühken R, Heitmann A, Jansen S, Schmidt-Chanasit J, Börstler J, Werner D, et al. Microsatellite typing of Aedes albopictus (Diptera: Culicidae) populations from Germany suggests regular introductions. Infect Genet Evol. 2020;81:104237.
Wilke ABB, Benelli G, Beier JC. Beyond frontiers: on invasive alien mosquito species in America and Europe. PLoS Negl Trop Dis. 2020;14:e0007864.
Ibáñez-Justicia A, Smitz N, den Hartog W, van de Vossenberg B, De Wolf K, Deblauwe I, et al. Detection of exotic mosquito species (Diptera: Culicidae) at international airports in Europe. Int J Environ Res Public Health. 2020;17:3450.
Becker N, Hoffmann D. First record of Culiseta longiareolata (Macquart) for Germany. Eur Mosquito Bulletin Eur Mosq Bull. 2011;29:143–50.
Cichocki Z. Środowisko Wrocławia. Wrocław: Argi; 2014.
Rydzanicz K, Lonc E. Species composition and seasonal dynamics of mosquito larvae in the Wrocław, Poland area. J Vector Ecol. 2003;28:255–66.
Słychan M. Ptaki pól irygacyjnych Wrocławia. Ptaki Śląska. 1996;11:133–50.
Fang Y, Shi W-Q, Zhang Y. Molecular phylogeny of Anopheles hyrcanus group (Diptera: Culicidae) based on mtDNA COI. Infect Dis Poverty. 2017;6:61.
Poncon N, Toty C, Kengne P, Alten B, Fontenille D. Molecular evidence for similarity between Anopheles hyrcanus (Diptera: Culicidae) and Anopheles pseudopictus (Diptera: Culicidae), sympatric potential vectors of malaria in France. J Med Entomol. 2008;45:576–80.
Bertola M, Mazzucato M, Pombi M, Montarsi F. Updated occurrence and bionomics of potential malaria vectors in Europe: a systematic review (2000–2021). Parasit Vectors. 2022;15:88.
Mihailović DT, Petrić D, Petrović T, Hrnjaković-Cvjetković I, Djurdjevic V, Nikolić-Đorić E, et al. Assessment of climate change impact on the malaria vector Anopheles hyrcanus, West Nile disease, and incidence of melanoma in the Vojvodina Province (Serbia) using data from a regional climate model. PLoS ONE. 2020;15:e0227679.
Tomazatos A, Jansen S, Pfister S, Török E, Maranda I, Horváth C, et al. Ecology of West Nile virus in the Danube Delta, Romania: phylogeography, xenosurveillance and mosquito host-feeding patterns. Viruses. 2019;11:1159.
Ponçon N, Toty C, L’Ambert G, Le Goff G, Brengues C, Schaffner F, et al. Biology and dynamics of potential malaria vectors in Southern France. Malar J. 2007;6:18.
Tomazatos A, Cadar D, Török E, Maranda I, Horváth C, Keresztes L, et al. Circulation of Dirofilaria immitis and Dirofilaria repens in the Danube Delta Biosphere Reserve, Romania. Parasit Vectors. 2018;11:392.
Stępień M. Malaria in Poland in 2014–2018. Przegl Epidemiol. 2019;73:201–9.
Alsarraf M, Dwużnik-Szarek D, Hildebrand J, Mierzejewska EJ, Kloch A, Kot K, et al. Occurrence of Dirofilaria repens in wild carnivores in Poland. Parasitol Res. 2023;122:1229–37.
Acknowledgements
In addition, we thank Piotr Jawien and the monitoring team for the collection of the mosquitoes in the field.
Funding
Open Access funding enabled and organized by Projekt DEAL. RL, FGS and KK were funded by the Federal Ministry of Education and Research of Germany (BMBF) under the project NEED (Grant No. 01Kl2022).
Author information
Authors and Affiliations
Contributions
NB and KR designed the study. NB, DD, KR and KK collected data. RL and FGS analyzed the data. RL, NB, FGS, KR and JSC drafted the initial manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lühken, R., Becker, N., Dyczko, D. et al. First record of Anopheles (Anopheles) hyrcanus (Pallas 1771) (Diptera: Culicidae) in Poland. Parasites Vectors 16, 345 (2023). https://doi.org/10.1186/s13071-023-05974-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05974-z