Abstract
Background
The distributions of ticks and tick-borne pathogens are thought to have changed rapidly over the last two decades, with their ranges expanding into new regions. This expansion has been driven by a range of environmental and socio-economic factors, including climate change. Spatial modelling is being increasingly used to track the current and future distributions of ticks and tick-borne pathogens and to assess the associated disease risk. However, such analysis is dependent on high-resolution occurrence data for each species. To facilitate such analysis, in this review we have compiled georeferenced tick locations in the Western Palearctic, with a resolution accuracy under 10 km, that were reported between 2015 and 2021
Methods
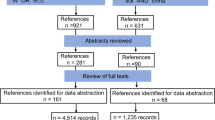
The PubMed and Web of Science databases were searched for peer-reviewed papers documenting the distribution of ticks that were published between 2015 and 2021, using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The papers were then screened and excluded in accordance with the PRISMA flow chart. Coordinate-referenced tick locations along with information on identification and collection methods were extracted from each eligible publication. Spatial analysis was conducted using R software (version 4.1.2).
Results
From the 1491 papers identified during the initial search, 124 met the inclusion criteria, and from these, 2267 coordinate-referenced tick records from 33 tick species were included in the final dataset. Over 30% of articles did not record the tick location adequately to meet inclusion criteria, only providing a location name or general location. Among the tick records, Ixodes ricinus had the highest representation (55%), followed by Dermacentor reticulatus (22.1%) and Ixodes frontalis (4.8%). The majority of ticks were collected from vegetation, with only 19.1% collected from hosts.
Conclusions
The data presented provides a collection of recent high-resolution, coordinate-referenced tick locations for use in spatial analyses, which in turn can be used in combination with previously collated datasets to analyse the changes in tick distribution and research in the Western Palearctic. In the future it is recommended that, where data privacy rules allow, high-resolution methods are routinely used by researchers to geolocate tick samples and ensure their work can be used to its full potential.
Graphical Abstract

Similar content being viewed by others
Background
Ticks are obligate hematophagous arthropods of global importance due to their public and veterinary health impacts; their blood-feeding can cause irritation, secondary infection, allergic reactions and, in some cases, paralysis [1]. However, their ability to transmit a wide range of pathogens, including viruses, protozoa and bacteria, makes them of particular importance [2]. For these reasons it is imperative to understand the distribution of individual tick species across the Western Palearctic, to guide future research as well as risk assessment and mitigation.
A total of 66 tick species, all belonging to the Ixodidae and Argasidae families, are endemic in the Western Palaearctic (11°W to 45°E and 29°S to 71°N). These ticks belong to five genera of Ixodidae, namely Ixodes (28 species), Hyalomma (9), Rhipicephalus (8), Haemaphysalis (7) and Dermacentor (2), and to two genera of Argasidae, namely Argas (5) and Ornithodoros (7) [3]. Although all are found in the Western Palearctic, the distribution of individual species varies according to their climatic niche. For example, Ixodes ricinus is a generalist that is present across much of the Western Palearctic, ranging from North Africa to Scandinavia and from Ireland to Russia [4]. Conversely, Hyalomma marginatum has a more restricted distribution around the Mediterranean basin [5]. However, a range of environmental and socio-economic factors, including climate change, have resulted in recent changes in the distribution and epidemiology of many tick species and tick-borne diseases. Tick species showing such changes in distribution include I. ricinus, whose range has expanded northwards in Sweden since the 1980s, from approximately 61°N to 66°N [6,7,8], and Dermacentor reticulatus, whose range has expanded across central and north-eastern Europe [9, 10]. The incidence of tick-borne disease has mirrored the range expansion of their vectors [11]. Since these changes have direct veterinary and public health implications, there has been a growing interest in surveillance to determine the current distribution of these ticks, as well as mechanistic and correlative models to assess likely future changes [12,13,14].
Geostatistical and spatial analysis, such as species distribution modelling, requires accurate species occurrence data. Previous efforts have been made to compile localised tick occurrence data, but several of these datasets provide tick distributions delimited by political boundaries, such as those provided by the European Centre for Disease Prevention and Control, which are not adequate for some spatial analyses. Furthermore, despite increases in the number of schemes encouraging the reporting of ticks and their locations by the public, the identification of these specimens may not be reliable. As a result, we designed the present systematic literature review to combine the results of existing peer-reviewed primary publications investigating tick distributions between 1970 and 2014 [15, 16], with the aim to create a secondary dataset of localised geographical occurrences in the Western Palearctic between 2015 and 2021. Our overall purpose is to provide a freely available updated set of records for tick species in the Western Palearctic for researchers investigating their spatial distribution.
Methods
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17] were followed in designing and performing this systematic review. The relevant literature was largely found by searching the Web of Science [18] and PubMed [19] databases, although we included other eligible literature when identified. The search was carried out in English using the key word string employed by European Food Safety Authority Panel on Animal Health and Welfare [20] to identify titles and/or abstracts from papers published between 1 January 2015 and 31 December 2021. All references were imported into a Microsoft Excel (Microsoft Corp., Redmond, WA, USA) spreadsheet for assessment by the lead author.
Criteria for inclusion and data extraction
Following the primary literature search of the databases and the identification of any additional relevant papers, all duplicates were removed. The literature was then initially screened for relevance based on the title and abstract, following which selected papers were downloaded and subject to a second screening to check their eligibility (see Table 1 for inclusion criteria). The following data were extracted from the eligible studies: (i) tick genus and species; (ii) identification method; (iii) country, named location and geographical longitude and latitude of found ticks (converted into degrees decimal if necessary); and (iv) the collection method and host information, if applicable. Data visualisation and analysis were carried out using R (v 4.1.2) [21].
Results
A total of 1489 publications were identified from the literature search of the two databases: 727 from Web of Science and 762 from PubMed. Two additional publications found during the search that fulfilled the eligibility criteria were also included. Of these 1491 publications, 570 were duplicates and removed. The remaining 921 publications were screened based on their title and abstract, resulting in the exclusion of a further 226 publications. The full texts of the remaining 695 publications were then screened for eligibility; of these, 310 publications were excluded due to the failure to provide coordinates and 152 were removed due to uncertainty in the coordinates provided. In the latter case, such coordinates were for centroids of large administrative divisions, cities or national parks, or the publication referred to different sampling sites with coordinates but failed to relate this information to which species were found at each sampling site. An additional 80 publications were discarded as they did not contain original records, 11 referenced ticks from migratory animals, 10 were not available in English and eight publications could not be obtained. Following the first and second screening, a total of 124 unique studies remained for analysis that fulfilled the inclusion criteria [10, 22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144] (Fig. 1; Additional file 1: Dataset S1).
A total of 2267 coordinate-referenced tick records from 33 tick species in 31 countries were regarded as eligible for inclusion in the final dataset (Fig. 2; Additional file 1: Dataset S1). Table 2 shows the number of records for each species, as well as the number of publications describing the location of each species. Ixodes was the predominant genus, accounting for 64.2% of records, followed by Dermacentor (22.5%), Hyalomma (6.6%), Haemaphysalis (3.5%) and Rhipicephalus (3.2%) (Figs. 3, 4, 5, 6, 7). In terms of individual species, the highest number of records were for I. ricinus (55%) (Fig. 6i), followed by D. reticulatus (22.1%) (Fig. 3b) and Ixodes frontalis (4.8%) (Fig. 6d). The number of records per species ranged from 1246 records for I. ricinus to just one for Ixodes gibbosus. All records documented as Rhipicephalus sanguineus sensu lato, Rhipicephalus sanguineus complex or simply Rhipicephalus sanguineus were combined and taken forward as Rhipicephalus sanguineus s.l.. Rhipicephalus sanguineus sensu stricto remained under this name.
Georeferenced records of Hyalomma species H. aegyptium (a), H. asiaticum (b), H. dromedarii (c), H. excavatum (d), H. impeltatum (e), H. lusitanicum (f), H. marginatum (g), H. rufipes (h) and H. truncatum (i) found in the literature published between 2015 and 2021. Filled blue circles indicate recorded collected sites
Georeferenced records of Ixodes species I. acuminatus (a), I. arboricola, (b), I. ariadnae (c), I. frontalis (d), I. gibbosus (e), I. inopinatus (f), I. lividus (g), I. persulcatus (h), I. ricinus (i), I. trianguliceps (j) and I. ventalloi (k) found in the literature published between 2015 and 2021. Filled blue circles indicate recorded collected sites
Georeferenced records of Rhipicephalus species R. annulatus (a), R. bursa (b), R. pulchellus (c), R. pusillus (d), R. sanguineus sensu lato (e), R. sanguineus sensu stricto (f) and R. turanicus (g) found in the literature published between 2015 and 2021. Filled blue circles indicate recorded collected sites
Most of the coordinate-referenced ticks were collected from vegetation (78.5%) and identified morphologically (81.2%). However, this was species dependent, with Ixodes persulcatus being solely found in vegetation while Rhipicephalus annulatus was exclusively collected from hosts (Table 2). Information on host order, genus or species was available for 433 records. The host order with the highest representation in this dataset was Passeriformes, followed by Artiodactyla and Rodentia.
Discussion
This systematic review provides an updated dataset of high-resolution tick occurrence records in the Western Palearctic between 2015 and 2021 for use in spatial statistics. This dataset can be used in combination with previously collated data to investigate the recent changes in tick distribution and research [15, 16]. Although 2246 records were found, the distribution of these distribution points was restricted compared to previously reported tick distributions [145, 146]. These gaps may not reflect true absence, but are more likely the result of insufficient data due to the narrower temporal range (2015–2021), reduced records as a result of uncertain georeferencing in publications (hence excluded from this dataset) and biases in sampling effort. Only 8.3% of publications found in the systematic search provided localised coordinates for tick occurrences. This discrepancy between distributions using all tick records and distributions using only coordinate-referenced records is apparent for Portugal: according to the REVIVE study [147], I. ricinus, Dermacentor marginatus and R. sanguineus were found throughout Portugal from 2011 to 2020, but this is not reflected in this dataset (Figs. 3a, 6i, 7e). There is exceptional value in documenting the localised coordinates of tick occurrences as these can be used for spatial analysis and, consequently, there should be a drive to include this practice in all tick sampling protocols.
An additional factor to consider is that there is bias within the georeferenced records. There are obvious biases towards tick species with greater public and veterinary health implications. Ixodes ricinus and D. reticulatus received the greatest attention in terms of sampling efforts, representing 55% and 22.1% of the dataset, respectively. These species are considered among the most important in the Western Palearctic, and this importance has fuelled research into their distributions [12]. There are also spatial biases in terms of only a few countries representing the majority of records. The country with the highest representation of records was Poland (15.2% of all records), followed by Lithuania (12.9%) and Germany (9.1%). The focus on tick distribution in Poland and Lithuania may be due to the north-east expansion of ticks, especially D. reticulatus, into these areas, leading to increased sampling efforts for monitoring purposes [10]. Additionally, due to the selective nature of this systematic review, there will be bias towards research groups with protocols that include the documentation of site coordinates. It must be considered that the records of a species are only reflective of the areas sampled and, consequently, are not always complete in the ecological context. There needs to be a concerted effort to accurately document all tick species and to sample both endemic and novel regions.
The reliability of data is an essential factor for the use of that data in further analysis; consequently, any possible sources of error must be noted. As the majority of these ticks were morphologically identified (81%), there may be errors associated with misidentification, for example, due to the subtle morphological differences between tick species, lack of expertise of the researcher or emergence of new species. A recent study showed that 29.6% of ticks in the Western Palearctic and North Africa had been misidentified by researchers, with the genus Rhipicephalus having the highest misidentification rate (54%) [148]. Furthermore, the emergence of “new” species which closely resemble well-established species raises the question of uncertainty in historical records. For example, the recent description of Ioxides inopinatus and its similarity to I. ricinus means that historical reports of I. ricinus within the I. inopinatus range may be misreported [141, 149]. Reassuringly, the members of the I. inopinatus haplogroup recorded in this dataset were identified genetically [141].
The overall results of the literature search reported here are in agreement with previously reported data. For example, the records for D. reticulatus and D. marginatus match the overall trend described by Rubel et al. [145]; that is, D. reticulatus present in central and northern Europe and D. marginatus with a more southernly range around the Mediterranean, albeit with a narrower distributional range (Fig. 3). Of all the species reported, I. ricinus had the greatest range in distribution, being dispersed over 29 countries, ranging longitudinally from Lisbon, Portugal (9.2°W) to south of Kyiv, Ukraine (30.6°E), and latitudinally from Djebel Zaghouan, Tunisa (36.4°N) to Dønna, Norway (66.2°N) (Fig. 6i) [119, 124, 128, 141]. The extensive distribution of I. ricinus matches previous attempts to map its range [4, 15].
Due to the human and veterinary impact of ticks, it is essential that up-to-date reliable information on their distribution is recorded. It is therefore crucial that, where data privacy regulations allow, high-resolution methods, such as site-specific pairs of coordinates, are adopted by more researchers to ensure their work can be used as secondary data and hence applied to its full potential. These localised data can then be used in combination with previous tick collections to examine the changes in tick distribution in a period of rapid change, as well as provide insight into hotspots of tick research and the locations where future efforts should be focused.
Availability of data and materials
Occurrence datasets are available in Supplementary Information.
References
Sonenshine DE, Roe RM. Biology of ticks, vol. 2. Oxford: Oxford University Press; 2013.
Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129:3–14.
Estrada-Peña A, Pfäffle M, Baneth G, Kleinerman G, Petney TN. Ixodoidea of the Western Palaearctic: a review of available literature for identification of species. Ticks Tick Borne Dis. 2017;8:512–25.
Alkishe AA, Peterson AT, Samy AM. Climate change influences on the potential geographic distribution of the disease vector tick Ixodes ricinus. PLoS ONE. 2017;12:1–14.
Fernández-Ruiz N, Estrada-Peña A. Towards new horizons: climate trends in Europe increase the environmental suitability for permanent populations of Hyalomma marginatum (Ixodidae). Pathogens. 2021;10:1–13.
Jaenson TGT, Talleklint L, Lundqvist L, Olsen B, Chirico J, Mejlon H. Geographical distribution, host associations, and vector roles of ticks (Acari: Ixodidae, Argasidae) in Sweden. J Med Entomol. 1994;31:240–56.
Tälleklint L, Jaenson TGT. Increasing geographical distribution and density of Ixodes ricinus (Acari: Ixodidae) in Central and Northern Sweden. J Med Entomol. 1998;35:521–6.
Jaenson TGT, Lindgren E. The range of Ixodes ricinus and the risk of contracting Lyme borreliosis will increase northwards when the vegetation period becomes longer. Ticks Tick Borne Dis. 2011;2:44–9.
Drehmann M, Springer A, Lindau A, Fachet K, Mai S, Thoma D, et al. The spatial distribution of Dermacentor ticks (Ixodidae) in Germany—evidence of a continuing spread of Dermacentor reticulatus. Front Vet Sci. 2020;7:578220.
Mierzejewska EJ, Estrada-Peña A, Alsarraf M, Kowalec M, Bajer A. Mapping of Dermacentor reticulatus expansion in Poland in 2012–2014. Ticks Tick Borne Dis. 2016;7:94–106.
Hvidsten D, Frafjord K, Gray JS, Henningsson AJ, Jenkins A, Kristiansen BE, et al. The distribution limit of the common tick, Ixodes ricinus, and some associated pathogens in north-western Europe. Ticks Tick Borne Dis. 2020;11:101388.
Cunze S, Glock G, Kochmann J, Klimpel S. Ticks on the move—climate change-induced range shifts of three tick species in Europe: current and future habitat suitability for Ixodes ricinus in comparison with Dermacentor reticulatus and Dermacentor marginatus. Parasitol Res. 2022;121:2241–52.
Uusitalo R, Siljander M, Lindén A, Sormunen JJ, Aalto J, Hendrickx G, et al. Predicting habitat suitability for Ixodes ricinus and Ixodes persulcatus ticks in Finland. Parasit Vectors. 2022;15:310.
Li S, Gilbert L, Harrison PA, Rounsevell MDA. Modelling the seasonality of Lyme disease risk and the potential impacts of a warming climate within the heterogeneous landscapes of Scotland. J R Soc Interface. 2016;13:20160140.
Estrada-Peña A, Farkas R, Jaenson TGT, Koenen F, Madder M, Pascucci I, et al. Association of environmental traits with the geographic ranges of ticks (Acari: Ixodidae) of medical and veterinary importance in the Western Palearctic. A digital dataset. Exp Appl Acarol. 2013;59:351–66.
Estrada-Peña A, De La Fuente J. Species interactions in occurrence data for a community of tick-transmitted pathogens. Sci Data. 2016;3:160056.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9.
Clarivate Plc. Web of Science. 2022. https://www.webofscience.com/ Accessed 5 Jan 2022.
National Center for Biotechnology Information (NCBI). PubMed. 2022. https://pubmed.ncbi.nlm.nih.gov/ Accessed 1 Dec 2022.
EFSA Panel on Animal Health and Welfare (AHAW). Scientific opinion on geographic distribution of tick-borne infections and their vectors in Europe and the other regions of the Mediterranean basin. EFSA J. 2010;8:1723.
R Core Team. R: A language and environment for statistical computing. 2021. Vienna: R Foundation for Statistical Computing. https://www.R-project.org/.
Akl T, Bourgoin G, Souq ML, Appolinaire J, Poirel MT, Gibert P, et al. Detection of tick-borne pathogens in questing Ixodes ricinus in the French Pyrenees and first identification of Rickettsia monacensis in France. Parasite. 2019;26:20.
Al-Khafaji AM, Bell-Sakyi L, Fracasso G, Luu L, Heylen D, et al. Isolation of Candidatus Rickettsia vini from Belgian Ixodes arboricola ticks and propagation in tick cell lines. Ticks Tick Borne Dis. 2020;11:101511.
Andersen NS, Bestehorn M, Chitimia-Dobler L, Kolmos HJ, Jensen PM, Dobler G, et al. Phylogenetic characterization of tick-borne encephalitis virus from Bornholm, Denmark. Ticks Tick Borne Dis. 2019;10:533–9.
Asghar N, Petersson M, Johansson M, Dinnetz P. Local landscape effects on population dynamics of Ixodes ricinus. Geospat Health. 2016;11:487.
Aureli S, Galuppi R, Ostanello F, Foley JE, Bonoli C, Rejmanek D, et al. Abundance of questing ticks and molecular evidence for pathogens in ticks in three parks of Emilia-Romagna region of Northern Italy. Ann Agric Environ Med. 2015;22:459–66.
Banović P, Díaz-Sánchez AA, Galon C, Foucault-Simonin A, Simin V, Mijatović D, et al. A One Health approach to study the circulation of tick-borne pathogens: a preliminary study. One Health. 2021;13:100270.
Bell-Sakyi L, Palomar AM, Kazimirova M. Isolation and propagation of a Spiroplasma sp. from Slovakian Ixodes ricinus ticks in Ixodes spp. cell lines. Ticks Tick Borne Dis. 2015;6:601–6.
Bertola M, Montarsi F, Obber F, Da Rold G, Carlin S, Toniolo F, et al. Occurrence and identification of Ixodes ricinus borne pathogens in northeastern Italy. Pathogens. 2021;10:1181.
Bestehorn M, Weigold S, Kern WV, Chitimia-Dobler L, Mackenstedt U, Dobler G, et al. Phylogenetics of tick-borne encephalitis virus in endemic foci in the upper Rhine region in France and Germany. PLoS ONE. 2018;13:10.
Blaňarová L, Stanko M, Miklisová D, Víchová B, Mošanský L, Kraljik J, et al. Presence of Candidatus Neoehrlichia mikurensis and Babesia microti in rodents and two tick species (Ixodes ricinus and Ixodes trianguliceps) in Slovakia. Ticks Tick Borne Dis. 2016;7:319–26.
Blazhev A, Atanasova M, Kostov K, Doychinova T, Blazheva S, Karcheva M. Estimation of Ixodes ricinus (Acari: Ixodidae) populations of Kaylaka Park in the town of Pleven, Bulgaria. Insects. 2021;12:9.
Boehnke D, Brugger K, Pfäffle M, Sebastian P, Norra S, Petne T, et al. Estimating Ixodes ricinus densities on the landscape scale. Int J Health Geogr. 2015;14:23.
Bona M, Blaňárová L, Stanko M, Mošanský L, Čepčeková E, Víchová B. Impact of climate factors on the seasonal activity of ticks and temporal dynamics of tick-borne pathogens in an area with a large tick species diversity in Slovakia, Central Europe. Biologia. 2021;77:1619–31.
Bonnet SI, Paul RE, Bischoff E, Cote M, Le Naour E. First identification of Rickettsia helvetica in questing ticks from a French Northern Brittany Forest. PLoS Negl Trop Dis. 2017;11:3.
Borşan SD, Toma-Naic A, Péter Á, Sándor AD, Peștean C, Mihalca AD. Impact of abiotic factors, habitat type and urban wildlife on the ecology of hard ticks (Acari: Ixodidae) in urban and peri-urban habitats. Parasit Vectors. 2020;13:476.
Boyer PH, Baldinger L, Degeilh B, Wirth X, Kamdem CM, Hansmann Y, et al. The emerging tick-borne pathogen Neoehrlichia mikurensis: first French case series and vector epidemiology. Emerg Microbes Infect. 2021;10:1731–8.
Cafiso A, Olivieri E, Floriano AM, Chiappa G, Serra V, Sassera D, et al. Investigation of tick-borne pathogens in Ixodes ricinus in a peri-urban park in Lombardy (Italy) reveals the presence of emerging pathogens. Pathogens. 2021;10:732.
Chitimia-Dobler L. Spatial distribution of Dermacentor reticulatus in Romania. Vet Parasitol. 2015;214:219–23.
Chvostáč M, Špitalská E, Václav R, Vaculová T, Minichová L, Derdáková M. Seasonal patterns in the prevalence and diversity of tick-borne Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Rickettsia spp. in an urban temperate forest in south western Slovakia. Int J Environ Res Public Health. 2018;15:994.
Cuber P, Andreassen Å, Vainio K, Asman M, Dudman S, Szilman P, et al. Risk of exposure to ticks (Ixodidae) and the prevalence of tick-borne encephalitis virus (TBEV) in ticks in Southern Poland. Ticks Tick Borne Dis. 2015;6:356–63.
Cull B, Hansford KM, McGinley L, Gillingham EL, Vaux AGC, Smith R, et al. A nationwide study on Borrelia burgdorferi sl infection rates in questing Ixodes ricinus: a six-year snapshot study in protected recreational areas in England and Wales. Med Vet Entomol. 2021;35:352–60.
Daniel M, Danielová V, Kříž B, Růžek D, Fialová A, Mal M, et al. The occurrence of Ixodes ricinus ticks and important tick-borne pathogens in areas with high tick-borne encephalitis prevalence in different altitudinal levels of the Czech Republic Part I. Ixodes ricinus ticks and tick-borne encephalitis virus. Epidemiol Mikrobiol Imunol. 2016;65:118–28.
Daniel M, Malý M, Danielová V, Kříž B, Nuttall P. Abiotic predictors and annual seasonal dynamics of Ixodes ricinus, the major disease vector of Central Europe. Parasit Vectors. 2015;8:478.
Didyk YM, Mangová B, Kraljik J, Stanko M, Spitalská E, Derdáková M. Rhipicephalus sanguineus sl detection in the Slovak Republic. Biologia. 2021;77:1523–9.
Drehmann M, Chitimia-Dobler L, Lindau A, Frank A, Mai S, Fachet K, et al. Ixodes frontalis: a neglected but ubiquitous tick species in Germany. Exp Appl Acarol. 2019;78:79–91.
Duscher GG, Hodžić A, Weiler M, Vaux AG, Rudolf I, Sixl W, et al. First report of Rickettsia raoultii in field collected Dermacentor reticulatus ticks from Austria. Ticks Tick Borne Dis. 2016;7:720–2.
Dwużnik-Szarek D, Mierzejewska EJ, Rodo A, Goździk K, Behnke-Borowczyk J, Kiewra D, et al. Monitoring the expansion of Dermacentor reticulatus and occurrence of canine babesiosis in Poland in 2016–2018. Parasit Vectors. 2021;14:267.
Dwużnik D, Mierzejewska EJ, Alsarraf M, Bajer A. A new focus of the tick Haemaphysalis concinna in Western Poland. Exp Appl Acarol. 2019;78:93–112.
Ehrmann S, Liira J, Gärtner S, Hansen K, Brunet J, Cousins SA, et al. Environmental drivers of Ixodes ricinus abundance in forest fragments of rural European landscapes. BMC Ecol. 2017;17:31.
El Mouden EH, Laghzaoui EM, Elbahi A, Abbad A. A case of massive infestation of a female Spur-thighed tortoise Testudo graeca by blood-sucking ticks Hyalomma aegyptium (Acari: Ixodidae). Int J Acarol. 2020;46:63–5.
Fares W, Dachraoui K, Cherni S, Barhoumi W, Slimane TB, Younsi H, et al. Tick-borne encephalitis virus in Ixodes ricinus (Acari: Ixodidae) ticks, Tunisia. Ticks Tick Borne Dis. 2021;12:101606.
Flaisz B, Sulyok KM, Kováts D, Kontschán J, Csörgő T, Csipak Á, et al. Babesia genotypes in Haemaphysalis concinna collected from birds in Hungary reflect phylogeographic connections with Siberia and the Far East. Ticks Tick Borne Dis. 2017;8:666–70.
Frank R, Kuhn T, Werblow A, Liston A, Kochmann J, Klimpel S. Parasite diversity of European Myotis species with special emphasis on Myotis myotis (Microchiroptera, Vespertilionidae) from a typical nursery roost. Parasit Vectors. 2015;8:101.
Giangaspero A, Marangi M, Papini R, Paoletti B, Wijnveld M, Jongejan F. Theileria sp. OT3 and other tick-borne pathogens in sheep and ticks in Italy: molecular characterization and phylogeny. Ticks Tick Borne Dis. 2015;6:75–83.
Gillingham EL, Hansford KM, Meadows S, Henney J, Wieckowski F, Hernández-Triana LM, et al. Ticks on the Channel Islands and implications for public health. Ticks Tick Borne Dis. 2020;11:101405.
Hekimoglu O, Ozer AN. Distribution and phylogeny of Hyalomma ticks (Acari: Ixodidae) in Turkey. Exp Appl Acarol. 2017;73:501–19.
Hauser G, Rais O, Morán Cadenas F, Gonseth Y, Bouzelboudjen M, Gern L. Influence of climatic factors on Ixodes ricinus nymph abundance and phenology over a long-term monthly observation in Switzerland (2000–2014). Parasit Vectors. 2018;11:289.
Heglasová I, Rudenko N, Golovchenko M, Zubriková D, Miklisová D, Stanko M. Ticks, fleas and rodent-hosts analyzed for the presence of Borrelia miyamotoi in Slovakia: the first record of Borrelia miyamotoi in a Haemaphysalis inermis tick. Ticks Tick Borne Dis. 2020;11:101456.
Hekimoglu O, Sahin MK, Ergan G, Ozer N. A molecular phylogenetic investigation of tick species in Eastern and Southeastern Anatolia. Ticks Tick Borne Dis. 2021;12:101777.
Hofmeester TR, Sprong H, Jansen PA, Prins HH, Van Wieren SE. Deer presence rather than abundance determines the population density of the sheep tick, Ixodes ricinus, in Dutch forests. Parasit Vectors. 2017;10:433.
Hofmeester TR, van der Lei PB, Van Leeuwen AD, Sprong H, van Wieren SE. New foci of Haemaphysalis punctata and Dermacentor reticulatus in the Netherlands. Ticks Tick Borne Dis. 2016;7:367–70.
Honig V, Carolan HE, Vavruskova Z, Massire C, Mosel MR, Crowder CD. Broad-range survey of vector-borne pathogens and tick host identification of Ixodes ricinus from Southern Czech Republic. FEMS Microbiol Ecol. 2017;93:129.
Hornok S, Krawczyk A. First record of Ixodes ariadnae in western Europe, Belgium. Acta Vet Hung. 2016;64:467–71.
Hornok S, Takács N, Szőke K, Kunz B. First record of Ixodes ariadnae in Germany. Acta Vet Hung. 2015;63:347–51.
Hvidsten D, Stordal F, Lager M, Rognerud B, Kristiansen BE, Matussek A, et al. Borrelia burgdorferi sensu lato-infected Ixodes ricinus collected from vegetation near the Arctic Circle. Ticks Tick Borne Dis. 2015;6:768–73.
Ivanova A, Geller J, Katargina O, Värv K, Lundkvist Å, Golovljova I. Detection of Candidatus Neoehrlichia mikurensis and Ehrlichia muris in Estonian ticks. Ticks Tick Borne Dis. 2017;8:13–7.
Jaenson TG, Wilhelmsson P. First records of tick-borne pathogens in populations of the taiga tick Ixodes persulcatus in Sweden. Parasit Vectors. 2019;12:559.
Jaenson TG, Värv K, Fröjdman I, Jääskeläinen A, Rundgren K, Versteirt V, et al. First evidence of established populations of the taiga tick Ixodes persulcatus (Acari: Ixodidae) in Sweden. Parasit Vectors. 2016;9:377.
Kar S, Rodriguez SE, Akyildiz G, Cajimat MN, Bircan R, Mears MC, et al. Crimean-Congo hemorrhagic fever virus in tortoises and Hyalomma aegyptium ticks in East Thrace, Turkey: potential of a cryptic transmission cycle. Parasit Vectors. 2020;13:201.
Karbowiak G, Biernat B, Werszko J, Rychlik L. The transstadial persistence of tick-borne encephalitis virus in Dermacentor reticulatus ticks in natural conditions. Acta Parasitol. 2016;61:201–3.
Karger A, Bettin B, Gethmann JM, Klaus C. Whole animal matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry of ticks–are spectra of Ixodes ricinus nymphs influenced by environmental, spatial, and temporal factors? PLoS ONE. 2019;2019:0210590.
Kazimírová M, Hamšíková Z, Kocianová E, Marini G, Mojšová M, Mahríková L, et al. Relative density of host-seeking ticks in different habitat types of south-western Slovakia. Exp Appl Acarol. 2016;69:205–24.
Kiewra D, Czułowska A, Dyczko D, Zieliński R, Plewa-Tutaj K. First record of Haemaphysalis concinna (Acari: Ixodidae) in Lower Silesia, SW Poland. Exp Appl Acarol. 2019;77:449–54.
Kiewra D, Stefańska-Krzaczek E, Szymanowski M, Szczepańska A. Local-scale spatio-temporal distribution of questing Ixodes ricinus L (Acari: Ixodidae)—a case study from a riparian urban forest in Wrocław, SW Poland. Ticks Tick Borne Dis. 2017;8:362–9.
Kjær LJ, Klitgaard K, Soleng A, Edgar KS, Lindstedt HEH, Paulsen KM, et al. Spatial data of Ixodes ricinus instar abundance and nymph pathogen prevalence, Scandinavia, 2016–2017. Sci Data. 2020;7:238.
Klitgaard K, Kjær LJ, Isbrand A, Hansen MF, Bødker R. Multiple infections in questing nymphs and adult female Ixodes ricinus ticks collected in a recreational forest in Denmark. Ticks Tick Borne Dis. 2019;10:1060–5.
Kohn M, Krücken J, McKay-Demeler J, Pachnicke S, Krieger K, von Samson-Himmelstjerna G. Dermacentor reticulatus in Berlin/Brandenburg (Germany): activity patterns and associated pathogens. Ticks Tick Borne Dis. 2019;10:191–206.
Kowalec M, Szewczyk T, Welc-Falęciak R, Siński E, Karbowiak G, Bajer A. Ticks and the city-are there any differences between city parks and natural forests in terms of tick abundance and prevalence of spirochaetes? Parasit Vectors. 2017;10:537.
Kubiak K, Sielawa H, Dziekońska-Rynko J, Kubiak D, Rydzewska M, Dzika E. Dermacentor reticulatus ticks (Acari: Ixodidae) distribution in north-eastern Poland: an endemic area of tick-borne diseases. Exp Appl Acarol. 2018;75:289–98.
Laghzaoui EM, Kasrati A, Abbad A, Leach D, Spooner-Hart R, El Mouden EH. Acaricidal properties of essential oils from Moroccan plants against immature ticks of Hyalomma aegyptium (Linnaeus, 1758); an external parasite of the spur-thighed tortoise (Testudo graeca). Int J Acarol. 2018;44:315–21.
Lesiczka PM, Daněk O, Modrý D, Hrazdilová K, Votýpka J, Zurek L. A new report of adult Hyalomma marginatum and Hyalomma rufipes in the Czech Republic. Ticks Tick Borne Dis. 2021;13:101894.
Li K, Stanojević M, Stamenković G, Ilić B, Paunović M, Lu M, et al. Insight into diversity of bacteria belonging to the order Rickettsiales in 9 arthropod species collected in Serbia. Sci Rep. 2019;9:18680.
Luu L, Bown KJ, Palomar AM, Kazimírová M, Bell-Sakyi L. Isolation and partial characterisation of a novel Trypanosoma from the tick Ixodes ricinus. Ticks Tick Borne Dis. 2020;11:101501.
Marchant A, Le Coupanec A, Joly C, Perthame E, Sertour N, Garnier M, et al. Infection of Ixodes ricinus by Borrelia burgdorferi sensu lato in peri-urban forests of France. PLoS ONE. 2017;12:0183543.
Matulaitytė V, Radzijevskaja J, Paulauskas A. First records of Ixodes lividus from sand martin (Riparia riparia) nests in Lithuania. J Vector Ecol. 2017;42:264–70.
Matysiak A, Wasielewski O, Włodarek J, Ondrejkova A, Tryjanowski P. First report of ticks in the subcutaneous tissue of the raccoon dog Nyctereutes procyonoides. Vet Med (Praha). 2018;63:571–4.
Medlock JM, Hansford KM, Vaux AGC, Cull B, Abdullah S, Pietzsch ME, et al. Distribution of the tick Dermacentor reticulatus in the United Kingdom. Med Vet Entomol. 2017;31:281–8.
Medlock JM, Hansford K, Vaux AG, Simonsen W, Jensen JK, Joensen C, et al. Surveillance for Ixodes ricinus ticks (Acari, Ixodidae) on the Faroe Islands. Ticks Tick Borne Dis. 2017;8:190–5.
Mehlhorn H, Mehlhorn T, Müller M, Vogt M, Rissland J. Tick survey for prevalent pathogens in peri-urban recreation sites in Saarland and Rhineland-Palatinate (Germany). Parasitol Res. 2016;115:1167–72.
Millins C, Leo W, MacInnes I, Ferguson J, Charlesworth G, Nayar D, et al. Emergence of Lyme disease on treeless islands, Scotland, United Kingdom. Emerg Infect Dis. 2021;27:538–46.
Mori E, Pisanu B, Zozzoli R, Solano E, Olivieri E, Sassera D, et al. Arthropods and associated pathogens from native and introduced rodents in Northeastern Italy. Parasitol Res. 2018;117:3237–43.
Mtierová Z, Derdáková M, Chvostáč M, Didyk YM, Mangová B, Rusňáková Tarageľová V, et al. Local population structure and seasonal variability of Borrelia garinii genotypes in Ixodes ricinus ticks, Slovakia. Int J Environ Res Public Health. 2020;17:3607.
Napoli E, Remesar S, Gaglio G, Giannetto S, Spadola F, Díaz P, et al. Ectoparasites of wild rabbit (Oryctolagus cuniculus) in Southern Italy. Vet Parasitol Reg Stud. 2021;24:100555.
Nava S, Beati L, Venzal JM, Labruna MB, Szabó MP, Petney T, et al. Rhipicephalus sanguineus (Latreille, 1806): neotype designation, morphological re-description of all parasitic stages and molecular characterization. Ticks Tick Borne Dis. 2018;9:1573–85.
Novakova M, Bulkova A, Costa FB, Kristin A, Krist M, Krause F, et al. Molecular characterization of ‘Candidatus Rickettsia vini’ in Ixodes arboricola from the Czech Republic and Slovakia. Ticks Tick Borne Dis. 2015;6:330–3.
Nováková M, Heneberg P, Heylen DJ, Medvecký M, Muñoz-Leal S, Šmajs D, et al. Isolated populations of Ixodes lividus ticks in the Czech Republic and Belgium host genetically homogeneous Rickettsia vini. Ticks Tick Borne Dis. 2018;9:479–84.
Nyrhilä S, Sormunen JJ, Mäkelä S, Sippola E, Vesterinen EJ, Klemola T. One out of ten: low sampling efficiency of cloth dragging challenges abundance estimates of questing ticks. Exp Appl Acarol. 2020;82:571–85.
Obiegala A, Pfeffer M, Pfister K, Karnath C, Silaghi C. Molecular examinations of Babesia microti in rodents and rodent-attached ticks from urban and sylvatic habitats in Germany. Ticks Tick Borne Dis. 2015;6:445–9.
Okely M, Anan R, Gad-Allah S, Samy AM. Hard ticks (Acari: Ixodidae) infesting domestic animals in Egypt: diagnostic characters and a taxonomic key to the collected species. Med Vet Entomol. 2021;35:333–51.
Olivieri E, Gazzonis AL, Zanzani SA, Veronesi F, Manfredi MT. Seasonal dynamics of adult Dermacentor reticulatus in a peri-urban park in southern Europe. Ticks Tick Borne Dis. 2017;8:772–9.
Olivieri E, Zanzani SA, Latrofa MS, Lia RP, Dantas-Torres F, Otranto D, et al. The southernmost foci of Dermacentor reticulatus in Italy and associated Babesia canis infection in dogs. Parasit Vectors. 2016;9:213.
Ouarti B, Hamzaoui BE, Stanko M, Laroche M, Mediannikov O, Parola P, et al. Detection of Rickettsia raoultii in Dermacentor reticulatus and Haemaphysalis inermis ticks in Slovakia. Biologia. 2021;77:1611–7.
Pajoro M, Pistone D, Varotto Boccazzi I, Mereghetti V, Bandi C, Fabbi M, et al. Molecular screening for bacterial pathogens in ticks (Ixodes ricinus) collected on migratory birds captured in northern Italy. Folia Parasitol. 2018;15:65.
Palomar AM, Portillo A, Santibáñez P, Mazuelas D, Roncero L, García-Álvarez L, et al. Detection of tick-borne Anaplasma bovis, Anaplasma phagocytophilum and Anaplasma centrale in Spain. Med Vet Entomol. 2015;29:349–53.
Da Silva LP, De Carvalho L, De SousaNorte RAC. First report of Ixodes lividus (Koch 1844 in sand martins Riparia riparia in Portugal. Syst Appl Acarol. 2020;2020:1883–8.
Paulauskas A, Galdikas M, Galdikaitė-Brazienė E, Stanko M, Kahl O, Karbowiak G, et al. Microsatellite-based genetic diversity of Dermacentor reticulatus in Europe. Infect Genet Evol. 2018;66:200–9.
Paulauskas A, Radzijevskaja J, Mardosaitė-Busaitienė D, Aleksandravičienė A, Galdikas M, Krikštolaitis R. New localities of Dermacentor reticulatus ticks in the Baltic countries. Ticks Tick Borne Dis. 2015;6:630–5.
Paulsen KM, Pedersen BN, Soleng A, Okbaldet YB, Pettersson JHO, et al. Prevalence of tick-borne encephalitis virus in Ixodes ricinus ticks from three islands in north-western Norway. APMIS. 2015;123:759–64.
Pedersen BN, Jenkins A, Paulsen KM, Okbaldet YB, Edgar KS, Lamsal A, et al. Distribution of Neoehrlichia mikurensis in Ixodes ricinus ticks along the coast of Norway: the western seaboard is a low-prevalence region. Zoonoses Public Health. 2020;67:130–7.
Pistone D, Pajoro M, Novakova E, Vicari N, Gaiardelli C, Vigano R, et al. Ticks and bacterial tick-borne pathogens in Piemonte region, Northwest Italy. Exp Appl Acarol. 2017;73:477–91.
Plantard O, Hoch T, Daveu R, Rispe C, Stachurski F, Boué F, et al. Where to find questing Ixodes frontalis ticks? Under bamboo bushes! Ticks Tick Borne Dis. 2021;12:101625.
Potkonjak A, Gutiérrez R, Savić S, Vračar V, Nachum-Biala Y, Jurišić A, et al. Molecular detection of emerging tick-borne pathogens in Vojvodina, Serbia. Ticks Tick Borne Dis. 2016;7:199–203.
Potkonjak A, Petrović T, Ristanović E, Lalić I, Vračar V, Savić S, et al. Molecular detection and serological evidence of tick-borne encephalitis virus in Serbia. Vector Borne Zoonotic Dis. 2017;17:813–20.
Radzijevskaja J, Mardosaitė-Busaitienė D, Aleksandravičienė A, Paulauskas A. Investigation of Babesia spp. in sympatric populations of Dermacentor reticulatus and Ixodes ricinus ticks in Lithuania and Latvia. Ticks Tick Borne Dis. 2018;9:270–4.
Răileanu C, Tauchmann O, Vasić A, Wöhnke E, Silaghi C. Borrelia miyamotoi and Borrelia burgdorferi (sensu lato) identification and survey of tick-borne encephalitis virus in ticks from north-eastern Germany. Parasit Vectors. 2020;13:106.
Ramos RAN, Campbell BE, Whittle A, Lia RP, Montarsi F, Parisi A, et al. Occurrence of Ixodiphagus hookeri (Hymenoptera: Encyrtidae) in Ixodes ricinus (Acari: Ixodidae) in Southern Italy. Ticks Tick Borne Dis. 2015;6:234–6.
Remesar S, Diaz P, Venzal JM, Prieto A, Estrada-Peña A, López CM, et al. Longitudinal study of infection with Borrelia spp. in questing ticks from North-Western Spain. Vector Borne Zoonotic Dis. 2019;19:785–92.
Rogovskyy AS, Nebogatkin IV, Scoles GA. Ixodid ticks in the megapolis of Kyiv, Ukraine. Ticks Tick Borne Dis. 2017;8:99–102.
Rogovskyy AS, Threadgill DW, Akimov IA, Nebogatkin IV, Rogovska YV, Melnyk MV, et al. Borrelia and other zoonotic pathogens in Ixodes ricinus and Dermacentor reticulatus ticks collected from the Chernobyl Exclusion Zone on the 30th Anniversary of the nuclear disaster. Vector Borne Zoonotic Dis. 2019;19:466–73.
Rudolf I, Venclíková K, Blažejová H, Betášová L, Mendel J, Hubálek Z, et al. First report of Rickettsia raoultii and Rickettsia helvetica in Dermacentor reticulatus ticks from the Czech Republic. Ticks Tick Borne Dis. 2016;7:1222–4.
Rybářová M, Široký P. Occurrence of Anaplasma phagocytophilum in three sympatric tick species in the South Moravia, Czech Republic. Biologia. 2017;72:365–9.
Saloña-Bordas MI, Bahillo de la Puebla P, Díaz Martín B, Sumner J, Perotti MA. Ixodes ricinus (Ixodidae), an occasional phoront on necrophagous and coprophagous beetles in Europe. Exp Appl Acarol. 2015;65:243–8.
Santos AS, de Bruin A, Veloso AR, Marques C, da Fonseca IP, de Sousa R, et al. Detection of Anaplasma phagocytophilum, Candidatus Neoehrlichia sp, Coxiella burnetii and Rickettsia spp. in questing ticks from a recreational park, Portugal. Ticks Tick Borne Dis. 2018;9:1555–64.
Schmuck HM, Chitimia-Dobler L, Król N, Kacza J, Pfeffer M. Collection of immature Dermacentor reticulatus (Fabricius, 1794) ticks from vegetation and detection of Rickettsia raoultii in them. Ticks Tick Borne Dis. 2020;11:101543.
Sidorenko M, Radzijevskaja J, Mickevičius S, Bratčikovienė N, Paulauskas A. Prevalence of tick-borne encephalitis virus in questing Dermacentor reticulatus and Ixodes ricinus ticks in Lithuania. Ticks Tick Borne Dis. 2021;12:101594.
Silaghi C, Weis L, Pfister K. Dermacentor reticulatus and Babesia canis in Bavaria (Germany)—a georeferenced field study with digital habitat characterization. Pathogens. 2020;9:541.
Soleng A, Edgar KS, Paulsen KM, Pedersen BN, Okbaldet YB, Skjetne IEB, et al. Distribution of Ixodes ricinus ticks and prevalence of tick-borne encephalitis virus among questing ticks in the Arctic Circle region of northern Norway. Ticks Tick Borne Dis. 2018;9:97–103.
Sormunen JJ, Klemola T, Hänninen J, Mäkelä S, Vuorinen I, Penttinen R, et al. The importance of study duration and spatial scale in pathogen detection—evidence from a tick-infested island. Emerg Microbes Infect. 2018;7:189.
Sormunen JJ, Klemola T, Vesterinen EJ, Vuorinen I, Hytönen J, Hänninen J, et al. Assessing the abundance, seasonal questing activity, and Borrelia and tick-borne encephalitis virus (TBEV) prevalence of Ixodes ricinus ticks in a Lyme borreliosis endemic area in Southwest Finland. Ticks Tick Borne Dis. 2016;7:208–15.
Špitalská E, Kraljik J, Miklisová D, Boldišová E, Sparagano OA, Stanko M. Circulation of Rickettsia species and rickettsial endosymbionts among small mammals and their ectoparasites in Eastern Slovakia. Parasitol Res. 2020;119:2047–57.
Špitalská E, Stanko M, Mošanský L, Kraljik J, Miklisová D, Mahríková L, et al. Seasonal analysis of Rickettsia species in ticks in an agricultural site of Slovakia. Exp Appl Acarol. 2016;68:315–24.
Sprong H, Moonen S, van Wieren SE, Hofmeester TR. Effects of cattle grazing on Ixodes ricinus-borne disease risk in forest areas of the Netherlands. Ticks Tick Borne Dis. 2020;11:101355.
Stańczak J, Cieniuch S, Lass A, Biernat B, Racewicz M. Detection and quantification of Anaplasma phagocytophilum and Babesia spp. in Ixodes ricinus ticks from urban and rural environment, northern Poland, by real-time polymerase chain reaction. Exp Appl Acarol. 2015;2015:63–81.
Stevanović O, Jurković D, Polkinghorne A, Ćeleš A, Ilić T, Dimitrijević S, et al. Molecular detection of Babesia divergens and Mycoplasma wenyonii infection in cattle from Bosnia and Herzegovina. Parasitol Res. 2020;119:1423–7.
Tarageľová VR, Mahríková L, Selyemová D, Václav R, Derdáková M. Natural foci of Borrelia lusitaniae in a mountain region of Central Europe. Ticks Tick Borne Dis. 2016;7:350–6.
Toma L, Khoury C, Bianchi R, Severini F, Mancini F, Ciervo A, et al. Preliminary investigation on tick fauna in the neighborhood of Tarquinia, Lazio, Italy. Ann Ist Super. 2015;51:67–70.
Torina A, Blanda V, Blanda M, Auteri M, La Russa F, Scimeca S, et al. A geographical information system based approach for integrated strategies of tick surveillance and control in the peri-urban natural reserve of Monte Pellegrino (Palermo, Southern Italy). Int J Environ Res Public Health. 2018;15:404.
Venclikova K, Mendel J, Betasova L, Hubalek Z, Rudolf I. First evidence of Babesia venatorum and Babesia capreoli in questing Ixodes ricinus ticks in the Czech Republic. Ann Agric Environ Med. 2015;22:212–4.
Weiner M, Żukiewicz-Sobczak W, Tokarska-Rodak M, Plewik D, Pańczuk A, Siłuch M, et al. Prevalence of in ticks from the Ternopil region in Ukraine. J Vet Res. 2018;62:275–80.
Younsi H, Fares W, Cherni S, Dachraoui K, Barhoumi W, Najjar C, et al. Ixodes inopinatus and Ixodes ricinus (Acari: Ixodidae) are sympatric ticks in North Africa. J Med Entomol. 2020;57:952–6.
Zając Z, Sędzikowska A, Maślanko W, Woźniak A, Kulisz J. Occurrence and Abundance of Dermacentor reticulatus in the habitats of the ecological corridor of the Wieprz river, eastern Poland. Insects. 2021;12:96.
Zajac Z, Wozniak A, Kulisz J. Infestation of dairy cows by ticks Dermacentor reticulatus (Fabricius, 1794) and Ixodes ricinus (Linnaeus, 1758) in eastern Poland. Ann Parasitol. 2020;66:87–96.
Zubriková D, Wittmann M, Hönig V, Švec P, Víchová B, Essbauer S, et al. Prevalence of tick-borne encephalitis virus and Borrelia burgdorferi sensu lato in Ixodes ricinus ticks in Lower Bavaria and Upper Palatinate, Germany. Ticks Tick Borne Dis. 2020;11:101375.
Rubel F, Brugger K, Pfeffer M, Chitimia-Dobler L, Didyk YM, Leverenz S, et al. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick Borne Dis. 2016;7:224–33.
Tokarevich NK, Tronin AA, Blinova OV, Buzinov RV, Boltenkov VP, Yurasova ED, et al. The impact of climate change on the expansion of Ixodes persulcatus habitat and the incidence of tick-borne encephalitis in the north of European Russia. Glob Health Action. 2011;4:8448.
Instituto Nacional de Saúde Dr. Ricardo Jorge. REVIVE. 2021. http://hdl.handle.net/10400.18/8004 Accessed 26 Jan 2023.
Estrada-Peña A, D’Amico G, Palomar AM, Dupraz M, Fonville M, Heylen D, et al. A comparative test of Ixodid tick identification by a network of European researchers. Ticks Tick Borne Dis. 2017;8:540–6.
Estrada-Peña A, Nava S, Petney T. Description of all the stages of Ixodes inopinatus n. sp. (Acari: Ixodidae). Ticks Tick Borne Dis. 2014;5:734–43.
Funding
This work was funded by MSD Animal Health UK.
Author information
Authors and Affiliations
Contributions
MN: conceptualisation, methodology, formal analysis, data curation, writing—original draft and visualisation. RW: writing—review and editing, supervision and funding acquisition. BM: writing—review and editing and supervision. HRV: conceptualisation, methodology, writing—review and editing, supervision and funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
RW and HRV have had research funded by a range of pharmaceutical companies and animal health charities. RW is director ESCCAP UK & Ireland.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Dataset S1
. Extracted data for coordinate-referenced tick records in Europe from 2015 to 2021.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Noll, M., Wall, R., Makepeace, B.L. et al. Distribution of ticks in the Western Palearctic: an updated systematic review (2015–2021). Parasites Vectors 16, 141 (2023). https://doi.org/10.1186/s13071-023-05773-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05773-6