Abstract
Background
The common tick Ixodes ricinus and the taiga tick I. persulcatus are the main tick vectors of Borrelia spirochaetes, TBE virus (TBEV) and of several other zoonotic pathogens in the western and eastern areas, respectively of the Palaearctic region. Recently, populations of the taiga tick were, for the first time, detected in northern Sweden. This prompted us to investigate if they harbour human pathogens.
Methods
A total of 276 I. persulcatus ticks (136 males, 126 females and 14 nymphs) and one I. ricinus nymph was collected by the cloth-dragging method in northern Sweden in July–August 2015 and May–July 2016. In addition, 8 males and 10 females of I. persulcatus were collected from two dogs (16 and 2 ticks, respectively) in two of the localities. All ticks were microscopically and molecularly identified to developmental stage and species and screened for B. burgdorferi (sensu lato), B. miyamotoi, Anaplasma phagocytophilum, Rickettsia spp., Neoehrlichia mikurensis, Babesia spp. and TBEV using real-time PCR followed by species identification by sequencing the PCR-products of conventional PCR assays.
Results
Of the ticks collected by the cloth-dragging method, 55% (152/277) were positive for Borrelia. There was no significant difference between the proportions of Borrelia-infected nymphs (33%, 5/15) and Borrelia-infected adult ticks (56%, 147/262), and no significant difference between the proportions of Borrelia-infected males (54%, 74/136) and Borrelia-infected females (58%, 73/126). Three different Borrelia species were identified. Borrelia afzelii was the predominant species and detected in 46% of all Borrelia-infected ticks followed by B. garinii, 35%, B. valaisiana, 1%, and mixed infections of different Borrelia species, 1%; 17% of all Borrelia-infections were untypeable. One I. persulcatus female contained Rickettsia helvetica, and one nymph contained Rickettsia sp. Of the 277 ticks analysed, all were negative for A. phagocytophilum, Babesia spp., Borrelia miyamotoi, N. mikurensis and TBEV. The ticks collected from the two dogs were negative for all pathogens examined except for Borrelia spp., that was detected in 5 out of 16 ticks removed from one of the dogs.
Conclusions
To our knowledge, this is the first time that I. persulcatus from Sweden has been analysed for the presence of tick-borne pathogens. The examined tick populations had a low diversity of tick-borne pathogens but a high prevalence of B. burgdorferi (s.l.).
Similar content being viewed by others
Background
Two closely related tick species, Ixodes ricinus and I. persulcatus, are the main vectors of Lyme borreliosis spirochaetes, of the tick-borne encephalitis virus (TBEV) and of several other tick-borne pathogens of humans in the western and eastern Palaearctic region, respectively. In Sweden, the common tick I. ricinus is the main vector of tick-borne pathogens to humans [1, 2]. It is abundant in the southern and south-central parts of the country. In inland northern Sweden and along the northern east coast bordering the Baltic Sea the distribution of I. ricinus is more scattered [1, 2]. Recently in 2015, permanent populations of the taiga tick, I. persulcatus were detected for the first time in northern Sweden [3]. Until then, its known geographical distribution was from eastern Latvia and Estonia, and Finland eastwards through Russia into Mongolia and parts of the Peoples Republic of China, Taiwan and North Korea to Japan [4, 5]. The vegetation type usually inhabited by I. persulcatus is mixed deciduous–coniferous forest [4, 5].
Several proven or putative pathogens of humans have been recorded from the taiga tick. They include Borrelia miyamotoi, B. burgdorferi (sensu stricto), B. afzelii, B. garinii, B. bavariensis, Rickettsia heilongjiangensis, R. helvetica, R. raoultii, R. sibirica, “Candidatus Rickettsia tarasevichiae”, Anaplasma phagocytophilum, Ehrlichia muris, Neoehrlichia mikurensis, Bartonella henselae, Babesia divergens, Ba. microti, Ba. venatorum, tick-borne encephalitis virus (TBEV) and Kemerovo virus [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20].
Lyme borreliosis (LB) is the most common tick-borne disease in the temperate regions of Europe, North America and Asia; 65,000 human cases of LB are estimated to occur annually in Europe [21]. LB is prevalent also in Asia [13]; and in the USA there are presumably > 300,000 human cases of LB each year [22]. However, the potentially most severe and most important of the Ixodes-vectored pathogens in the Palaearctic region is the TBEV, which during 1990–2009 caused between 6000 and 12,000 human cases annually in Europe including Russia [9].
This prompted us to investigate the potential occurrence of the TBEV, different genospecies of B. burgdorferi (s.l.), Borrelia miyamotoi, Rickettsia spp., Anaplasma phagocytophilum, N. mikurensis and Babesia spp. in the recently discovered populations of the taiga tick in northern Sweden. The main aim was to analyse the potential medical importance of I. persulcatus in the study area.
Methods
Climate and vegetation
As described by Jaenson et al. [3], the locations from where we collected ticks are situated in the Bothnian Bay area, province of Norrbotten in northern Sweden. The region belongs to the middle boreal subzone [23]. Norrbotten is to the west bordered by the Swedish province Lappland, which together with Norrbotten form the northernmost provinces of Sweden. This is a typical taiga region, strongly dominated by forests but also in part by mires. It reaches in altitude to about to 150–200 m a.s.l. in Norrbotten. In the Bothnian Bay area, where we found I. persulcatus at several localities, the length of the vegetation period is about 140–150 days and the annual precipitation 600–700 mm. The July mean temperature is 15–16 °C. The human population density is low compared with southern Sweden. Most of the 500,000 inhabitants of the Bothnian Bay area reside in the coastal regions and the river valleys.
Tick collection localities
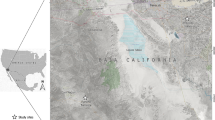
We contacted dog-owners and hunters living in the coastal areas of the provinces of northern Sweden and asked if they and/or their dogs had become infested by ticks in the Baltic Bay area. We then asked for the name(s) of the presumed locality/localities where any tick infestation had occurred. Based on this information we searched for ticks at a total of 49 localities in the Bothnian Bay area, province of Norrbotten, northern Sweden in July–August 2015 and May–July 2016. Ticks were detected by the cloth-dragging method at seven mixed woodland, island localities in the Bothnian Bay, province of Norrbotten, northern Sweden (Fig. 1): Axelsvik (65°46′31″N, 23°23′11″E); Båtskärsnäs (65°47′05.88″N, 23°25′13.85″E); Seskarö (65°42′20″N, 23°43′30″E); Stora Hamnskär (65°42′21.45″N, 24°6′35.75″E and 65°42′21,50″N, 24°6′27,92″E); Västra Knivskär (65°4′33″N, 24°6′46″E); Östra Knivskär (65°40′3″N, 24°8′59″E and 65°40′33″N, 24°8′54″E); and Ytterstlandet (65°42′34″N, 23°17′20″E).
Map showing the seven localities where ticks were collected by cloth-dragging method. Ticks [adult males (AM); adult females (AF); nymphs (N)] were collected in the Bothnian Bay area, province of Norrbotten, northern Sweden in July-August 2015 and May-July 2016. Numbers (1–7) correspond to the following localities: 1, Ytterstlandet (AM = 114; AF = 108; N = 4); 2, Axelsvik (AM = 1; AF = 1; N = 1); 3, Båtskärsnäs (AM = 2); 4, Seskarö (AF = 1); 5. Stora Hamnskär (AM = 1); 6, Västra Knivskär (AM = 18; AF = 16; N = 8); 7, Östra Knivskär (N = 2)
Tick collection
All sampling occasions took place during daytime when the ground vegetation was not wet due to recent or ongoing rainfall. At each sampling locality a white woolen 1 m2 cloth was pulled at slow walking pace over the ground vegetation and/or field vegetation for a total of 300 m. At every 10 m of dragging, a stop was done and both sides of the cloth were inspected with the help of a magnifying lens. Any tick detected was put in a numbered plastic vial containing 80% ethanol. A total number of 277 ticks was collected by the cloth-dragging method. In addition, 18 ticks were removed and collected from two dogs at Ytterstlandet (n = 16), and Stora Hamnskär (n = 2), respectively.
Tick identification
All tick specimens were identified to stage and species by morphological characteristics as described in more detail in [3]. A Leitz Wild M10 stereomicroscope was used together with keys and illustrations in [4, 5] and [24].
Nucleic acid extraction and cDNA synthesis from ticks
Collected ticks were homogenized individually by bead-beating in 2 ml safe-lock microcentrifuge tubes (Eppendorf AG, Hamburg, Germany) with a 5-mm stainless steel bead (Qiagen, Hilden, Germany) in 350 µl RLT buffer (Qiagen), supplemented with 1% 2-mercaptoethanol (Sigma-Aldrich, Stockholm, Sweden), using a TissueLyser II (Qiagen) for 2 min at 25 Hz. After centrifugation at 20,000× g for 3 min, 300 µl supernatant were transferred to new microcentrifuge tubes for total nucleic acid (NA) extraction, using MagAttract® Viral RNA M48 kit (Qiagen) in a BioRobot M48 workstation (Qiagen), using a 65-µl elution volume. Each batch of 24 samples consisted of 22 ticks, one positive control [5 µl of B. burgdorferi (sensu stricto) B31 ATCC 35210 (108 cells/ml), and 5 µl inactivated TBEV strain K23, Encepur®, Chiron Vaccines, Marburg, Germany] and one negative control (H2O) that were extracted simultaneously.
The eluted NA was reverse-transcribed to cDNA using illustra™ Ready-to-Go RT-PCR Beads kit (GE Healthcare, Amersham Place, UK). Twenty microliters NA and 10 µl pd(N)6 random hexamer primers (0.25 µg/µl) were incubated for 5 min at 97 °C and then mixed with one RT-PCR bead dissolved in 20 µl RNase-free water. The mixture was incubated for 30 min at 42 °C, followed by 5 min at 97 °C, producing 50 µl cDNA.
Molecular identification of tick species
Morphological identification of tick species was confirmed by molecular identification, using a species-specific duplex TaqMan real-time PCR assay, as previously described [25]. The primers IXO-I2-F4 and IXO-I2-R4 are designed to target the Ixodes spp. internal transcribed spacer (ITS2) to amplify genus-specific segments. The species-specific probes Iri-I2-P4 and Ipe-I2-P4 are designed to target either of the tick species (I. ricinus or I. persulcatus, respectively) (Table 1).
A 20-µl reaction consisted of 10 µl Maxima® Probe qPCR Master Mix (2X) (Thermo Fisher Scientific, Stockholm, Sweden), 0.4 µl of each primer (10 µM; Invitrogen; Table 1), 0.3 µl of probe Iri-I2-P4 (10 µM; Invitrogen; Table 1), 0.2 µl of probe Ipe-I2-P4 (10 µM; Invitrogen; Table 1), 6.7 µl RNase-free water and 2 µl cDNA template. The PCR reactions were performed on a C1000™ Thermal Cycler, CFX96™ Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA) using an activation step at 95 °C for 5 min, and 45 cycles of 95 °C for 10 s and 60 °C for 1 min.
Detection of Borrelia burgdorferi (sensu lato) and determination of species
Detection of Borrelia burgdorferi (sensu lato) was done using a genus-specific LUX real-time PCR assay, as previously described [26]. The primers B16S_FL and B16S_R are designed to target the Borrelia spp. 16S rRNA gene to amplify a 131 bp long amplicon (Table 1).
A 20-µl reaction consisted of 10 µl Platinum qPCR SuperMix uracil-D-glycosylase (UDG) (Invitrogen), 0.4 µl of each primer (10 µM; Invitrogen; Table 1), 7.2 µl RNase-free water and 2 µl cDNA template. The PCR reactions were performed on a C1000™ Thermal Cycler, CFX96™ Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.) using an activation step at 50 °C for 2 min followed by 95 °C for 2 min, and then subjected to 45 cycles of 95 °C for 15 s, 58 °C for 30 s, and 72 °C for 30 s. Immediately after completion of PCR, melting curve analyses were performed by heating to 95 °C for 15 s, followed by cooling to 60 °C for 1 min, and subsequent heating to 95 °C at 0.8 °C min−1 with continuous fluorescence recording.
To determine Borrelia burgdorferi (s.l.) species of the samples positive in the LUX real-time PCR assay, a nested, conventional PCR assay using primers targeting the intergenic spacer region (IGS) between 5S and 23S rRNA genes (Table 1), was applied as previously described [26, 27]. Samples that failed to produce PCR products with this assay were denoted as ‘untypeable’.
Detection of Borrelia miyamotoi
Detection of B. miyamotoi was done using a species-specific TaqMan real-time PCR assay, as previously described [28]. The primers Bm_F and Bm_R, and the probe Bm_P are designed to target the B. miyamotoi flagellin B gene (flaB) to amplify a 156-bp long amplicon (Table 1).
A 20-µl reaction consisted of 10 µl Maxima® Probe qPCR Master Mix (2X) (Thermo Fisher Scientific), 0.4 µl of each primer (10 µM; Invitrogen; Table 1), 0.4 µl of probe (10 µM; Life Technologies; Table 1), 3.8 µl RNase-free water and 5 µl cDNA template. The PCR reactions were performed on a C1000™ Thermal Cycler, CFX96™ Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.) using an activation step at 95 °C for 10 min, and 45 cycles of 95 °C for 5 s and 60 °C for 35 s, and finally one cycle of 37 °C for 20 s. As a positive control, a synthetic plasmid containing the target sequence of the TaqMan real-time PCR assay was used. The plasmid contained the target sequence, spanning the nucleotides 510–665 of the B. miyamotoi flagellin (flaB) gene (GenBank: KT932823), synthesized and cloned into Eurofins standard vector carrying the ampicillin selection marker (Eurofins Genomics, Ebersberg, Germany).
Detection of TBEV
Detection of TBEV was done using a duplex TaqMan real-time PCR assay, as previously described [29]. The primers and probes are designed to target all three subtypes of TBEV to amplify a 68-bp and a 88-bp long amplicon, respectively [30, 31] (Table 1).
A 20-µl reaction consisted of 10 µl Maxima® Probe qPCR Master Mix (2X) (Thermo Scientific™), 0.4 µl of each primer and probe (10 µM; Invitrogen; Table 1), 5.6 µl RNase-free water and 2 µl cDNA template. The PCR reactions were performed on a C1000™ Thermal Cycler, CFX96™ Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.) using an activation step at 95 °C for 5 min, and 45 cycles of 95 °C for 10 s and 60 °C for 1 min.
Detection of Neoehrlichia mikurensis
Detection of N. mikurensis was done using a SYBR green real-time PCR assay, as previously described [32]. The primers Neo_16S_F and Neo_16S_R are designed to target the N. mikurensis 16S rRNA gene to amplify a 107-bp long amplicon (Table 1).
A 20-µl reaction consisted of 10 µl SYBR™ Green PCR Master Mix (Thermo Fisher Scientific), 0.4 µl of each primer (10 µM; Invitrogen; Table 1), 7.2 µl RNase-free water and 2 µl cDNA template. The PCR reactions were performed on a C1000™ Thermal Cycler, CFX96™ Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.) using an activation step at 95 °C for 3 min, and 45 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s. Immediately after completion of PCR, melting curve analyses were performed by heating to 95 °C for 15 s, followed by cooling to 60 °C for 1 min, and subsequent heating to 95 °C at 0.8 °C min-1 with continuous fluorescence recording. As a positive control, cDNA samples positive for N. mikurensis confirmed by sequencing in an earlier study [32] were used in each run.
Detection of Anaplasma phagocytophilum
Detection of A. phagocytophilum was done using a TaqMan real-time PCR assay, as previously described [33]. The primers ApF and ApR, and the probe ApM are designed to target the A. phagocytophilum citrate synthase gene (gltA) to amplify a 64-bp long amplicon (Table 1).
A 25-µl reaction consisted of 12.5 µl of 10 µl Maxima® Probe qPCR Master Mix (2×) (Thermo Fisher Scientific), 1.5 µl of each primer and 0.375 µl of probe (10 µM; Invitrogen; Table 1), 7.125 µl RNase-free water and 2 µl cDNA template. The PCR reactions were performed on a C1000™ Thermal Cycler, CFX96™ Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.) using an activation step at 95 °C for 10 min, and 40 cycles of 95 °C for 15 s and 60 °C for 1 min. As a positive control, a synthetic plasmid containing the target sequence of the TaqMan real-time PCR assay was used. The plasmid contained the target sequence, spanning the nucleotides 304–420 of the A. phagocytophilum gltA gene (GenBank: AF304137), synthesized and cloned into pUC57 vector (GenScript, Piscataway, NJ, USA).
Detection of Rickettsia and determination of species
Detection of Rickettsia spp. was done using a TaqMan real-time PCR assay, as previously described [34]. The primers CS-F and CS-R, and probe CS-P are designed to target the Rickettsia spp. citrate synthase gene (gltA) to amplify a 74-bp long amplicon (Table 1).
A 20-µl reaction consisted of 10 µl Maxima® Probe qPCR Master Mix (2×) (Thermo Fisher Scientific), 0.4 µl of each primer and probe (10 µM; Invitrogen; Table 1), 6.8 µl RNase-free water and 2 µl cDNA template. The PCR reactions were performed on a C1000™ Thermal Cycler, CFX96™ Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.) using an activation step at 95 °C for 5 min, and 60 cycles of 95 °C for 20 s and 60 °C for 40 s. As a positive control, a synthetic plasmid containing the target sequence of the TaqMan real-time PCR assay was used. The plasmid contained the target sequence, spanning the nucleotides 1102–1231 of the Rickettsia rickettsii gltA gene (GenBank: U59729), synthesized and cloned into pUC57 vector (GenScript).
To determine Rickettsia species of the samples positive in the TaqMan real-time PCR assay, conventional PCR assays were used to amplify a fragment of the genes coding for the outer membrane protein B, ompB, 17-kDa gene or using semi-nested PCR targeting the gltA gene (Table 1), as previously described [35,36,37].
Detection of Babesia species
Detection of Babesia spp. was done using a SYBR green real-time PCR assay, as previously described [38]. Primers BJ1 and BN2 were designed to target the Babesia 18S rRNA gene to amplify a 411–452 bp long amplicon depending on the species of Babesia [39] (Table 1).
A 20-µl reaction consisted of 10 µl SYBR™ Green PCR Master Mix (Thermo Fisher Scientific), 0.4 µl of each primer (10 µM; Invitrogen; Table 1), 7.2 µl RNase-free water and 2 µl cDNA template. The PCR reactions were performed on a C1000™ Thermal Cycler, CFX96™ Real-Time PCR Detection System (Bio-Rad Laboratories Inc.) using an activation step at 94 °C for 10 min, and 35 cycles of 94 °C for 1 min, 55 °C for 1 min and 72 °C for 2 min, and finally one cycle of 72 °C for 5 min. Immediately after completion of PCR, melting curve analyses were performed by heating to 95 °C for 15 s, followed by cooling to 60 °C for 1 min, and subsequent heating to 95 °C at 0.8 °C min−1 with continuous fluorescence recording. As a positive control, a synthetic plasmid containing the target sequence of the SYBR green real-time PCR assay was used. The plasmid contained the target sequence, spanning the nucleotides 467–955 of the B. divergens 18S rRNA gene (GenBank: AJ439713), synthesized and cloned into pUC57 vector (GensSript).
Nucleotide sequencing of PCR-products
Nucleotide sequencing of the PCR products amplified by conventional PCR assays to determine species of Borrelia and Rickettsia was performed by Macrogen Inc. (Amsterdam, The Netherlands). All sequences were confirmed by sequencing both strands. The obtained chromatograms were initially edited and analyzed using BioEdit Software v7.0 (Tom Hall, Ibis Therapeutics, Carlsbad, CA), and the sequences were examined using Basic Local Alignment Tool (BLAST). The appearance of dual peaks in chromatograms was regarded as a mixed infection of at least two strains of the same species and thus denoted as ‛mixedʼ. An additional file shows all the aligned sequences (see Additional file 1).
Statistical analyses
Data were presented as percentage for categorical variables, i.e. prevalence of Borrelia-positive ticks. The categorical variables were analysed using Chi-square test, but when the expected frequency was < 5 in at least one of the cells of the contingency table we used Fisher’s exact test. The Mann–Whitney test was used to compare cycle threshold (Cq)-values obtained by the real-time PCR assay for the Borrelia-positive samples that could be determined to species with the Borrelia-positive samples that were denoted as untypeable. Results were reported as median values with interquartile range (IQR). Statistical analyses were performed using GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, CA). P-values ≤ 0.05 were considered statistically significant.
Results
Tick collection
In July–August 2015 and May–July 2016, a total of 277 tick specimens was collected by the cloth-dragging method at seven mixed woodland, island localities in the Bothnian Bay area, province of Norrbotten, northern Sweden (Table 2): Axelsvik (n = 3); Båtskärsnäs (n = 2); Seskarö (n = 1); Stora Hamnskär (n = 1); Västra Knivskär (n = 42); Östra Knivskär (n = 2); and Ytterstlandet (n = 226). Of the 277 tick specimens, 276 ticks were morphologically and molecularly identified as I. persulcatus and one tick (a nymph) was identified as I. ricinus. Among all ticks (including the I. ricinus nymph), 5.4% were nymphs (n = 15), and 94.6% were adult ticks (n = 262; 136 adult males, 126 adult females). A significantly higher proportion of nymphs was collected at Västra Knivskär (19%, 8/42) compared to Ytterstlandet (2%, 4/226, P < 0.0001). No other significant geographical differences between the proportions of developmental stages were detected.
From the dog at Ytterstlandet, 8 adult male ticks and 8 adult female ticks were collected. All were morphologically and molecularly identified as I. persulcatus. We noted that all adult female ticks contained blood, most likely ingested from the dog. One of the adult male ticks also contained blood in the gut. From the dog at Stora Hamnskär, two adult female ticks were collected, and both were morphologically and molecularly identified as I. persulcatus. We noted that these two adult females contained blood, most likely ingested from the dog.
Prevalence of Borrelia bacteria in the ticks
Of the ticks collected by the cloth-dragging method, 55% (152/277) were positive for Borrelia (Table 2). There was no significant difference between the proportions of Borrelia-infected nymphs (33%, 5/15) and Borrelia-infected adult ticks (56%, 147/262), and no significant difference between the proportions of Borrelia-infected adult males (54%, 74/136) and Borrelia-infected adult females (58%, 73/126).
Borrelia-infected ticks were detected only at Västra Knivskär and at Ytterstlandet. A significantly higher proportion of Borrelia-infected adult ticks was detected at Ytterstlandet (64%, 142/222) compared to Västra Knivskär (15%, 5/34, P < 0.0001). There was no significant difference between the proportion of Borrelia-infected nymphs at Ytterstlandet (50%, 2/4) compared to that at Västra Knivskär (38%, 3/8).
Of the adult ticks removed from the dog at Ytterstlandet, 31% (5/16) were positive for Borrelia: 38% (3/8) of the adult males; and 25% (2/8) of the adult females. Of the two adult female ticks detached from the dog at Stora Hamnskär, both were negative for borreliae.
Prevalence of Borrelia species in the ticks
Of the ticks collected by the cloth-dragging method, three different Borrelia species were identified by sequence analysis of the 5S–23S IGS (Table 2). B. afzelii was the predominant species and detected in 46% (70/152) of all Borrelia-infected ticks followed by B. garinii (35%, 53/152), B. valaisiana (1%, 1/152), and mixed infections of different Borrelia species (1%, 2/152). Seventeen percent (26/152) of all Borrelia-infections were untypeable. Of the samples that were determined to Borrelia species, no significant differences in prevalence of Borrelia species between nymphs and adult ticks or between adult male ticks and adult female ticks were detected. Furthermore, no significant geographical differences in prevalence of Borrelia species were detected.
Of the five Borrelia-positive adult ticks removed from the dog at Ytterstlandet, one adult female and one adult male contained B. afzelii while the other three Borrelia-positive samples were untypeable.
The Borrelia-positive samples that were defined as untypeable had a significantly higher Cq-value in the real-time PCR analyses (n = 29, median 34.3, IQR 30.4–36.0) compared to Borrelia-positive samples that could be determined to species (n = 128, median 25.9, IQR 23.6–28.9, P < 0.0001).
Prevalence of Rickettsia bacteria in the ticks
Of the ticks collected by cloth-dragging, 0.7% (2/277; one adult female tick and one nymphal tick) were positive for Rickettsia (Table 2). The Rickettsia-positive adult female tick was collected in July 2016 at Västra Knivskär. Our analysis of the amplified ompB and 17-kDa sequences revealed a 100% signature match of Rickettsia helvetica, as compared with R. helvetica sequences deposited in GenBank (AF123725 and LC379447, respectively). The Rickettsia-positive nymph was collected in August 2015 at Östra Knivskär. The amplified ompB sequences revealed a 95% signature match of Rickettsia sp., as compared with Rickettsia sp. sequences detected from the host Adalia bipunctata ῾the two-spotted ladybug beetleʼ (AJ582613-AJ582615 and AJ628731) deposited in GenBank. Our attempts to amplify fragments of the genes coding for 17-kDa and gltA of this Rickettsia-positive sample were unsuccessful.
None of the Rickettsia-positive I. persulcatus ticks were positive for any other analysed tick-borne microorganisms. The ticks collected from the two dogs (n = 18) were all negative for Rickettsia spp.
Prevalence of other tick-borne microorganisms in the ticks
Of the 295 ticks analysed (including the 18 ticks collected from the dogs), all were negative for TBEV, Borrelia miyamotoi, A. phagocytophilum, N. mikurensis and Babesia spp.
Discussion
Borrelia infection prevalence
This is the first time that specimens of I. persulcatus from Sweden have been analysed for the presence of Borrelia species and other potentially human-pathogenic microorganisms. In comparison with previous investigations on the Borrelia infection prevalence (36%) of adult female I. ricinus in southern and central Sweden [40] the present, significantly higher prevalence (58%) in adult female I. persulcatus is noteworthy. Even more so is the difference in Borrelia infection prevalence between I. ricinus nymphs (25%; [40]) compared to that of adult females of I. persulcatus (58%) in this study. The reason for comparing the infection prevalence of I. ricinus nymphs with that of I. persulcatus adult females is, as explained below, because the nymphal stage of I. ricinus and the female adult stage of I. persulcatus are the main vectors of human pathogens to humans in northern Europe [1, 3, 4, 13].
Males of I. persulcatus may attach to humans and imbibe blood [4]. Our observation of one blood-fed male of I. persulcatus collected from a dog at Ytterstlandet, confirms this statement of Pomerantzev (1950) [4].
In a study on I. ricinus ticks that had bitten humans in southern and central Sweden and on the Åland archipelago, Finland, Wilhelmsson et al. [40] found that 26% of the ticks were Borrelia-infected; they recorded seven different Borrelia species; B. afzelii was the predominant species and found in 50% of all Borrelia-infected ticks, followed by B. garinii (19%) and B. valaisiana (7%); B. afzelii was more prevalent in nymphs (67%) than in adult ticks (46%) whereas B. garinii and B. valaisiana were more prevalent in adult ticks (30% and 11%, respectively) than in nymphs (20% and 6%, respectively).
In a more recent study on I. ricinus and I. persulcatus in Finland, Laaksonen et al. [14] found B. burgdorferi (s.l.) in 18.1% of 1451 I. persulcatus ticks analysed and in 16.2% of 2014 I. ricinus ticks analysed. In I. persulcatus, B. garinii (62.6%) was the predominant genospecies followed by B. afzelii (35%) and B. valaisiana (2.5%). Borrelia burgdorferi (s.s.) was not found in our study nor in that of Laaksonen et al. [14]. This supports the view that B. burgdorferi (s.s.) is a relatively rare species in I. persulcatus [13, 21]. The prevalence of B. garinii (63%) in I. persulcatus [14] was even higher than the corresponding prevalence (35%) in our study. However, a relatively large proportion of our Borrelia-positive specimens were not possible to identify to genospecies; if they had been possible to identify, it is likely that some of them might have been B. garinii.
Of the Borrelia-positive ticks, 17% were infected with Borrelia that could not be determined to species. These ticks were, in general, infected with a lower amount of Borrelia bacteria as indicated by a significantly higher Cq-value, compared to ticks infected by typeable Borrelia species. This may, at least partly, explain why PCR products, used to determine Borrelia species, were not amplified in the conventional PCR assays.
The lower diversity of potentially human-pathogenic microorganisms recorded in the present populations of I. persulcatus in the Baltic Bay area is likely due to several factors: the small total tick sampling area covered; the isolated nature of the islands in the Baltic Bay; the generally low biodiversity, which is a characteristic feature of the Boreal region compared to more southern regions of Sweden and Finland; and the relatively small sample size investigated. The prevalence of TBEV in ticks is usually very low. However, a possible explanation for the negative results obtained in the present study may be the fact that we preserved our ticks in ethanol, which may have permitted degradation of RNA before extracting total NA.
The present study and that of Laaksonen et al. [14] reveal high prevalence of B. garinii in I. persulcatus in northern Sweden and northern Finland. Neuroborreliosis is usually due to a B. garinii infection and is, in general, the most serious of the disease syndromes caused by members of the B. burgdorferi (s.l.) complex. Consequently, the high prevalence of B. garinii in the taiga tick populations in northern Sweden and Finland may constitute a potentially serious public health threat in areas where the taiga tick is abundant.
Vector behaviour
It is well known that it is usually the nymphs of I. ricinus but the adult females of I. persulcatus, which attack humans and transmit pathogens to humans [1, 3, 4, 13]. It is also well known that the prevalence of borreliae and the TBEV, in general, is significantly lower in I. ricinus than in I. persulcatus [13, 14]. There is presumably more than one explanation for these differences. One may be that the questing behaviour of the immature ticks might differ between I. ricinus and I. persulcatus. For instance, in the Nearctic different nymphal questing behaviours of I. scapularis, especially regarding the questing height of nymphs, between northern and southern allopatric populations in the eastern USA, seem to explain the higher Lyme disease risk in areas inhabited by the northern tick population, having nymphs which tend to quest above the leaf litter [41]. Similar differences might exist between the immatures of I. persulcatus and I. ricinus.
An additional explanation may be that the “host preferences”, i.e. the force of attraction to particular host species may differ between the two Palaearctic tick species. Small and medium-sized mammals constitute the main vertebrate reservoir for B. afzelii whereas birds constitute the main vertebrate reservoir for B. garinii [42, 43]. Since I. persulcatus immature ticks seem to “avoid” or rarely feed on large mammals, which in general are incompetent reservoirs for B. burgdorferi (s.l.), it may be concluded that, most likely, the pre-adult ticks of I. persulcatus feed on small and medium-sized mammals and ground-inhabiting birds, which in general are reservoir-competent for B. burgdorferi (s.l.). This may be the most likely explanation for the generally higher Borrelia infection rate in I. persulcatus than in I. ricinus.
In I. ricinus, on the other hand, larvae, nymphs and adult males and females can usually be found together on large and medium-sized mammals. Therefore, since the immature ticks of I. ricinus to a much greater extent, compared to the immatures of I. persulcatus, feed on both reservoir-competent and reservoir-incompetent hosts the force of infection of B. burgdorferi (s.l.) will be weaker, i.e. more “diluted” in I. ricinus than in I. persulcatus.
In I. persulcatus, the adult female tick is the most important vector of pathogens to humans. The adult females of I. persulcatus have been described as exhibiting an “aggressive” behaviour, i.e. showing strong attraction towards humans and other large mammals and as being significantly more active than females of I. ricinus; this behaviour likely renders them more competent vectors of human pathogens [44].
In contrast to the immature stages of I. ricinus, the larvae and nymphs of I. persulcatus are rarely found on humans [13] but are usually infesting small mammals and ground-frequenting birds; the nymphs can also be found on medium-sized mammals [13]. Therefore, these “host preferences” of I. persulcatus differ from those of its close relative I. ricinus.
Since both nymphs and adult females of I. ricinus are strongly attracted to feed on humans (and other large mammals) it is nymphs of I. ricinus, due to their relatively higher abundance compared to that of adult female ticks, which are the main vectors of pathogens to humans. In I. persulcatus, since the nymphs rarely feed on humans it is the adult females, which are strongly attracted to humans, which are the main vectors of pathogens to humans.
Borrelia afzelii was the most prevalent (46%) of the Borrelia-infected ticks. Small and medium-sized mammals constitute the main vertebrate reservoir for B. afzelii [42, 43] and the larvae and nymphs of I. persulcatus feed, in general, on small and medium-sized mammals and ground-inhabiting birds. The fauna of small and medium-sized mammals at Ytterstlandet and the other islands, where tick sampling took place, has not been studied in detail but these abundant small mammal species, presumably occur there: Myodes glareolus, M. rufocanus, Microtus agrestis and Sorex araneus [45]. One or more of these small mammal species are proven competent reservoirs and transmission hosts for B. afzelii [42, 46] and other pathogens vectored by I. persulcatus. It is concluded that the feeding behaviour of immatures of I. persulcatus, i.e. that small mammals constitute a “preferred” blood source, and that they, in general, are competent reservoirs and transmission hosts for B. afzelii, explain the high prevalence of B. afzelii in I. persulcatus in the study area.
Geese are presumably a main tick host and a main vertebrate Borrelia reservoir
Dense populations of Greylag goose (Anser anser) and Canada goose (Branta canadensis) breed in the same area of Ytterstlandet where most of the I. persulcatus ticks were found. The seasonal taiga tick activity peak, i.e. about April–June, coincides with the breeding time of the geese. Moreover, the first author was told by one of the inhabitants of the island that “a goose that was shot in May 2015 was teeming with ticks”. People who have summer cottages at Ytterstlandet regard the relatively recent massive infestation of the island by the taiga tick to be due to the abundance of geese, which use certain parts of the island for breeding and feeding during April, May and June. These particular parts of the island are the ones most heavily infested by the taiga tick (T.G.T. Jaenson, unpublished observation). Borrelia garinii is known to have different birds as its vertebrate reservoir [42, 43, 46]. Together, these observations suggest that geese are a main vertebrate reservoir for B. garinii and a main blood host of I. persulcatus at Ytterstlandet and some neighbouring islands.
Conclusions
To our knowledge, this is the first time that I. persulcatus from Sweden has been analysed for the presence of tick-borne pathogens. The examined tick populations had a low diversity of tick-borne pathogens but a high prevalence of B. afzelii and B. garinii.
Availability of data and materials
The data supporting the conclusions of this article are included within the article. Raw data can be shared with researchers upon a specific request. An additional file shows the aligned Borrelia and Rickettsia nucleotide sequences (see Additional file 1).
References
Jaenson TGT, Tälleklint L, Lundqvist L, Olsen B, Chirico J, Mejlon H. Geographical distribution, host associations, and vector roles of ticks (Acari: Ixodidae, Argasidae) in Sweden. J Med Entomol. 1994;31:240–56.
Jaenson TG, Jaenson DG, Eisen L, Petersson E, Lindgren E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit Vectors. 2012;10:8.
Jaenson TG, Värv K, Fröjdman I, Jääskeläinen A, Rundgren K, Versteirt V, et al. First evidence of established populations of the taiga tick Ixodes persulcatus (Acari: Ixodidae) in Sweden. Parasit Vectors. 2016;1:377.
Pomerantzev BI. Fauna of the USSR. Arachnida. Ixodid ticks (Ixodidae). Moscow: Zoological Institute of the Academy of Sciences of the USSR; 1950.
Yamaguti N, Tipton VJ, Keegan HL, Toshika S. Ticks of Japan, Korea, and the Ryuku Islands. Brigham Young University Science Bulletin, vol. XV. Provo: Brigham Young University Press; 1971.
Alekseev AN, Semenov AV, Dubinina HV. Evidence of Babesia microti infection in multi-infected Ixodes persulcatus ticks in Russia. Exp Appl Acarol. 2003;29:345–53.
Alekseev AN, Dubinina HV, Jushkova OV. First report on the coexistence and compatibility of seven tick-borne pathogens in unfed adult Ixodes persulcatus Schulze (Acarina: Ixodidae). Int J Med Microbiol. 2004;293:104–8.
Inokuma H, Ohashi M, Tanabe S, Miyahara K. Prevalence of tick-borne Rickettsia and Ehrlichia in Ixodes persulcatus and Ixodes ovatus in Tokachi district, Eastern Hokkaido, Japan. J Vet Med Sci. 2007;69:661–4.
Süss J. Tick-borne encephalitis 2010: epidemiology, risk areas, and virus strains in Europe and Asia—an overview. Ticks Tick Borne Dis. 2011;2:2–15.
Hubalek Z, Rudolf I. Tick-borne viruses in Europe. Parasitol Res. 2012;111:9–36.
Karnath C, Obiegala A, Speck S, Essbauer S, Derschum H, Scholz H, et al. Detection of Babesia venatorum, Anaplasma phagocytophilum and Neoehrlichia mikurensis in Ixodes persulcatus ticks from Mongolia. Ticks Tick Borne Dis. 2016;7:357–60.
Katargina O, Geller J, Vasilenko V, Kuznetsova T, Jarvekulg L, Vene S, et al. Detection and characterization of Babesia species in Ixodes ticks in Estonia. Vector Borne Zoonotic Dis. 2011;11:923–8.
Korenberg EI, Gorelova NB, Kovalevskii YI. Ecology of Borrelia burgdorferi sensu lato in Russia. In: Gray JS, Kahl O, Lane RS, Stanek G, editors. Lyme borreliosis biology epidemiology and control. 1st ed. Oxon: CABI Publishing; 2002. p. 175–200.
Laaksonen M, Klemola T, Feuth E, Sormunen JJ, Puisto A, Mäkelä S, et al. Tick-borne pathogens in Finland: comparison of Ixodes ricinus and I. persulcatus in sympatric and parapatric areas. Parasit Vectors. 2018;11:556.
Nuttall PA. Tick-borne viruses. In: Sonenshine DE, Roe RM, editors. Biology of ticks. 2nd ed. New York City: Oxford University Press; 2013. p. 180–210.
Pfäffle MP, Petney TN, Jaenson TGT. Ixodes persulcatus Schulze, 1930. In: Jaenson TGT, editor. Ticks of Europe and North Africa. A guide to species identification. Cham: Springer International Publishing; 2017. p. 197–201.
Rar VA, Fomenko NV, Dobrotvorsky AK, Livanova NN, Rudakova SA, Fedorov EG, et al. Tickborne pathogen detection, western Siberia, Russia. Emerg Infect Dis. 2005;11:1708–15.
Rar V, Livanova N, Tkachev S, Kaverina G, Tikunov A, Sabitova Y, et al. Detection and genetic characterization of a wide range of infectious agents in Ixodes pavlovskyi ticks in Western Siberia, Russia. Parasit Vectors. 2017;10:258.
Shpynov SN, Rudakov NV, Iastrebov VK, Leonova GN, Khazova TG, Egorova NV, et al. New evidence for the detection of Ehrlichia and Anaplasma in Ixodes ticks in Russia and Kazakhstan (In Russian). Med Parazitol (Mosk). 2004;2:10–4.
Uspensky I. The taiga tick Ixodes persulcatus (Acari: Ixodidae), the main vector of Borrelia burg-dorferi sensu lato in Eurasia. In: Lyme disease. Dover: SM Group; 2016. https://smjournals.com/ebooks/lyme-disease/chapters/LD-16-02.pdf. Accessed 26 May 2019.
Rizzoli A, Hauffe HC, Carpi G, Vourch GI, Neteler M, Rosà R. Lyme borreliosis in Europe. Euro Surveill. 2011;16:19906.
CDC (Centers for Disease Control and Prevention). 2019. How many people get Lyme disease? https://www.cdc.gov/lyme/stats/humancases.html. Accessed 26 May 2019.
Sjörs H. The background: geology, climate and zonation. In: Rydin H, Snoeijs P, Diekmann M, editors. Swedish Plant Geography, Vol. 84. Uppsala: Acta Phytogeographica Suecica 84 (Svenska Växtgeografiska Sällskapet); 1999. p. 5–14.
Filippova NA. Ixodidae, ticks of subfamily of Ixodinae. Fauna USSR. Arachnidea, Vol 4. Leningrad: Nauka; 1977. (In Russian)
Sormunen JJ, Penttinen R, Klemola T, Hänninen J, Vuorinen I, Laaksonen M, et al. Tick-borne bacterial pathogens in southwestern Finland. Parasit Vectors. 2016;9:168.
Wilhelmsson P, Fryland L, Börjesson S, Nordgren J, Bergström S, Ernerudh J, et al. Prevalence and diversity of Borrelia species in ticks that have bitten humans in Sweden. J Clin Microbiol. 2010;48:4169–76.
Postic D, Assous MV, Grimont PA, Baranton G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)-rrl (23S) intergenic spacer amplicons. Int J Syst Bacteriol. 1994;44:743–52.
Hovius JW, de Wever B, Sohne M, Brouwer MC, Coumou J, Wagemakers A, et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;17(382):658.
Lindblom P, Wilhelmsson P, Fryland L, Sjöwall J, Haglund M, Matussek A, et al. Tick-borne encephalitis virus in ticks detached from humans and follow-up of serological and clinical response. Ticks Tick Borne Dis. 2014;5:21–8.
Schwaiger M, Cassinotti P. Development of a quantitative real-time RT-PCR assay with internal control for the laboratory detection of tick-borne encephalitis virus (TBEV) RNA. J Clin Virol. 2003;27:136–45.
Gäumann R, Mühlemann K, Strasser M, Beuret CM. High-throughput procedure for tick surveys of tick-borne encephalitis virus and its application in a national surveillance study in Switzerland. Appl Environ Microbiol. 2010;76:4241–9.
Labbé Sandelin L, Tolf C, Larsson S, Wilhelmsson P, Salaneck E, Jaenson TG, et al. Candidatus Neoehrlichia mikurensis in ticks from migrating birds in Sweden. PLoS One. 2015;24(10):e0133250.
Henningsson AJ, Hvidsten D, Kristiansen BE, Matussek A, Stuen S, Jenkins A. Detection of Anaplasma phagocytophilum in Ixodes ricinus ticks from Norway using a realtime PCR assay targeting the Anaplasma citrate synthase gene gltA. BMC Microbiol. 2015;15:153.
Stenos J, Graves SR, Unsworth NB. A highly sensitive and specific real-time PCR assay for the detection of spotted fever and typhus group rickettsiae. Am J Trop Med Hyg. 2005;73:1083–5.
Carl M, Tibbs CW, Dobson ME, Paparello S, Dasch GA. Diagnosis of acute typhus infection using the polymerase chain reaction. J Infect Dis. 1990;161:791–3.
Choi YJ, Lee SH, Park KH, Koh YS, Lee KH, Baik HS, et al. Evaluation of PCR-based assay for diagnosis of spotted fever group rickettsiosis in human serum samples. Clin Diagn Lab Immunol. 2005;12:759–63.
Wallménius K, Pettersson JH, Jaenson TG, Nilsson K. Prevalence of Rickettsia spp, Anaplasma phagocytophilum, and Coxiella burnetii in adult Ixodes ricinus ticks from 29 study areas in central and southern Sweden. Ticks Tick Borne Dis. 2012;3:100–6.
Andersson MO, Víchová B, Tolf C, Krzyzanowska S, Waldenström J, Karlsson ME. Co-infection with Babesia divergens and Anaplasma phagocytophilum in cattle (Bos taurus). Ticks Tick Borne Dis. 2007;8:933–5.
Casati S, Sager H, Gern L, Piffaretti JC. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann Agric Environ Med. 2006;13:65–70.
Wilhelmsson P, Lindblom P, Fryland L, Ernerudh J, Forsberg P, Lindgren PE. Prevalence, diversity, and load of Borrelia species in ticks that have fed on humans in regions of Sweden and Åland Islands, Finland with different Lyme borreliosis incidences. PLoS ONE. 2013;21(8):e81433.
Arsnoe I, Tsao JI, Hickling GJ. Nymphal Ixodes scapularis questing behavior explains geographic variation in Lyme borreliosis risk in the eastern United States. Ticks Tick Borne Dis. 2019;10:553–63.
Franke J, Hildebrandt A, Dorn W. Exploring gaps in our knowledge on Lyme borreliosis spirochaetes—updates on complex heterogeneity, ecology, and pathogenicity. Ticks Tick Borne Dis. 2013;4:11–25.
Piesman J, Gern L. Lyme borreliosis in Europe and North America. In: Bowman AS, Nuttall PA, editors. Ticks. Biology, disease and control. New York City: Cambridge University Press; 2008. p. 220–52.
Alekseev AN, Jensen PM, Dubinina HV, Smirnova LA, Makrouchina NA, Zharkov SD. Peculiarities of behaviour of taiga (Ixodes persulcatus) and sheep (Ixodes ricinus) ticks (Acarina: Ixodidae) determined by different methods. Folia Parasitol. 2000;47:147–53.
Bjärvall A, Ullström S. Däggdjur. Alla våra vilda arter i Sverige. Stockholm: Bonnier Fakta; 2010.
Coipan EC, Sprong H. Ecology of Borrelia burgdorferi sensu lato. In: Braks MAH, van Wieren SE, Takken W, Sprong H, editors. Ecology and prevention of Lyme borreliosis. Ecology and control of vector-borne diseases. 1st ed. Wageningen: Wageningen Academic Publishers; 2016. p. 41–61.
Acknowledgements
We are grateful to Eszter Tompa for skilful laboratory assistance. For information about the potential occurrence of ticks in certain localities we are indebted to Torbjörn Forsberg, Nysätra; Stig Olof Lövgren, Påläng (Kalix); Lisbeth Pantzare, Haparanda; Lennart Johansson, Renholmen, Piteå; Tommy Westin, Seskarö; Gunnar Eriksson, Kalix-Nyborg; Ida Svanberg, Haparanda; Erkki and Eine Partanen, Haparanda; Allan Bergström, Måttsund, Luleå; Tore Wikström, Ytterstlandet; Jens and Anneli Nilsson, Ytterstlandet; Ellinor Rönnkvist, Vitgrundet; Kjell Strömbäck, Lutskärsgrundet; and Jan Morin, Lägenön who all provided information of particular value to this project.
Open access funding provided by Linköping University.
Funding
This study was supported by Carl Tryggers stiftelse, Helge Ax:son Johnsons stiftelse, Längmanska kulturfonden, Magnus Bergvalls stiftelse, and the Medical Research Council of Southeast Sweden (FORSS, 657881), and the Division of Laboratory Medicine, Region Jönköping County. The work was carried out under the auspices of ESGBOR (the European Study Group on Lyme Borrelioses) and VectorNet, a European network for sharing data on the geographic distribution of arthropod vectors, transmitting human and animal disease agents (framework contract OC/EFSA/AHAW/2013/02-FWC1) funded by the European Food Safety Authority (EFSA) and the European Centre for Disease prevention and Control (ECDC).
Author information
Authors and Affiliations
Contributions
TGTJ planned the study and organised the collection of ticks. TGTJ morphologically determined species, developmental stage and sex of the ticks. PW performed the laboratory analyses and processed the data. The authors interpreted the data and wrote the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable. Ethical approval is not required for this study, because the analyses were performed on nucleic acid from ticks collected in field and from dogs.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Aligned Borrelia and Rickettsia nucleotide sequences based on PCR-products.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jaenson, T.G.T., Wilhelmsson, P. First records of tick-borne pathogens in populations of the taiga tick Ixodes persulcatus in Sweden. Parasites Vectors 12, 559 (2019). https://doi.org/10.1186/s13071-019-3813-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-019-3813-0