Abstract
Background
Aedes aegypti is a vector that transmits various viral diseases, including dengue and Zika. The radiation-based sterile insect technique (SIT) has a limited effect on mosquito control because of the difficulty in irradiating males without reducing their mating competitiveness. In this study, the insect sex pheromone heptacosane was applied to Ae. aegypti males to investigate whether it could enhance the mating competitiveness of irradiated males.
Methods
Heptacosane was smeared on the abdomens of Ae. aegypti males that were allowed to mate with untreated virgin females. The insemination rate was used to assess the attractiveness of heptacosane-treated males to females. The pupae were irradiated with different doses of X-rays and γ-rays, and the emergence, survival time, egg number, and hatch rate were detected to find the optimal dose of X-ray and γ-ray radiation. The males irradiated at the optimal dose were smeared with heptacosane, released in different ratios with untreated males, and mated with females. The effect of heptacosane on the mating competitiveness of irradiated mosquitoes was then evaluated by the hatch rate, induced sterility, and mating competitiveness index.
Results
Applying heptacosane to Ae. aegypti males significantly increased the insemination rate of females by 20%. Pupal radiation did not affect egg number but significantly reduced survival time and hatch rate. The emergence of the pupae was not affected by X-ray radiation but was affected by γ-ray radiation. Pupae exposed to 60 Gy X-rays and 40 Gy γ-rays were selected for subsequent experiments. After 60 Gy X-ray irradiation or 40 Gy γ-ray irradiation, the average hatch rate was less than 0.1%, and the average survival time was more than 15 days. Moreover, at the same release ratio, the hatch rate of the irradiated group perfumed with heptacosane was lower than that of the group without heptacosane. Conversely, the male sterility and male mating competitiveness index were significantly increased due to the use of heptacosane.
Conclusions
The sex pheromone heptacosane enhanced the interaction between Ae. aegypti males and females. Perfuming males irradiated by X-rays or γ-rays with heptacosane led to a significant increase in mating competitiveness. This study provided a new idea for improving the application effect of SIT.
Graphical Abstract

Similar content being viewed by others
Background
Aedes aegypti is a vector responsible for transmitting various viral diseases, including dengue, Zika, chikungunya, and yellow fever, which pose a great threat to human health [1]. The use of chemical insecticides to control mosquitoes remains the most effective means of combating these mosquito-borne diseases [2]. However, the extensive use of chemical pesticides can make mosquitoes resistant to pesticides, and pesticide residues pose a threat to humans and non-target organisms [3, 4]. Thus, the need to find new mosquito control methods is urgent.
The sterile insect technique (SIT) involves releasing a large number of irradiated sterile male mosquitoes to compete with wild males for female mates to suppress the mosquito population [5]. This technique is species-specific, environmentally friendly, and suitable for large-scale control [6]. For better control of disease-transmitting mosquitoes, the Food and Agriculture Organization of the United Nations and the International Atomic Energy Agency have increased efforts to comprehensively develop and improve SIT packages for the area-wide management of mosquitoes to support their member states [7]. Several countries are currently conducting pilot trials to evaluate the efficacy of SIT. For example, the National Institute of Public Health in Mexico is evaluating the possibility of SIT as an additional control measure for local Ae. aegypti and Ae. albopictus in the most affected areas of the country [8]. Furthermore, feasibility studies on the use of SIT against invasive Ae. albopictus started in 2000, including several field tests on the release of irradiated males [9, 10]. In addition, radiation-based sterile insect techniques were combined with Wolbachia-induced insect incompatibility techniques to control mosquitoes in the field (known as insect incompatibility technique/SIT [IIT-SIT]) [5]. After sex separation and radiation, any female with Wolbachia accidentally released would not be able to reproduce, thus minimizing any unwanted establishment of Wolbachia in the wild. The use of IIT-SIT has successfully controlled Ae. albopictus in field trials in China [5] and suppressed the natural population of Ae. aegypti in Singapore [11], Thailand [12], and Mexico [13].
Gamma rays are the most commonly used radiation source in SIT, with high photon energy and strong penetrating ability, but with the stricter management of radioactive substances in most countries, the acquisition of γ-ray sources has become increasingly difficult. In contrast, X-ray equipment is easier to obtain, simpler to operate, and safer, but its penetrating power is weaker than that of γ-rays [14]. After male mosquitoes are irradiated by X-rays or γ-rays, their survival time and ability to compete with unirradiated male mosquitoes for mating with females are significantly affected, which also dramatically limits the wide application of this technology [15].
Pheromones are chemical signals used in communication between individuals of the same species, and their roles include attraction, aggression, aphrodisiac, anti-aphrodisiac, aggregation, kin recognition, and alarm signal recognition [16, 17]. In many Diptera insect species, such as housefly, fruit fly, vinegar fly, and mosquito, long-chain diolefins and mono-olefins (cuticular hydrocarbons, CHCs) found on the surface of the epidermis act as attractants and aphrodisiacs, affecting mate choice and inducing courtship [18]. The CHCs tricosane and heptacosane have been isolated and identified from epidermal extracts of mature males of Anopheles stephensi, and it was demonstrated that heptacosane enhances the attractiveness of sexually mature males to courtship females [19]. However, little is known about these pheromones in Aedes mosquitoes. Therefore, we wondered whether the sex pheromone of Anopheles mosquitoes affects the mating activity of Aedes mosquitoes. In this study, we compared the effects of CHC components (tricosane and heptacosane) on the mating success of male adult Ae. aegypti mosquitoes. In addition, we also combined pheromone with SIT (X-ray and γ-ray irradiation of male mosquitoes) to explore whether pheromone can enhance the competitiveness of sterile male mosquitoes and achieve a better inhibitory effect on mosquito populations.
Methods
Mosquito
Aedes aegypti mosquitoes from Zhanjiang City, Guangdong Province, China, were collected by the Guangdong Provincial Center for Disease Control and Prevention. This colony was maintained under conditions of 28 ± 1 °C, 80 ± 5% relative humidity, and a light/dark cycle of 16 h/8 h. The mosquito larvae were fed daily with turtle food, and adults were provided with 10% glucose solution ad libitum. Kunming mouse blood (provided by the Animal Experiment Center of Anhui Medical University) was allowed to feed adult females to lay eggs.
Radiation instruments
The Varian Clinac 23EX linear accelerator (Varian, Palo Alto, CA, USA) and Biobeam GM2000 γRay irradiation device (Gamma-Service Medical GmbH, Leipzig, Germany) were used for X- and γ-ray radiation, respectively.
Effect of heptacosane and tricosane on the mating activity of Ae. aegypti
Heptacosane and tricosane were dissolved in n-hexane at a concentration of 75 µg/ml and applied to the abdomen of 2-day-old Ae. aegypti males using paintbrushes as described by Wang et al. [19]. The solvent n-hexane was used as a control. Forty-eight hours later, 20 treated males and 20 virgin females were introduced into a cage (25 × 35 × 25 cm) and allowed to mate overnight. Then, the female spermathecae were dissected, and the insemination status was examined. When sperm was detected in at least one of the three spermathecae, the mosquito was considered successfully inseminated. Each treatment was replicated three times, with 20 mosquitoes per replicate, and the mating activity tests were repeated three times.
Effects of pupal irradiation on emergence, survival, egg number, and hatch rate
Depending on the size and color of the pupae, smaller and lighter Ae. aegypti male pupae were selected. Then, the selected male pupae (12–24 h old, 150–200/tray) were placed in the center of the tray (diameter: 9 cm). The excess water in the tray was removed with a straw. The tray was placed at the bottom of the X-ray or γ-ray irradiation chamber. Mosquito pupae were exposed to X-rays or γ-rays at a dose rate of 200 Gy/h. After 20 Gy, 40 Gy, or 60 Gy irradiation, pupae were transferred to clean cages (25 × 35 × 25 cm). After 48 h, the non-emerged dead pupae were counted to evaluate the emergence rate. Moreover, 30 unirradiated and virgin females were added to each cage (holding 30 irradiated males) for mating overnight and then blood-feeding. Blood-fed females were placed in individual 70-ml tubes containing wet filter papers for oviposition. The females were fed Kunming mouse blood only once, and the same was true for collecting eggs. After 5 days, the filter papers holding the eggs were left to dry for 24 h under ambient conditions and then placed in a water basin for 7 days for hatching. Female fertility was calculated by recording the individual numbers of eggs under the microscope and the hatch rates. In addition, pupae exposed to 20 Gy, 40 Gy, and 60 Gy were chosen to examine the effects of X-ray or γ-ray radiation on the survival time of male adults. Three replicates of 30 pupae or adult mosquitoes were used in each treatment, including the controls (unirradiated groups). Males were fed a 10% glucose solution.
Effect of heptacosane on the mating competitiveness of irradiated mosquitoes
In combination with the above experimental results (relatively little impact on the survival time of males and a better effect on reducing the hatch rate), pupae exposed to 60 Gy X-rays and 40 Gy γ-rays were chosen to examine the effects of heptacosane (while tricosane did not significantly enhance the insemination rate) on the male mating competitiveness of irradiated mosquitoes.
Male pupae (12–24 h old) were irradiated with 60 Gy X-ray or 40 Gy γ-ray and then transferred to clean cages for emergence. After 48 h, heptacosane was smeared on the abdomen of the males. Then, 30, 30, 90, 150, and 210 treated males were moved to different cages containing 0, 30, 30, 30, and 30 unirradiated males. The release ratios [(irradiated + heptacosane)/unirradiated; I-H/U] were 1:0, 1:1, 3:1, 5:1, and 7:1. Irradiated males without heptacosane and unirradiated male mosquitoes were released in the same release ratios (irradiated/unirradiated; I/U) as controls. After 24 h, 30 female mosquitoes (unirradiated virgins, 5–7 days old) were placed in each cage for mating competition for 3 days. Then, females were fed Kunming mouse blood. After 5 days, eggs were collected and allowed to hatch for 7 days. The hatch rate for each group was recorded. Three replicates were performed for each group. The induced sterility (IS) and the male mating competitiveness index (C) were calculated according to the following formulas [20, 21]:
where Hs is the hatch rate of the irradiated control group, Hc is the hatch rate of the competition group (a mixed ratio of unirradiated and irradiated males), Hn is the hatch rate of the unirradiated control group, N is the number of unirradiated males, and S is the number of irradiated males.
Statistical analysis
Statistical analyses were performed using IBM SPSS version 20. Differences in emergence and hatch rates among groups were compared using Pearson's Chi-square test and Bonferroni test. Analysis of variance (ANOVA) and Tukey's post hoc test were used to compare differences in egg number, IS, and C between groups. Kaplan‒Meier analysis was performed to determine relative differences in survival time among groups. Values of P < 0.05 were considered statistically significant.
Results
Effect of heptacosane and tricosane on the mating activity of Aedes mosquitoes
The average insemination rate of female Ae. aegypti mosquitoes in the heptacosane-treated group was 60.6 ± 1.6%, which was significantly higher than the 36.1 ± 2.3% in the tricosane group (χ2 = 21.535, df = 1, P < 0.001) and 40.6 ± 2.5% in the n-hexane control group (χ2 = 14.402, df = 1, P < 0.001). However, there was no significant difference in the insemination rate of Ae. aegypti females between the n-hexane group and the tricosane control group (χ2 = 0.752, df = 1, P = 0.386). Therefore, applying tricosane to mature male Ae. aegypti mosquitoes did not affect their mating activities, while applying heptacosane significantly increased the insemination rate of Ae. aegypti females by 20%.
Effects of pupal irradiation on emergence, egg number, and hatch rate
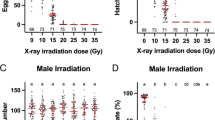
There was no effect of radiation dose on the number of eggs laid per female, regardless of the kind of used radiation ray (χ2 = 6.584, df = 3, P = 0.086) (Table 1). However, when the γ-ray radiation dose reached 40 Gy or 60 Gy, the pupal emergence rate was significantly lower than that of the control group (χ2 = 39.155, df = 3, P < 0.001) (Table 1).
Moreover, male pupae radiation (including X-ray and γ-ray radiation) was not correlated with female egg number (F = 0.592, df = 6, P = 0.737). In contrast, the hatch rate decreased significantly with increasing radiation dose (χ2 = 3635.000, df = 6, P < 0.001) (Fig. 1). After 60 Gy X-ray or more than or equal to 40 Gy γ-ray radiation, the hatch rate was less than 0.1%. Furthermore, no egg was hatched after 60 Gy γ-ray irradiation (Table 1).
Effect of pupal radiation on the survival of male mosquitoes
The average survival time of the males in the control groups was 24.7 ± 1.0 days. At the same time, the average survival times of adult males were 23.0 ± 0.9, 19.8 ± 0.8, and 15.6 ± 0.6 days after exposure to 20 Gy, 40 Gy, and 60 Gy X-rays, respectively (Table 2). The survival of male mosquitoes tended to decrease with increasing X-ray radiation dose. Compared with the control group, 40 Gy and 60 Gy X-ray irradiation of pupae significantly reduced the survival time of adult male mosquitoes (Fig. 2A). However, no significant difference in male longevity was observed between 20 Gy X-ray irradiation of pupae and the control group. In addition, the average survival times of male adults were 22.1 ± 0.7, 17.3 ± 0.6, and 12.6 ± 0.4 days after exposure to 20 Gy, 40 Gy, and 60 Gy γ-rays, respectively. Here, again, the survival time was inversely proportional to the radiation dose (Fig. 2B). The survival times for all γ-ray irradiation doses were significantly lower than that of the control.
Effect of heptacosane on the mating competitiveness of irradiated mosquitoes
Pupae exposed to 60 Gy X-rays and 40 Gy γ-rays were used to study the effect of heptacosane on the mating competitiveness of irradiated mosquito males. After mating with irradiated males, the hatch rate of wild females decreased with the increase in release ratio, and the difference was significant (X-ray irradiation: χ2 = 1556.853, df = 5, P < 0.001; γ-ray irradiation: χ2 = 1739.767, df = 5, P < 0.001). These trends were also observed in experiments releasing heptacosane-coated male mosquitoes (X-ray irradiation: χ2 = 1692.237, df = 5, P < 0.001; γ-ray irradiation: χ2 = 1826.224, df = 5, P < 0.001). Moreover, the hatch rate of the group coated with heptacosane was lower than that of the group without heptacosane at the same release ratio. When the release ratios were 1:1, 5:1, and 7:1 in the X-ray radiation experiment and 1:1 and 5:1 in the γ-ray radiation experiment, the difference was statistically significant (Table 2).
Furthermore, the induced sterility increased with the increase in the release ratio, and when at the same release ratio, the induced sterility of the male mosquitoes perfused with heptacosane was significantly higher than that of the untreated group (Fig. 3). Similarly, at the same release ratio, the mating competitiveness index (C) of male mosquitoes perfumed with heptacosane was significantly higher than that of male mosquitoes not perfumed with heptacosane (Fig. 4).
Discussion
Aedes aegypti is a highly efficient vector of the dengue virus and Zika virus [22]. It likes to go out in the early morning and the evening before dusk to find the host to suck blood [7]. This means that insecticide-treated mosquito nets are ineffective in preventing dengue and Zika transmission, unlike malaria. SIT, a concept proposed by Edward Knipling in the 1950s, is a species-specific, pollution-free, and eco-friendly method for pest control [23, 24]. Radiation-based SIT has been successfully used to control mosquitoes and reduce the incidence of vector-borne diseases [25]. Most laboratory studies and field trials have used γ-rays, and X-rays were rarely used to irradiate mosquitoes [26,27,28]. In our study, Ae. aegypti pupae were irradiated with X-rays and γ-rays, and the differences in emergence, survival time, egg number, and hatch rate of the two types of radiation were compared.
The emergence of the adults was not affected by X-ray radiation but was affected by γ-ray radiation. The additional deaths may be due to the greater penetration of γ-rays, which destroy more of the mosquito's somatic cells. Similar findings were reported for Ae. albopictus. X-rays did not affect the emergence rate of Ae. albopictus, but γ-rays significantly reduced the emergence rate of Ae. albopictus [29, 30].
Moreover, in the present study, pupal radiation did not affect the egg number of females but significantly reduced the hatch rate, especially when γ-ray radiation was used. For example, 40 Gy γ-ray radiation had the same effect on the hatch rate as 60 Gy X-ray radiation. The destructive ability of γ-rays on mosquito sperm cells was stronger than that of X-rays. Similarly, the survival time of male mosquitoes that emerged after pupae irradiation was significantly reduced. The effect of γ-rays on the survival of male mosquitoes is higher than that of X-rays at the same doses. Moreover, Chen et al. and Shetty et al. irradiated Ae. aegypti with different doses of γ-rays. They also found that high-dose radiation did not affect the fecundity of females but had a negative impact on the survival time and mating competitiveness of irradiated male mosquitoes [31, 32]. Similarly, Rodriguez et al. proved that X-ray radiation did not affect egg numbers, but the survival time of Ae. aegypti males decreased with increasing radiation dose [33]. A study by Yamada et al. showed that X-rays generated by Raycell Mk2 irradiators induced comparable sterility levels for Ae. aegypti males compared to γ-rays [34]. In addition, in our previous study, we used different doses of X-rays and γ-rays to irradiate Ae. albopictus and obtained similar results as Ae. aegypti [35]. Based on the above results, although male mosquitoes could be sterile by radiation, the mating ability of the irradiated males was significantly affected, which greatly reduced the application effect of SIT.
Chemical pheromones play an important role in female mosquitoes' search for, identification of, and selection of mating males [36]. For example, the pheromone heptacosane (a cuticular hydrocarbon) enhanced the interaction between Anopheles males and females [19]. Heptacosane is also a contact and volatile pheromone that promotes the mating activity of the tea weevil Myllocerinus aurolineatus [37]. In addition, heptacosane was one of the main components of pheromone in the termite Reticulitermes speratus, which could induce long-term aggregation at new nesting and feeding sites [38]. In the present study, applying heptacosane to mature Ae. aegypti males significantly increased the insemination rate of females. Therefore, we used this sex pheromone in combination with SIT, hoping to enhance the population-suppressing effect of releasing irradiated male mosquitoes. Surprisingly, we found that the induced sterility and mating competitiveness index of the male mosquitoes smeared with heptacosane was significantly higher than that of the non-smeared group. When the release ratio of irradiated male mosquitoes smeared with heptacosane to normal male mosquitoes was 5:1, the sterility effect was equivalent to that of the non-smeared group at a release ratio of 7:1. These results suggested that perfuming heptacosane to sterile mosquitoes can enhance the inhibitory effect on the mosquito population with the same release amount. In addition, field releases of Wolbachia-infected Ae. aegypti are being implemented in multiple countries. Insect sex pheromones heptacosane may also be used in IIT or IIT-SIT in the future to enhance the population inhibition effect of sterile mosquitoes released in the field.
However, although our results demonstrate the good effect of SIT in combination with heptacosane at the laboratory level, there are still many problems to be solved before the actual application of mosquito control in the field. First, heptacosane has strong volatility, and how can its continuous effect on releasing males be ensured? Sun et al. reported that 10 μg/ml heptacosane solution applied to females of Myllocerinus aurolineatus could attract males for mating within 12.04 h on average [34]. Heptacosane may lose its effect on enhancing mating competitiveness after 12 h. It may be necessary to explore suitable formulations for the slow release and sustained action of heptacosane. Moreover, Fawaz et al. reported that excitation and attraction were observed when Ae. aegypti females were exposed to the pheromones 2,6,6-trimethylcyclohex-2-ene-1,4-dione, 2,2,6 trimethylcyclohexane-1,4-dione, or 1-(4-ethylphenyl) ethanone [39]. Perhaps in the future, we can find the long-range male sex pheromones that females use to find and identify males to attract females to actively mate with sterile males in the field. A recent study by Mozöraitis et al. highlighted the presence of five volatile compounds (acetoin, sulcatone, octanal, nonanal, and decanal) that Anopheles males released. According to the authors, these might be related to aggregation behaviors that attract males and females and increase the insemination rate [40]. Nevertheless, a laboratory study failed to demonstrate the long-range male sex pheromones associated with swarm detection and recognition by females [41]. They repeated the protocol by Mozūraitis et al. and found that acetoin was absent from almost all the male samples. The other four compounds (sulcatone, octanal, nonanal, and decanal) were detected but did not differ significantly from the control group [41]. The high quantitative variability found in the laboratory and the fact that these compounds are often found in the control indicate that they may be uncontrollable laboratory and/or human pollution. Overall, our study provides a new idea for applying sterile insect technology in the future. The combination of insect sex pheromones and irradiated mosquitoes may enhance the effect of sterile insects in the field.
Conclusion
In conclusion, we confirmed that the sex pheromone heptacosane perfumed on the abdomen of Aedes aegypti male mosquitoes could enhance their attractiveness to female mosquitoes and thus increase their insemination rate. In addition, when male mosquitoes irradiated by X-rays or γ-rays were smeared with heptacosane, the mating competitiveness of the irradiated male mosquitoes could be significantly enhanced. Although more evidence is needed for applying the sex pheromone in SIT in the field, our experiments undoubtedly provide new ideas for the broader application of SIT.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Change history
20 December 2023
This article has been corrected since original publication; please see the linked erratum for further details.
11 December 2023
A Correction to this paper has been published: https://doi.org/10.1186/s13071-023-06078-4
Abbreviations
- SIT:
-
Sterile insect technique
- IIT:
-
Insect incompatibility technique
- CHC:
-
Cuticular hydrocarbon
- I-H/U:
-
(Irradiated + heptacosane)/unirradiated
- I/U:
-
Irradiated/unirradiated
- IS:
-
Induced sterility
- C :
-
Mating competitiveness index
References
Matthews BJ. Aedes aegypti. Trends Genet. 2019;35:470–1. https://doi.org/10.1016/j.tig.2019.03.005.
Jones RT, Ant TH, Cameron MM, Logan JG. Novel control strategies for mosquito-borne diseases. Philos Trans R Soc Lond B Biol Sci. 1818. https://doi.org/10.1098/rstb.2019.0802.
Kamgang B, Wilson-Bahun TA, Yougang AP, Lenga A, Wondji CS. Contrasting resistance patterns to type I and II pyrethroids in two major arbovirus vectors Aedes aegypti and Aedes albopictus in the Republic of the CCgo Central Africa. Infect Dis Poverty. 2020;1:23. https://doi.org/10.1186/s40249-020-0637-2.
Yougang AP, Kamgang B, Bahun TAW, Tedjou AN, Nguiffo-Nguete D, Njiokou F, et al. First detection of F1534C knockdown resistance mutation in Aedes aegypti (Diptera: Culicidae) from Cameroon. Infect Dis Poverty. 2020;9:152. https://doi.org/10.1186/s40249-020-00769-1.
Zheng X, Zhang D, Li Y, Yang C, Wu Y, Liang X, et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature. 2019;572:56–61. https://doi.org/10.1038/s41586-019-1407-9.
de Castro PL, Dos Anjos FA, de Oliveira DA, Rebechi D, de Oliveira RN, Chitolina RF, et al. Novel sterile insect technology program results in suppression of a field mosquito population and subsequently to reduced incidence of dengue. J Infect Dis. 2021;224:1005–14. https://doi.org/10.1093/infdis/jiab049.
FAO/IAEA. Thematic Plan for the Development and Application of the Sterile Insect Technique (SIT) and Related Genetic and Biological Control Methods for Disease Transmitting Mosquitoes, Vienna, Austria. 93 pp. https://www.iaea.org/sites/default/files/21/07/mosquitoes_thematic_plan_report_final.pdf. 2019.
Marina CF, Bond JG, Hernández-Arriaga K, Valle J, Ulloa A, Fernández-Salas I, et al. Population dynamics of Aedes aegypti and Aedes albopictus in Two Rural Villages in Southern Mexico: Baseline Data for an Evaluation of the Sterile Insect Technique. Insects. 2021. https://doi.org/10.3390/insects12010058.
Bellini R, Carrieri M, Balestrino F, Puggioli A, Malfacini M, Bouyer J. Field Competitiveness of Aedes albopictus (Diptera: Culicidae) Irradiated Males in Pilot Sterile Insect Technique Trials in Northern Italy. J Med Entomol. 2021;58:807–13. https://doi.org/10.1093/jme/tjaa235.
Bellini R, Medici A, Puggioli A, Balestrino F, Carrieri M. Pilot field trials with Aedes albopictus irradiated sterile males in Italian urban areas. J Med Entomol. 2013;50:317–25. https://doi.org/10.1603/me12048.
Ching NL. Wolbachia-mediated sterility suppresses Aedes aegypti populations in the urban tropics. medRxiv. 2021. https://doi.org/10.1101/2021.06.16.21257922.
Kittayapong P, Ninphanomchai S, Limohpasmanee W, Chansang C, Chansang U, Mongkalangoon P. Combined sterile insect technique and incompatible insect technique: the first proof-of-concept to suppress Aedes aegypti vector populations in semi-rural settings in Thailand. PLoS Negl Trop Dis. 2019;13:e000771. https://doi.org/10.1371/journal.pntd.0007771.
Martín-Park A, Che-Mendoza A, Contreras-Perera Y, Pérez-Carrillo S, Puerta-Guardo H, Villegas-Chim J, et al. Pilot trial using mass field-releases of sterile males produced with the incompatible and sterile insect techniques as part of integrated Aedes aegypti control in Mexico. PLoS Negl Trop Dis. 2022;16:e0010324. https://doi.org/10.1371/journal.pntd.0010324.
Helinski ME, Parker AG, Knols BG. Radiation biology of mosquitoes. Malar J. 2009. https://doi.org/10.1186/1475-2875-8-s2-s6.
Dame DA, Curtis CF, Benedict MQ, Robinson AS, Knols BG. Historical applications of induced sterilisation in field populations of mosquitoes. Malar J. 2009. https://doi.org/10.1186/1475-2875-8-s2-s2.
Yew JY, Chung H. Insect pheromones: an overview of function, form, and discovery. Prog Lipid Res. 2015;59:88–105. https://doi.org/10.1016/j.plipres.2015.06.001.
Engl T, Kaltenpoth M. Influence of microbial symbionts on insect pheromones. Nat Prod Rep. 2018;35:386–97. https://doi.org/10.1039/c7np00068e.
Wicker-Thomas C. Pheromonal communication involved in courtship behavior in Diptera. J Insect Physiol. 2007;53:1089–100. https://doi.org/10.1016/j.jinsphys.2007.07.003.
Wang G, Vega-Rodríguez J, Diabate A, Liu J, Cui C, Nignan C, et al. Clock genes and environmental cues coordinate Anopheles pheromone synthesis, swarming, and mating. Science. 2021;371:411–5. https://doi.org/10.1126/science.abd4359.
Fried M. Determination of Sterile-Insect Competitiveness. J Econ Entomol. 1971;64:869–72.
Yamada H, Vreysen MJ, Gilles JR, Munhenga G, Damiens DD. The effects of genetic manipulation, dieldrin treatment and irradiation on the mating competitiveness of male Anopheles arabiensis in field cages. Malar J. 2014;13:318. https://doi.org/10.1186/1475-2875-13-318.
Barrera R. New tools for Aedes control: mass trapping. Curr Opin Insect Sci. 2022;52:100942. https://doi.org/10.1016/j.cois.2022.100942.
Bushland RC, Lindquist AW, Knipling EF. Eradication of screw-worms through release of sterilized males. Science. 1955;122:287–8. https://doi.org/10.1126/science.122.3163.287.
Klassen W, Curtis CF. History of the sterile insect technique. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile insect technique: principles and practice in area-wide integrated pest management. Dordrecht: Springer; 2005. p. 3–36.
Lees RS, Gilles JR, Hendrichs J, Vreysen MJ, Bourtzis K. Back to the future: the sterile insect technique against mosquito disease vectors. Curr Opin Insect Sci. 2015;10:156–62. https://doi.org/10.1016/j.cois.2015.05.011.
de Morais LMO, Jussiani EI, Zequi JAC, Dos Reis PJ, Andrello AC. Morphological study of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) eggs by X-ray computed microtomography. Micron. 2019;126:102734. https://doi.org/10.1016/j.micron.2019.102734.
Bouyer J, Culbert NJ, Dicko AH, Pacheco MG, Virginio J, Pedrosa MC, et al. Field performance of sterile male mosquitoes released from an uncrewed aerial vehicle. Sci Robot. 2020. https://doi.org/10.1126/scirobotics.aba6251.
Gato R, Menendez Z, Prieto E, Argiles R, Rodriguez M, Baldoquin W, et al. Sterile insect technique: successful suppression of an Aedes aegypti Field Population in Cuba. Insects. 2021. https://doi.org/10.3390/insects12050469.
Du W, Hu C, Yu C, Tong J, Qiu J, Zhang S, et al. Comparison between pupal and adult X-ray radiation, designed for the sterile insect technique for Aedes albopictus control. Acta Trop. 2019;199:105110. https://doi.org/10.1016/j.actatropica.2019.105110.
Ricardo Machi A, Rodrigues Mayne R, Adriani Gava M, Bergamin Arthur P, Arthur V. Gamma Radiation Sterilization Dose of Adult Males in Asian Tiger Mosquito Pupae. Insects. 2019. https://doi.org/10.3390/insects10040101.
Chen C, Aldridge RL, Gibson S, Kline J, Aryaprema V, Qualls W, et al. Developing the radiation-based sterile insect technique (SIT) for controlling Aedes aegypti: identification of a sterilizing dose. Pest Manag Sci. 2023;79:1175–83. https://doi.org/10.1002/ps.7303.
Shetty V, Shetty NJ, Harini BP, Ananthanarayana SR, Jha SK, Chaubey RC. Effect of gamma radiation on life history traits of Aedes aegypti (L.). Parasite Epidemiol Control. 2016;1:26–35. https://doi.org/10.1016/j.parepi.2016.02.007.
Rodriguez SD, Brar RK, Drake LL, Drumm HE, Price DP, Hammond JI, et al. The effect of the radio-protective agents ethanol, trimethylglycine, and beer on survival of X-ray-sterilized male Aedes aegypti. Parasit Vectors. 2013;6:211. https://doi.org/10.1186/1756-3305-6-211.
Yamada H, Kaboré BA, Bimbilé Somda NS, Ntoyi NL, de Beer CJ, Bouyer J, et al. Suitability of Raycell MK2 Blood X-ray Irradiator for the Use in the Sterile Insect Technique: Dose Response in Fruit Flies Tsetse Flies and Mosquitoes. Insects. 2023. https://doi.org/10.3390/insects14010092.
Wang L-M, Li N, Ren C-P, Peng Z-Y, Lu H-Z, Li D, et al. Sterility of Aedes albopictus by X-ray Irradiation as an Alternative to γ-ray Irradiation for the Sterile Insect Technique. Pathogens. 2023. https://doi.org/10.3390/pathogens12010102.
Clements AN. The biology of mosquitoes. Volume 1. Development, nutrition and reproduction. London: Chapman & Hall; 1992.
Sun X, Zhang X, Wu G, Li X, Liu F, Xin Z, et al. n-Pentacosane Acts as both Contact and Volatile Pheromone in the tea Weevil. Myllocerinus aurolineatus J Chem Ecol. 2017;43:557–62. https://doi.org/10.1007/s10886-017-0857-5.
Mitaka Y, Matsuyama S, Mizumoto N, Matsuura K, Akino T. Chemical identification of an aggregation pheromone in the termite Reticulitermes speratus. Sci Rep. 2020;10:7424. https://doi.org/10.1038/s41598-020-64388-4.
Fawaz EY, Allan SA, Bernier UR, Obenauer PJ, Diclaro JW 2nd. Swarming mechanisms in the yellow fever mosquito: aggregation pheromones are involved in the mating behavior of Aedes aegypti. J Vector Ecol. 2014;39:347–54. https://doi.org/10.1111/jvec.12110.
Mozūraitis R, Hajkazemian M, Zawada JW, Szymczak J, Pålsson K, Sekar V, et al. Male swarming aggregation pheromones increase female attraction and mating success among multiple African malaria vector mosquito species. Nat Ecol Evol. 2020;4:1395–401. https://doi.org/10.1038/s41559-020-1264-9.
Poda SB, Buatois B, Lapeyre B, Dormont L, Diabaté A, Gnankiné O, et al. No evidence for long-range male sex pheromones in two malaria mosquitoes. Nat Ecol Evol. 2022. https://doi.org/10.1038/s41559-022-01869-x.
Acknowledgements
Not applicable.
Funding
This research was supported by the National Natural Science Foundation of China (82102432), Anhui Provincial Natural Science Foundation Project (2108085QH347), and Research Fund Project of Anhui Medical University (2020xkj005) to DSQ.
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology, DSQ; investigation, WLM, MZ, TQ, LHZ, ZQY, NJX, XL, PZY, DYN, RCP, and ZC; writing—original draft preparation, DSQ, and WLM; writing—review and editing, DSQ and WDQ; supervision, DSQ, WDQ, and LM; funding acquisition, DSQ. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lin-Min Wang, Ni Li and Mao Zhang are co-first authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, LM., Li, N., Zhang, M. et al. The sex pheromone heptacosane enhances the mating competitiveness of sterile Aedes aegypti males. Parasites Vectors 16, 102 (2023). https://doi.org/10.1186/s13071-023-05711-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05711-6