Abstract
Background
Apicomplexan haemoparasites are protozoans that infect a variety of domestic and wild animal species, as well as humans. Data regarding haemoprotozoans in domestic cats are limited; therefore, the aim of this study was to assess the occurrence of Babesia spp., Cytauxzoon spp., and Hepatozoon spp. in domestic cats in Romania using molecular tools.
Methods
Blood samples from 371 domestic cats were screened for the presence of piroplasmids. All samples that yielded a visible band in agarose gels were subsequently tested by specific assays targeting the 18S rDNA of Babesia spp., Cytauxzoon spp., and Hepatozoon spp. Moreover, nested PCR assays targeting mitochondrial genes of Babesia spp. were used for screening of all Babesia spp. 18S rDNA-positive samples.
Results
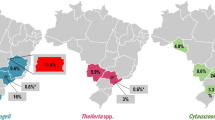
From the total number of sampled cats, 19.4% were positive in the PCR assay targeting piroplasmids. Babesia spp. were identified in 15.1% of cats, while 0.5% were positive for Hepatozoon spp. Molecular analyses confirmed the presence of Babesia canis. No samples were positive for Cytauxzoon spp.
Conclusions
The high infection rates of domestic cats with Babesia spp. and the need for species differentiation highlight the importance of mitochondrial genes as targets for molecular protocols.

Similar content being viewed by others
Background
Apicomplexan haemoparasites are protozoans that infect a wide variety of domestic and wild animals, as well as humans [1, 2]. The complex interactions between domestic animals, wild reservoirs and arthropod vectors favour tick-borne pathogen transmission and increase their geographical distribution [3]. During the last decades, several studies have focused on the detection and characterization of haemoprotozoans in domestic dog populations worldwide [4,5,6,7]. However, in domestic cats, data regarding the presence of haemoprotozoans are scarce.
Genus Babesia includes more than 100 species, with intraerythrocytic localization in the vertebrate host, causing mild to severe haemolytic diseases [2, 8]. Several ixodid ticks are thought to be involved in their transmission, although vector competence has not been confirmed in all cases [9]. Feline babesiosis is a relatively newly recognized clinical entity, with most available studies originating from South Africa [10]. Non-specific clinical signs such as anaemia, lethargy, and anorexia are described, whereas icterus and fever are inconsistently found [11]. While several Babesia spp. have been documented in domestic cats, the species most commonly associated with clinical cases is Babesia felis [10]. In Europe, Babesia microti [12, 13] and dog-related species such as Babesia canis [14, 15], Babesia vogeli [15, 16], and Babesia vulpes (formerly known as Theileria annae) [14, 17] have been detected in domestic cats. Nevertheless, the European cases were rarely associated with clinical manifestations. Recently, Babesia pisicii was described in European wild cats, Felis silvestris in Romania [18], but its presence in domestic cats has not been documented yet.
Hepatozoon spp. are haemogregarines with a life-cycle shared between a wide range of vertebrates as intermediate hosts and various haematophagous arthropods as definitive hosts [19]. The main transmission pathway is represented by the ingestion of the arthropod definitive host containing mature sporozoites by the intermediate host [20]. In domestic cats, Hepatozoon spp. were reported for the first time in India [21]. Since then, Hepatozoon infections have been found in domestic cats and various wild felids worldwide [22]. In Europe, Hepatozoon felis is recognized as the main agent infecting domestic and wild felines [15, 16, 23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Hepatozoon canis has also been reported in domestic cats in Europe [28, 29, 37,38,39]. Recently, a novel species, Hepatozoon silvestris, was described in European wild cats from Bosnia and Herzegovina [30] and was further reported in domestic [29, 35, 40] and wild felids [31] in Europe. Feline hepatozoonosis is mostly subclinical, with no significant inflammatory response in association with the presence of meronts in muscle tissue [26, 31].
Feline cytauxzoonosis, first described in the 1970s [41], is a tick-borne disease affecting both domestic and wild felids [42]. Five Cytauxzoon species have been described so far in felids Cytauxzoon felis, Cytauxzoon manul, Cytauxzoon europaeus, Cytauxzoon banethi, and Cytauxzoon otrantorum. Cytauxzoon felis is considered endemic to North America, causing a highly fatal disease in domestic cats, in both natural and experimental infections [43]. Bobcats (Lynx rufus) are the natural reservoirs [3]. In 2005, C. manul was described from Pallas’ cats (Otocolobus manul) imported from Mongolia to the USA [44, 45]. In Europe, unnamed isolates of Cytauxzoon have been documented in the past decade in domestic cats [28, 35, 39, 46,47,48,49,50,51,52,53], Iberian lynx (Lynx pardinus) [54,55,56,57,58,59], Eurasian Lynx (Lynx lynx) [60], and European wild cats [31, 60,61,62]. In a breeding centre in Russia, Cytauxzoon spp. were identified in a serval, a bobcat, seven Amur wild cats, and two domestic cats [63]. Recently, three Cytauxzoon species were described in European wild cats: C. europaeus, which was identified in several central, eastern, and southern European countries [64,65,66,67], and C. banethi and C. otrantorum, which to date had been identified only in Romania [64]. Additionally, C. europaeus was identified in domestic and stray cats in Switzerland [65]. However, no data are available on the clinical significance of these species for domestic cats.
No previous studies are available on apicomplexan haemoparasites in domestic cats in Romania, and data from Eastern Europe is generally very limited. Therefore, the aim of this study was to investigate the occurrence of Babesia spp., Cytauxzoon spp., and Hepatozoon spp. in domestic cats in Romania using highly specific polymerase chain reaction (PCR) protocols and to identify the potential risk factors associated with these infections.
Methods
Sample and data collection
Blood samples from 371 domestic cats were collected between October 2017 and May 2019. The animals included in the study were client-owned (referred to urban private veterinary clinics or from rural areas), stray (from animal shelters), or feral (living in cat colonies). Whole blood samples were collected into sterile tubes containing anticoagulant (ethylenediaminetetraacetic acid [EDTA] or citrate) after obtaining informed consent for patient enrolment from the owners. The samples were stored at −20 °C until further analysis.
Outdoor access and age (cats older than 4 months) were considered as inclusion criteria. When available, epidemiological data (sex, age, breed, lifestyle, habitat, and ecoregion) were noted for each animal.
Molecular and phylogenetic analyses
Genomic DNA was isolated using the Isolate II Genomic DNA Kit (Meridian Bioscience, London, UK) from 200 µl of whole blood, following the manufacturer’s instructions. Each DNA sample was stored at −20 °C until further use.
A highly sensitive nested PCR protocol targeting a 561–613-base pair (bp) fragment of 18S ribosomal DNA (18S rDNA) of piroplasmids (Cytauxzoon spp., Babesia spp., Theileria spp., and Hepatozoon spp.) was used for initial screening. Due to the high number of weak bands and the low quality of the sequences obtained, all positive or dubious samples were subsequently screened by specific nested PCR assays targeting the 18S rDNA of Babesia spp., Cytauxzoon spp., and Hepatozoon spp. Moreover, nested PCR assays targeting the cytochrome b (Cytb) and cytochrome c oxidase subunit I (COI) genes of Babesia spp. were used for screening of all Babesia spp.-positive samples. Primers and PCR conditions are detailed in Table 1.
First-round reactions were carried out in a total volume of 15 μl containing 7.5 μl of 2× PCRBIO Taq Mix Red (PCR Biosystems, London, UK), 400 nM of each primer, and 1 μl of template DNA, except for the amplification of the partial 18S rDNA of piroplasmids that was performed using 2 μl of DNA. Amplification of the second round was carried out in a 25 μl reaction mixture consisting of 12.5 μl of 2× PCRBIO Taq Mix Red (PCR Biosystems, London, UK), 400 nM of each primer, and 1 μl of the first PCR round as the template. One positive control, consisting of DNA from carnivores previously confirmed as positive for the targeted pathogens [18, 64, 68], and a negative control represented by sterile water were included in each reaction set. Amplicons were visualized on 1.5% agarose gels stained with ECO Safe Nucleic Acid Staining Solution (PacificImage Electronics, New Taipei City, Taiwan).
Products of expected size were cut from gels and purified using the Gel/PCR DNA Fragments Extraction Kit (Geneaid Biotech Ltd., New Taipei City, Taiwan). PCR products were sequenced with Sanger sequencing technology in both directions (performed at Macrogen Europe, Amsterdam, the Netherlands) using the amplification primers. Chromatograms were assembled and edited using Geneious 4.8.5 software [69], and consensus sequences were compared to homologous sequences available in the GenBank® database using the NCBI Basic Local Alignment Search Tool (BLASTn) analysis. Protein coding gene sequences were translated to corresponding amino acids, based on the protozoan mitochondrial genetic code, to guide nucleotide alignment.
To investigate the relations among Babesia spp., phylogenetic analysis of the 18S rDNA and Cytb genes was performed using MEGA X software [70] based on all unique sequences obtained in the present study and available sequences of Babesia sensu stricto clade VI, according to Schnittger et al. [2], longer than 300 bp. In the case of Cytb, B. pisicii was also included in the analysis. Two 18S rDNA sequences of H. felis and two Cytb sequences of Theileria parva were used as outgroups. In the case of both datasets, the sequences were aligned using the ClustalW algorithm, resulting in a final alignment of 47 sequences for the 18S rDNA and 25 for the Cytb gene, respectively. The phylogenetic trees were inferred by the maximum likelihood method. The best-fit substitution models, with the lowest Bayesian information criterion (BIC) scores, as calculated by the software, were used: Kimura 2-parameter, using a discrete gamma distribution (K2+G) for the 18S rDNA, and Tamura 3-parameter, using a discrete gamma distribution (T92+G) for the Cytb gene, respectively. Branch support was estimated using 1000 bootstrap replicates. The resulting tree topologies were visualized and edited in FigTree v1.4.4 and Inkscape 0.94.
Statistical analyses
Statistical analyses were performed using R software v. 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria). The prevalence and its 95% confidence interval (CI), overall and differentiated by different epidemiological data, were calculated, and the existence of a statistical association between PCR positivity rates and explanatory variables (sex, age, breed, lifestyle, habitat, and ecoregion) was evaluated by Fisher’s exact test. P-values less than 0.05 were considered statistically significant.
Results
From the total number of sampled cats, 72 (19.4%, 95% CI 15.4–23.4) showed a visible band in the PCR targeting the 18S rDNA of piroplasmida. From these, 56 samples (15.1%, 95% CI 11.5–18.7) yielded an amplicon in the assay targeting the 18S rDNA of Babesia spp. The sequences represented five unique haplotypes. The most common haplotype (BHF014) was detected in 52 samples. The remaining four haplotypes were represented by one sample each, differing from the main haplotype by three single-nucleotide polymorphism (SNP) sites and one indel (1.12%; 4/356 nucleotides [nt]), in the case of the sample represented by ARF008, and one SNP site in the case of the other three haplotypes, respectively. The BLASTn analysis of the obtained haplotypes showed 98.9–100% nucleotide sequence identity with B. canis from dogs from Lithuania (GenBank accession numbers: MN078319-MN078323), Iran (MN173223), or Bosnia and Herzegovina (MK107800-MK107806). All sequences represented by unique haplotypes were deposited in GenBank (accession numbers OL342311-OL342315).
The amplification of the Cytb gene of Babesia spp. was successful in six samples, while no positivity was noticed in the assay targeting the Babesia spp. COI gene. Two haplotypes with 99.8% identity were detected in the six samples (1 SNP/477 nt). The sequences displayed a 99.6–99.8% identity to B. canis from the USA (KC207822) or B. canis reported in a dog from Poland (MK024727) and 99.4–99.6% identity to B. canis detected in European wild cats in Romania (MW938761). The two sequences were deposited in the GenBank database under the accession numbers OL355016 and OL355017.
From the 72 samples positive in the initial screening, two yielded an amplicon in the assays targeting the 1600-bp fragment of the 18S rDNA of Hepatozoon spp., resulting in an overall prevalence of 0.5% (95% CI 0.0–1.3). However, direct sequencing resulted in four short sequences (two forward and two reverse), that showed an overall identity of 99–100% to Hepatozoon sp. identified in ocelots from Brazil (KX776299, KX776303) or reptiles from Spain (MG787243), or H. felis reported in lions from India (KX01729, ON075470) or wild cats from Hungary (OM422756). The two samples were also positive for Babesia sp. The remaining 16 samples tested negative in all specific protocols. All sampled domestic cats were PCR negative for Cytauxzoon spp.
Statistical analyses showed that the Babesia spp. infection rate was statistically higher in cats from the Pannonian ecoregion than in the rest of the country, at a P-value of 0.03. No associations were found between the presence of the pathogens and other categorical variables (Table 2).
Phylogenetic analyses of both 18S rDNA (Fig. 1) and Cytb (Fig. 2) sequences confirmed the affiliation of our sequences to the B. canis clade, clustering together with other B. canis sequences from dogs, European wild cats or ticks, in a sister clade to B. vogeli, in the case of 18S rDNA, and to B. pisicii and Babesia rossi (bootstrap value: 98), in the case of Cytb.
Maximum likelihood tree based on partial 18S rDNA sequences obtained in the current study (in bold) and sequences of Babesia sensu stricto species (clade VI sensu Schnittger et al. [2]). Only bootstrap values above 75% are displayed. The scale bar indicates the number of nucleotide substitutions per site. The GenBank accession number, assigned name, host, and country of origin are indicated for each sequence, if available
Maximum likelihood tree based on Cytb sequences obtained in the current study (highlighted in bold) and sequences of Babesia sensu stricto species (clade VI sensu Schnittger et al. [2]). Only bootstrap values above 75% are displayed. The scale bar indicates the number of nucleotide substitutions per site. The GenBank accession number, assigned name, host, and country of origin are indicated for each sequence, if available
Discussion
The results of the current study confirmed for the first time that B. canis and Hepatozoon spp. are circulating in domestic cats in Romania. The 18S rDNA, a highly conserved region, is the primary PCR target used in studies addressing the diagnosis of piroplasmids [71, 72]. However, several studies have questioned its ability to differentiate between closely related species [2, 18, 64, 73, 74]. In the present study, BLAST analyses of the 18S rDNA sequences showed 99–100% identity with different sequences of B. canis, although the presence of this species was confirmed in only six samples by Cytb gene amplification and analyses. Babesia canis was previously reported in domestic cats in Europe, the data being supported exclusively by relatively short 18S rDNA fragments [14, 15]. Therefore, as previously highlighted [18], our recommendation remains to avoid using protocols targeting the 18S rDNA for piroplasmid species differentiation, and samples amplified by these protocols should be considered as Babesia sp. PCR protocols based on mitochondrial gene detection can be successfully used for species confirmation, but have shown lower sensitivity than 18S rDNA amplification protocols (100 to 1000 times lower sensitivity than the protocol targeting the 376-bp fragment of B. canis 18S rDNA) [18, 74].
Several Babesia species have been documented in domestic cats worldwide: B. felis [10], B. leo [75], B. lengau [76], and Babesia sp. cat Western Cape [75] in Africa; B. hongkongensis [77], B. canis presentii [78], B. panickeri [79], and B. vogeli [80, 81] in Asia; and B. vogeli and B. gibsoni in the Americas [82]. In Europe, epidemiological data on Babesia infection in domestic cats are limited to a few molecular studies targeting the 18S rDNA, with B. canis reported in Spain and Portugal [14, 15], B. vogeli in Portugal [15, 16], and B. microti in Italy [12, 13]. Babesia canis was also recently reported in wild cats from Romania [18].
Traditionally, the naming of Babesia spp. has been based on their assumed host specificity and morphological characters [2]. However, as already discussed by others [18, 83], the presence of B. canis DNA was detected in several other non-canid hosts, such as bats [84] and horses [85]. Furthermore, B. canis was molecularly detected in mice experimentally fed Dermacentor reticulatus-positive ticks, raising the possibility of oral transmission through vectors [83]. This hypothesis was also put forward by Hornok et al. [86] when B. canis DNA was identified in insectivorous bat faeces. Cats can ingest ticks, either with their prey (immature D. reticulatus feed on micromammals such as mice and voles [87]) or due to their grooming behaviour.
In wild cats in Romania, a recent study noted a prevalence of Babesia spp. infection of 39.2% [18]. However, in this previous study on wild cats [18], B. canis was confirmed in only one sample by mitochondrial marker assay, while in three samples, a novel species, B. pisicii, was described. The presence of this species was not confirmed in domestic cats.
The geographical distribution of Hepatozoon spp. in domestic cats is apparently wide in Europe, with reports originating mainly from Mediterranean countries, such as Spain [23, 24, 28, 37], Portugal [15, 16], Italy [29, 32, 35], Cyprus [27], Greece [36], and France [39], but also from Central Europe: Austria [33] and Switzerland [40]. In our study, the exact identity of the species involved could not be established due to the low quality of the obtained sequences. The low parasitaemia level observed in domestic cats during other studies [25, 35], as well as the predominance of subclinical manifestations in Hepatozoon spp. infection [26, 35], most likely contributed to these impediments. Cloning procedures presumably have the ability to improve the molecular results.
In recent decades, Hepatozoon infection in felids has been increasingly reported worldwide, usually with low infection rates, but ranging up to 37.9% in Cyprus [27]. The overall prevalence of Hepatozoon spp. in the present study was 0.5%, similar to that found in Spain [37] or Italy [32].
Piroplasms of the genus Cytauxzoon have gained increased interest in recent years in Europe, due to the high prevalence observed in wild felids [54,55,56,57,58,59,60,61,62,63,64,65,66,67] and occasional clinical reports [48,49,50,51, 53, 65]. Despite the diversity and common occurrence of Cytauxzoon infection in European wild cats [64], no positive domestic cat was found in this study. Similar results have been obtained in other large-scale surveys conducted on outdoor, stray, or feral cats in Italy [13, 88, 89] and Greece [90]. The reports of Cytauxzoon sp. in domestic cats from Europe either are clinical case reports [48,49,50,51, 53] or represent findings in asymptomatic cats from Mediterranean regions: Spain [28, 46], France [39], or Italy [35, 47, 52]. At the moment, no report of Cytauxzoon is available from healthy cats in other parts of Europe.
Conclusions
To the authors’ knowledge, this is the first report of Babesia and Hepatozoon spp. in domestic cats in Romania. Moreover, the study shows high infection rates with Babesia spp. in domestic cat populations, confirms the presence of B. canis by using a specific genetic marker, and highlights the importance of using mitochondrial genes as targets for PCR analyses that are aimed at piroplasmid species differentiation. Nevertheless, no Cytauxzoon spp.-positive samples were identified. Further studies are required to develop highly sensitive PCR assays targeting the Cytb or COI gene of Babesia spp., and to clarify the clinical implication of this pathogen in domestic cats.
Availability of data and materials
The datasets supporting the conclusions of this study are included in this published article.
References
Gray J, Zintl A, Hildebrandt A, Hunfeld KP, Weiss L. Zoonotic babesiosis: overview of the disease and novel aspects of pathogen identity. Ticks Tick Borne Dis. 2010;2010:3–10.
Schnittger L, Rodriguez AE, Florin-Christensen M, Morrison DA. Babesia: a world emerging. Infect Genet Evol. 2010;12:1788–809.
Alvarado-Rybak M, Solano-Gallego L, Millán J. A review of piroplasmid infections in wild carnivores worldwide: importance for domestic animal health and wildlife conservation. Parasit Vectors. 2016;2016:1–19.
Ewing SA, Panciera RJ. American canine hepatozoonosis. Clin Microbiol Rev. 2003;16:688–97.
Irwin PJ. Canine babesiosis. Vet Clin North Am Small Anim Pract. 2010;40:1141–56.
Solano-Gallego L, Baneth G. Babesiosis in dogs and cats—expanding parasitological and clinical spectra. Vet Parasitol. 2011;181:48–60.
Ghauri HN, Ijaz M, Farooqi SH, Ali A, Ghaffar A, Saleem S, et al. A comprehensive review on past, present and future aspects of canine theileriosis. Microb Pathog. 2019;126:116–22.
Mihalca AD, Cozma V, Şuteu E, Marinculic A, Boireau P. The quest for piroplasms: from Babeş and Smith to molecules. Sci Parasitol. 2010;11:14–9.
Bonnet SI, Nadal C. Experimental infection of ticks: An essential tool for the analysis of Babesia species biology and transmission. Pathogens. 2021;10:1403.
Penzhorn BL, Oosthuizen MC. Babesia species of domestic cats: molecular characterization has opened Pandora’s Box. Front Vet Sci. 2020;7:134.
Futter GJ, Belonje PC. Studies on feline babesiosis. 2. Clinical observations. J S Afr Vet Assoc. 1980;51:143–6.
Pennisi MG, Alongi A, Agnone A, Vitale F, Reale S, Torina A. Cats as reservoir of Babesia microti. Parassitologia. 2007;49:100.
Spada E, Proverbio D, Galluzzo P, Perego R, Bagnagatti De Giorgi G, Roggero N, et al. Frequency of piroplasms Babesia microti and Cytauxzoon felis in stray cats from northern Italy. BioMed Res Int. 2014. https://doi.org/10.1155/2014/943754.
Criado-Fornelio A, Martinez-Marcos A, Buling-Sarana A, Barba-Carretero JC. Presence of Mycoplasma haemofelis, Mycoplasma haemominutum and piroplasmids in cats from southern Europe: a molecular study. Vet Microbiol. 2003;93:307–17.
Vilhena H, Martinez-Díaz VL, Cardoso L, Vieira L, Altet L, Francino O, et al. Feline vector-borne pathogens in the north and centre of Portugal. Parasit Vectors. 2013;6:1–6.
Maia C, Ramos C, Coimbra M, Bastos F, Martins Â, Pinto P, et al. Bacterial and protozoal agents of feline vector-borne diseases in domestic and stray cats from southern Portugal. Parasit Vectors. 2014;7:1–8.
Fritz D, Derré G. A case of babesiosis due to Babesia annae in a cat. Summa Animali da Compagnia. 2011;28:61–5.
Panait LC, Hrazdilová K, Ionică AM, Deak G, Chişamera GB, Adam C, et al. Babesia pisicii n. sp. and Babesia canis infect European wild cats, Felis silvestris, in Romania. Microorganisms. 2021;9:1474.
Smith TG. The genus Hepatozoon (Apicomplexa: Adeleina). J Parasitol. 1996. https://doi.org/10.2307/3283781.
Baneth G, Samish M, Shkap V. Life cycle of Hepatozoon canis (Apicomplexa: Adeleorina: Hepatozoidae) in the tick Rhipicephalus sanguineus and domestic dog (Canis familiaris). J Parasitol. 2007;93:283–99.
Patton WS. The haemogregarines of mammals and reptiles. Parasitology. 1908;1908:318–21.
Salakij C, Salakij J, Narkkong NA, Sirinarumitr T, Pattanarangsan R. Hematologic, cytochemical, ultrastructural, and molecular findings of Hepatozoon-infected flat-headed cats (Prionailurus planiceps). Vet Clin Pathol. 2008;37:31–41.
Ortuño A, Castellà J, Criado-Fornelio A, Buling A, Barba-Carretero JC. Molecular detection of a Hepatozoon species in stray cats from a feline colony in North-eastern Spain. Vet J. 2008;177:134–5.
Tabar MD, Altet L, Francino O, Sánchez A, Ferrer L, Roura X. Vector-borne infections in cats: molecular study in Barcelona area (Spain). Vet Parasitol. 2008;151:332–6.
Baneth G. Perspectives on canine and feline hepatozoonosis. Vet Parasitol. 2011;181:3–11.
Baneth G, Sheiner A, Eyal O, Hahn S, Beaufils JP, Anug Y, et al. Redescription of Hepatozoon felis (Apicomplexa: Hepatozoidae) based on phylogenetic analysis, tissue and blood form morphology, and possible transplacental transmission. Parasit Vectors. 2013;6:1–10.
Attipa C, Papasouliotis K, Solano-Gallego L, Baneth G, Nachum-Biala Y, Sarvani E, et al. Prevalence study and risk factor analysis of selected bacterial, protozoal and viral, including vector-borne, pathogens in cats from Cyprus. Parasit Vectors. 2017;10:1–14.
Díaz-Regañón D, Villaescusa A, Ayllón T, Rodríguez-Franco F, Baneth G, Calleja-Bueno L, et al. Molecular detection of Hepatozoon spp. and Cytauxzoon sp. in domestic and stray cats from Madrid. Spain Parasit Vectors. 2017;10:1–9.
Giannelli A, Latrofa MS, Nachum-Biala Y, Hodžić A, Greco G, Attanasi A, et al. Three different Hepatozoon species in domestic cats from southern Italy. Ticks Tick Borne Dis. 2017;8:721–4.
Hodžić A, Alić A, Prašović S, Otranto D, Baneth G, Duscher GG. Hepatozoon silvestris sp. nov.: morphological and molecular characterization of a new species of Hepatozoon (Adeleorina: Hepatozoidae) from the European wild cat (Felis silvestris silvestris). Parasitology. 2017;144:650–61.
Hodžić A, Alić A, Duscher GG. High diversity of blood-associated parasites and bacteria in European wild cats in Bosnia and Herzegovina: a molecular study. Ticks Tick Borne Dis. 2018;9:589–93.
Otranto D, Napoli E, Latrofa MS, Annoscia G, Tarallo VD, Greco G, et al. Feline and canine leishmaniosis and other vector-borne diseases in the Aeolian islands: pathogen and vector circulation in a confined environment. Vet Parasitol. 2017;236:144–51.
Basso W, Görner D, Globokar M, Keidel A, Pantchev N. First autochthonous case of clinical Hepatozoon felis infection in a domestic cat in Central Europe. Parasitol Int. 2019;72:101945.
Diakou A, Dimzas D, Astaras C, Savvas I, Di Cesare A, Morelli S, et al. Clinical investigations and treatment outcome in a European wildcat (Felis silvestris silvestris) infected by cardio-pulmonary nematodes. Vet Parasitol Reg Stud. 2020;19:100357.
Grillini M, Simonato G, Tessarin C, Dotto G, Traversa D, Cassini R, et al. Cytauxzoon sp. and Hepatozoon spp. in domestic cats: a preliminary study in North-Eastern Italy. Pathogens. 2021;10:1214.
Morelli S, Diakou A, Traversa D, Di Gennaro E, Simonato G, Colombo M, et al. First record of Hepatozoon spp. in domestic cats in Greece. Ticks Tick Borne Dis. 2021;12:101580.
Criado-Fornelio A, Ruas JL, Casado N, Farias NAR, Soares MP, Müller G, et al. New molecular data on mammalian Hepatozoon species (Apicomplexa: Adeleorina) from Brazil and Spain. J Parasitol. 2006;92:93–9.
Criado-Fornelio A, Buling A, Cunha-Filho NA, Ruas JL, Farias NAR, Rey-Valeiron C, et al. Development and evaluation of a quantitative PCR assay for detection of Hepatozoon sp. Vet Parasitol. 2007;150:352–6.
Criado-Fornelio A, Buling A, Pingret JL, Etievant M, Boucraut-Baralon C, Alongi A, et al. Hemoprotozoa of domestic animals in France: prevalence and molecular characterization. Vet Parasitol. 2009;159:73–6.
Kegler K, Nufer U, Alic A, Posthaus H, Olias P, Basso W. Fatal infection with emerging apicomplexan parasite Hepatozoon silvestris in a domestic cat. Parasit Vectors. 2018;11:1–5.
Wagner JE. Cytauxzoonosis in domestic cats (Felis domestica) in Missouri. J Am Vet Med Assoc. 1975;167:874.
Lloret A, Addie DD, Boucraut-Baralon C, Egberink H, Frymus T, Gruffydd-Jones T, et al. Cytauxzoonosis in cats: ABCD guidelines on prevention and management. J Feline Med Surg. 2015;17:637–41.
Greene CE, Meinkoth J, Kocan AA. Cytauxzoonosis. In: Greene CE, editor. Infectious diseases of the dog and the cat. St. Louis: Saunders; 2006. p. 716–22.
Ketz-Riley CJ, Reichard MV, Van Den Bussche RA, Hoover JP, Meinkoth J, Kocan AA. An intraerythrocytic small piroplasm in wild-caught Pallas’s cats (Otocolobus manul) from Mongolia. J Wildl Dis. 2003;39:424–30.
Reichard MV, Van Den Bussche RA, Meinkoth JH, Hoover JP, Kocan AA. A new species of Cytauxzoon from Pallas’ cats caught in Mongolia and comments on the systematics and taxonomy of piroplasmids. J Parasitol. 2005;91:420–6.
Criado-Fornelio A, Gónzalez-del-Rıo MA, Buling-Saraña A, Barba-Carretero JC. The “expanding universe” of piroplasms. Vet Parasitol. 2004;119:337–45.
Carli E, Trotta M, Chinelli R, Drigo M, Sinigoi L, Tosolini P, et al. Cytauxzoon sp infection in the first endemic focus described in domestic cats in Europe. Vet Parasitol. 2012;183:343–52.
Carli E, Trotta M, Bianchi E, Furlanello T, Caldin M, Pietrobelli M, et al. Cytauxzoon sp. infection in two free ranging young cats: clinicopathological findings, therapy and follow up. Türkiye Parazitol Derg. 2014;38:185.
Alho AM, Silva J, Fonseca MJ, Santos F, Nunes C, de Carvalho LM, et al. First report of Cytauxzoon sp. infection in a domestic cat from Portugal. Parasit Vectors. 2016;9:1–5.
Legroux JP, Halos L, René-Martellet M, Servonnet M, Pingret JL, Bourdoiseau G, et al. First clinical case report of Cytauxzoon sp. infection in a domestic cat in France. BMC Vet Res. 2017;13:1–7.
Nentwig A, Meli ML, Schrack J, Reichler IM, Riond B, Gloor C, et al. First report of Cytauxzoon sp infection in domestic cats in Switzerland: natural and transfusion-transmitted infections. Parasit Vectors. 2018;11:1–13.
Ebani VV, Guardone L, Marra F, Altomonte I, Nardoni S, Mancianti F. Arthropod-borne pathogens in stray cats from northern Italy: a serological and molecular survey. Animals. 2020;10:2334.
Panait LC, Stock G, Globokar M, Balzer J, Groth B, Mihalca AD, et al. First report of Cytauxzoon sp. infection in Germany: organism description and molecular confirmation in a domestic cat. Parasitol Res. 2020;119:3005–11.
Luaces I, Aguirre E, García-Montijano M, Velarde J, Tesouro MA, Sánchez C, et al. First report of an intraerythrocytic small piroplasm in wild Iberian lynx (Lynx pardinus). J Wildl Dis. 2005;41:810–5.
Millán J, Naranjo V, Rodríguez A, De La Lastra JP, Mangold AJ, De La Fuente J, et al. Prevalence of infection and 18S rRNA gene sequences of Cytauxzoon species in Iberian lynx (Lynx pardinus) in Spain. Parasitology. 2007;134:995–1001.
Millán J, Candela MG, Palomares F, Cubero MJ, Rodríguez A, Barral M, et al. Disease threats to the endangered Iberian lynx (Lynx pardinus). Vet J. 2009;182:114–24.
Meli ML, Cattori V, Martínez F, López G, Vargas A, Simón MA, et al. Feline leukemia virus and other pathogens as important threats to the survival of the critically endangered Iberian lynx (Lynx pardinus). PLoS ONE. 2009;4:e4744.
García-Bocanegra I, Dubey JP, Martínez F, Vargas A, Cabezón O, Zorrilla I, et al. Factors affecting seroprevalence of Toxoplasma gondii in the endangered Iberian lynx (Lynx pardinus). Vet Parasitol. 2010;167:36–42.
León CI, García-Bocanegra I, McCain E, Rodríguez E, Zorrilla I, Gómez AM, et al. Prevalence of selected pathogens in small carnivores in reintroduction areas of the Iberian lynx (Lynx pardinus). Vet Rec. 2017;180:252.
Gallusová M, Jirsová D, Mihalca AD, Gherman CM, D’Amico G, Qablan MA, et al. Cytauxzoon infections in wild felids from Carpathian-Danubian-Pontic space: further evidence for a different Cytauxzoon species in European felids. The J Parasitol. 2016;102:377–80.
Barandika JF, Espí A, Oporto B, Del Cerro A, Barral M, Povedano I, et al. Occurrence and genetic diversity of piroplasms and other apicomplexa in wild carnivores. Parasitol Open. 2016. https://doi.org/10.1017/pao.2016.4.
Veronesi F, Ravagnan S, Cerquetella M, Carli E, Olivieri E, Santoro A, et al. First detection of Cytauxzoon spp. infection in European wildcats (Felis silvestris silvestris) of Italy. Ticks Tick Borne Dis. 2016;7:853–8.
Naidenko SV, Erofeeva MN, Sorokin PA, Gershov SO, Yakovenko NP, Botvinovskaya AS, et al. The First Case of Cytauxzoon spp. in Russia: the parasite conquers Eurasia. Animals. 2022;12:593.
Panait LC, Mihalca AD, Modrý D, Juránková J, Ionică AM, Deak G, et al. Three new species of Cytauxzoon in European wild felids. Vet Parasitol. 2021;290:109344.
Willi B, Meli ML, Cafarelli C, Gilli UO, Kipar A, Hubbuch A, et al. Cytauxzoon europaeus infections in domestic cats in Switzerland and in European wildcats in France: a tale that started more than two decades ago. Parasit Vectors. 2022;15:1–7.
Hornok S, Boldogh SA, Takács N, Kontschán J, Szekeres S, Sós E, et al. Molecular epidemiological study on ticks and tick-borne protozoan parasites (Apicomplexa: Cytauxzoon and Hepatozoon spp.) from wild cats (Felis silvestris), Mustelidae and red squirrels (Sciurus vulgaris) in central Europe Hungary. Parasit Vectors. 2022;15:174.
Unterköfler MS, Harl J, Barogh BS, Spergser J, Hrazdilová K, Müller F, et al. Molecular analysis of blood-associated pathogens in European wildcats (Felis silvestris silvestris) from Germany. Int J Parasitol Parasites Wildl. 2022;19:128–37.
Daskalaki AA, Ionică AM, Jeetah K, Gherman CM, Mihalca AD. Molecular confirmation of Hepatozoon canis in Mauritius. Acta Trop. 2018;17:116–7.
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–9.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9.
Lempereur L, Beck R, Fonseca I, Marques C, Duarte A, Santos M, et al. Guidelines for the detection of Babesia and Theileria parasites. Vector Borne Zoonotic Dis. 2017;17:51–65.
Uilenberg G, Gray J, Kahl O. Research on Piroplasmorida and other tick-borne agents: are we going the right way? Ticks Tick Borne Dis. 2018;9:860–3.
Allsopp MT, Allsopp BA. Molecular sequence evidence for the reclassification of some Babesia species. Ann NY Acad Sci. 2006;1081:509–17.
Hrazdilová K, Myśliwy I, Hildebrand J, Buńkowska-Gawlik K, Janaczyk B, Perec-Matysiak A. Paralogs vs. genotypes? Variability of Babesia canis assessed by 18S rDNA and two mitochondrial markers. Vet Parasitol. 2019;266:103–10.
Bosman AM, Penzhorn BL, Brayton KA, Schoeman T, Oosthuizen MC. A novel Babesia sp. associated with clinical signs of babesiosis in domestic cats in South Africa. Parasit Vectors. 2019;12:1–12.
Bosman AM, Oosthuizen MC, Venter EH, Steyl JC, Gous TA, Penzhorn BL. Babesia lengau associated with cerebral and haemolytic babesiosis in two domestic cats. Parasit Vectors. 2013;6:1–6.
Wong SS, Poon RW, Hui JJ, Yuen KY. Detection of Babesia hongkongensis sp. nov. in a free-roaming Felis catus cat in Hong Kong. J Clin Microbiol. 2010;50:2799–803.
Baneth G, Kenny MJ, Tasker S, Anug Y, Shkap V, Levy A, et al. Infection with a proposed new subspecies of Babesia canis, Babesia canis subsp. presentii, in domestic cats. J Clin Microbiol. 2004;42:99–105.
Panicker VP, Sreedharannair AK, Narayanan A, George S, Hameed SV. Molecular identification of a novel species, Babesia panickeri sp. nov., from a naturally infected domestic cat of India and its comparison with canine Babesia isolates. Acta Parasitol. 2020;65:913–8.
Alho AM, Lima C, Latrofa MS, Colella V, Ravagnan S, Capelli G, et al. Molecular detection of vector-borne pathogens in dogs and cats from Qatar. Parasit Vectors. 2017;10:1–5.
Do T, Kamyingkird K, Chimnoi W, Inpankaew T. Evaluation of hematological alteration of vector-borne pathogens in cats from Bangkok. Thailand BMC Vet Res. 2021;17:1–9.
Kelly PJ, Köster L, Li J, Zhang J, Huang K, Branford GC, et al. Survey of vector-borne agents in feral cats and first report of Babesia gibsoni in cats on St Kitts. West Indies BMC Vet Res. 2017;13:1–6.
Corduneanu A, Ursache TD, Taulescu M, Sevastre B, Modrý D, Mihalca AD. Detection of DNA of Babesia canis in tissues of laboratory rodents following oral inoculation with infected ticks. Parasit Vectors. 2020;13:1–7.
Corduneanu A, Sándor AD, Mihalca AD, Hrazdilová K, Modrý D, Hornok S. Molecular evidence of canine pathogens in tissues of European bats. In: Proceedings of the 17th International Bat Research Conference. Durban, South Africa. 2016. 50–51
Zanet S, Bassano M, Trisciuoglio A, Taricco I, Ferroglio E. Horses infected by piroplasms different from Babesia caballi and Theileria equi: species identification and risk factors analysis in Italy. Vet Parasitol. 2017;236:38–41.
Hornok S, Estók P, Kováts D, Flaisz B, Takács N, Szőke K, et al. Screening of bat faeces for arthropod-borne apicomplexan protozoa: Babesia canis and Besnoitia besnoiti-like sequences from Chiroptera. Parasit Vectors. 2015;8:1–6.
Földvári G, Široký P, Szekeres S, Majoros G, Sprong H. Dermacentor reticulatus: a vector on the rise. Parasit Vectors. 2016;9:1–29.
Persichetti MF, Pennisi MG, Vullo A, Masucci M, Migliazzo A, Solano-Gallego L. Clinical evaluation of outdoor cats exposed to ectoparasites and associated risk for vector-borne infections in southern Italy. Parasit Vectors. 2018;11:1–11.
Morganti G, Veronesi F, Stefanetti V, Di Muccio T, Fiorentino E, Diaferia M, et al. Emerging feline vector-borne pathogens in Italy. Parasit Vectors. 2019;12:1–9.
Mylonakis ME, Schreeg M, Chatzis MK, Pearce J, Marr HS, Saridomichelakis MN, et al. Molecular detection of vector-borne pathogens in Greek cats. Ticks Tick Borne Dis. 2018;9:171–5.
Criado-Fornelio A, Martinez-Marcos A, Buling-Sarana A, Barba-Carretero JC. Molecular studies on Babesia, Theileria and Hepatozoon in southern Europe: part II. Phylogenetic analysis and evolutionary history. Vet Parasitol. 2003;114:173–94.
Zintl A, Finnerty EJ, Murphy TM, de Waal T, Gray JS. Babesias of red deer (Cervus elaphus) in Ireland. Vet Res. 2011;42:1–6.
Bonnet S, Jouglin M, Malandrin L, Becker C, Agoulon A, Hostis l’M, et al. Transstadial and transovarial persistence of Babesia divergens DNA in Ixodes ricinus ticks fed on infected blood in a new skin-feeding technique. Parasitology. 2007;134:197–207.
Sgroi G, Iatta R, Veneziano V, Bezerra-Santos MA, Lesiczka P, Hrazdilová K, et al. Molecular survey on tick-borne pathogens and Leishmania infantum in red foxes (Vulpes vulpes) from southern Italy. Ticks Tick Borne Dis. 2021;12:101669.
Hrazdilová K, Červená B, Blanvillain C, Foronda P, Modrý D. Quest for the type species of the genus Hepatozoon–phylogenetic position of hemogregarines of rats and consequences for taxonomy. Syst Biodivers. 2021;9:622–31.
Acknowledgements
The authors are grateful to the veterinarians who have collaborated in this study: Bilţi Luciana, Bobu Cristian, Boncea Radu, Copos Teodora, Cotuţiu Vlad, Debreczeni Daiana, Seni Robert, Solyom Levente, Ţeculescu Adina and Udvari Margareta.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
LCP performed the study design, sample collection, DNA isolation, and molecular analyses, analysed and interpreted the data, and wrote the initial manuscript. AMI performed the molecular analyses, analysed and interpreted the data and revised the manuscript. CDC analysed and interpreted the data and revised the manuscript. MC, AMD, AMB and CM performed the sample collection and revised the manuscript. ADM performed the study design, supervised the study, acquired funding and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with legal regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Panait, L.C., Ionică, A.M., Cazan, C.D. et al. Apicomplexan haemoparasites in domestic cats in Romania. Parasites Vectors 16, 56 (2023). https://doi.org/10.1186/s13071-023-05683-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05683-7