Abstract
Background
Hard ticks (Ixodidae) are hematophagous ectoparasites that transmit various pathogens to a variety of hosts including humans. Transhumant herds have been involved in the spread of ticks and associated Rickettsia spp., and studies on this neglected topic have been unexplored in many regions including Pakistan. This study aimed to investigate ticks infesting transhumant herds of sheep (Ovis aries) and goats (Capra hircus) in district Shangla, Khyber Pakhtunkhwa, Pakistan.
Methods
Of the 144 examined animals, 112 hosts (68 sheep and 44 goats) of transhumant herds were infested by 419 ticks of different life stages including nymphs (105; 25%), males (58; 14%) and females (256; 61%). For molecular analyses, DNA was extracted from 64 collected ticks and subjected to PCR for the amplification of tick 16S rDNA and ITS2 partial sequences and for the amplification of rickettsial gltA and ompA gene sequences.
Results
All tick specimens were identified as Ixodes kashmiricus based on morphological features. The obtained 16S rDNA and ITS2 sequences showed 95.7% and 95.3% identity, respectively, with Ixodes kazakstani reported from Kyrgyzstan. In the phylogenetic tree, the sequences clustered with members of the Ixodes ricinus species complex, including I. kazakstani and Ixodes apronophorus. Additionally, rickettsial gltA and ompA partial sequences were 99.7% identical to Rickettsia sp. endosymbiont of Ixodes spp. from Panama and Costa Rica and 99.2% with Rickettsia endosymbiont from the USA. Phylogenetically, the rickettsial gltA and ompA partial sequences from I. kashmiricus clustered with various haplotypes of Rickettsia endosymbiont, which were sister cladded to Rickettsia monacensis.
Conclusions
This is the first genetic report of I. kashmiricus and associated Rickettsia sp. Large-scale tick surveillance studies across the country are needed to investigate Ixodes ticks and associated pathogens.
Graphical Abstract

Similar content being viewed by others
Background
Hard ticks (Ixodida: Ixodidae) are hematophagous ectoparasites that adversely affect vertebrate hosts including amphibians, reptiles, birds and mammals, including humans [1,2,3,4]. Among ticks, several species spread and adopt to novel geographical regions with transhumant herds [5]. Globally, 18 subgenera comprised of 255 Ixodes species have been described [4, 6, 7]. Among them, subgenus Ixodes have closely related species, such as Ixodes nipponensis, Ixodes kazakstani, Ixodes kashmiricus, Ixodes scapularis, Ixodes hyatti, Ixodes redikorzevi, Ixodes hymalayensis, Ixodes nuttallianus, Ixodes ricinus, Ixodes persulcatus, Ixodes pacificus, Ixodes pavlovskyi, Ixodes inopinatus, Ixodes minor and Ixodes granulatus, included in the I. ricinus species complex distributed mostly in the Oriental, Nearctic and Palearctic regions [4, 7,8,9,10,11]. Evolutionarily, Ixodes ticks are considered a more basal lineage of the Ixodidae family [6].
Ixodes ticks mainly infest small ruminants, rodents, birds, carnivores and humans in cold deciduous, mixed forests and vegetative regions where rainfall is abundant and relatively more humid (> 80% relative humidity) [1, 6,7,8,9,10,11,12,13,14,15]. Environmental fluctuations, such as low temperature at high latitudes have been considered to limit the spread of Ixodes ticks and to a certain extent become a severe threat to novel hosts [4, 5, 16, 17].
A number of tick-borne rickettsial bacteria have been reported in Ixodes ticks: Rickettsia helvetica, Rickettsia monacensis, Rickettsia japonica, Rickettsia sibirica, Rickettsia buchneri, Rickettsia cooleyi, “Candidatus Rickettsia vini”, “Candidatus Rickettsia mendelii”, “Candidatus Rickettsia uralica”, “Candidatus Rickettsia thierseensis” and other Rickettsia spp. in different regions. Many of these Rickettsia spp. agents are probably endosymbionts of Ixodes spp., whereas a few have been associated with human rickettsiosis [2, 11, 18,19,20,21,22,23,24,25,26]. Most of the Ixodes-related Rickettsia spp. belong to more basal groups of rickettsiae, while a few are part of the Spotted Fever group [2].
Pakistan is an agricultural country where livestock has an important place in the national economy. The climate and geography of Pakistan offer suitable conditions for the establishment of a variety of tick species, likely increasing the risk of transmission of tick-borne pathogens [3, 27,28,29]. The tick fauna of Pakistan includes species of the argasid genera Argas, Carios and Ornithodoros, the ixodid Metastriata genera Rhipicephalus, Haemaphysalis, Hyalomma, Amblyomma and Nosomma, and the ixodid Prostriata genus Ixodes [1, 3, 27, 28, 30,31,32,33,34]. For the latter genus, only two species, Ixodes hyatti and Ixodes redikorzevi, have been identified in Pakistan, both based only on morphology [35,36,37]. Studies on Rickettsia spp. associated with Prostriata ticks infesting transhumant herds have been neglected in the country.
In this study, we report I. kashmiricus ticks infesting transhumant herds (sheep, Ovis aries; goats, Capra hircus) and screened the collected ticks for Rickettsia spp. in Shangla district, Khyber Pakhtunkhwa (KP), Pakistan. Genetic characterization of an Ixodes species from Pakistan was performed for the first time to our knowledge.
Methods
Study area
Shangla district (34°47′32.1"N, 72°41′26.4"E) is situated 331 km northwest of Islamabad, the capital of Pakistan, covering 1586 km2. Shangla is surrounded by the Kohistan district to the north, Torghar and Battagram to the east, Swat to the west and Buner to the south. This is a hilly and mountainous region, with an elevation of 3440 m above sea level. Temperature ranges between 17 °C and 30 °C in summer and − 5 °C to 10 °C in winter and annual precipitation remains above 1200 mm (worldweatheronline.com). Ticks were collected from four mountainous regions in two tehsils (subdivision of a district), including Puran (Singoor, Towa and Garai Sar) and Chakesar (Koo) in Shangla district. “Google Earth Pro v 7.3” was used to find the exact geographic coordinates of collection sites, and the study map was designed via ArcGIS v 10.3.1 (Fig. 1).
Tick collection and preservation
Tick samples were collected from transhumant herds of sheep and goats from February 2019 to November 2021. Tweezers were used to safely remove tick species without any damage. Relevant information such as date of collection, location, temperature, humidity and geo-coordinates of collection points were noted. The collected specimens were cleaned with distilled water and preserved in 100% ethanol.
Morphological identification
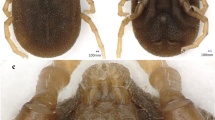
Collected ticks were identified by using a Keyence microscope (Illinois, VHX 900F, USA, Itasca) with 50–200× magnification following the current literature [8, 38]. The specimens were photographed via scanning electron microscope (JSM5910, JEOL, Japan) in Centralized Resource Laboratory (CRL) at the University of Peshawar (Fig. 2).
Female (upper row) and male (lower row) of Ixodes kashmiricus. A: female dorsal (I: scutal margin, II: marginal groove), B: female dorsal (I: posterior margin of basis capituli), C: female ventral (I: genital aperture, II: anal groove), D: female ventral (I: coxa I internal spur), E: male dorsal (I: capitulum, II: marginal groove), F: male dorsal (I: posterior margin of basis capituli), G: male ventral (I: anal groove), H: male ventral (I: coxa I internal spur)
DNA extraction and PCR
A total of 64 tick specimens (32 nymphs, 17 males and 15 females) were selected randomly from hosts of all regions and were individually subjected to the extraction of genomic DNA by using the phenol-chloroform method [39]. The concentration of the extracted DNA samples was quantified by using Nanodrop (Nano-Q, Optizen, Daejeon, South Korea).
The extracted DNA was used for the amplification of fragments of the mitochondrial 16S ribosomal RNA gene and the nuclear second internal transcribed spacer (ITS2). Conventional PCR assays were performed in microtubes containing 20 µl reaction mixture containing: 1 µl (10 µM) of each (forward and reverse) primer (Table 1), 2 µl DNA (50–100 ng/µl), 4 µl PCR water (nuclease free) and 12 µl DreamTaq Green MasterMix (2X) (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Conditions for thermocycling were: initial denaturation was 95 °C for 3 min, followed by 35 cycles of 95 °C for 30 s, 55 °C for 30 s (16S rRNA), 55 °C for 60 s (ITS2) and extension 72 °C for 30 s and final extension at 72 °C for 7 min. The PCR contained negative (PCR water) and positive (Rhipicephalus microplus DNA) control. The PCR products were run in a 2% agarose gel, dyed with 4 µl ethidium bromide and observed in Gel Documentation System (BioDoc-It™ Imaging Systems UVP, LLC, Upland, CA, USA).
Screening for rickettsial DNA
Extracted DNA was used for the detection of rickettsial DNA by the amplification of a short fragment of the citrate synthase (gltA) gene using specific primers (Table 1) in a real-time PCR (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA) [42]. Positive specimens for rickettsial DNA were further screened by the amplification of a fragment of the rickettsial 190-kDa outer membrane protein (ompA) and a larger fragment of the rickettsial gltA gene using specific primers (Table 1) in conventional PCR assays (BIOER, China). PCR reaction mixture of 20 µl contained 1 µl (10 µM) of each primer (Table 1), 2 µl extracted DNA (50–100 ng/µl), 4 µl PCR water and 12 µl DreamTaq Green MasterMix (2×) (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cycling conditions for the amplification of rickettsial DNA were: initial denaturation at 95 °C for 3 min, followed by 40 cycles at 95 °C for 20 s, 56 °C for 30 s (gltA), 55 °C for 30 s (ompA), extension at 72 °C for 30 s and final extension at 72 °C for 7 min. In each PCR reaction, PCR water and Rickettsia massiliae DNA were used as a negative and positive control, respectively. PCR products were electrophoresed in 2% agarose gel, and the results were visualized under ultraviolet light using a Gel Documentation System (UVP BioDoc-It Imaging system, UVP, LLC, Upland, CA, USA).
All PCR-positive products of ticks (16S rRNA and ITS2) and associated Rickettsia (gltA and ompA) were purified by ExoSAP-IT Kit (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s protocol. Purified PCR products were sent for DNA sequencing bidirectionally (Macrogen Inc., Seoul, South Korea) using the same primers used in the PCR amplification.
DNA sequencing and phylogenetic analyses
The obtained sequences were trimmed to remove primer sequences and contaminated and poor-quality nucleotides in the flanks by using SeqMan V 5.0 (DNASTAR Inc., Madison, WI, USA). The purified or cropped sequences were subjected to a Basic Local Alignment Search Tool (BLAST) [44] at the National Center for Biotechnology Information (NCBI). Homologous sequences along with ancestor species as an outgroup were downloaded in FASTA format based on their high percentage identity. The downloaded sequences were subjected to ClustalW Multiple alignment [45] in BioEdit Sequence Alignment Editor V 7.0.5 (Raleigh, NC, USA) [46]. The phylogenetic tree was constructed separately for tick sequences (16S rDNA and ITS2) and Rickettsia sequences (gltA and ompA) by Molecular Evolutionary Genetics Analysis (MEGA-X), aligned by the MUSCLE algorithm [47], and used the maximum likelihood method based on the Tamura-Nei model with bootstrapping value at 1000 replications [48].
Results
Tick morphological identification
A total of 419 hard ticks (Table 2) were collected and morphologically identified as I. kashmiricus, a member of the I. ricinus species complex. During collection, no other tick species were found infesting the small ruminants.
Briefly, morphological identification of adult female ticks relies on a shorter inner spur on coxa I; all coxae have a short external spur; scutum is covered with uniform, larger punctuations; setae of the scutum are sparse. Scapulae have the shape of slightly long or pointed teeth. The posterior valve is convex. The stigmas are irregularly oval and elongated (Fig. 2A–D).
Males lack auriculae, idiosoma broadly oval, the posterior pair of teeth of the hypostome is relatively short and poorly developed, and the posterior margin of the basis capituli forms a rounded line on the ventral side. Punctuations on the conscutum are smoothed, larger, deepened punctuations. Conscutum is flattened with long setae; the longest setae are located on the lateral parts of the conscutum and on the marginal carina. Scapulae are small and lateral carinae absent. The stigmas are relatively small, roundish or oval, elongated in the longitudinal direction (Fig. 2E–H).
Nymphs contained a broadly oval, rounded scutum with several setae, posterior margin slightly kinked; alloscutum setae longer than those of the scutum. Hypostome widest at base, wedge-shaped, narrowed, apex rounded; shorter than in I. pavlovskyi, but longer than in I. kazakstani, which are the main features of I. kashmiricus.
Hosts
Overall, 144 small ruminants (74 sheep and 70 goats) were examined in four villages (38 animals in Singoor, 37 in Koo, 30 in Towa and 39 in Garai Sar) of Shangla district (Table 2). Of them, 112 (78%) hosts including 68 (92%) sheep and 44 (63%) goats were infested by 419 I. kashmiricus of different life stages. Among them, sheep (68 out of 74 hosts) were more infested, with a significantly higher (P < 0.01) prevalence. Moreover, the number of infested sheep was higher than infested goats in each of the four sampled locations (Singoor, Koo, Towa, Garai Sar) (Table 2).
Molecular analyses of ticks
A total of 64 tick specimens were tested by molecular analyses and generated amplicons of the expected size through PCR assays targeting the 16S rRNA and ITS2 genes. Partial sequences of the 16S rDNA mitochondrial gene were generated for 31 nymphs, 16 males and 12 females, which were identical to each other; the remaining PCR-positive ticks (1 nymph, 1 male, 3 females) did not generate high-quality sequences. By BLAST analysis, the partial 16S rDNA sequence (415 bp) of I. kashmiricus from Pakistan was most similar (95.7%) to the sequences of I. kazakstani from Kyrgyzstan (MF095803-MF095806) and then 94.7% similar to several sequences of Ixodes apronophorus from Russia (MH790193-MH790198). A consensus partial sequence of the ITS2 nuclear gene (754 bp) was generated for five ticks, which by BLAST analysis was the most similar (95.3%) to the sequences of I. kazakstani from Kyrgyzstan (MF095819-MF095822) and then 85.3–85.8% to some sequences of I. apronophorus reported from Russia (MH784894-MH784898).
Among the 64 ticks tested by molecular analyses, rickettsial DNA was detected in 48 (75%) ticks (22 nymphs, 11 males, 15 females), including specimens collected from sheep or goats and from each of the four locations (Table 2). These ticks generated identical gltA sequences, which by BLAST analysis were most similar (99.7%; 349/350 bp) to Rickettsia sp. endosymbiont of Ixodes boliviensis from Panama (MW699695) and Costa Rica (KU529481) and Ixodes tapirus from Panama (MW699691). The same tick specimens generated identical ompA sequences, which by BLAST analysis were the most similar (99.2%; 366/369 bp) to Rickettsia sp. endosymbiont of Ixodes pacificus (KX505847) from the USA.
Phylogenetic analyses
A total of 27 and 30 partial sequences of the 16S rDNA and ITS2, respectively, of Ixodes spp. were downloaded from GenBank. In the case of Rickettsia spp., 30 and 33 sequences of gltA and ompA genes, respectively, were downloaded and used in the alignments.
In the phylogenetic tree inferred from partial sequences of the 16S rDNA gene, I. kashmiricus from Pakistan (MW578839) clustered with I. kazakstani (MF095806) and I. apronophorus (MH790195) reported from Kyrgyzstan and Russia, respectively (Fig. 3). Similarly, in the ITS2 phylogenetic tree, the partial sequence of Pakistan (OM987271) clustered again with I. kazakstani (MF095821) and I. apronophorus (MH784894, MH784896 and MG542676) reported from Kyrgyzstan and Russia, respectively (Fig. 4). In both phylogenetic trees, the 16S rDNA and ITS2 partial sequences clustered within a large clade composed by Ixodes species of the I. ricinus species complex.
Maximum likelihood phylogenetic tree based on 16S rDNA partial sequence of Ixodes kashmiricus. The Ixodes trianguliceps, Ixodes uriae and Ixodes ovatus sequences were taken as an outgroup. The levels of bootstrap support (> 50%) for phylogenetic groupings are given at each node; the accession numbers are followed by the species name and location. The obtained sequence was represented by black circle (MW578839)
Maximum Likelihood phylogenetic tree based on ITS2 partial sequence of Ixodes kashmiricus. The Ixodes turdus and Ixodes ovatus sequences were taken as an outgroup. The levels of bootstrap support (> 50%) for phylogenetic groupings are given at each node; the accession numbers are followed by the species name and location. The obtained sequence was represented by black circle (OM987271)
In the phylogenetic trees inferred from partial sequences of the rickettsial genes, the gltA (MW592991) and ompA (ON125553) partial sequences from I. kashmiricus in Pakistan formed a clade with different haplotypes of Rickettsia endosymbionts of Ixodes spp. from Costa Rica, Panama and the USA, which were sister to R. monacensis, a species associated primarily with I. ricinus (Figs. 5 and 6). These sequences were sister or basal to the Rickettsia species typically belonging to the spotted fever group.
Maximum likelihood phylogenetic tree based on gltA sequence for Rickettsia sp. from Ixodes kashmiricus. The Rickettsia canadensis sequences were taken as an outgroup. The levels of bootstrap support (> 50%) for phylogenetic groupings are given at each node; the accession numbers are followed by the species name and location. The obtained sequence was represented by a black circle (MW592991)
Maximum likelihood phylogenetic tree based on ompA sequence of Rickettsia sp. from Ixodes kashmiricus. The Rickettsia australis sequence was taken as an outgroup. The levels of bootstrap support (> 50%) for phylogenetic groupings are given at each node; the accession numbers are followed by the species name and location. The obtained sequence was represented by a black circle (ON125553)
Discussion
Ixodes ticks have been observed as vector reservoirs for tick-borne relapsing fever, tick-borne encephalitis, Lyme borreliosis and babesiosis [2, 11, 21, 49]. The diversity of Ixodes ticks reported in various regions is still unexplored in Pakistan. In Pakistan, the local population faces large economic and health problems due to the lack of knowledge about ticks and associated disease-causing agents. Based on morphology, I. hyatti from Peshawar and I. redikorzevi from Kaghan and an Ixodes sp. identified at genus level from Swat have been reported in Pakistan [35,36,37]. Until the present study, Ixodes species had never been genetically identified in Pakistan. In this study, ticks collected from transhumant herds in district Shangla were morphologically and genetically characterized as I. kashmiricus and associated Rickettsia sp. for the first time to our knowledge.
During this study, large ruminants such as cattle, Asian water buffaloes and equids were also observed (data not shown); however, I. kashmiricus was found infesting only small ruminants (sheep and goats) of transhumant herds. Among the small ruminants, the significantly high infestation on sheep suggests the preference of this tick to sheep as a suitable host. Many Ixodes spp. of the I. ricinus species complex are associated with small ruminants [1, 8, 10], which might graze in areas with favorable environmental conditions for the survival of ticks [6, 13]. Likewise, the district Shangla is a hilly and high mountainous region having mild summer (17 °C to 30 °C), cold winter (− 5 °C to 10 °C), relative humidity approximately 80%, heavy rainfall in all seasons (spring, summer, autumn and winter) and annual precipitation above 1200 mm. It is important to mention that these transhumant herds annually migrate to northern regions between April and September at the frontiers of the country in Gilgit Baltistan, Kohistan (Dassu) and Chitral. These regions share borders with Afghanistan to the north, China to the northeast, India (Jammu and Kashmir) to the southeast and AJK (Azad Jammu and Kashmir) to the south of Pakistan. Moreover, these boundaries have been linked to the former Union of Soviet Socialist Republics (USSR). The USSR countries have been associated with the distribution of some ancestral Ixodes species because of the occurrence of favorable environmental conditions [8, 9].
To date, Ixodes ticks have not been genetically characterized from Pakistan. The genetic identification of the collected ticks validated the morphological compatibility, as the 16S rDNA and ITS2 sequences clustered in a phylogenetic tree with the sequences of I. kazakstani and I. apronophorus (I. ricinus species complex) reported from Kyrgyzstan and Russia, respectively. The topology of the phylogenetic trees of I. kashmiricus were compared with the species of the I. ricinus group [7, 50]. The phylogenetic tree based on the 16S rDNA and ITS2 partial sequences were congruent and confirmed that I. kashmiricus belongs to the I. ricinus species complex. In the pairwise alignment, the 16S rDNA and ITS2 partial sequences of I. kashmiricus showed a minimum nucleotide difference of 18 and 27 bp, respectively, which showed 5% genetic difference with I. kazakstani followed by I. apronophorus of the I. ricinus species complex.
Ixodes kashmiricus was described by Pomerantzev in 1948 and has been reported only from India-Jammu and Kashmir, Vardvan-Maru River and the northern stream of Chenab River region in India [8, 10]. This location is ≈300 km away from the present locality in Pakistan. Until the present study, there were no molecular genetic data or DNA sequence for I. kashmiricus. Herein, we genetically characterized I. kashmiricus based on 16S rDNA and ITS2 partial sequences for the first time to our knowledge; they shared high identity with two available sequences of I. ricinus species complex—I. kazakstani and I. apronophorus.
Ixodes ticks are commonly reported as infected by rickettsial endosymbionts, although a few Ixodes species have also been implicated as a vector of rickettsial agents, like for example R. australis, R. monacensis and R. helvetica [2, 18, 23, 43]. The present study reported Rickettsia sp. in I. kashmiricus that grouped with Rickettsia endosymbionts of Ixodes spp. of the I. ricinus species complex. The pathogenicity of the Rickettsia sp. detected in this study remains to be investigated given the importance of this bacterial group as an agent of emerging infectious tick-borne diseases. However, the high infection rate (75%) of Rickettsia sp. in I. kashmiricus is compatible with an endosymbiont.
Conclusions
This study genetically characterized I. kashmiricus and associated Rickettsia sp. for the first time to our knowledge. Morphological and phylogenetic analyses of the collected ticks showed close resemblance to I. kashmiricus and clustered with members of the I. ricinus species complex, including I. kazakstani and I. apronophorus. A Rickettsia sp. was detected in I. kashmiricus and shown to be genetically related to Rickettsia sp. endosymbiont of other Ixodes spp. of the I. ricinus species complex. These results may assist our understanding of the epidemiology of Ixodes ticks and associated Rickettsia sp. and reinforce country-wide tick surveillance programs.
Availability of data and materials
The datasets to support the conclusions of this article are given within the article.
Abbreviations
- BLAST:
-
Basic Local Alignment Search Tool
- CRL:
-
Centralized Resource Laboratory
- gltA :
-
Citrate synthase
- ITS2:
-
Internal transcribed spacer 2
- KP:
-
Khyber Pakhtunkhwa
- MEGA:
-
Molecular evolutionary genetics analysis
- NCBI:
-
National Center for Biotechnology Information
- ompA :
-
Outer membrane protein
- PCR:
-
Polymerase chain reaction
- rDNA:
-
Ribosomal DNA
References
Hoogstraal H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol. 1979;15:307–417.
Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, Kernif T, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev. 2013;26:657–702.
Ali A, Khan MA, Zahid H, Yaseen PM, Khan MQ, Nawab J, et al. Seasonal dynamics, record of ticks infesting humans, wild and domestic animals, and molecular phylogeny of Rhipicephalus microplus in Khyber Pakhtunkhwa Pakistan. Front Physiol. 2019;10:793.
Guglielmone AA, Petney TN, Robbins RG. Ixodidae (Acari: Ixodoidea): descriptions and redescriptions of all known species from 1758 to December 31, 2019. Zootaxa. 2020;4871:1–322.
Gray JS, Dautel H, Estrada-Peña A, Kahl O, Lindgren E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip Perspect Infect Dis. 2009;2009:1–12.
Beati L, Klompen H. Phylogeography of ticks (Acari: Ixodida). Annu Rev Entomol. 2018;64:379–97.
Rar V, Yakimenko V, Tikunov A, Vinarskaya N, Tancev A, Babkin I, et al. Genetic and morphological characterization of Ixodes apronophorus from Western Siberia. Russia Ticks Tick Borne Dis. 2020;11:101284.
Pomerantzev BI. New ticks of the family Ixodidae. Parazitol Sborn Zool Inst Akad Nauk SSSR. 1948;10:20–4 (in Russian).
Filippova NA. Forms of sympatry and possible ways of microevolution of closely related species of the group Ixodes ricinus-persulcatus (Ixodidae). Acta Zool Litu. 2002;12:215–27.
Filippova NA. Type specimens of argasid and ixodid ticks (Ixodoidea: Argasidae, Ixodidae) in the collection of the Zoological Institute, Russian Academy of Sciences (St. Petersburg). Entomol Rev. 2008;88:1002–11.
Chitimia-Dobler L, Rieß R, Kahl O, Wölfel S, Dobler G, Nava S, et al. Ixodes inopinatus occurring also outside the Mediterranean region. Ticks Tick Borne Dis. 2018;9:196–200.
Wilhelmsson P, Lindblom P, Fryland L, Nyman D, Jaenson TG, Forsberg P, et al. Ixodes ricinus ticks removed from humans in Northern Europe: seasonal pattern of infestation, attachment sites and duration of feeding. Parasit Vectors. 2014;6:1–11.
Gilbert L, Aungier J, Tomkins JL. Climate of origin affects tick (Ixodes ricinus) host-seeking behavior in response to temperature: implications for resilience to climate change? Ecol Evol. 2014;4:1186–98.
Estrada-Peña A, Mihalca AD, Petney TN. Ticks of Europe and North Africa: a guide to species identification. Cham: Springer; 2018. p. 404.
Cicculli V, Capai L, Quilichini Y, Masse S, Fernández-Alvarez A, Minodier L, et al. Molecular investigation of tick-borne pathogens in ixodid ticks infesting domestic animals (cattle and sheep) and small rodents (black rats) of Corsica. France Ticks Tick Borne Dis. 2019;10:606–13.
Lindgren E, Tälleklint L, Polfeldt T. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ Health Perspect. 2000;10:119–23.
Pfäffle M, Littwin N, Muders SV, Petney TN. The ecology of tick-borne diseases. Int J Parasitol. 2013;43:1059–77.
Balraj P, Karkouri KE, Vestris G, Espinosa L, Raoult D, Renesto P. RickA expression is not sufficient to promote actin-based motility of Rickettsia raoultii. PLoS ONE. 2008;3:e2582.
Billings AN, Teltow GJ, Weaver SC, Walker DH. Molecular characterization of a novel Rickettsia species from Ixodes scapularis in Texas. Emerg Infect Dis. 1998;4:305.
Cumbie AN, Walters EL, Gaff HD, Hynes WL. First report of Candidatus Rickettsia mendelii in Ixodes brunneus from the United States. Ticks Tick Borne Dis. 2020;11:101309.
Rizzoli A, Silaghi C, Obiegala A, Rudolf I, Hubálek Z, Földvári G, et al. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: new hazards and relevance for public health. Front Public Health. 2014;2:251.
Król N, Obiegala A, Kretschmar FM, Hamel D, Pfeffer M. Tick-borne pathogens in the European polecat, Mustela putorius and in attached Ixodes hexagonus ticks from Germany. Ticks Tick Borne Dis. 2019;10:594–7.
Kurtti TJ, Felsheim RF, Burkhardt NY, Oliver JD, Heu CC, Munderloh UG. Rickettsia buchneri sp. nov, a rickettsial endosymbiont of the blacklegged tick Ixodes scapularis. Int J Syst Evol. 2015;65:965.
Alowaysi M, Chen J, Stark S, Teague K, LaCourse M, Proctor J, et al. Isolation and characterization of a Rickettsia from the ovary of a Western black-legged tick Ixodes pacificus. Ticks Tick Borne Dis. 2019;10:918–23.
Grochowska A, Milewski R, Pancewicz S, Dunaj J, Czupryna P, Milewska AJ, et al. Comparison of tick-borne pathogen prevalence in Ixodes ricinus ticks collected in urban areas of Europe. Sci Rep. 2020;10:1–9.
Igolkina Y, Rar V, Yakimenko V, Tikunov A, Tikunova N. “Candidatus Rickettsia uralica” and “Candidatus Rickettsia thierseensis” are genetic variants of one species. Ticks Tick Borne Dis. 2022;13:101933.
Karim S, Budachetri K, Mukherjee N, Williams J, Kausar A, Hassan MJ, et al. A study of ticks and tick-borne livestock pathogens in Pakistan. PLOS Negl Trop Dis. 2017;11:e0005681.
Ali A, Shehla S, Zahid H, Ullah F, Zeb I, Ahmed H, et al. Molecular survey and spatial distribution of Rickettsia spp. In ticks infesting free-ranging wild animals in Pakistan (2017–2021). Pathogens. 2022;11:162.
Ali A, Zahid H, Zeb I, Tufail M, Khan S, Haroon M, et al. Risk factors associated with tick infestations on equids in Khyber Pakhtunkhwa, Pakistan, with notes on Rickettsia massiliae detection. Parasit Vectors. 2021;14:1–12.
Zahid H, Muñoz-Leal S, Khan MQ, Alouffi AS, Labruna MB, Ali A. Life cycle and genetic identification of Argas persicus infesting domestic fowl in Khyber Pakhtunkhwa. Pakistan Front Vet Sci. 2021;8:302.
Kamran K, Ali A, Villagra C, Siddiqui S, Alouffi AS, Iqbal A. A cross-sectional study of hard ticks (acari: Ixodidae) on horse farms to assess the risk factors associated with tick-borne diseases. Zoonoses Public Health. 2021;68:247–62.
Kamran K, Ali A, Villagra CA, Bazai ZA, Iqbal A, Sajid MS. Hyalomma anatolicum resistance against ivermectin and fipronil is associated with indiscriminate use of acaricides in southwestern Balochistan. Pakistan Parasitol Res. 2021;120:15–25.
Aiman O, Ullah S, Chitimia-Dobler L, Nijhof AM, Ali A. First report of Nosomma monstrosum ticks infesting Asian water buffaloes (Bubalus bubalis) in Pakistan. Ticks Tick Borne Dis. 2022;13:101899.
Ali A, Numan M, Khan M, Aiman O, Muñoz-Leal S, Chitimia-Dobler L, et al. Ornithodoros (Pavlovskyella) ticks associated with a Rickettsia sp. in Pakistan. Parasit Vectors. 2022;15:1–3.
Begum F, Wisseman CL Jr, Casals J. Tick-borne viruses of west Pakistan: II. Hazara virus, a new agent isolated from Ixodes redikorzevi ticks from the Kaghan valley, w. Pakistan. Am J Epidemiol. 1970;92:192–4.
Begum F, Wisseman CL, Traub R. Tick-borne viruses of West Pakistan: I. Isolation and general characteristics. Am J Epidemiol. 1970;92:180–91.
Clifford CM, Hoogstraal H, Kohls GM. Ixodes hyatti, n sp., and I. shahi, n. sp. (Acarina: Ixodidae), parasites of pikas (Lagomorpha: Ochotonidae) in the Himalayas of Nepal and West Pakistan. J Med Entomol. 1971;8:430–8.
Filippova NA. Ixodid ticks (Ixodinae). Fauna USSR New Ser 4 (4). Nauka Moscow Leningrad. 1977;1:3–22.
Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. New York: Cold Spring Harbor Laboratory Press; 1989.
Mangold AJ, Bargues MD, Mas-Coma S. Mitochondrial 16S rDNA sequences and phylogenetic relationships of species of Rhipicephalus and other tick genera among Metastriata (Acari: Ixodidae). Parasitol Res. 1998;84:478–84.
Zahler M, Gothe R, Rinder H. Genetic evidence against a morphologically suggestive conspecificity of Dermacentor reticulatus and D. marginatus (Acari: Ixodidae). Int J Parasitol. 1995;25:1413–9.
Labruna MB, Whitworth T, Bouyer DH, McBride J, Camargo LMA, Camargo EP, et al. Rickettsia bellii and Rickettsia amblyommii in Amblyomma ticks from the state of Rondônia, Western Amazon. Brazil J Med Entomol. 2004;41:1073–81.
Roux V, Fournier PE, Raoult D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J Clin Microbiol. 1996;34:2058–65.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10.
Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80.
Hall T, Biosciences I, Carlsbad C. BioEdit: an important software for molecular biology. GERF Bull Biosci. 2011;2:60–1.
Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–7.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA-X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9.
Kjær LJ, Soleng A, Edgar KS, Lindstedt HEH, Paulsen KM, Andreassen ÅK, et al. Predicting and mapping human risk of exposure to Ixodes ricinus nymphs using climatic and environmental data, Denmark, Norway, and Sweden, 2016. Euro Surveill. 2019;4:1800101.
Kovalev SY, Fedorova SZ, Mukhacheva TA. Molecular features of Ixodes kazakstani: first results. Ticks Tick Borne Dis. 2018;9:759–61.
Acknowledgements
We appreciate the financial support offered by the Pakistan Science Foundation (PSF) and Higher Education Commission (HEC) of Pakistan.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AA and MN designed the study. MN, AA, SZS, NI and MA collected the ticks. AA, MBL, NI and MN performed molecular work and phylogenetic analyses. LCD contributed to the morphological identification, photographed the tick and read the manuscript. MN carried out the statistics and designed the location map. AA acquired the budget. The authors carried out a critical review and authorized the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present work was approved by Advanced Study Research Board (ASRB) (Dir/A&R/AWKUM/2018/1410) members, Abdul Wali Khan University Mardan, Khyber Pakhtunkhwa, Pakistan. Consent was obtained from the animal's owners for screening of the animals and for tick collection.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Numan, M., Islam, N., Adnan, M. et al. First genetic report of Ixodes kashmiricus and associated Rickettsia sp.. Parasites Vectors 15, 378 (2022). https://doi.org/10.1186/s13071-022-05509-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05509-y