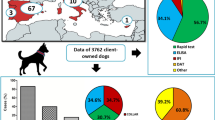
Abstract
Background
Canine vector-borne disease (CVBD) has been an area of increasing interest in Europe over the last few decades, and there have been changes in the prevalence and distribution of many of these diseases. Monitoring CVBD infections in Europe is often done by individual countries, but aggregated data for the European countries are helpful to understand the distribution of CVBDs.
Methods
We used an extensive retrospective database of results from point-of-care rapid enzyme-linked immunosorbent assay (ELISA) tests on dogs across Europe to identify distribution and seropositivity in animals tested for selected CVBDs (Anaplasma spp., Ehrlichia spp., Borrelia burgdorferi, Leishmania spp., and Dirofilaria immitis) from 2016 through 2020. Geographic distribution of positive tests and relative percent positive values were mapped by the Nomenclature of Territorial Units for Statistics classification for regions with sufficient test results for reporting.
Results
A total of 404,617 samples corresponding to 1,134,648 canine results were available from dogs tested in 35 countries over the 5-year study period. Over this period the number of test results per year increased whereas test positivity decreased. Leishmania spp. had the largest increase in total test results from 25,000 results in 2016 to over 60,000 results in 2020. Test positivity for Leishmania spp. fell from 13.9% in 2016 to 9.4% in 2020. Test positivity fell for Anaplasma spp. (7.3 to 5.3%), Ehrlichia spp. (4.3 to 3.4%), and Borrelia burgdorferi (3.3 to 2.4%). Dirofilaria immitis test positivity trended down with a high of 2.7% in 2016 and low of 1.8% in 2018. Leishmania spp. test positivity was highest in endemic areas and in several non-endemic countries with low numbers of test results. Co-positivity rates were significantly higher than expected for all pathogen test positive pairs except for Ehrlichia spp. with Borrelia burgdorferi and D. immitis with Borrelia burgdorferi.
Conclusions
This study represents the largest data set on CVBD seropositivity in Europe to date. The increase in the number of test results and decreasing test positivity over the study period may reflect changes in testing behavior and increased screening of healthy animals. The Europe-wide mapping of CVBD provides expected test positivity that can help inform veterinarians’ decisions on screening and improve prevention and identification of these important, sometimes zoonotic, diseases.
Graphical Abstract

Similar content being viewed by others
Background
Canine vector-borne disease (CVBD) has been an area of increasing interest in Europe over the last few decades, and there have been changes in the prevalence and distribution of many of these diseases [1,2,3,4,5,6]. Many CVBDs (including Borrelia burgdorferi complex, Anaplasma phagocytophilum and Anaplasma platys, Ehrlichia canis and Ehrlichia ewingii, Dirofilaria immitis and Dirofilaria repens, and Leishmania infantum) have been reported to infect both humans and dogs [7,8,9]. For Leishmania infantum and Dirofilaria. spp., dogs are an essential reservoir species for the parasites in endemic areas.
The distribution of CVBDs across Europe has also shifted over recent decades. Changes to the climate and land use have affected the ranges and population size of many insect and tick vectors, and wildlife reservoirs (e.g. rodents, tick transport hosts, migratory birds, wolves, jackals, foxes, wildcats, etc.) [10,11,12,13,14,15,16,17,18,19,20,21]. Additionally, tourism, travel with dogs and importation of dogs from endemic areas have contributed to introduction of CVBDs to new areas [10, 11]. The range for Ixodes ricinus, the primary European tick vector for Borrelia burgdorferi, Anaplasma spp., and the tick-borne encephalitis virus, has expanded to northern regions and to higher elevations resulting in increased infections in those areas [12, 20, 22,23,24]. Another example is Rhipicephalus sanguineus (vector for multiple CVBDs), which is being introduced to non-endemic countries (i.e. Germany and Poland) and can establish temporary or permanent populations associated with human habitation or in the environment [12, 17, 25,26,27,28,29]. Increased D. immitis infections have been caused by range expansion of both native mosquitos and imported Aedes species, climatic changes, and importation of dogs from endemic areas [4, 5, 10, 11, 30]. Canine L. infantum infections in northern Europe are thus far almost all related to the importation of infected animals from, or travel to, endemic areas. Uncommon autochthonous non-vector spread has been reported in non-endemic areas, including Germany, UK, Hungary, and Finland [3, 31,32,33,34]. Vertical or horizontal transmission through blood transfusion or mating is the most common cause for autochthonous infections in non-endemic areas [1, 3, 10, 34, 35], but rare cases of unexplained horizontal transmission have been reported [31]. Recently, Phlebotomus genus sand flies have been identified in regions of northern Europe where they had not previously been found and could potentially result in local transmission of Leishmania parasites under the right conditions [33, 36, 37] but the competence of these vectors for transmitting L. infantum is still questionable [38]. Climate change may lead to L. infantum infections decreasing in some currently endemic areas of southern Europe as they become too hot and dry for Phlebotomus sand flies [39].
Monitoring CVBD infections in Europe is often done on a regional level in individual countries, but aggregated data for the European countries can be helpful in understanding the distribution of CVBDs on a broader scale. We used an extensive retrospective database of results from point-of-care rapid enzyme-linked immunosorbent assay (ELISA) tests on dogs across Europe to identify distribution and seropositivity in animals tested for selected VBDs from 2016 through 2020.
Methods
Source of data
Results from 2016 to 2020 were generated using point-of-care test kits (IDEXX Laboratories, Inc.) and included: SNAP® 4Dx® Plus Test kit, an in-clinic enzyme-linked immunosorbent assay (ELISA) for detection of D. immitis antigen and canine antibodies to B. burgdorferi, Ehrlichia spp. (E. canis, E. ewingii), and Anaplasma spp. (A. phagocytophilum and A. platys); SNAP® HW RT Test kit, an in-clinic ELISA for the detection of D. immitis antigen; SNAP® Leishmania Test kit, an in-clinic ELISA for the detection of antibodies to L. infantum. These SNAP® tests can be run on serum, plasma, or whole blood. The sample used in individual patients was not captured. The performance of each test has been reported previously [40,41,42,43].
Test results were collated directly from veterinary practices testing patients in their clinic (SNAP® Heartworm RT Test, SNAP® Leishmania Test, and SNAP® 4Dx® Plus Test). Test results were stored in IDEXX VetLab® Instrumentation and Software and were entered automatically by the IDEXX SnapShot Dx® Instrument or SNAP Pro® Analyzer or manually by clinic staff. All sample results were obtained from practicing veterinarians in the course of their regular care of the dogs with the consent of the animal owner. To ensure data privacy, results were collected without owner information or canine patient identification; thus, repeat testing events or translocated dogs (i.e. dogs with a travel history to another region) could not be identified or omitted. Similarly, no data were collected about the reason for CVBD testing or about vaccination or prophylaxis usage. All results from veterinary clinics on the European continent and from associated overseas territories owned by European countries were included in the analysis.
Data analysis
Data analysis and mapping were done using R version 4.0.4 and various R packages [44].,Footnote 1,Footnote 2,Footnote 3
Test positive percentages are reported with 95% confidence intervals calculated using the binomial exact method. Specific tests for differences were not conducted because the intention of this study was to describe pathogen test positivity, not to test hypotheses about differences in test positivity. Positive percentages were presented in tables at country level only if the country had at least 135 results. This threshold was set to ensure precision in estimates.
Co-positivity percentages were estimated for each pair of infections as the percentage of samples that tested positive for multiple pathogens out of all samples that were tested for the respective pathogens. A series of chi-square tests of independence were conducted to determine whether the percentage of co-positives was higher than expected because of chance alone using an alpha of 0.05. P-values from the ten pairwise comparisons were adjusted for multiple comparisons using the Holm-Bonferroni method.
Generation of regional test positivity maps
Mapping of test positivity was done using the Nomenclature of Territorial Units for Statistics (NUTS) classification for Europe.Footnote 4 NUTS classifications have different levels of division within each European country [45]. NUTS 0 represents the boundaries of the country. NUTS 1 represents large regions within a country. NUTS 1 regions are further subdivided into NUTS 2 level regions and then further subdivided into NUTS 3. Each result was assigned to its NUTS 0 (country level) through NUTS 3 units for analysis.
The preference was to display data at the smallest appropriate NUTS level for each region. To balance the desire to provide meaningful granular regional data with unequal distribution of data across different regions, the following system was used to determine which NUTS level would be displayed. First, a minimum of three clinics with at least one result each was required within each NUTS region to qualify for inclusion in the display at that NUTS level. This restriction was included to ensure privacy of the clinics. Second, at least 50% of the smaller NUTS regions within an individual larger NUTS region had to qualify for display (at least 3 clinics and at least 135 total results for the region) at the smaller NUTS region level. If < 50% of the smaller regions qualified for display, the corresponding next larger region was evaluated for inclusion. Mapping of Leishmania spp. positives in France and Germany was conducted at NUTS level 1, even though < 50% of these regions qualified for display. This was done because most tests in these countries were from a few geographical areas, and very few tests were from outside of these areas. To present these limited data at the NUTS level 0 (country level) was considered to be misrepresentative.
For regions that qualified for inclusion, test positivity rate over the 5-year study period was displayed on a gradient from green (lower test positivity) to red (higher test positivity). Regions that did not qualify for inclusion are colored pale gray. NUTS classification is not available in Russia. Russia was not included in the maps because of the low number of samples, which were not considered representative of CVBD. The Canary Islands of Spain were included in the map for D. immitis because they have been previously found to be hyperendemic for these infections (prevalence of 58%) since 1995 with an important decrease in the last decades (prevalence around 18%) [46, 47] and because travel to island territories with pets can spread disease.
Results
Summary
A total of 404,617 samples corresponding to 1,134,648 results were available from dogs tested in 26 European countries over the 5-year period summarized in the current paper (Table 1). This represents results from > 251,000 tests for antigen of D. immitis, 211,000 tests for antibodies to L. infantum, and 224,000 tests for antibodies to each of B. burgdorferi, Ehrlichia spp., and Anaplasma spp. Geographic distribution of positive tests and relative percent positive values are shown by NUTS classification in Figs. 1, 2, 3, 4, 5. Overall, each disease showed a decrease in percent positive results (Fig. 6) as the total tests performed increased (Table 1).
Dirofilaria immitis antigen test positivity for NUTS regions or country over the study period (2016–2020). NUTS levels are shown with the most geographic detail allowed by the regional data. Gray regions did not have sufficient results for evaluation of region-specific test positivity analysis. Test positivity in the Canary Islands (a high endemic area) was added to the figure (not to scale) for reference
Leishmania spp. antibody test positivity for NUTS regions or country over the study period (2016–2020). NUTS levels are shown with the most geographic detail allowed by the regional data. Gray regions did not have sufficient results for evaluation of region-specific test positivity analysis. Results from Germany and France are shown at NUTS level 1 since test results were restricted to a few areas within the country
Anaplasma spp., Ehrlichia spp., and Borrelia burgdorferi
The total number of results for tick-borne diseases in Europe increased each year (Table 1) with a trend of declining rates of test positivity for each pathogen (Fig. 6). Similar trends of declining test positivity were noted for each of the pathogens. Annual European test positivity rates decreased from 7.3% in 2016 to 5.3% in 2020 for Anaplasma spp., from 4.3% in 2016 to 3.4% in 2020 for Ehrlichia spp., and from 3.3% in 2016 to 2.4% in 2020 for Borrelia burgdorferi (Table 2).
Anaplasma spp. antibody test positivity rates for the study period varied regionally and were higher (> 10%) in Austria, Bosnia and Herzegovina, Czech Republic, Germany, Poland, Slovakia, Slovenia, and Sweden and lower (< 5%) in Andorra, Belgium, Finland, France, Hungary, Italy, Malta, Norway, Portugal, Russia, Spain, Switzerland, and the UK (Table 3). Remaining countries had test positivity between 5 and 10%. Geographic distribution of positive test results using the NUTS classification is displayed for regions with sufficient test results for reporting (Fig. 1).
Ehrlichia spp. antibody test positivity rates were higher (> 3%) in Greece, Italy, Lithuania, The Netherlands, Portugal, Romania, Russia, Spain, and Switzerland and lower (< 1%) in Denmark, Estonia, Finland, Hungary, Norway, Slovakia, Slovenia, and Sweden (Table 3). Remaining countries had test positivity between 1 and 3%. Greece showed the highest percent positive results (19.6%) while Denmark, Estonia, Hungary, and Slovenia all reported < 0.5% positive results during the study period (Fig. 2).
Borrelia burgdorferi antibody positivity was concentrated in Northern and Eastern Europe with higher rates of positivity (> 5%) in Austria, Czech Republic, Estonia, Finland, Germany, Lithuania, The Netherlands, Norway, Poland, Slovenia, Sweden, and Switzerland and lowest rates (< 1%) in Andorra, Croatia, Greece, Hungary, Italy, Malta, Portugal, Romania, and Spain (Table 3). Remaining countries had test positivity between 1 and 5%. The highest test positivity was seen in Sweden (13.3%) and lowest in Greece (< 0.1%). Test positivity based on NUTS classification in the EU and UK is presented in Fig. 3.
Dirofilaria immitis
The total number of D. immitis test results in Europe increased steadily over the study period with 1.8 × more tests run in 2020 than in 2016 (Table 1). The yearly percent positive results for D. Immitis antigen trended down over the 5-year study period (Fig. 6) with a high of 2.7% in 2016 and low of 1.8% in 2018 (Table 4).
Dirofilaria immitis positivity rates varied regionally with > 9% positive tests in Hungary, Romania, and Russia and < 1% positive results in 12 countries, Czech Republic, Denmark, Estonia, Finland, France, Germany, Malta, Norway, Poland, Slovakia, Slovenia, and Sweden (Table 3). Remaining countries had between 1 and 9% test positivity. Malta and Norway each had < 0.1% positive results (Table 3).
Leishmania spp
Leishmania spp. infection showed a similar trend of increasing result numbers each year in Europe and decreasing test positivity. The yearly percent of positive Leishmania antibody test results decreased from 13.9% in 2016 to 9.4% in 2020 (Table 2). Substantial year-over-year decreases with non-overlapping confidence intervals suggest significant decreases for each pair of sequential years except 2017–2018 (Fig. 6).
Leishmania spp. results were primarily available from endemic areas in southern Europe (Table 3, Fig. 5). There was wide variability in the number of test results from different countries. Italy and Spain each had > 90,000 test results and test positivity of 11.9% and 10.7%, respectively. All other endemic countries had < 10,000 test results. Greece and Malta had the highest rate of test positivity in the endemic countries with 18.5% and 15.9%, respectively. All other endemic areas, other than Romania, had test positivity rates > 7%. Within France, most test results originated from endemic areas in southern regions. Only three non-endemic countries, The Netherlands, Switzerland, and Germany, had sufficient tests to report test positivity. These countries had high test positivity and < 700 test results (Table 3).
Co-positivity
Co-positivity was evaluated on a Europe-wide scale. Actual co-positivity rates were significantly higher than expected for all pathogen test positive pairs except for Ehrlichia spp. and Borrelia burgdorferi co-positivity and D. immitis and Borrelia burgdorferi (Table 4). The overall total rate of co-positives was 2–3 times more than the expected for most pairings. The highest percentage of co-positivity was for Ehrlichia spp. and Leishmania spp. (1.44%), Anaplasma spp. and Ehrlichia spp. (0.81%), and Anaplasma spp. and Leishmania spp. (0.78%).
Discussion
In this study we used data from point-of-care ELISA testing to map distribution and test positivity of CVBDs across Europe. This is to date the most extensive study done on vector-borne infection in dogs in Europe with > 1.1 million test results over a 5-year period. It provides data about the current state of vector-borne infection in tested dogs but is not an unbiased random sample representing prevalence in all dogs in Europe. Over the study period, the yearly number of tests performed increased by a factor of 1.7–2.5 from 2016 to 2020 for each pathogen, while the percentage of positive test results declined. Testing behavior likely impacted these trends. In this study, we did not have access to clinical information and cannot determine how many dogs were sampled as part of wellness screening or because of clinical illness. Substantial variation exists between veterinarians and countries in whether CVBD testing is performed as part of preventative care or primarily in cases with clinical illness [48, 49]. For instance, perceptions of veterinarians around the prevalence of Leishmania in their area of practice is related to their likelihood to test and to prescribe preventative measures to their patients [49, 50]. Substantial differences have previously been demonstrated in test positivity in CVBDs when healthy and clinically suspect dogs are tested [51]. The European Scientific Counsel Companion Animal Parasites (ESCCAP) recommends serologic screening for CVBDs endemic in the animal’s home region and within 3–6 months following travel to areas where other CVBDs are endemic. This bias due to different testing criteria in endemic and non-endemic areas was most notable with Leishmania. Although Spain and Germany had similar test positivity, 98,737 test results were available for Spain and only 686 tests for Germany, where testing is primarily performed on dogs with a travel or importation history [52]. Additionally, increasing adoption of screening testing in some regions during the 4-year study period may have impacted the results by identifying infected animals who are not sick and reduced the expected percentage of positive test results [53,54,55,56].
In addition to changes in testing behavior over the study period, increased use of preventatives and other efforts to decrease exposure to vectors, and newer treatment modalities may have decreased new exposure of dogs to CVBDs or the likelihood of positive results. For Leishmania spp. infections increased usage of insect repellents with proven efficacy to prevent sand fly bites [57, 58] and antiparasite products [59] likely contribute to this decline. Additionally, Leishmania test positivity could also have been reduced by long-term reductions in antibody levels of patients treated with domperidone [60, 61] and the use of other immunomodulators [62, 63]. For other CVBDs, the more modest declines may be similarly multi-factorial with changes in screening behavior and preventative use in combination with year-to-year climate effects on vector populations. Information about tick, mosquito, and sandfly populations was not collected as part of this study, so it was not possible to identify how or whether changes in vector populations impacted test positivity. Determining the contribution of potential causal factors in these declines would require additional study.
Differences in the number of test results in a particular region were also reflected in the regional resolution of the maps. The NUTS system was used since it is a convenient system for mapping subregions of European countries that is tied to the population in each subregion. Countries (NUTS 0) are divided, when population size allows, into large regions with 3–7 million residents (NUTS 1), which are subdivided into smaller regions with 800,000–3 million residents (NUTS 2), which are then subdivided again into regions of 150,000–800,000 residents (NUTS 3). Since different regions had different numbers of tests available and the goal of the study was to show clinically meaningful data for as many regions as possible, the granularity of regional data presented varies. Where possible, NUTS 3 classification was used to generate regional maps, but in some instances the results were presented at country level when the number of results did not support finer resolution.
Using these data, it was not possible to draw information about whether there was an expansion of CVBDs into new areas since we cannot identify where dogs were infected by a particular pathogen. Positive test results outside of endemic areas could represent imported cases or expansion of CVBDs into new areas.
This study focused on test positivity from point-of-care testing and did not confirm positive tests. Rarely, false-positive results on the SNAP HW RT tests, but not the SNAP 4Dx Plus test, have been reported in Angiostrongylus vasorum-positive dogs [64]. This potential for false positives due to A. vasorum infection is not likely to have substantially impacted the data since almost all D. immitis results in the data set were from SNAP 4Dx Plus tests. Similar false positives due to other CVBD infections have not been reported for the other tests included in this study. The VlsE C6 peptide (C6) utilized to detect B. burgdorferi antibodies does not cross-react with other Borrelia spp. or Lyme vaccination (regardless of the vaccine type) [65,66,67,68].
Co-positivity was higher than expected by chance for almost all pairs of pathogens. Results are presented in aggregate representing tests from all available European counties. They do not account for differences in regional risk of infection for different CVBDs or identify whether co-positives were higher in some regions. Additionally, since no clinical information was collected, we cannot identify dogs with previous exposure from those that are clinically ill. The number of dogs positive for two pathogens was relatively low, but dogs positive for one pathogen should be tested for other CVBD pathogens. High co-positivity rates for Leishmania spp. and Ehrlichia spp. and for Leishmania spp. and Anaplasma spp. have previously been described in dogs in, or imported from, southern Europe [51, 69,70,71,72,73]. For example, dogs imported to Germany showed a significant rate of co-positivity for Leishmania spp. and Ehrlichia spp. (617 out of 15,955 tested dogs), and to a lesser but still significant extent for Leishmania spp. and Babesia canis, Ehrlichia spp. and Babesia canis and for Leishmania spp. and Anaplasma spp. [10]. Dogs with clinical illness associated with CVBDs are more likely to have co-positive results for other CVBDs [51, 72, 73] and may have more severe clinical presentations. The cause for the higher than expected co-positivity is not clear, and potential contributing factors for increased co-infection risk may include changes in distribution or life cycle of CVBDs and associated vectors, outdoor dog housing and associated increased exposure to vectors, lack of effective insect repellents or antiparasitic treatments, and immunocompromised status [74,75,76].
Conclusions
This study provides CVBD test positivity and geographic test positivity at the most granular scale possible for countries in Europe from 2016 to 2020. During the study period, increasing numbers of test results were available each year even as the proportion of positive tests decreased. The most substantial decline was in Leishmania spp. test positivity. Increases in use of effective preventatives and routine screening and preventative care of animals without clinical leishmaniosis also likely play roles in the increasing total number of tests and decreasing test positivity. This study represents the largest data set on test positivity for CVBDs for European countries and can help inform veterinarians on the results in their geography and improve prevention of these important clinical and zoonotic diseases.
Notes
R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Wickham, H. 2021. https://CRAN.R-project.org/package=tidyverse.
Lahti, L et al. 2021. Tools for Eurostat Open Data. https://CRAN.R-project.org/package=eurostat.
Abbreviations
- CVBD:
-
Canine vector-borne disease
- IFA:
-
Immunofluorescense assay
- qELISA:
-
Quantitative enzyme linked immunosorbent assay
References
Bogdan C, Schonian G, Banuls A-L, Hide M, Pratlong F, Lorenz E, et al. Visceral leishmaniasis in a German child who had never entered a known endemic area: case report and review of the literature. Clin Infect Dis. 2001;32:302–6.
Traversa D, Di Cesare A. Cardio-pulmonary parasitic nematodes affecting cats in Europe: unraveling the past, depicting the present, and predicting the future. Front Vet Sci. 2014;1. https://doi.org/10.3389/fvets.2014.00011/abstract. Accessed 22 Feb 2021.
Tánczos B, Balogh N, Király L, Biksi I, Szeredi L, Gyurkovsky M, et al. First record of autochthonous canine leishmaniasis in Hungary. Vector-Borne Zoonotic Dis. 2012;12:588–94.
Morchón R, Carretón E, González-Miguel J, Mellado-Hernández I. Heartworm disease (Dirofilaria immitis) and their vectors in Europe—new distribution trends. Front Physio. 2012;3. https://doi.org/10.3389/fphys.2012.00196/abstract. Accessed 18 Feb 2021.
Genchi C, Mortarino M, Rinaldi L, Cringoli G, Traldi G, Genchi M. Changing climate and changing vector-borne disease distribution: the example of Dirofilaria in Europe. Vet Parasitol. 2011;176:295–9.
Beugnet F, Marié J-L. Emerging arthropod-borne diseases of companion animals in Europe. Vet Parasitol. 2009;163:298–305.
Reddy MV. Human dirofilariasis: an emerging zoonosis. Trop Parasitol. 2013;3:2–3.
Simón F, López-Belmonte J, Marcos-Atxutegi C, Morchón R, Martín-Pacho JR. What is happening outside North America regarding human dirofilariasis. Vet Parasitol. 2005;133:181–9.
Otranto D, Dantas-Torres F, Breitschwerdt EB. Managing canine vector-borne diseases of zoonotic concern: part one. Trends Parasitol. 2009;25:157–63.
Vrhovec MG, Pantchev N, Failing K, Bauer C, Travers-Martin N, Zahner H. Retrospective analysis of canine vector-borne diseases (CVBD) in Germany with emphasis on the endemicity and risk factors of leishmaniosis. Parasitol Res. 2017;116:131–44.
Pantchev N, Etzold M, Daugschies A, Dyachenko V. Diagnosis of imported canine filarial infections in Germany 2008–2010. Parasitol Res. 2011;109:61–76.
Pantchev N, Pluta S, Huisinga E, Nather S, Scheufelen M, Vrhovec MG, et al. Tick-borne diseases (borreliosis, anaplasmosis, babesiosis) in German and Austrian dogs: status quo and review of distribution, transmission, clinical findings. Diagn Prophyl Parasitol Res. 2015;114:19–54.
Palfreyman J, Graham-Brown J, Caminade C, Gilmore P, Otranto D, Williams DJL. Predicting the distribution of Phortica variegata and potential for Thelazia callipaeda transmission in Europe and the United Kingdom. Parasit Vectors. 2018;11:272.
Deplazes P, Hegglin D, Gloor S, Romig T. Wilderness in the city: the urbanization of Echinococcus multilocularis. Trends Parasitol. 2004;20:77–84.
Rosà R, Andreo V, Tagliapietra V, Baráková I, Arnoldi D, Hauffe H, et al. Effect of climate and land use on the spatio-temporal variability of tick-borne bacteria in Europe. IJERPH. 2018;15:732.
Gray JS, Dautel H, Estrada-Peña A, Kahl O, Lindgren E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip Perspect Infect Dis. 2009;2009:1–12.
Buczek A, Buczek W. Importation of ticks on companion animals and the risk of spread of tick-borne diseases to non-endemic regions in Europe. Animals. 2020;11:6.
Mendoza-Roldan J, Benelli G, Panarese R, Iatta R, Furlanello T, Beugnet F, et al. Leishmania infantum and Dirofilaria immitis infections in Italy, 2009–2019: changing distribution patterns. Parasites Vectors. 2020;13:193.
Otranto D, Capelli G, Genchi C. Changing distribution patterns of canine vector borne diseases in Italy: leishmaniosis vs. dirofilariosis. Parasites Vectors. 2009;2:S2.
Lindgren E, Tälleklint L, Polfeldt T. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ Health Perspect. 2000;108:119–23.
Semenza JC, Suk JE. Vector-borne diseases and climate change: a European perspective. FEMS microbiology letters. 2018;365. https://doi.org/10.1093/femsle/fnx244/4631076. Accessed 3 Feb 2022.
Jore S, Vanwambeke SO, Viljugrein H, Isaksen K, Kristoffersen AB, Woldehiwet Z, et al. Climate and environmental change drives Ixodes ricinus geographical expansion at the northern range margin. Parasit Vectors. 2014;7:11.
Jaenson TG, Jaenson DG, Eisen L, Petersson E, Lindgren E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasites Vectors. 2012;5:8.
Martello E, Mannelli A, Ragagli C, Ambrogi C, Selmi M, Ceballos LA, et al. Range expansion of Ixodes ricinus to higher altitude, and co-infestation of small rodents with Dermacentor marginatus in the Northern Apennines. Italy Ticks Tick-borne Dis. 2014;5:970–4.
Dongus H, Zahler M. Gothe R [The brown dog tick, Rhipicephalus sanguineus (Ixodidae), in Germany: an epidemiologic study and control measures]. Berl Munch Tierarztl Wochenschr. 1996;109:245–8.
Snellgrove AN, Krapiunaya I, Ford SL, Stanley HM, Wickson AG, Hartzer KL, et al. Vector competence of Rhipicephalus sanguineus sensu stricto for Anaplasma platys. Ticks Tick-borne Dis. 2020;11:101517.
Földvári G, Široký P, Szekeres S, Majoros G, Sprong H. Dermacentor reticulatus: a vector on the rise. Parasites Vectors. 2016;9:314.
Drehmann M, Springer A, Lindau A, Fachet K, Mai S, Thoma D, et al. The spatial distribution of Dermacentor ticks (Ixodidae) in Germany—evidence of a continuing spread of Dermacentor reticulatus. Front Vet Sci. 2020;7:578220.
Zahler M, Schein E, Rinder H, Gothe R. Characteristic genotypes discriminate between Babesia canis isolates of differing vector specificity and pathogenicity to dogs. Parasitol Res. 1998;84:544–8.
Pantchev N, Norden N, Lorentzen L, Rossi M, Rossi U, Brand B, et al. Current surveys on the prevalence and distribution of Dirofilaria spp. in dogs in Germany. Parasitol Res. 2009;105:63–74.
Wright I, Baker S. Leishmaniosis in a dog with no history of travel outside the UK. Vet Rec. 2019;184:387–8.
Wright I, Moral-Gant JM. Possible vertical transmission of Leishmania in a dog. Vet rec. 2020;187:35–35.
Naucke TJ, Menn B, Massberg D, Lorentz S. Sandflies and leishmaniasis in Germany. Parasitol Res. 2008;103:65–8.
Karkamo V, Kaistinen A, Näreaho A, Dillard K, Vainio-Siukola K, Vidgrén G, et al. The first report of autochthonous non-vector-borne transmission of canine leishmaniosis in the Nordic countries. Acta Vet Scand. 2014;56:84.
Dujardin J-C, Campino L, Cañavate C, Dedet J-P, Gradoni L, Soteriadou K, et al. Spread of vector-borne diseases and neglect of leishmaniasis. Europe Emerg Infect Dis. 2008;14:1013–8.
Melaun C, Krüger A, Werblow A, Klimpel S. New record of the suspected leishmaniasis vector Phlebotomus (Transphlebotomus) mascittii Grassi, 1908 (Diptera: Psychodidae: Phlebotominae)—the northernmost phlebotomine sandfly occurrence in the Palearctic region. Parasitol Res. 2014;113:2295–301.
Oerther S, Jöst H, Heitmann A, Lühken R, Krüger A, Steinhausen I, et al. Phlebotomine sand flies in Southwest Germany: an update with records in new locations. Parasites Vectors. 2020;13:173.
Obwaller AG, Karakus M, Poeppl W, Töz S, Özbel Y, Aspöck H, et al. Could Phlebotomus mascittii play a role as a natural vector for Leishmania infantum? New Data Parasites Vectors. 2016;9:458.
Trájer AJ. The effect of climate change on the potential distribution of the European Phlebotomus species. Appl Ecol Env Res. 2013;11:189–208.
Stillman BA, Monn M, Liu J, Thatcher B, Foster P, Andrews B, et al. Performance of a commercially available in-clinic ELISA for detection of antibodies against Anaplasma phagocytophilum, Anaplasma platys, Borrelia burgdorferi, Ehrlichia canis, and Ehrlichia ewingii and Dirofilaria immitis antigen in dogs. J Am Vet Med Assoc. 2014;245:80–6.
Atkins CE. Comparison of results of three commercial heartworm antigen test kits in dogs with low heartworm burdens. J Am Vet Med Assoc. 2003;222:1221–3.
Marcondes M, Biondo AW, Gomes AAD, Silva ARS, Vieira RFC, Camacho AA, et al. Validation of a Leishmania infantum ELISA rapid test for serological diagnosis of Leishmania chagasi in dogs. Vet Parasitol. 2011;175:15–9.
Ferroglio E, Centaro E, Mignone W, Trisciuoglio A. Evaluation of an ELISA rapid device for the serological diagnosis of Leishmania infantum infection in dog as compared with immunofluorescence assay and Western blot. Vet Parasitol. 2007;144:162–6.
Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, et al. Welcome to the Tidyverse. JOSS. 2019;4:1686.
Eurostat. Postcodes and NUTS. https://doi.org/10.2807/1560-7917.ES.2020.25.3.2000045. Accessed 22 Apr 2021.
Montoya-Alonso JA, Carretón E, Morchón R, Silveira-Viera L, Falcón Y, Simón F. The impact of the climate on the epidemiology of Dirofilaria immitis in the pet population of the Canary Islands. Vet Parasitol. 2016;216:66–71.
Montoya JA, Morales M, Ferrer O, Molina JM, Corbera JA. The prevalence of Dirofilaria immitis in Gran Canaria, Canary Islands, Spain (1994–1996). Vet Parasitol. 1998;75:221–6.
Nichol GK, Weese JS, Evason M, Clow KM. Assessing knowledge, attitudes, and practices of Canadian veterinarians with regard to Lyme disease in dogs. J Vet Intern Med. 2021;35:294–302.
Zini E, Muscardin L, D’Anna N, Fondati A, Gradoni L, Lubas G, et al. Preventive measures of canine leishmaniosis in Italy: attitudes of veterinarians based on a questionnaire. Prev Vet Med. 2020;183:105148.
Le Rutte EA, van Straten R, Overgaauw PAM. Awareness and control of canine leishmaniosis: a survey among Spanish and French veterinarians. Vet Parasitol. 2018;253:87–93.
Cardoso L, Mendão C, Madeira de Carvalho L. Prevalence of Dirofilaria immitis, Ehrlichia canis, Borrelia burgdorferi sensu lato, Anaplasma spp and Leishmania infantum in apparently healthy and CVBD-suspect dogs in Portugal—a national serological study. Parasites Vectors. 2012;5:62.
Mencke N. The importance of canine leishmaniosis in non-endemic areas, with special emphasis on the situation in Germany. Berl Munch Tierarztl Wochenschr. 2011;124:434–42.
Baneth G, Koutinas AF, Solano-Gallego L, Bourdeau P, Ferrer L. Canine leishmaniosis—new concepts and insights on an expanding zoonosis: part one. Trends Parasitol. 2008;24:324–30.
Lladró S, Picado A, Ballart C, Portús M, Gállego M. Management, prevention and treatment of canine leishmaniosis in north-eastern Spain: an online questionnaire-based survey in the province of Girona with special emphasis on new preventive methods (CaniLeish vaccine and domperidone). Vet Rec. 2017;180:47–47.
Velez R, Ballart C, Domenech E, Abras A, Fernández-Arévalo A, Gómez SA, et al. Seroprevalence of canine Leishmania infantum infection in the Mediterranean region and identification of risk factors: the example of North-Eastern and Pyrenean areas of Spain. Prev Vet Med. 2019;162:67–75.
Gálvez R, Montoya A, Cruz I, Fernández C, Martín O, Checa R, et al. Latest trends in Leishmania infantum infection in dogs in Spain, Part I: mapped seroprevalence and sand fly distributions. Parasites Vectors. 2020;13:204.
Miró G, Montoya A, Roura X, Gálvez R, Sainz A. Seropositivity rates for agents of canine vector-borne diseases in Spain: a multicentre study. Parasites Vectors. 2013;6:117.
Miró G, Petersen C, Cardoso L, Bourdeau P, Baneth G, Solano-Gallego L, et al. Novel areas for prevention and control of canine leishmaniosis. Trends Parasitol. 2017;33:718–30.
Animal’s Health. La prevención frente a Leishmania en perros se dispara en España. https://www.animalshealth.es/empresas/prevencion-leishmania-perros-espana-dispara. Accessed 1 Jun 2021.
Gómez-Ochoa P, Castillo JA, Gascón M, Zarate JJ, Alvarez F, Couto CG. Use of domperidone in the treatment of canine visceral leishmaniasis: a clinical trial. Vet J. 2009;179:259–63.
Sabaté D, Llinás J, Homedes J, Sust M, Ferrer L. A single-centre, open-label, controlled, randomized clinical trial to assess the preventive efficacy of a domperidone-based treatment programme against clinical canine leishmaniasis in a high prevalence area. Prev Vet Med. 2014;115:56–63.
Segarra S, Miró G, Montoya A, Pardo-Marín L, Teichenné J, Ferrer L, et al. Prevention of disease progression in Leishmania infantum-infected dogs with dietary nucleotides and active hexose correlated compound. Parasites Vectors. 2018;11:103.
Segarra S, Miró G, Montoya A, Pardo-Marín L, Boqué N, Ferrer L, et al. Randomized, allopurinol-controlled trial of the effects of dietary nucleotides and active hexose correlated compound in the treatment of canine leishmaniosis. Vet Parasitol. 2017;239:50–6.
Schnyder M, Deplazes P. Cross-reactions of sera from dogs infected with Angiostrongylus vasorum in commercially available Dirofilaria immitis test kits. Parasites Vectors. 2012;5:258.
Stillman BA, Thatcher B, Beall MJ, Lappin M, O’Connor TP, Chandrashekar R. Borrelia burgdorferi antibody test results in dogs administered 4 different vaccines. Top Companion Anim Med. 2019;37:100358.
Margos G, Pantchev N, Globokar M, Lopez J, Rodon J, Hernandez L, et al. First cases of natural infections with Borrelia hispanica in two dogs and a cat from Europe. Microorganisms. 2020;8:1251.
Liang FT, Jacobson RH, Straubinger RK, Grooters A, Philipp MT. Characterization of a Borrelia burgdorferi VlsE invariable region useful in canine Lyme disease serodiagnosis by enzyme-linked immunosorbent assay. J Clin Microbiol. 2000;38:4160–6.
O’Connor TP, Esty KJ, Hanscom JL, Shields P, Philipp MT. Dogs vaccinated with common lyme disease vaccines do not respond to IR 6, the conserved immunodominant region of the VlsE surface protein of Borrelia burgdorferi. Clin Vaccine Immunol. 2004;11:458–62.
Schäfer I, Volkmann M, Beelitz P, Merle R, Müller E, Kohn B. Retrospective evaluation of vector-borne infections in dogs imported from the Mediterranean region and southeastern Europe (2007–2015). Parasites Vectors. 2019;12:30.
Montoya-Alonso JA, Morchón R, Costa-Rodríguez N, Matos JI, Falcón-Cordón Y, Carretón E. Current distribution of selected vector-borne diseases in dogs in Spain. Front Vet Sci. 2020;7:564429.
Díaz-Regañón D, Roura X, Suárez ML, León M, Sainz Á. Serological evaluation of selected vector-borne pathogens in owned dogs from northern Spain based on a multicenter study using a commercial test. Parasites Vectors. 2020;13:301.
Attipa C, Solano-Gallego L, Papasouliotis K, Soutter F, Morris D, Helps C, et al. Association between canine leishmaniosis and Ehrlichia canis co-infection: a prospective case-control study. Parasites Vectors. 2018;11:184.
Baxarias M, Álvarez-Fernández A, Martínez-Orellana P, Montserrat-Sangrà S, Ordeix L, Rojas A, et al. Does co-infection with vector-borne pathogens play a role in clinical canine leishmaniosis? Parasites Vectors. 2018;11:135.
Ballart C, Alcover MM, Picado A, Nieto J, Castillejo S, Portús M, et al. First survey on canine leishmaniasis in a non classical area of the disease in Spain (Lleida, Catalonia) based on a veterinary questionnaire and a cross-sectional study. Prev Vet Med. 2013;109:116–27.
Cortes S, Vaz Y, Neves R, Maia C, Cardoso L, Campino L. Risk factors for canine leishmaniasis in an endemic Mediterranean region. Vet Parasitol. 2012;189:189–96.
Martín-Sánchez J, Morales-Yuste M, Acedo-Sánchez C, Barón S, Díaz V, Morillas-Márquez F. Canine leishmaniasis in southeastern Spain. Emerg Infect Dis. 2009;15:795–8.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
GM—data review, writing, editing; IW—data review, editing; HM—data review, writing, editing; WB—study planning, data review, writing, editing; EH—study planning, data generation, data analysis and review, writing, editing; JR—data review, writing, editing; JB—study planning, data review, editing; NP—data review, writing, editing; GSH—data review, editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
HM, WB, EH, JR, JB, and NP are full-time employees of IDEXX Laboratories, Inc.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Miró, G., Wright, I., Michael, H. et al. Seropositivity of main vector-borne pathogens in dogs across Europe. Parasites Vectors 15, 189 (2022). https://doi.org/10.1186/s13071-022-05316-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05316-5