Abstract
Background
This report describes L. infantum infection seroprevalence in dogs in Spain through data obtained from peer-reviewed literature and a cross-sectional serological survey assessing epidemiological and habitat variables as risk factors for infection. The study also provides preliminary sand fly species distribution data and indicates factors affecting their distribution and density.
Methods
Three different studies were conducted in Spain: (i) a peer-reviewed literature seroprevalence survey (1985–2019); (ii) a cross-sectional serological survey (2011–2016); and (iii) a preliminary entomological survey (2013–2014). In the cross-sectional serological survey, 1739 dogs from 74 different locations including 25 Spanish provinces were tested for L. infantum by indirect immunofluorescence antibody test (IFAT) (antibody titre ≥ 1:100). Seroprevalence of L. infantum infection was analysed by province and bioclimatic zone. Statistics were used to analyse relationships between several dog- and environment-related variables and L. infantum seroprevalence. In parallel, during 2013–2014, sand flies were collected across the Iberian Peninsula and the Balearic Islands using CDC light traps to examine relationships between habitat-related factors and sand fly species densities (number of sand flies per trap per hour).
Results
The literature review revealed that the provinces showing the highest seroprevalence were Balearic Islands (57.1%), Ourense (35.6%), Málaga (34.6%) and Cáceres (34.2%), and those showing the lowest seroprevalence were Vizcaya (0%), Cantabria (2.0%) and Álava (3.3%). In our survey, anti-Leishmania IgG antibodies were detected in 176 of the 1739 dogs rendering a seroprevalence of 10.12%. Percentage seroprevalence distributions significantly varied among bioclimatic belts. Seropositivity for L. infantum was related to size (large breed dogs versus small) and were significantly higher in younger dogs (≤ 1 years-old). In the entomological survey, 676 sand flies of five species were captured: 562 (83.13%) Phlebotomus perniciosus; 64 (9.47%) Sergentomyia minuta; 38 (5.62%) P. ariasi: 6 (0.89%) P. sergenti; and 6 (0.89%) P. papatasi. Phlebotomus perniciosus showed a greater density in the thermo-Mediterranean than in the meso-Mediterranean zone. Densities of S. minuta and P. ariasi were significantly higher in rural habitats.
Conclusions
This updated seroprevalence map of L. infantum infection in dogs in Spain defines non-endemic, hypoendemic, endemic and hyperendemic areas, and confirms P. perniciosus as the most abundant sand fly vector in Spain.

Similar content being viewed by others
Background
Leishmaniosis caused by Leishmania infantum is a widespread zoonotic disease that may be transmitted to animals and humans by their vectors, blood-sucking phlebotomine sand flies [1, 2]. Other non-sand fly routes of L. infantum transmission include vertical and horizontal routes (from blood donors, venereal transmission and direct dog-to-dog transmission through bites or wounds) [3]. In endemic areas, a population subset with subclinical infection acts as a disease reservoir [3]. In Spain, CanL is an endemic and dynamic disease with an overall seroprevalence and transmission risk that vary according to local environmental and climatic conditions [4, 5]. In the Mediterranean basin, the dog is the main reservoir for L. infantum, and it is estimated that close to 2.5 million dogs are infected [6]. In Spain, CanL shows a broad seroprevalence range varying according to the geographical area [7]. In the northern provinces of the Iberian Peninsula traditionally classified as non-endemic, seroprevalence is lower [8]. Climate and environmental changes provoked by human activities may have caused the expansion of L. infantum infection in dogs towards such areas historically considered disease-free [9]. Regarding sand fly status in Spain, Sergentomyia minuta is the most abundant species, followed by two vector species of L. infantum: P. perniciosus, which is more widespread and less affected by climatic conditions, and P. ariasi, which shows a preference for humid, cold areas [2].
There is great variability in how L. infantum manifests in a dog due to both individual factors (e.g. breed, age, immune status) and environmental factors (e.g. climate, land use) [3, 10, 11].
Control strategies should be based on local epidemiological information [1]. The updated data provided here on the seroprevalence of CanL and on the ecology of sand fly vectors in Spain will be useful for the design of targeted control measures.
This study is Part I of a larger investigation addressing the situation of CanL in Spain. In Part II, we examined how CanL is currently managed via a multicentre questionnaire completed by veterinarians and compared the data obtained with a similar survey conducted in 2005.
Methods
Study area
The study area was mainland Spain and the Balearic and Canary Islands. Nine bioclimatic zones have been traditionally defined for the Iberian Peninsula and Balearic Islands [12]. Five of these areas occupying 46 × 106 ha were surveyed: supratemperate and mesotemperate within the Eurosiberian region, and supra-Mediterranean, meso-Mediterranean and thermo-Mediterranean within the Mediterranean region. The zones not surveyed were the four highest regions occupying 1 × 106 ha (alpine and subalpine in the Eurosiberian region, and cryoro-Mediterranean and oro-Mediterranean in the Mediterranean region; mean altitudes of 2396, 1882, 2548 and 1757 meters above sea level, respectively) because climatic conditions are not suitable for sand fly development.
Leishmania infantum seroprevalence study
Literature review
Scientific works published from 1985 to 2019 reporting CanL seroprevalences for mainland Spain and the Balearic Islands were identified. Inclusion criteria were seroprevalence studies conducted on randomly sampled dog populations in which the humoral response was assessed by detecting antibodies. According to the antibody titre cut-off established in each study, seroprevalence ranges were calculated for each province. These data were used to prepare a seroprevalence map of L. infantum in the dog.
Cross-sectional serological survey
During 2011–2016, a cross-sectional seroprevalence study was performed in 25 Spanish provinces without taking into account the clinical status of dogs. Epidemiological variables recorded included geographical location, habitat, age, sex, breed, weight, travel history and presence of clinical signs. A 5 ml blood sample was obtained from each dog by cephalic venipuncture and sera were separated and kept at − 20 °C until analysis. Serodiagnosis was conducted by detecting specific antibodies against L. infantum using the indirect immunofluorescence antibody test (IFAT) for anti-Leishmania-specific immunoglobulin G (IgG) antibodies according to standard procedures [13]. Serological analyses were conducted at the Pet Parasite Lab (Animal Health Department Veterinary Faculty, UCM, Spain). The cut-off indicating a positive result was 1:100. Seroprevalence was calculated as the percentage of dogs testing positive for anti-L. infantum antibodies.
Entomological survey
Sand flies were collected from the wild using CDC light traps set up in the afternoon and recovered early in the morning. Sand flies were trapped in 2013–2014 seasons (from May to October). Captured sand flies were transferred to labelled 1.5 ml tubes containing 70% ethanol and identified to the species level. Females were cleared in Mark-André medium [14] and mounted on glass slides in Hoyerʼs medium [15]. Sand flies were identified according to taxonomic keys [16]. Sand fly densities were calculated according to the following formula: Sand fly density = No. of sand flies/(No. of traps × hours).
Sites were geocoded by locality using ArcGis Pro v.2.3.3 [Environmental Systems Research Institute (ESRI), Redlands, CA, USA].
Statistical analysis
For the cross-sectional serological survey, Chi-square test and Student’s t-test were used to identify significant associations between L. infantum seroprevalence and age, sex, breed, size, habitat, use given and bioclimate. Seroprevalence was calculated separately for every province and bioclimatic zone. Kruskal-Wallis and Wilcoxon signed rank tests were used to examine relationships between sand fly species density (number of sand flies per trap per hour) and the variables bioclimatic zone, habitat and presence of animals (domestic, farm or wild fauna). Significance was set at P ≤ 0.05. All statistical tests were performed using the SPSS 25 package (SPSS Inc., Chicago, IL, USA).
Results
Forty-one scientific works reporting CanL seroprevalences for mainland Spain and the Balearic Islands were identified. The techniques used were IFI, ELISA and rapid tests. The review of reported seroprevalences of L. infantum revealed that of the 50 Spanish provinces there are published data for 31 of them. The provinces showing the highest seroprevalence were Balearic Islands (57.1%), Ourense (35.6%), Málaga (34.6%) and Cáceres (34.2%), followed by Gerona (24.6%), Córdoba (23.7%), Granada (19.3%) and Alicante (19.1%). Provinces showing the lowest seroprevalence were Vizcaya (0%), Cantabria (2%) and Álava (3.3%). Seroprevalence reported for the remaining provinces varied between 5–16% (Table 1 and Fig. 1).
Seroprevalence of canine L. infantum infection in Spain by province based on a review of the literature published from 1985 to 2019. References: Miró et al. [8]; Gálvez et al. [20]; Vélez et al. [21]; Sáez de Santamaría et al. [34]; Benito et al. [35]; Alonso et al. [36]; Sanchís Marín et al. [37]; Rosado et al. [38]; Asencio et al. [39]; Seguí [40]; Pujol et al. [41]; Solano-Gallego et al. [42]; Alcover et al. [43]; Botet et al. [44]; Corachan et al. [45]; Encinas Grandes et al. [46]; Nieto et al. [47]; Rosado et al. [48]; Morales-Yuste et al. [49]; Arnedo Pena et al. [50]; Martínez-Cruz et al. [51]; Reyes Magaña et al. [52, 53]; Acedo Sánchez et al. [54, 55]; Martín-Sánchez et al. [56]; Lepe et al. [57]; Celaya [58]; Amela et al. [59]; Castañeda et al. [60, 61]; García Nieto et al. [62]; Miró et al. [63, 64]; Morillas et al. [65]; Sesma et al. [66]; Amusátegui et al. [67]; Ariza-Astolfi et al. [68]; Portús et al. [69]; Fisa et al. [70, 71]; Fisa et al. [72, 73]; Benito-Hernández et al. [74]; Couto et al. [75]; Castillo Hernández et al. [76]
The features of the cross-sectional seroprevalence study dog population are provided in Table 2. The animals surveyed (n = 1739) were included into the following dog populations: (i) municipal animal shelter (35%, 608 stray dogs); (ii) hunting animal shelter (47%, 813 hunting dogs, 2 pets, 1 guard dog and 1 shepherd dog); (iii) housed dogs (13.3%, 229 pets and 2 guard dogs); and (iv) farms (4.7%, 21 shepherd dog, 11 pets, 8 hunting dogs and 1 guard dog). The study was conducted in 74 different locations in 25 Spanish provinces (see Table 3 and Fig. 2). Eleven new provinces without literature data were surveyed: A Coruña, Guipúzcoa, La Rioja, Las Palmas, Lérida, Lugo, Murcia, Pontevedra, Santa Cruz de Tenerife, Segovia, Valencia and Zamora.
Overall, 176 of the 1739 dogs examined tested positive for L. infantum (10.1%). Significant differences were detected between longer deviated seroprevalence values in relation to bioclimatic belt (χ2 = 51.9968, df = 4, P < 0.0001): supratemperate (5.4%), mesotemperate (3.5%), supra-Mediterranean (13.1%), meso-Mediterranean (11.9%), and thermo-Mediterranean (20.9%).
Significant differences were also detected when comparing seroprevalences by age group (χ2 = 21.5852, df = 5, P = 0.0006) and seroprevalence rates were higher in the younger dogs (< 1 year-old). Dog size data also revealed significant differences (χ2 = 12.4160, df = 2, P = 0.0020) and seroprevalence was higher in larger-sized dogs. Significant differences were observed when comparing seroprevalences for the different habitats (χ2 = 10.5837, df = 2, P = 0.005) and seroprevalence was higher in rural habitats. No differences were observed according to sex (χ2 = 2.4730, df = 1, P = 0.1158) or use category given to the dogs (χ2 = 2.7534, df = 4, P = 0.5999).
Cross-sectional survey data, together with published data for seroprevalence of L. infantum reveal that there are available data for 42 of the 50 provinces in Spain. A map of overall L. infantum infection seroprevalence was constructed (Fig. 3) by combining the data obtained in the literature review (Fig. 1) and the present survey (Fig. 2) based on seroprevalence records and the new areas reported here. This map provides seroprevalence data extrapolated to the entire country defining zones classified according to the seroprevalence range as: Zone 1 (non-endemic, low risk); Zone 2 (hypoendemic, intermediate risk); Zone 3 (endemic, intermediate-high risk); and Zone 4 (hyperendemic, high risk).
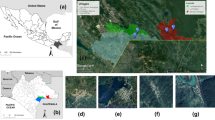
In the entomological survey conducted, fifty sites were sampled in 20 localities across the Mediterranean region (see Additional file 1: Table S1 and Fig. 4). The number of sites surveyed in each bioclimatic area was proportional to the size of the zone such that the largest bioclimatic belts, meso-Mediterranean and supra-Mediterranean were the most surveyed (22 sampling sites each) followed by the thermo-Mediterranean belt (6 sampling sites). A total of 676 specimens of five species were captured and further identified as follows: 562 (83.13%) P. perniciosus; 64 (9.47%) S. minuta; 38 (5.62%) P. ariasi; 6 (0.89%) P. sergenti; and 6 (0.89%) P. papatasi. Sand fly sites were georeferenced and depicted as a pie chart reflecting the proportion of each sand fly species (Fig. 4). Traps were set in rural (20 sites, 60%) and periurban habitats (20 sites, 40%).
Over the 2-year study period, densities of S. minuta (Z = − 2.7485, P = 0.0084) and P. ariasi (Z = − 2.2811, P = 0.0269) were increased in rural habitats. Phlebotomus perniciosus showed a greater density increase in the thermo-Mediterranean compared with the meso-Mediterranean zone (Z = − 2.75663, P = 0.00584). In addition, the densities of the vector species (P. perniciosus and P. ariasi) (Z = − 2.58737, P = 0.00967) and of all phlebotomines (Z = − 2.70698, P = 0.00679) also rose significantly in the thermo-Mediterranean compared with the meso-Mediterranean belt. New records were also observed for P. ariasi in Burgos, P. papatasi in Huelva and P. perniciosus in Segovia.
Discussion
In a CanL endemic country like Spain with high densities of sand fly vectors and reservoirs, L. infantum infection spreads quickly amongst dog populations. The overall seroprevalence of L. infantum infection in the dogs surveyed was 10.1% (176/1739 animals), taking ≥ 1:100 as the cut-off antibody titre. In areas where CanL is endemic, such a seroprevalence value of around 10% represents both dogs that develop the disease and a fraction of clinically healthy but persistently infected dogs [3]. In fact, CanL is only the tip of the iceberg in endemic areas, where part of the population is exposed and becomes infected without showing clinical evidence of disease [11]. In the survey, twelve provinces not sampled before were included. In future studies, canine L. infantum seroprevalence distributions need to be determined for the as yet unsampled provinces (in alphabetical order): Ávila, Burgos, Cuenca, Huesca, León, Palencia, Soria and Teruel. Differences between seroprevalence distributions in relation to bioclimatic belt were significant. The thermo-Mediterranean belt yielded the highest seroprevalence (20.9%) and may be considered of high-risk; the belts supra-Mediterranean (13.12%) and meso-Mediterranean (11.9%) of intermediate risk; and the belts supratemperate (5.4%) and mesotemperate (3.5%) of low risk.
In northern Spain, where the seroprevalence was low compared to the rest of the Iberian Peninsula, we found elevated prevalence (24.3%) in Ourense [8]. Despite the lower seroprevalence in northern Spain, the climatic conditions of the Galician province of Ourense should be highlighted as highly suitable for the presence of L. infantum [8]. This province features exceptionally adequate climate conditions for the expansion of leishmaniosis and it belongs to the supra-Mediterranean and not the temperate bioclimatic zone, like the northern region of Spain. Indeed, data from northern areas of Spain have already shown the expansion of L. infantum infection [8, 17].
According to Figs. 1 and 2, reported L. infantum seroprevalence in Spain ranges from 2% to 57.1% depending on the geographical region. Leishmania infantum seroprevalence variation between Spanish provinces might be explained by geographical differences in climate and seasonality associated with the bioclimatic and ecological requirements of the sand fly vectors [18]. In the European Union there is a zoonotic cutaneous and visceral leishmaniosis caused by L. infantum throughout the Mediterranean region [9]. It has been proposed that environmental changes and global warming are having an impact on the geographical distribution of CanL infection and its vectors all over Europe [9, 18, 19]. The map in Fig. 3 shows a snapshot of L. infantum seroprevalence that shows the northward emergence of CanL in Spain. These compendium maps of L. infantum seroprevalence will be useful for the implementation of control programmes. While it is desirable to standardize the data source of the surveys upon which we created Fig. 3, we have to take into account that they can present many varying factors such as dog selection procedures, serological techniques and antibody titre cut-offs used, different periods (from 1985 up to date) and sample sizes, among many others.
In the present cross-sectional study, differences were detected in L. infantum infection seroprevalence according to animal age such that seroprevalences were significantly higher among younger dogs (< 1 year-old). As already reported, this could be related to an immature immune system in these dogs making them more vulnerable to infection in their first or second year of life [2, 11, 20, 21]. No significant impact of sex on infection seroprevalence was detected. In a study examining risk factors for canine leishmaniosis in endemic areas, sex did not emerge as a risk factor [22]. In the present cross-sectional study, seroprevalence was found to vary according to animal size and was higher in dogs weighing over 20 kg. The explanation for this could be that a greater weight translates to an increased risk of infection either because of the greater body surface susceptible to sand fly bites or because medium-sized and large dogs are often used for work activities and remain outdoors for long periods of time [20, 22]. No significant relationship was found with factors such as habitat (rural, periurban or urban) or use given to the animal (e.g. hunting, guard, pet) of L. infantum infection. It should be noted that the individual immune response of the dog and virulence of the L. infantum isolate are important contributing factors [23].
The sand fly vectors of L. infantum identified here (P. perniciosus and P. ariasi) accounted for 88.75% of the total number of specimens identified. The wide distribution of these species, especially of P. perniciosus (83.13%), may indicate both an animal health and public health risk. Phlebotomus ariasi was distributed in zones of altitudes belonging to the supra-Mediterranean and meso-Mediterranean climates, as this species has a preference for wet and mountainous zones [9, 24,25,26]. Notwithstanding, it should be underscored that six specimens of this species were found at an altitude of 144 m above the sea level, close to the coast in Carboneras (Almería), although in a study conducted in Hérault (France), 8 individuals were captured at 80 metres of altitude 35 km from the coast [27].
Phlebotomus papatasi and P. sergenti were detected in the thermo-Mediterranean and meso-Mediterranean belts. This may be due to the fact that both species need environments with some aridity along with warm temperatures for their development [28]. Similarly, these two species are epidemiologically important in other world zones. Thus, P. papatasi is a vector of L. major in regions of Africa and Asia, while P. sergenti is a vector of L. tropica in North Africa, causing cutaneous leishmaniosis in both cases [29]. Fortunately, these two species of Leishmania have not been detected yet in Spain.
It should be noted that different species of sand flies were found here in places not described to date (P. ariasi in Burgos, P. papatasi in Huelva and P. perniciosus in Segovia). These observations reveal the lack of systematic surveys for the Iberian Peninsula and Balearic Islands, thus generating gaps in the data available or, in other words, a lack of representation of all the species present in a given zone.
According to the statistical treatment of the data, some variables were found to have a significant impact on the density of certain sand fly species. First, a higher density of S. minuta was observed in rural habitats. This was expected as this species is clearly a herpetophile [30]. Notwithstanding, in a study by Benito-de Martín et al. [31] in Zaragoza, a greater density of this species was observed in periurban rather than in rural settings.
Phlebotomus ariasi was also detected at higher densities in rural habitats. The explanation for this is that this species is exophilic, and consequently, prefers natural environments and daytime resting places far from human settlements [30].
Finally, P. perniciosus showed greater densities in the thermo-Mediterranean compared with the meso-Mediterranean belt. While this is a cosmopolitan species that thrives in different bioclimatic zones [2, 30], its preferred thermo-Mediterranean region features a warm climate with long summers allowing for two abundance peaks, one in early summer and the other at the beginning of autumn [2, 32]. In addition, compared with the meso-Mediterranean belt, the thermo-Mediterranean belt also showed significantly higher densities of both vector species (P. perniciosus and P. ariasi) and total sand fly species. However, this trend noted is clearly biased as P. perniciosus represented 83.13% of all species. Phlebotomine vectors of L. infantum are sensitive to climate variations, and in the Mediterranean subregion were identified in the period of June-October [32]. Bioclimate predetermines the time needed for completion of sand fly development and life-cycle progression of the parasite within the invertebrate [33].
Conclusions
This latest knowledge of seroprevalence distributions of canine L. infantum infection in Spain defines non-endemic, hypoendemic, endemic and hyperendemic areas. As far as we are aware this is the first time to propose a CanL map for the whole territory of Spain. Along with the distributions of the parasiteʼs sand fly vectors, this information is key to approaching the control of this significant zoonosis. Further, these detailed maps of CanL in Spain including more than three decades (1985–2019) are an important resource for future eco-epidemiological analyses at the national and regional levels. Both bibliography and survey maps combine the information needed for improved management of Canl and could exhibit an accurate prevalence predictive values. The methodology used to build the figure compendium can be extrapolated to other countries or even at the European level.
Availability of data and materials
All data generated or analysed during this study are included in this published article. Raw data are available from the corresponding author upon reasonable request.
Abbreviations
- CanL:
-
canine leishmaniosis
- IFAT:
-
indirect immunofluorescence antibody test
References
Gálvez R, Montoya A, Fontal F, Martínez De Murguia L, Miró G. Controlling phlebotomine sand flies to prevent canine Leishmania infantum infection: a case of knowing your enemy. Res Vet Sci. 2018;121:94–103.
Gálvez R, Descalzo MA, Miró G, Jiménez MI, Martín O, Dos Santos-Brandao F, et al. Seasonal trends and spatial relations between environmental/meteorological factors and leishmaniosis sand fly vector abundances in central Spain. Acta Trop. 2010;115:95–102.
Solano-Gallego L, Miró G, Koutinas A, Cardoso L, Pennisi MG, Ferrer L, et al. LeishVet guidelines for the practical management of canine leishmaniosis. Parasites Vectors. 2011;4:86.
Short EE, Caminade C, Thomas BN. Climate change contribution to the emergence or re-emergence of parasitic diseases. Infect Dis. 2017;10:1178633617732296.
Fouque F, Reeder JC. Impact of past and on-going changes on climate and weather on vector-borne diseases transmission: a look at the evidence. Infect Dis Poverty. 2019;8:51.
Moreno J, Alvar J. Canine leishmaniasis: epidemiological risk and the experimental model. Trends Parasitol. 2002;18:399–405.
Checa R, Gálvez R, Montoya A, Marino V, Betancor A, Fernández C, et al. New seroprevalence for mapping L. infantum infection and epidemiological data affecting dogs in Spain. In: 6 World congress on leishmaniasis, 16–20 May, Toledo, Spain; 2017. p. 1453.
Miró G, Checa R, Montoya A, Hernández L, Dado D, Gálvez R. Current situation of Leishmania infantum infection in shelter dogs in northern Spain. Parasites Vectors. 2012;5:60.
Ready PD. Leishmaniasis emergence in Europe. Euro Surveill. 2010;15:19505.
Miró G, Cardoso L, Pennisi MG, Oliva G, Baneth G. Canine leishmaniosis—new concepts and insights on an expanding zoonosis: part two. Trends Parasitol. 2008;24:371–7.
Baneth G, Koutinas AF, Solano-Gallego L, Bourdeau P, Ferrer L. Canine leishmaniosis—new concepts and insights on an expanding zoonosis: part one. Trends Parasitol. 2008;24:324–30.
Rivas-Martínez S. Pisos bioclimáticos de España. Lazaroa. 1983;5:33–43.
Mancianti F, Meciani N. Specific serodiagnosis of canine leishmaniasis by indirect immunofluorescence, indirect hemagglutination, and counterimmunoelectrophoresis. Am J Vet Res. 1988;49:1409–11.
Abonnenc E. Les phlébotomes de la région éthiopienne (Diptera, Psychodidae). Mémoires ORSTOM. 1972;55:289.
Upton MS. Aqueous gum-chloral slide mounting media: an historical review. Bull Entomol Res. 1993;83:267–74.
Gil Collado J, Morillas Marquez F, Sanchis Marin MC. Phlebotomus in Spain. Rev Sanid Hig Publica. 1989;63:15–34.
Ballart C, Alcover MM, Picado A, Nieto J, Castillejo S, Portus M, et al. First survey on canine leishmaniasis in a non classical area of the disease in Spain (Lleida, Catalonia) based on a veterinary questionnaire and a cross-sectional study. Prev Vet Med. 2013;109:116–27.
Reisen WK. Landscape epidemiology of vector-borne diseases. Annu Rev Entomol. 2010;55:461–83.
Ready PD. Leishmaniasis emergence and climate change. Rev Sci Tech. 2008;27:399–412.
Gálvez R, Miró G, Descalzo MA, Nieto J, Dado D, Martín O, et al. Emerging trends in the seroprevalence of canine leishmaniosis in the Madrid region (central Spain). Vet Parasitol. 2010;169:327–34.
Velez R, Ballart C, Domenech E, Abras A, Fernández-Arévalo A, Gómez SA, et al. Seroprevalence of canine Leishmania infantum infection in the Mediterranean region and identification of risk factors: the example of north-eastern and Pyrenean areas of Spain. Prev Vet Med. 2019;162:67–75.
Muniesa A, Peris A, Castillo JA, de Blas I. Variations in seroprevalences of canine leishmaniasis: could it be a consequence of the population structure? Vet Parasitol. 2016;226:5–9.
Miró G, Petersen C, Cardoso L, Bourdeau P, Baneth G, Solano-Gallego L, et al. Novel areas for prevention and control of canine leishmaniosis. Trends Parasitol. 2017;33:718–30.
Aransay AM, Testa JM, Morillas-Márquez F, Lucientes J, Ready PD. Distribution of sandfly species in relation to canine leishmaniasis from the Ebro Valley to Valencia, northeastern Spain. Parasitol Res. 2004;94:416–20.
Gállego M, Rioux JA, Rispail P, Guilvard E, Gállego J, Portús M, et al. Primera denuncia de flebotomos (Diptera, Psychodidae, Phlebotominae) en la provincia de Lérida (España, Cataluña). Rev Iber Parasitol. 1990;50:123–7.
Lucientes Curdi J, Benito de Martín I, Castillo Hernández JA, Orcajo-Teresa J. Seasonal dynamics of Larroussius species in Aragon (N.E. Spain). Parassitologia. 1991;33:381–6.
Rioux JA, Golvan YJ, Maistre O. Presence de Phlebotomus (Larroussius) ariasi Tonnoir, 1921 dans Ies departements de l’Aveyron, des Bouches-du-Rhone, du Gard., de I’HerauIt, des Pyrenees-Orientales et du Vaucluse. Ann Parasitol Hum Comp. 1961;36:706–7.
Martínez Ortega E, Conesa Gallego E. Fenología de los flebotomos del subgénero Larroussius (Dip. Psychodidae, Phlebotomus) en el sureste de la Península Ibérica. Bol Asoc Esp Entomol. 1987;11:293–300.
Ready PD. Biology of phlebotomine sand flies as vectors of disease agents. Annu Rev Entomol. 2013;58:227–50.
Lladó Villalonga T, Rotger Sureda MJ. Estudio del flebotomo como vector de la leishmaniasis en la isla de Mallorca. Palma de Mallorca: Conselleria de Sanitat i Seguretat Social Govern Balear; 1990.
Benito-De Martín MI, Lucientes-Curdi J, Orcajo-Teresa J, Castillo-Hernández JA. Seasonal dynamics of Sergentomyia minuta (Rondani, 1843) populations in Aragon (N.E. Spain). Parassitologia. 1991;33(Suppl.):89–97.
Alten B, Maia C, Afonso MO, Campino L, Jimenez M, Gonzalez E, et al. Seasonal dynamics of phlebotomine sand fly species proven vectors of Mediterranean leishmaniasis caused by Leishmania infantum. PLoS Negl Trop Dis. 2016;10:e0004458.
Maia C, Cardoso L. Spread of Leishmania infantum in Europe with dog travelling. Vet Parasitol. 2015;213:2–11.
Sáez de Santamaría M, Benito A, Eraso E, Beltrán de Heredia F, Estíbalez JJ, Guisantes JA. Estudio seroepidemiológico de la leishmaniosis canina en Álava, España. Acta Parasitol Port. 1997;4:160.
Benito A, Alvar, J. Estudio preliminar de la leishmaniosis en la Comunidad de Castilla la Mancha. In: VI Congreso Nacional de Parasitología, 25–29 September, Cáceres, Spain; 1989; p. 194.
Alonso F, Gimenez Font P, Manchon M, Ruiz de Ybanez R, Segovia M, Berriatua E. Geographical variation and factors associated to seroprevalence of canine leishmaniosis in an endemic Mediterranean area. Zoonoses Public Health. 2010;57:318–28.
Sanchís Marín MC, Martín Sánchez J, Amate P, Acedo Sánchez C, Miras N, Mostapha L, et al. Estudio epidemiológico de la leishmaniosis en la comarca del Campo de Níjar (Almería). Ars Pharm. 1997;38:53–61.
Rosado D, Suero F, Garrudo RM, Mirón C, García Alonso M, Navarrete I, et al. La leishmaniosis en Zafra (Badajoz, España) del reservorio canino y del agente vectorial. Acta Parasitol Port. 1997;4:160.
Asencio MA, Herraez O, Tenias JM, Garduno E, Huertas M, Carranza R, et al. Seroprevalence survey of zoonoses in Extremadura, southwestern Spain, 2002–2003. Jpn J Infect Dis. 2015;68:106–12.
Seguí MG. Estudi epidemiòlogic de la leishmaniosi a l´illa de Menorca. Revista de Ciencia. 1991;9:91–101.
Pujol A, Cortés E, Ranz A, Vela C, Aguiló C, Martí B. Estudio de seroprevalencia de leishmaniosis (L. infantum) y de ehrlichiosis (E. canis) en la isla de Mallorca mediante técnicas inmunológicas. In: Congreso de la Asociación de Veterinarios Especialistas en Diagnóstico de Laboratorio (AVEDILA), 22–23 September, Palma de Mallorca, Spain; 2005. p. 9–12.
Solano-Gallego L, Llull J, Osso M, Hegarty B, Breitschwerdt E. A serological study of exposure to arthropod-borne pathogens in dogs from northeastern Spain. Vet Res. 2006;37:231–44.
Alcover MM, Ballart C, Serra T, Castells X, Scalone A, Castillejo S, et al. Temporal trends in canine leishmaniosis in the Balearic Islands (Spain): a veterinary questionnaire. Prospective canine leishmaniosis survey and entomological studies conducted on the Island of Minorca, 20 years after first data were obtained. Acta Trop. 2013;128:642–51.
Botet J, Serra T, Portus M, Mora R, Gallego M. Incidencia de la leishmaniosis en el área de Barcelona. Rev Ibérica Parasitol. 1987;extra:51–54.
Corachan M, Portus M, Filella E, Olle JE. Leishmaniasis in Catalonia. Preliminary study. Rev Sanid Hig Publica. 1987;61:1229–36.
Encinas Grandes A, Gómez-Bautista M, Martín Novo M, Simón Martín F. Leishmaniasis in the province of Salamanca, Spain. Prevalence in dogs and seasonal dynamics of vectors. Ann Parasitol Hum Comp. 1988;63:387–97.
Nieto CG, Navarrete I, Habela M, Hernández-Rodríguez S. Seroprevalence of canine leishmaniasis around Cáceres, Spain. Prev Vet Med. 1992;13:173–8.
Rosado D, Arrieta E, Verdugo SG, García Alonso M, Pérez Martín E. Estado actual de la Leishmaniosis canina en la ciudad de Cáceres. Rev Exp Anim. 1995;6:93.
Morales-Yuste M, Morillas-Marquez F, Díaz-Saez V, Barón-López S, Acedo-Sánchez C, Martín-Sánchez J. Epidemiological implications of the use of various methods for the diagnosis of canine leishmaniasis in dogs with different characteristics and in differing prevalence scenarios. Parasitol Res. 2012;111:155–64.
Arnedo Pena A, Bellido Blasco JB, González Morán F, Arias Sánchez A, Calvo Mas C, Safont Adsuara L, et al. Leishmaniasis in Castellon: an epidemiological study of human cases, the vector and the canine reservoir. Rev Sanid Hig Publica. 1994;68:481–91.
Martínez-Cruz MS, Martínez-Moreno A, Martínez-Moreno FJ, Martínez-Gómez F, Hernández-Rodríguez S. Epidemiología de la leishmaniosis canina en Córdoba. Rev Ibérica Parasitol. 1990;50:1–7.
Reyes Magaña A, Morillas-Márquez F, Valero-López A, González-Castro J, Benavides-Delgado I, Sanchís-Marín MC. Encuesta sobre la leishmaniosis canina en las comarcas naturales de la provincia de Granada (Sur de España). Rev Ibérica Parasitol. 1988;48:233–40.
Reyes Magaña A, Morillas Márquez F, Montes Ramírez E, Sanchís Marín MC, Benavides Delgado I, González Castro J. La leishmaniosis en la provincia de Granada: estudio de la enzootia canina. Ars Pharm. 1989;30:35–44.
Acedo Sánchez C, Martín Sánchez J, Velez Bernal ID, Sanchís Marín MC, Louassini M, Maldonado JA, et al. Leishmaniasis eco-epidemiology in the Alpujarra region (Granada Province, southern Spain). Int J Parasitol. 1996;26:303–10.
Acedo Sánchez C, Morillas-Márquez F, Sanchiz-Marin MC, Martín-Sánchez J. Changes in antibody titres against Leishmania infantum in naturally infected dogs in southern Spain. Vet Parasitol. 1998;75:1–8.
Martín-Sánchez J, Morales-Yuste M, Acedo-Sánchez C, Barón S, Díaz V, Morillas-Márquez F. Canine leishmaniasis in southeastern Spain. Emerg Infect Dis. 2009;15:795–8.
Lepe JA, Ubeda JM, Morillas F, Guevara DC, Martín-Sánchez J, Guerrero FJ, Sanchos-Marín MC, Perea R. Epidemiology of leishmaniosis in the nature reserve of Sierra de Aracena and Picos de Aroche (Southwest Spain). Res Rev Parasitol. 2000;60:113–9.
Celaya C. Estudio epidemiológico descriptivo de la leishmaniasis canina en un entorno periurbano de la ciudad de Madrid. Veterinaria en Madrid. 1993;5:13–5.
Amela C, Méndez I, Torcal JM, Medina G, Pachón I, Cañavate C, et al. Epidemiology of canine leishmaniasis in the Madrid region, Spain. Eur J Epidemiol. 1995;11:157–61.
Castañeda R, Carpio I, Asensio A, Alberdi JC, García S, Domínguez T, et al. Estudio de seroprevalencia de la leishmaniosis canina en perros censados en Madrid. Consulta de Difusión Veterinaria. 1999;7:836–41.
Castañeda R, García A, Miró G, Carpio I, Caballero MJ, Alberdi JC, et al. Estudio de seguimiento de la leishmaniosis canina en perros vagabundos de la Comunidad de Madrid. Pequeños Animales. 2000;25:16–27.
García Nieto A, Taveira Jimenez JA, Castañeda López R, Moreno Alcalde S, Escacena Saínz C, Martínez Cortés M. Prevalencia de la leishmaniosis canina en el sistema de vigilancia en perros vagabundos de la Comunidad de Madrid durante el periodo 11-1996 a 4-2001. Profesión Veterinaria. 2002;53:56–61.
Miró G, Montoya A, Mateo M, Alonso A, García S, García A, et al. A leishmaniosis surveillance system among stray dogs in the region of Madrid: ten years of serodiagnosis (1996–2006). Parasitol Res. 2007;101:253–7.
Miro G, Muller A, Montoya A, Checa R, Marino V, Marino E, et al. Epidemiological role of dogs since the human leishmaniosis outbreak in Madrid. Parasites Vectors. 2017;10:209.
Morillas F, Sánchez Rabasco F, Ocaña J, Martín-Sánchez J, Ocana-Wihelmi J, Acedo C, et al. Leishmaniosis in the focus of the Axarquia region, Malaga Province, southern Spain: a survey of the human, dog, and vector. Parasitol Res. 1996;82:569–70.
Sesma B, Barricarte A. Leishmaniasis en Navarra: Revisión de actuaciones. Anales Sis San Navarra. 1997;20:209–16.
Amusátegui I, Sainz A, Aguirre E, Tesouro MA. Seroprevalence of Leishmania infantum in northwestern Spain, an area traditionally considered free of leishmaniasis. Ann NY Acad Sci. 2004;1026:154–7.
Ariza-Astolfi C, Úbeda-Ontiveros JM, Guevara-Benítez D, Cutillas-Barrios C, de Rojas-Álvarez M, Reina-Mulero F. Epidemiología de la leishmaniosis canina en la Provincia de Sevilla (España). In: X Congreso Ibérico de Parasitología, 15–20 July, Madrid, Spain; 2007. p. 49.
Portús M, Fisa R, Serra T, Gállego M, Mora R. Estudios seroepidemiológicos sobre la leishmaniosis canina en Cataluña. Med Vet. 1987;4:569–75.
Fisa R, Gallego M, Portús M, Gallego J. Evolución de la leishmaniosis canina en zona endémica a través de su seguimiento serológico. Ciencias Veterinarias. 1991;4:69–76.
Fisa R, Portús M, Gallego M, Valls D, Alisa MJ. El diagnóstico serológico de la leishmaniosis canina en la comarca del Priorat (Tarragona). Clínica Veterinaria de Pequeños Animales. 1992;12:231–6.
Fisa R, Gallego M, Riera C, Aisa MJ, Valls D, Serra T, de Colmenares M, Castillejo S, Portús M. Serological diagnosis of canine leishmaniasis by dot-ELISA. J Vet Diagn Invest. 1997;9:50–5.
Fisa R, Gállego M, Castillejo S, Aisa MJ, Serra T, Riera C, Carrio J, Gállego J, Portús M. Epidemiology of canine leishmaniosis in Catalonia (Spain) the example of the Priorat focus. Vet Parasitol. 1999;83:87–97.
Benito Hernández M, Pérez-Díaz JV, Osuna-Calvet B, Domínguez MT, Vega-García S. The importance of a continued serovigilance in the control of canine leishmaniosis. A comparison of two methods of control. Épidemiol Santé Anima. 2004;45:87–90.
Couto CG, Lorentzen L, Beall MJ, Shields J, Bertolone N, Couto JI, et al. Serological study of selected vector-borne diseases in shelter dogs in central Spain using point-of-care assays. Vector Borne Zoonotic Dis. 2010;10:885–8.
Castillo Hernández JA, Sánchez Acedo C, Gutiérrez Galindo J, Lucientes Curdi J, Estrada Peña A et al. Evaluación de diversas pruebas en el diagnóstico de la leishmaniasis canina. In: IV Congreso Nacional de Parasitología. 9–13 July, Tenerife, Spain; 1985. p. 31.
Acknowledgements
The authors wish to thank the owners of the dogs for their valuable collaboration. Publication of this paper was sponsored by Bayer Animal Health in the framework of the 15th CVBD® World Forum Symposium.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
RG and GM conceived and coordinated the study. AM, RCH, CF, VM and OM participated on the completion and reporting of the serological surveys. IC, CCH and SM participated on the design, completion and reporting of the entomological survey. RG and GM were involved in the drafting of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was carried out in accordance with international guidelines for the Care and Use of Experimental Animals and Spanish Legislation (RD 53/2013).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Detailed information about sites surveyed for sand flies across the Iberian Peninsula and Balearic Islands.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gálvez, R., Montoya, A., Cruz, I. et al. Latest trends in Leishmania infantum infection in dogs in Spain, Part I: mapped seroprevalence and sand fly distributions. Parasites Vectors 13, 204 (2020). https://doi.org/10.1186/s13071-020-04081-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-020-04081-7