Abstract
Background
Ixodes barkeri, a tick with a distinctive ventrolateral horn-like projection on palpal segment 1, was described in 2019 from two male ticks from the Wet Tropics of Far North Queensland, Australia. However, females lie at the core of the taxonomy and subgenus classification of Ixodes; hence, we sought specimens of female ticks, successfully recovering females, plus nymphs and larvae. Mitochondrial genomes are also desirable additions to the descriptions of species of ticks particularly regarding subgenus systematics. So, we sequenced the mt genomes of I. barkeri Barker, 2019, and the possible relatives of I. barkeri that were available to us (I. australiensis Neumann, 1904, I. fecialis Warburton & Nuttall, 1909, and I. woyliei Ash et al. 2017) with a view to discovering which if any of the subgenera of Ixodes would be most suitable for I. barkeri Barker, 2019.
Results
The female, nymph, larva and mitochondrial genome of Ixodes barkeri Barker, 2019, are described for the first time and the male of I. barkeri is redescribed in greater detail than previously. So far, I. barkeri is known only from a monotreme, the short-beaked echidna, Tachyglossus aculeatus (Shaw, 1792), from the highland rainforests of the Wet Tropics of Far North Queensland, Australia.
Conclusions
Our phylogeny from entire mitochondrial genomes indicated that I. barkeri and indeed I. woyliei Ash et al., 2017, another tick that was described recently, are best placed in the subgenus Endopalpiger Schulze, 1935.
Graphical Abstract

Similar content being viewed by others
Background
Ixodes barkeri Barker, 2019, was described from two male ticks collected from the short-beaked echidna Tachyglossus aculeatus (Shaw, 1792). One of these ticks was from rainforest of the Peeramon Scrub, Atherton Tableland, Far North Queensland (FNQ), whereas the other male was from an unknown locality in the vicinity of the Atherton Tableland. Ixodes barkeri is distinctive among Australian ticks, especially for its ventrolateral palpal projection, which is found only in an echidna tick from Papua New Guinea, I. zaglossi Kohls, 1960. Ixodes zaglossi, however, has syncoxae, whereas I. barkeri does not, and the ventrolateral palpal projection in I. barkeri is much bigger [1]. However, this extraordinary species, I. barkeri, could not be placed within a higher taxonomic framework, such as the subgenus classification of Ixodes, largely because of the lack of females. Since [1], we have acquired other specimens of I. barkeri: 6 males, 5 females, 34 nymphs and 2 larvae (Table 1, Fig. 1), allowing us to describe the female, nymph and larvae for the first time and to redescribe the male in greater detail to compare and contrast the morphology of I. barkeri with other species of the Australasian Ixodes clade (sensu [2]). We have also described the mitochondrial genome of I. barkeri, enabling inferences of phylogenetic relationships of this species with others in the genus.
The subgenera of Ixodes are morphologically ambiguous and in need of further refinement and testing with genetic data. In this regard, mitochondrial (mt) genomes have been remarkably instructive about the evolutionary history (phylogeny) of ticks (e.g. [3,4,5,6,7]). Thus, we sequenced the mt genomes of I. barkeri and its possible relatives that were available to us (I. australiensis Neumann, 1904, I. fecialis Warburton & Nuttall, 1909, and I. woyliei Ash et al. 2017) with a view to discovering which if any of the subgenera of Ixodes would be most suitable for I. barkeri Barker, 2019; [8] and [1] were not able to place I. woyliei and I. barkeri in a subgenus, respectively.
Methods
Material examined
Only field-collected ticks were available for study. The specimens were from the Barker and Barker Collection at the University of Queensland (Qld), the Queensland Museum (QM), the Australian National Insect Collection (ANIC) and the Field Museum of Natural History, Chicago, Illinois, USA (FM) (Table 1).
Microscopy methods
Ticks were studied using a stereoscopic microscope (Nikon SMZ800N, Nikon Corp., Tokyo, Japan, and Olympus SZX16, Olympus Corp., Tokyo, Japan), compound microscope (Olympus BX53, Olympus Corp., Tokyo, Japan) and scanning electron microscope (JEOL JSM6610LV, JEOL Ltd., Tokyo, Japan). An ocular micrometre was used to measure ticks. Measurements are in millimetres for the adults, micrometres for the juveniles, and are given as the range followed by the mean and the number of specimens measured (n) in parentheses. Colour digital images were taken with a Canon 6D camera (Canon Corp., Tokyo, Japan).
Adobe Photoshop® software was used to correct images for broken legs and other damaged parts of the tick and to polish the image.
Sequencing and assembly of mitochondrial genomes
Mitochondrial genomes were sequenced and assembled in two ways. First, the mt genome of I. barkeri Barker, 2019, was sequenced at Novogene Singapore and then assembled at the University of Queensland (UQ) by the protocol of [5]. DNA was prepared by us at the University of the Sunshine Coast and the University of Queensland. We extracted DNA from individual ticks and from various pools (groups of up to 3 ticks) of females, males, nymphs and larvae in an effort get adequate DNA for our experiments from all life-stages i.e. females, males, nymphs and larvae (Below we report that adequate DNA was obtained from a male and a pool of 3 nymphs but not from females nor larvae since the females and larvae had not been preserved well). Ticks were cut in half and then incubated at 56 °C for 62 h with Proteinase K to lyse the cells. The QIAGEN DNeasy Blood and Tissue kit was used to extract genomic DNA. The amount of DNA recovered was measured with Nanodrop and Qbit instruments. Groups of ticks that yielded > 200 nanograms (ng) of DNA were sent to Novogene Singapore for de novo library construction and next-generation Illumina sequencing. Groups of ticks with < 200 ng were combined with DNA from a different organism, usually a bird, to reach the minimum threshold of 200 ng of DNA required by Novogene Singapore. At Novogene Singapore, DNA was sonicated to fragment the DNA, and then fragments were end-polished, A-tailed and ligated with Illumina adaptors. DNA fragments were amplified with PCR, using P5 and P7 oligos, to create genomic libraries, which were purified with AMPure XP system. The Illumina Novaseq 6000 sequencing platform was used to generate two giga-bases of nucleotide sequence data (PE 150). We then constructed de novo contig assemblies of Illumina sequences in Geneious Prime [9] by the default assembler of Geneious Prime. Blast-searches of contigs revealed mt genes of ticks; these gene sequences were then assembled until entire mt genomes had been assembled.
Second, the mt genomes of I. australiensis, I. fecialis and I. woylie were sequenced at the Hokkaido University, Japan, and then assembled at the University of Queensland by the protocol of [5]. DNA was extracted from ticks with the NucleoSpin® DNA Insect (Macherey-Nagel, Germany). Entire mt genomes were then amplified in two overlapping fragments (long-range and short PCRs). Long-range PCR was used to amplify fragments that comprised about 12–13 kb of the mitogenome with the universal primers: mtG_K23 (5’-TCCTACATGATCTGAGTTYAGACCG-3’) and mtG_K25 (5’-AAAATTCWTAGGGTCTTCTTGTCC-3’) or mtG_K26 (5’-ACGGGCGATATGTRCATATTTTAGAGC-3’). Short PCRs were then used to amplify 1.5–2.5 kb of mt genomes with genus-specific primers. PrimeSTAR® GXL DNA Polymerase (Takara-Bio, Shiga, Japan) was used to amplify the long mt PCR products, whereas Tks Gflex™ DNA Polymerase (Takara-Bio) was used to amplify short mt gene fragments as well as nuclear rRNA genes. PCR conditions for PrimeSTAR® GXL DNA Polymerase were: 45 cycles of 98 °C for 10 s, 60 °C for 15 s and 68 °C for 10 min. PCR conditions for Tks Gflex™ DNA Polymerase were: 94 °C for 60 s, 45 cycles of 98 °C for 10 s, 55 °C for 15 s, 68 °C for 60 s and a final extension of 68 °C for 5 min. PCR products were examined on 1.2% agarose gels stained with Gel-Red™ (Biotium, Hayward, CA). PCR products were purified with a NucleoSpin Gel and PCR Clean Up Kit (Takara-Bio). Illumina sequencing libraries were constructed from the PCR fragments from the long-range and short PCR reactions with the Nextera DNA Library Prep Kit (Illumina, Hayward, CA) and were sequenced with the Illumina MiSeq platform with the MiSeq reagent kit v3 for 600 cycles.
Annotation of mitochondrial genomes
Mitochondrial genomes were annotated with Geneious Prime. Protein-coding genes were identified by searches with BLAST [10] for open reading frames. Regions between protein-coding genes were searched with BLAST [10] to find rRNA genes, tRNA genes and control regions. The tRNA that we expected to find but did not find with BLAST was found with the aid of the tRNAscan-SE Search Server v1.21 [11] and the MITOS Web Server [12]. The nucleotide sequences of tRNA genes were confirmed by studying the putative secondary structure of transcripts, as implemented in Geneious Prime [9].
Phylogenetic methods
Phylogenies were inferred by both maximum likelihood (ML) and Bayesian inference (BI) methods implemented in the RAXML-HPC2 v 8.2.12 [13] and MrBayes v3.2.2 [14], respectively. JmodelTest2 v2.1.6 [15] was used to find the optimal substitution model for the nucleotide dataset. The GTR + G + I model was found to be the best fit for our dataset. In all ML and BI runs (experiments), genes were partitioned. Rapid bootstrapping of 1000 replicates of our data was executed in RAXML-HPC2 v 8.2.12 [13]. There were two simultaneous BI runs: 10 million generations sampled every 1000 MCMC steps. For every BI run, four MCMC chains (three heated and one cold) were executed. The first 25% of steps was discarded as burn-in. Tracer v 1.5 [16] was used to observe the effective sample size (ESS) and convergence of independent runs. Phylogenetic trees were displayed in FigTree v 1.4.4 [17]. Branch support in the phylogenetic trees generated by RAXML-HPC2 v 8.2.12 [12] and MrBayes v 3.2.2 [14] was assessed by the bootstrap values and posterior probability values, respectively. All phylogenies were inferred through the CIPRES Science Gateway v.3.3 [18]. Ixodes pavlovskyi Pomerantzev, 1946, a species from the clade of the “other Ixodes” (sensu [2]) was the out-group.
Results
Systematics
Family Ixodidae Murray, 1877
Genus Ixodes Latreille, 1795
Subgenus Endopalpiger Schulze, 1935
Figures 2, 3, 4, 5, 6, 7, 8, 9, 10
Ixodes barkeri Barker, 2019
Male. (Based on 6 specimens, 3 measured—refer to Table 1; Figs. 2, 3, 8) Idiosoma (Fig. 2) broadly elongate-oval with broadly rounded posterior margin, widest slightly posterior to mid-length; length from apices of scapulae to posterior body margin 2.17–2.59 mm (2.42 mm), width 1.53–1.72 (1.61), ratio 1.42–1.56 (1.49). Lateral groove distinct. Conscutum (Figs. 2, 8) length 2.12–2.56 (2.38), width 1.37–1.48 (1.44), ratio 1.54–1.73 (1.65); laterally and postero-laterally with a distinct narrow non-sclerotised band; scapulae short, blunt; lateral carinae absent; cervical grooves indistinct; dense moderately large punctations evenly distributed over conscutum, except for pseudoscutum area; pseudoscutum with indistinct punctations; setae moderately dense, very short (c. 0.01) and indistinct (Fig. 2). Venter plate outlines as illustrated (Fig. 2); median plate: length 1.06–1.18 (1.10), width 0.74–0.84 (0.81), ratio 1.26–1.43 (1.37); adanal plate: length 0.74–0.82 (0.79), width 0.42–0.48 (0.45), ratio 1.67–1.95 (1.77); anal plate: 0.54–0.60 (0.58), width 0.32–0.36 (0.34), ratio 1.67–1.71 (1.69). All ventral plates with dense, moderately large punctations (Figs. 1, 2). Genital aperture (Fig. 2C) located at level of posterior margin of coxae II; posterior margin of genital apron deeply concave. Ventral setae (Fig. 2) moderately dense, very short, evenly distributed on all plates; length of setae on median plate c. 0.01. Anal groove (Fig. 2C) straight anteriorly and open posteriorly. Spiracular plate (Fig. 2A) broadly oval, longer than wide, length 0.34–0.42 (0.38), width 0.28–0.34 (0.31), ratio 1.21–1.25 (1.23).
Ixodes barkeri Barker, 2019, scanning electron micrographs of male. A Spiracular plate (arrows show orientation of spiracular plate: a, anterior; d, dorsal). B Gnathosoma, dorsal view. C Gnathosoma, ventral view. D Gnathosoma, anteroventral view. E Coxae. F Trochanter I, dorsal view. Scale bars: A–D, F 0.1 mm; E, 0.2 mm
Gnathosoma (Figs. 3, 8) length from palpal apices to cornual apices dorsally 0.45–0.48 (0.46), width between lateral projection of palpal segments I 0.43–0.48 (0.46), ratio 0.98–1.04 (1.01). Dorsal basis capituli (Fig. 3A) length from medial insertion of palpal segment I to cornual apices 0.21–0.25 (0.23), width 0.32–0.35 (0.33), ratio width to length 1.41–1.52 (1.46), subrectangular, posterior margin nearly straight; cornua long, ratio total length of basis capituli, including cornua, to cornual length 4.83–5.78 (5.39), triangular with narrowly rounded apex. Ventral basis capituli (Fig. 3C) subrectangular; lateral margins with slight constrictions at mid-length; auriculae absent; short converging ridges in auricular areas. Palpi (Fig. 3B) short, length dorsally (segments II and III) 0.23–0.24 (0.23), maximum width (in dorsolateral plane) 0.18–0.19 (0.19), ratio 1.22–1.29 (1.26), length of palpal segment I ventrally 0.16 (n = 1), maximum width ventrally 0.17 (n = 1), ratio 0.94 (n = 1); segment I greatly enlarged, greatest dimension in anteromedian-posterolateral direction; dorsally segment I subrectangular; ventrally segment I subtriangular, posterior margin with very long and narrow spur with sharply pointed apex; segments II and III fused together with indistinct suture between them, narrower proximally and abruptly widening to broadly rounded apex. Hypostome (Fig. 3C) length 0.18–0.19 (0.18), width 0.08–0.10 (0.09), ratio 1.75–2.10 (1.95); club-shaped, widening to broadly rounded apex with medial indentation; base of hypostome at level of base of palpal segment I; dental formula 3/3, basal half of hypostome without denticles, denticles sharply pointed.
Legs moderately long, slender. Coxae (Figs. 2E, 9): coxae I–IV with long and narrow external spur with narrowly rounded to sharply pointed apex; spur on coxae I–III subequal, spur on coxa IV nearly twice shorter those on coxae I–III; coxae I–IV without syncoxae. Trochanter I with long, triangular spur with sharply pointed apex; trochanters I–IV with long, narrow, with sharply pointed apex spur ventrally. Tarsus I: length 0.56–0.60 (0.58); tarsus IV length 0.53–0.58 (0.55); tarsi only slightly humped subapically.
Female. (Based on 1 to 5 specimens—refer to Table 1; Figs. 4, 5, 9) Idiosoma (Figs. 4A, 9) length from scapular apices to posterior body margin in moderately engorged specimen 4.1 (n = 1), width in moderately engorged 2.5 (n = 1), ratio 0.16 (n = 1), broadly suboval, widest approximately at mid-length. Scutum (Figs. 4, 9) length 1.05–1.18 (1.13; n = 4), width 1.40–1.58 (1.49; n = 4), ratio 0.73–0.79 (0.75; n = 4); lateral margins diverging for approximately 2/3 of scutum length, broadly rounded posteriorly; lateral carinae lacking; cervical grooves shallow; dense, small punctations evenly distributed throughout scutum; setae (Fig. 4) relatively sparse, very short (c. 0.005), indistinct and nearly equal to those on alloscutum, distributed as figured. Alloscutum (Fig. 4) as illustrated; setae of alloscutum (Fig. 4C) numerous, evenly distributed, very short, length of setae in central field c. 0.01, indistinct. Venter (Fig. 4E) as illustrated; genital aperture (Fig. 4E) medial to coxae III; genital groove (Fig. 4E) well developed; anal groove (Fig. 4E) oval with open posterior margin; ventral setae numerous, length of preanal setae c. 0.01, evenly distributed. Spiracular plates (Fig. 5A) length 0.27–0.32 (0.30; n = 4), width 0.39–0.47 (0.44; n = 4), ratio 0.68–0.70 (0.69; n = 4); broadly oval; marginal row of perforations in groove anteriorly.
Ixodes barkeri Barker, 2019, scanning electron micrographs of female. A Idiosoma, dorsal view. B Scutum, dorsal view. C Scutum, dorsolateral view. D Idiosoma showing scutum and alloscutum with punctations and setae, dorsal centrolateral portion. E Idiosoma, ventral view. Scale bars: A, E 0.5 mm; B, C 0.2 mm; D 0.1 mm
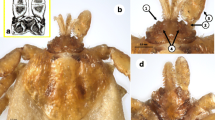
Ixodes barkeri Barker, 2019, scanning electron micrographs of female. A Spiracular plate (arrows show orientation of spiracular plate: a, anterior; d, dorsal). B Gnathosoma, dorsal view. C Gnathosoma, ventral view (I, palpal article 1; II, palpal article 2; ss the strongly salient part of palpal article 1). D Gnathosoma, anteroventral view. E Coxae. F Trochanter I, dorsal view. Scale bars: A, F, 0.1 mm; B–E, 0.2 mm
Gnathosoma (Fig. 5B) length from palpal apices to cornual apices dorsally 0.60–0.70 (0.65; n = 4), width between lateral projection of palpal segments I 0.66–0.74 (0.71; n = 4), ratio 0.89–0.95 (0.92; n = 4). Dorsal basis capituli (Fig. 5B) length from medial insertion of palpal segment I to cornual apices 0.22–0.25 (0.24; n = 4), width 0.51–0.55 (0.54; n = 4), ratio width to length 2.20–2.32 (2.26; n = 4), subrectangular, posterior margin nearly straight; cornua short, ratio of total length of basis capituli (including cornua) to cornual length 17.50–20.00 (19.12; n = 4), triangular with broadly rounded apex; subcircular porose areas poorly distinct, not indented, separated by distance nearly equal to their own width, several openings arranged in discrete punctations. Ventral basis capituli (Fig. 5C) subrectangular; lateral margins with slight constrictions at mid-length; auriculae absent; short converging ridges in auricular areas. Palpi (Fig. 5B) short, length dorsally (segments II and III) 0.41–0.50 (0.46; n = 4), maximum width (in dorsolateral plane) 0.21–0.25 (0.23; n = 4), ratio 1.85–2.10 (1.95; n = 4), length of palpal segment I ventrally 0.31–0.36 (0.34; n = 4), maximum width ventrally 0.19–0.20 (0.20; n = 4), ratio 1.64–1.75 (1.68; n = 4); segment I greatly enlarged, greatest dimension in anteromedian-posterolateral direction; dorsally segment I subrectangular with convex medial margin; ventrally segment I subtriangular, posterior margin with long moderately narrow spur with narrowly rounded apex; segments II and III fused together with indistinct suture between them, narrower proximally and abruptly widening to broadly rounded apex. Hypostome (Fig. 5C) length 0.36–0.40 (0.38; n = 4), width 0.12–0.16 (0.14; n = 4), ratio 2.46–2.85 (2.68; n = 4); club-shaped, widening to broadly rounded apex with medial indentation; base of hypostome approximately at level of base of palpal segment II; dental formula 4/4 (few rows may be 3/3), basal half of hypostome without denticles, denticles sharply pointed.
Legs moderately long, slender. Coxae (Figs. 5E, 9): coxae I–IV with moderately long and narrow external spur with narrowly rounded apex; spur on coxae I–III subequal, spur on coxa IV nearly twice shorter than those on coxae I–III; coxae I–IV without syncoxae. Trochanter I with moderately long, triangular spur with sharply pointed apex; trochanters I–IV without spur ventrally. Tarsus I: length 0.66–0.76 (0.73; n = 4); tarsus IV length 0.57–0.66 (0.63; n = 4); tarsi only slightly humped subapically.
Nymph. (Based on 34 specimens—refer to Table; Fig. 6) Scutum (Fig. 6A) length 515–520 (518; n = 2), width 690–700 (695; n = 2), ratio 0.74–0.75 (0.75; n = 2); lateral margins diverging for approximately half of scutum length, broadly rounded posteriorly; posterolateral margin with slight indentations; lateral carinae lacking; cervical grooves shallow; moderately dense, small punctations evenly distributed throughout scutum; setae (Fig. 6A) relatively sparse, very short: length in central field of scutum 10 (n = 2), indistinct and nearly equal to those on alloscutum, distributed as figured. Setae of alloscutum numerous, evenly distributed, very short, length of setae in central field 10–13 (11; n = 2), indistinct. Anal groove oval with open posterior margin; ventral setae numerous, evenly distributed. Spiracular plates (Fig. 6B) broadly oval; marginal row of perforations in groove anteriorly.
Ixodes barkeri Barker, 2019, scanning electron micrographs of nymph. A Scutum. B Spiracular plate (arrows show orientation of spiracular plate: a, anterior; d, dorsal). C Gnathosoma, dorsal view. D Gnathosoma, ventral view. E Gnathosoma, anteroventral view. F Coxae. Scale bars: A, C–F, 0.1 mm; B, 0.05 mm
Gnathosoma (Fig. 6C) length from palpal apices to cornual apices dorsally 285 (n = 2), width between lateral projection of palpal segments I 375–380 (378; n = 2), ratio 0.75–0.76 (0.76; n = 2). Dorsal basis capituli (Fig. 6C) length from medial insertion of palpal segment I to cornual apices 108 (n = 2), width 238–240 (239; n = 2), ratio width to length 2.21–2.23 (2.22; n = 2), subrectangular, posterior margin nearly straight; cornua moderately long, triangular with narrowly rounded apex. Ventral basis capituli (Fig. 6D) subrectangular; lateral margins with slight constrictions at mid-length; auriculae absent; short converging ridges in auricular areas. Palpi (Fig. 6C) short, length dorsally (segments II and III) 190–193 (191; n = 2), width 73–85 (79; n = 2), ratio 2.24–2.66 (2.45; n = 2), length of palpal segment I ventrally 140–150 (145; n = 2), maximum width ventrally 125–130 (128; n = 2), ratio 1.12–1.15 (1.14; n = 2); segment I greatly enlarged, greatest dimension in anteromedian-posterolateral direction; dorsally segment I subrectangular with convex medial margin; ventrally segment I subtriangular, posterior margin with long moderately broad spur with narrowly rounded apex; segments II and III fused together with indistinct suture between them, narrower proximally and abruptly widening to broadly rounded apex. Hypostome (Fig. 6D) length 150 (n = 1), width 73 (n = 1), ratio 2.07 (n = 1); club-shaped, widening to broadly rounded apex with medial indentation; base of hypostome approximately at level of base of palpal segment II; dental formula 3/3 (few basal rows 2/2), basal half of hypostome without denticles, denticles sharply pointed.
Legs moderately long, slender. Coxae (Fig. 6F): coxae I–IV with external spur; spur on coxae I–III moderately long, nearly subequal; spur on coxa I with broadly rounded apex, spur on coxae II and III with narrowly rounded to sharply pointed apex; spur on coxa IV very short; coxae I–IV with syncoxae occupying approximately 1/3, 1/4, 1/5 and 1/6 respectively of coxal width. Trochanters I–IV without spur ventrally. Tarsus I: length 320–335 (328; n = 2); tarsus IV length 298–310 (304; n = 2); tarsi only slightly humped subapically.
Larva. (Based on 2 specimens—refer to Table 1; Figs. 7, 10) Scutum (Fig. 7A) length 238 (n = 1), width 360 (n = 1), ratio 0.66 (n = 1); lateral margins diverging for approximately half of scutum length, broadly rounded posteriorly; posterolateral margin with slight indentations; lateral carinae lacking; cervical grooves shallow; setae 3 pairs, length of Sc1 11 (n = 1); length of Sc4 14 (n = 1). Dorsal setae of alloscutum undetermined in number since we only had larvae that were engorged to examine: it was impossible to confidently count and associate setae of the idiosoma dorsally and ventrally. Length of Cd1 17 (n = 1), length of Md1 23 (n = 1). Ventral setae undetermined number; 1 pair on anal valves; 3 pairs of sternals, length of St1 19 (n = 1); 2 pairs of preanals, length of Pa1 22 (n = 1), length of Pa2 32 (n = 1).
Gnathosoma (Fig. 7B–D) length from palpal apices to cornual apices dorsally 123 (n = 1), width between lateral projection of palpal segments I 155 (n = 1), ratio 0.79 (n = 1). Dorsal basis capituli (Fig. 7B) width 135 (n = 1), subrectangular, posterior margin nearly straight; cornua practically indistinct. Ventral basis capituli (Fig. 7C) subrectangular; lateral margins with slight constrictions at mid-length; auriculae absent. Post-hypostomal setae two pairs, length of Ph1 4 (n = 1), length of Ph2 4 (n = 1); distance between Ph1 37 (n = 1), distance between Ph2 27 (n = 1). Palpi (Fig. 7B) short, length dorsally (segments II and III) 77 (n = 1), maximum width (in dorsolateral plane) 42 (n = 1), ratio 1.83 (n = 1), maximum width of palpal segment I ventrally 50 (n = 1); segment I greatly enlarged, greatest dimension in anteromedian-posterolateral direction; dorsally segment I subrectangular with convex medial margin; ventrally segment I subtriangular, posterior margin with long moderately broad spur with narrowly rounded apex; segments II and III fused together with indistinct suture between them, narrower proximally and abruptly widening to broadly rounded apex; segment I lacking setae, segments II and III combined with nine dorsal and three ventral setae. Hypostome (Fig. 7C) length 83 (n = 1), width 34 (n = 1), ratio 2.44 (n = 1); club-shaped, widening to broadly rounded apex with medial indentation; base of hypostome approximately at level of mid-length of palpal segment I; dental formula 2/2, approximately five denticles in files; basal half of hypostome without denticles, denticles sharply pointed.
Legs moderately long, slender. Coxae (Fig. 7E): coxae I–III with external spur; spur on coxa I moderately long, on coxa II and III short to very short; spur on coxae I–III with broadly rounded apex; coxae I–III with syncoxae occupying approximately 1/3, 1/4 and 1/5 respectively posteromedian portion of coxal width. Trochanters I–IV without spur ventrally. Tarsus I: length 183 (n = 1); tarsus III length 166 (n = 1); tarsi only slightly humped subapically.
Remarks
By having a greatly enlarged palpal segment I that extends inwardly and anteriorly, all active life stages of I. barkeri most closely resemble those of the Endopalpiger species of Australasia: I. acer, I. australiensis, I. giluwensis, I. hydromyidis, I. luxuriosus, I. mirzai, I. planiscutatus, I. steini, I. stellae, I. tasmani, I. victoriensis, I. woyliei and I. zaglossi (refer to [8, 19, 20]).
The males of I. australiensis, I. tasmani, I. victoriensis and I. zaglossi have been described [19, 21,22,23,24]. The male of I. barkeri is easily distinguished from the males of the Endopalpiger species by the absence of the syncoxal areas on all coxae (vs. well-developed syncoxae on coxae I–IV in all those species).
The female of I. barkeri resembles only that of I. woyliei by the absence of syncoxal areas on coxae (vs. females of all other Endopalpiger with well-developed syncoxae). The female of I. barkeri can be differentiated from I. woyliei by the scutum and basis capituli dorsally and ventrally without lateral carinae and other longitudinal ridges (vs. lateral carinae and longitudinal ridges present in I. woyliei), the considerably smaller palpal segment I with a long spur on its posterior margin (vs. greatly enlarged palpal segment I with shorter spur on its posterior margin in I. woyliei), 4/4 dental formula on hypostome (vs. 6/6 in I. woyliei) and the long spur on trochanter I dorsally (vs. indistinct spur in I. woyliei).
The nymph of I. australiensis, I. hydromyidis, I. luxuriosus ([25] wrote that the nymph of I. luxuriosus had not been described, although there is a brief description of it in [26]), I. steini, I. tasmani, I. victoriensis and I. woyliei have been described [8, 19, 22, 24, 26]. Unfortunately, all of these published descriptions and illustrations are too brief for confident comparison. Nonetheless, we note that the nymph of I. barkeri has a scutum without lateral carinae (vs. distinct carinae in I. victoriensis), a scutum and basis capituli dorsally and ventrally without distinct longitudinal ridges (vs. with distinct, sharp ridges in I. woyliei), a distinct cornua (vs. no cornua in I. australiensis, I. hydromyidis, I. tasmani and I. woyliei), mostly 3/3 dental formula on the hypostome (vs. 2/2 dental formula in I. hydromyidis and I. tasmani, 4/4 in I. australiensis), external spurs on coxae I–IV (vs. apparently no spurs on coxae in I. hydromyidis, I. luxuriosus, I. steini and I. tasmani) and tarsi I–IV slightly humped subapically, without a notch (vs. strongly humped tarsi with distinct notch in I. victoriensis).
The larvae of I. hydromyidis, I. tasmani and I. victoriensis have been described [22, 24, 27, 28]. Unfortunately, as with the nymphs, all of these published descriptions and illustrations of larvae are too brief for confident comparison. Nonetheless, we note that the larva of I. barkeri has indistinct cornua on the basis capituli dorsally (vs. distinct cornua in I. victoriensis) and has external spurs on coxae I–III (vs. no spurs on coxae in I. hydromyidis and I. tasmani).
Our diagnoses may be broadened and improved once the nymphs and larvae of the other Australasian species of Endopalpiger are redescribed and illustrated accurately.
Mitochondrial genomes and phylogeny
Five entire mt genomes are presented here for the first time: I. australiensis (OL597990, B4930, from 1 female tick), I. barkeri (OM302450, B5322, 1 male; OL597991, B5321, pool of 3 nymphs), I. fecialis (OL597993, B4928a 1 female) and I. woyliei (OL597992, B4928b 1 female) (Fig. 9). These mt genomes have the gene arrangement that is typical of the Australasian Ixodes clade ([29], Fig. 1) except that in I. fecialis the main cluster of tRNA genes has the arrangement ARNESF rather than ARNSEF: ARNSEF has been found in all other Ixodidae, Argasidae, Nuttalliellidae and Holothyrida studied so far [3, 4, 5, 6, 7 and Fig. 1 of 29]. Moreover, in I. fecialis we found a 60-bp insertion between tRNA-Asn (N) and tRNA-Glu (E) and a 57-bp insertion between tRNA-Glu (E) and tRNA-Ser (S); neither of these insertions is similar to any other motifs in the mitochondrial genome of I. fecialis.
Mitochondrial genomes of Ixodes (Endopalpiger) australiensis, I. (Endo.) barkeri, I. (Endo.) woyliei and I. (Exopalpiger) fecialis. Protein-coding genes are shown in green, tRNAs are in yellow, rRNAs are in red, and the two control regions are in blue. Protein-coding genes are labelled by their four-character abbreviations, tRNAs are labelled by their one-letter amino acid abbreviations, and the two control regions are labelled as CR1 and CR2. Mitochondrial genome size variation is indicated in parentheses. The arrangement of genes in these four species is identical except that the main cluster of tRNA genes has the arrangement ARNSEF in the three species of Endopalpiger [I. (Endo.) australiensis, I. (End.) barkeri and I. (End.) woyliei], whereas in the one species of Exopalpiger [I. (Exo.) fecialis] the arrangement is ARNESF. The arrangement in I. (Exo.) fecialis is the first known arrangement in an Ixodidae tick that is different from ARNSEF. Thus, ARNESF might be a synapomorphy for the subgenus Exopalpiger
The phylogeny from these mt genomes indicates that I. barkeri and I. woyliei are best placed in the subgenus Endopalpiger Schulze, 1935, since I. barkeri and I. woyliei were in a lineage with I. (Endopalpiger) australiensis and I. (Endopalpiger) tasmani to the exclusion of species from the subgenera Ceratixodes, Exopalpiger and Sternalixodes: I. (Ceratixodes) uriae, I. (Exopalpiger) fecialis and I. (Sternalixodes) holocyclus (Fig. 10).
Maximum likelihood (ML) phylogenetic tree from entire mt genomes (14,935 bps). The sequence alignment was put though Gblocks to remove regions with alignment gaps. Tip labels indicate NCBI accession numbers and (Barker & Barker Collection reference nos.). Numbers above branches show maximum likelihood bootstrap support, whereas numbers below branches show the Bayesian posterior probability support. Ixodes pavlovskyi Pomerantzev, 1946, one of the species “Other Ixodes” (sensu Barker & Murrell, 2004), for which an entire mitochondrial (mt) genome was available in GenBank, was set as the outgroup. The scale bar indicates 0.06 nucleotide substitutions per nucleotide site for the 14,935 nucleotide sites in our alignment of theses entire mt genomes. So, for example, there were about 896 nucleotide substitutions along the branch that leads to I. (Ceratixodes) uriae plus I. (Sternalixodes) holocyclus plus I. (Exopalpiger) fecialis, which is marked with an asterisk [i.e. 0.06 nucleotide substitutions per nucleotide site × 14,935 nucleotide sites (bps) = 1896 nucleotide substitutions]. Ticks in bold were sequenced in the present study
Discussion
We had hoped to extract sufficient DNA for our experiments from all of the putative life-stages of I. barkeri [1] i.e. females, males, nymphs and larvae. Adequate DNA for our experiments, however, was obtained from only a male and a pool of three nymphs but not from females nor larvae since the females and larvae had not been preserved well enough. The mt genomes sequences of the male (GenBank OM302450) and the pool of three nymphs (OL597991) were > 99.96 % identical; thus, the nymphs were certainly I. barkeri [1]. Although, we do not have mt genome sequences from the females or the larvae the evidence that the females and larva are also attributable to I. barkeri [1] is strong on account of the morphological similarity of the females and larvae to the males and nymphs (above and Figs. 2, 3, 4, 5, 6, 7, 8, 9). The subgeneric classification of Ixodes is complex and sometimes ignored, probably because some subgenera are defined ambiguously, making species difficult to place in a subgenus. However, the names of the subgenera are valid, represent hypotheses of relationships and deserve closer attention. Previously, neither [8] nor [1] attempted to place I. woylie and I. barkeri, respectively, in a subgenus. We, however, conclude that I. woylie and I. barkeri are best placed in the subgenus Endopalpiger Schulze, 1935 (Fig. 10). Alas, mt genomes from the other species of Endopalpiger were not available to us: (i) I. victoriensis Nuttall, 1916, and I. hydromyidis Swan, 1931, from Australia; (ii) I. acer Apanaskevich, 2020; I. giluwensis Apanaskevich 2020; I. luxuriosus Schulze, 1935; I. mirzai Apanaskevich, 2020; I. planiscutatus Apanaskevich, 2020; I. steini Schulze, 1935; I. stellae Apanaskevich, 2020; and I. zaglossi Kohls, 1960, from Papua New Guinea.
Paul Schulze was a prolific German taxonomist whose life works were reviewed recently [26]. He described 17 entities that are presently considered as subgenera [30], including Endopalpiger in 1935, with Ixodes luxuriosus Schulze, 1935, as the type species (redescribed by [20]). The subgenus Endopalpiger was based mainly on their prominent and distinctive palps. Later, Schulze [31] gave generic status to Endopalpiger, thus emphasizing the very unusual form of the palps. [32] and [19] considered the subgenus Endopalpiger to be valid, but [33] and [34] presented the subgenus Endopalpiger as a synonym of Exopalpiger Schulze, 1935, but without evidence or argument. Here, our phylogenetic trees show that Endopalpiger and Exopalpiger are not closely related. Rather, Exopalpiger is much closer to Sternalixodes and Ceratixodes than it is to Endopalpiger (Fig. 10).
The four species of Endopalpiger in our tree formed a monophyletic group (barkeri, tasmani, woylie, australiensis); indeed, a monophyletic group with 100% bootstrap support and a posterior probability of 1.0, the highest possible posterior probability (Fig. 10). This is the first phylogenetic tree from entire mt genomes (about 15,000 bps) or any similarly large number of nucleotides. [The only other tree that had more than one species of Endopalpiger was by [8] (Fig. 10; ca. 800 bps of cox1)]. Therefore, we found strong support for Endopalpiger, albeit with a limited set of taxa. The unique nature of palpal segment (article) I is a morphological synapomorphy of Endopalpiger. As described by [19] (p. 13), the female palpal segment I (“I” in Fig. 5C) is greatly enlarged and projects inwardly and forwardly so that it ensheathes each side of the base of the mouthparts, and ventrally palpal segment I is strongly salient (“ss” in Fig. 5C). The only similar palp morphology in adults is that of Exopalpiger, which, in the words of [19] (p. 13), sounds more like that of Endopalpiger than it actually is. According to [19] (p. 13), the female palpal segment 1 of Exopalpiger is also greatly enlarged, being attached at right angles to the transverse axis of the basis, but does not project inwardly or forwardly and it does not ensheathe any part of the base of the mouthparts; ventrally palpal segment I is salient but not as salient as in Endopalpiger.
Availability of data and materials
The data supporting the conclusions of this article are in the article. The mitochondrial genomes published for the first time in this paper have been submitted to GenBank database, accession numbers OL597990-OL597993, and OM302450.
Abbreviations
- ANIC:
-
Australian National Insect Collection
- mt:
-
Mitochondrial
References
Barker D. Ixodes barkeri n. sp. (Acari: Ixodidae) from the short-beaked echidna, Tachyglossus aculeatus, with a revised key to the male Ixodes of Australia, and list of the subgenera and species of Ixodes known to occur in Australia. Zootaxa. 2019;4658:331–42. https://doi.org/10.11646/zootaxa.4658.2.7.
Barker SC, Murrell A. Systematics and evolution of ticks with a list of valid genus and species names. Parasitology. 2004;129(Suppl):S15-36.
Shao R, Aoki Y, Mitani H, Tabuchi N, Barker SC, Fukunaga M. The mitochondrial genomes of soft ticks have an arrangement of genes that has remained unchanged for over 400 million years. Insect Mol Biol. 2004;13(3):219–24. https://doi.org/10.1111/j.0962-1075.2004.00447.x.
Burger TD, Shao R, Barker SC. Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species. Mol Phylogenet Evol. 2014;76:241–53. https://doi.org/10.1016/j.ympev.2014.03.017.
Kelava S, Mans BJ, Shao R, Moustafa MAM, Matsuno K, Takano A, et al. Phylogenies from mitochondrial genomes of 120 species of ticks: Insights into the evolution of the families of ticks and of the genus Amblyomma. Ticks Tick Borne Dis. 2021;12:101577. https://doi.org/10.1016/j.ttbdis.2020.101577.
Mans B, Featherston J, Kvas M, Pillay KA, de Klerk DG, Pienaar R, et al. Argasid and ixodid systematics: implications for soft tick evolution and systematics, with a new argasid species list. Ticks Tick Borne Dis. 2019;10(1):219–40. https://doi.org/10.1016/j.ttbdis.2018.09.010.
Mans BJ, Kelava S, Pienaar R, Featherston J, de Castro MH, Quetglas J, et al. Nuclear (18S-28S rRNA) and mitochondrial genome markers of Carios (Carios) vespertilionis (Argasidae) support Carios Latreille, 1796 as a lineage embedded in the Ornithodorinae: re-classification of the Carios sensu Klompen and Oliver (1993) clade into its respective subgenera. Ticks Tick Borne Dis. 2021;21:101688.
Ash A, Elliot A, Godfrey S, Burmej H, Abdad MY, Northover A, et al. Morphological and molecular description of Ixodes woyliei n. sp. (Ixodidae) with consideration for co-extinction with its critically endangered marsupial host. Parasites Vectors. 2017;10:70. https://doi.org/10.1186/s13071-017-1997-8.
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–9. https://doi.org/10.1093/bioinformatics/bts199.
Chen Y, Ye W, Zhang Y, Xu Y. High speed BLASTN: an accelerated MegaBLAST search tool. Nucleic Acids Res. 2015;43(16):7762–8. https://doi.org/10.1093/nar/gkv784.
Lowe TM, Chan PP. tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016. https://doi.org/10.1093/nar/gkw413.
Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, et al. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 2013;69(2):313–9. https://doi.org/10.1016/j.ympev.2012.08.023.
Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–3. https://doi.org/10.1093/bioinformatics/btu033.
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, et al. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61(3):539–42. https://doi.org/10.1093/sysbio/sys029.
Diego D, Guillermo LT, Ramón D, David P. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9:772. https://doi.org/10.1038/nmeth.2109.
Rambaut A: TRACER v1. 5. 2009. http://tree.bio.ed.ac.uk/software/tracer/.
Rambaut A: FigTree v1. 4. 2012. http://tree.bio.ed.ac.uk/software/FigTree/.
Miller MA, Pfeiffer W, Schwartz T: Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: 2010 Gateway Computing Environments Workshop (GCE): 14–14 Nov. 2010 2010: 1–8.
Roberts FHS. Ticks of Australia. Melbourne: CSIRO; 1970.
Apanaskevich DA, Schenk JJ. Description of five new species of Ixodes Latreille, 1795 (Acari: Ixodidae) and redescription of I. luxuriosus Schulze, 1935, I. steini Schulze, 1935 and I. zaglossi Kohls, 1960, parasites of marsupials, rodents and echidnas in New Guinea Island. Syst Parasitol. 2020;97(3):223–66. https://doi.org/10.1007/s11230-020-09909-5.
Kohls GM. Ixodes (Endopalpiger) zaglossi, n. sp. from the long-beaked echidna of New Guinea (Acarina, Ixodidae). Acarologia. 1960;2:447–52.
Roberts FHS. A systematic study of the Australian species of the genus Ixodes (Acarina: Ixodidae). Aust J Zool. 1960;8:392–485.
Roberts FHS. The males of Ixodes pterodromae Arthur and Ixodes australiensis Neumann (Acarina : Ixodidae). J Entomol Soc Queensl. 1964;3:75–8.
Weaver HJ. Redescription of Ixodes victoriensis Nuttall, 1916 (Ixodida: Ixodidae) from marsupials in Victoria. Australia Syst Appl Acarol. 2016;21(6):820–9. https://doi.org/10.11158/saa.21.6.9.
Guglielmone AA, Petney TN, Richard GR. Ixodidae (Acari: Ixodoidea): descriptions and redescriptions of all known species from 1758 to December 31, 2019. Zootaxa. 2020;4871(1):1–322.
Schulze P. Zur vergleichenden anatomie der zecken. (Das sternale, die mundwerkzeuge, analfurchen und analbeschilderung, ihre bedeutung, ursprünglichkeit und luxurieren). Z Morphol Okol Tiere. 1935;30(1):1–40.
Roberts FHS. The larvae of Australian Ixodidae (Acarina: Ixodoidea). Aust J Entomol. 1969;8:37–78.
Senevet G, Ripert C. Les larves des espèces du genre Ixodes. Ann Parasitol Hum Comp. 1967;42:79–121.
Barker D, Seeman OD, Barker SC. The development of tick taxonomy and systematics in Australia and Papua New Guinea through the works of five late 20th century contributors and with comments on the place of Australasia in the study of the phylogeny and evolution of the ticks. Syst Appl Acarol. 2021;26(10):1793–832.
Guglielmone AA, Petney TN, Mastropaolo M, Robbins RG. Genera, subgenera, species and subspecies of hard ticks (Acari: Ixodidae) described, named, renamed or given new rank by Paul Schulze (1887–1949) and their current status. Zootaxa. 2017;4325:1–66. https://doi.org/10.11646/zootaxa.4325.1.1.
Schulze P. Eine neue Art der neuguineisch-autralischen Zeckengatt-ung Endopalpiger aus Deutschland. Zoologischer Anzeiger Leipzig. 1939;125:35–9.
Clifford CM, Sonenshine DE, Keirans JE, Kohls GM. Systematics of the subfamily lxodinae (Acarina: Ixodidae). l. The subgenera of Ixodes. Ann Entomol Soc Am. 1973;66(3):489–500.
Camicas JL, Morel PC. Position systematique et classification des tiques (Acarida: Ixodida). Acarologia. 1977;18(3):410–20.
Camicas JL, Hervy JP, Adam F, Morel PC. Les Tiques du monde. Nomenclature, stades decrits, hotes, repartition. The Ticks of the World Nomenclature, described stages, hosts, distrubtion. (Acarida, Ixodida). France: Orstom Editions Publisher; 1998.
Acknowledgements
We sincerely thank Brodie Foster (University of Queensland) and Geoff Thompson (Queensland Museum) for the colour figures; William McGuire and Maria Apanaskevich for exquisite polishing with Adobe Photoshop of the colour and scanning electron microscopy (SEM) images, respectively. Ian Beveridge, Amy Shima, Anne-Marie Horwitz and David Forshaw very kindly gave us ticks. We are especially grateful to Ian Beveridge for giving us larvae of I. barkeri. Dr. Bruce Halliday lent us ticks from the Australian National Tick Collection, Canberra—thank you. We thank our three reviewers for helpful suggestions.
Funding
RS acknowledges the funding support of GeneCology Research Centre, University of the Sunshine Coast. RN acknowledges the funding support of JSPS KAKENHI (19H03118, 20K21358 and 20KK0151).
Author information
Authors and Affiliations
Contributions
Conception and design of the study that led to the paper and/or the paper: DB, ODS, MK, SCB, DAA. Acquisition of research data where the acquisition required significant intellectual judgement, planning, design and/or input: DB, SK, RS, RN, SCB, DAA. Analysis and interpretation of the data on which the research output is based: DB, ODS, SCB, DAA. Drafting and/or critical review and re-drafting of the paper so as to contribute to the interpretation: DB, ODS, MK, SCB, DAA. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Barker, D., Kelava, S., Shao, R. et al. Description of the female, nymph and larva and mitochondrial genome, and redescription of the male of Ixodes barkeri Barker, 2019 (Acari: Ixodidae), from the short-beaked echidna, Tachyglossus aculeatus, with a consideration of the most suitable subgenus for this tick. Parasites Vectors 15, 117 (2022). https://doi.org/10.1186/s13071-022-05165-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05165-2