Abstract
Background
The tribe Rhodniini is a monophyletic group composed of 24 species grouped into two genera: Rhodnius and Psammolestes. The genus Psammolestes includes only three species, namely P. coreodes, P. tertius and P. arthuri. Natural hybridization events have been reported for the Rhodniini tribe (for genus Rhodnius specifically). Information obtained from hybridization studies can improve our understanding of the taxonomy and systematics of species. Here we report the results from experimental crosses performed between P. tertius and P. coreodes and from subsequent analyses of the reproductive and morphological aspects of the hybrids.
Methods
Crossing experiments were conducted between P. tertius and P. coreodes to evaluate the pre- and post-zygotic barriers between species of the Rhodniini tribe. We also performed cytogenetic analyses of the F1 hybrids, with a focus on the degree of pairing between the homeologous chromosomes, and morphology studies of the male gonads to evaluate the presence of gonadal dysgenesis. Lastly, we analyzed the segregation of phenotypic characteristics.
Results
Interspecific experimental crosses demonstrated intrageneric genomic compatibility since hybrids were produced in both directions. However, these hybrids showed a high mortality rate, suggesting a post-zygotic barrier resulting in hybrid unviability. The F1 hybrids that reached adulthood presented the dominant phenotypic segregation pattern for P. tertius in both directions. These insects were then intercrossed; the hybrids were used in the cross between P. tertius ♀ × P. coreodes ♂ died before oviposition, and the F1 hybrids of P. coreodes ♀ x P. tertius ♂ oviposited and their F2 hybrids hatched (however, all specimens died after hatching, still in first-generation nymph stage, pointing to a hybrid collapse event). Morphological analyses of male gonads from F1 hybrids showed that they did not have gonadal dysgenesis. Cytogenetic analyses of these triatomines showed that there were metaphases with 100% pairing between homeologous chromosomes and metaphases with pairing errors.
Conclusion
The results of this study demonstrate that Psammolestes spp. have intrageneric genomic compatibility and that post-zygotic barriers, namely unviability of hybrid and hybrid collapse, resulted in the breakdown of the hybrids of P. tertius and P. coreodes, confirming the specific status of species based on the biological concept of species.
Graphical abstract

Similar content being viewed by others
Background
Chagas disease is a neglected disease which affects about 8 million people worldwide, with approximately 25 million people at risk of infection. Treatment with the anti-trypanosomatids benznidazole and nifurtimox is more effective in the acute phase of the disease than in the chronic phase [1, 2]. Chagas disease is caused by the protozoan Trypanosoma cruzi (Chagas, 1909) (Kinetoplastida, Trypanosomatidae) and the main form of transmission is through hematophagous insects known as triatomines [2]. Currently, 156 species of triatomines, divided into 18 genera and five tribes [3,4,5], have been identified, and all are considered to be potential Chagas disease vectors.
The tribe Rhodniini Pinto, 1926 is a monophyletic group composed of 24 species [3, 5,6,7] grouped into two genera that are morphologically and ecologically distinct: the members genus genus Rhodnius Stål, 1859 have long thin legs and a long head, and live mainly in palm trees, while members of genus Psammolestes Bergroth, 1911 have a short head, strong legs, wide femora, a very wide rostrum (the widest in the subfamily) and live in nests of birds of the family Furnariidae [8]. The inclusion of these two genera within the Rhodniini tribe is based on their mainly arboreal behavior and the presence of post-ocular tuberosities [7].
Rhodnius is a paraphyletic genus formed by 21 species divided into the trans-Andean Rhodnius clade (pallescens group) and the cis-Andean Rhodnius clade (pictipes + prolixus groups) [5,6,7, 9]. The event of paraphilia is supported by the greater evolutionary proximity of the species of the prolixus group with the genus Psammolestes which groups these species into a single clade [6, 10]. Based on this phenomenon, Hypsa et al. [11] proposed changing the name of the genus including Psammolestes spp. to Rhodnius: P. arthuri (Pinto, 1926) for R. arthuri (Pinto, 1926), P. coreodes Bergroth, 1911 for R. coreodes (Bergroth, 1911) and P. tertius Lent & Jurberg, 1965 for R. tertius (Lent & Jurberg, 1965).
The distribution of species included in genus Psammolestes is restricted to Latin America, with P. coreodes being reported in Argentina, Bolivia, Brazil and Paraguay, P. tertius in Brazil and Peru and P. arthuri in Colombia and Venezuela [12, 13]. Phylogenetic and cytogenetic analyses suggest that this genus is monophyletic [14, 15]. Monteiro et al. [14] suggested that perhaps Psammolestes should be regarded as a specialized lineage of Rhodnius from the prolixus group because the genus Psammolestes and species of the prolixus group share a common ancestral form, which highlights the paraphyly of the genus Rhodnius. Soares et al. [9] also suggested that Psammolestes is derived from an ancestral form similar to R. robustus Larrousse, 1927, and de Paula et al. [16] suggested that the species P. coreodes and P. tertius originated by vicariance.
de Paula et al. [16] also suggested that hydrological connections between the Araguaia–Amazon basins in the Early and Middle Miocene [20.4–9.0 million years ago (Mya)] would be a possible event that split the cis-Andean + trans-Andean clades of the R. domesticus Neiva & Pinto, 1923 + Psammolestes + prolixus group (15.2 Mya). These authors indicated that this connection would incorporate the Pantanal system in the Upper Miocene–Lower Miocene (10.0–4.5 Mya), resulting in the separation of the Pantanal system from the Atlantic Forest system, an event that may have contributed to the formation of Psammolestes species at a time when P. coreodes and P. tertius originated (4.98 Mya). On the other hand, Soares et al. [9] suggested that Psammolestes spp. spread from the Amazon region northward into the llanos of Venezuela (where P. arthuri is abundant in Furnariidae nests) and southeastward into the Caatinga–Cerrado path of Central Brazil (with subsequent differentiation of P. tertius along a north–south cline and P. coreodes in the Chaco region of Argentina and Paraguay) [14, 17].
Events of natural hybridization have been reported for the Rhodniini tribe (for the genus Rhodnius specifically) [18]. Information gained from hybridization studies can further our understanding of the taxonomy and systematics of species, and be used to analyze the isolating mechanisms that limit gene flow between species, and experimental crosses can be employed to establish the role of natural hybridization in generating new genetic variants (that may lead to adaptive evolution and/or in founding new evolutionary lineages) [19, 20]. In this context, we performed, for the first time, experimental crosses between P. tertius and P. coreodes and analyzed the reproductive and morphological aspects of the hybrids in order to characterize the possible barriers reproductive and the segregation of phenotypic characters, respectively.
Methods
Experimental crosses
To evaluate the pre- and post-zygotic barriers between the species of the Rhodniini tribe, we performed crossing experiments between P. tertius and P. coreodes (Table 1).
The crossing experiments were conducted in the Triatominae insectary of the School of Pharmaceutical Sciences, São Paulo State University (UNESP), Araraquara, São Paulo, Brazil, according to the experiments described by Mendonça et al. [21] and Neves et al. [22]. The insects were sexed at the fifth-instar nymph stage (N5) [23], and males and females were kept separately until they reached the adult stage in order that only adult virgins were used in the crosses. For the crossing experiments, three couples from each direction were kept in plastic jars [5 (diameter) ×10 cm (height)] and kept at room temperature. Intraspecific crosses were also performed as controls (Table 1). The eggs were collected weekly throughout the females’ oviposition periods, and the egg fertility rate and mortality rate of the hybrids were calculated (Table 1). After the hybrids from the first generation (F1) reached N5, a hybrid pair F1 was formed for each direction (Table 1) and the same parameters described above were used in the evaluation of these crosses.
Cytogenetic analysis
After the experimental crosses, the F1 males were dissected and the testes removed and stored in a methanol:acetic acid solution (3:1). Slides were prepared by the cell-crushing technique (as described by Alevi et al. [24]), and cytogenetic analyses were performed to characterize spermatogenesis, with emphasis on the degree of pairing between the homeologous chromosomes [21], using the lacto-acetic orcein technique [24, 25]. The slides were examined under a light microscope (Jenamed; Carl Zeiss, Jena, Germany) that was coupled to a digital camera, with a 1000-fold increase; AxioVision LE version 4.8 imaging software (Carl Zeiss) was used for analysis.
Morphology of the gonads
The morphology of the male gonads of the F1 hybrids was analyzed under a stereomicroscope microscope (model MZ APO; Leica Microsystems GmbH, Wetzlar, Germany) fitted with the Motic Advanced 3.2 Plus Image Analysis System (Motic, Hong Kong) to evaluate the presence of gonadal dysgenesis (which may be uni- or bilateral) [26].
Segregation of phenotypic characteristics
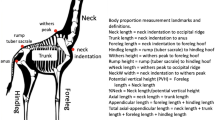
The head of F1 male hybrids were measured (MZ APO stereomicroscope and Motic Advanced 3.2 Plus Image Analysis System) to analyze the segregation of phenotypic characteristics, based on the main parameter used in the taxonomic key of Lent and Wygodzinsky [8]. Psammolestes tertius is characterized by an anteocular region that is 2- to 2.5-fold longer than the post-ocular region, and P. coreodes is characterized by an anteocular region that is no longer than twofold the length of the post-ocular region.
Results and discussion
It is estimated that the ancestors of the Rhodniini and Triatomini Jeannel, 1919 tribes diverged around 48.9–64.4 Mya, at about the time when South America was beginning to separate from Antarctic and Australia during the Lower Tertiary period [27]. It is believed that radiation of the genus Rhodnius occurred from the Amazon region and resulted in three main evolutionary lineages: those in the south (Brazilian Cerrado), in the north (Venezuela) and in the northwest (passing through the Andean Cordillera into the Magdalena valley in Colombia) [28]. The colonization of bromeliads, palms trees and bird nests represent important events for the speciation of these taxa [16]. In addition, Psammolestes spp. adapted to exploit bird nest microhabitats and currently occur over the open ecoregions north and south of the moist Amazon forests: P. arthuri in the Orinoco and Venezuelan coastal basins, P. tertius primarily in the Cerrado-Caatinga and P. coreodes primarily in the Chaco [14, 29].
Interspecific experimental crosses between P. tertius and P. coreodes (Fig. 1a) demonstrated intrageneric genomic compatibility since hybrids were produced in both directions (Table 1; Fig. 1b, c). Likewise, interspecific crosses between members of genus Rhodnius (R. prolixus Stål, 1859 × R. neglectus Lent, 1954, R. prolixus × R. robustus, R. prolixus × R. pictipes Stål, 1872 and R. pallescens Barber, 1932 × R. colombiensis Mejia, Galvão & Jurberg, 1999) also resulted in the production of hybrids in at least one of the directions [30,31,32]. According to the biological concept of species proposed by Mayr [33], i.e. “groups of natural populations that actually or potentially intersect and are reproductively isolated from other groups”, this feature is extremely important from an evolutionary point of view because it demonstrates that evolutionary events resulting in total pre-zygotic isolation between species have not yet been established in the Rhodniini tribe.
Interspecific experimental crosses between P.coreodes and P. tertius, and resulting hybrids. a Crosses between P. tertius ♀ and P. coreodes ♂, b adult hybrids from the experimental cross between P. coreodes ♀ and P.tertius ♂, c adult hybrids from the experimental cross between P. tertius ♀ and P. coreodes ♂. Bar: 1 cm
The main mechanisms of pre-zygotic reproductive isolation observed in the subfamily Triatominae are ecological isolation and mechanical isolation. The first prevents the formation of hybrids between Triatoma infestans (Klug, 1834) and T. platensis Neiva, 1913, two species that are phylogenetically related [6] but present in different habits (T. infestans is associated with domiciliary regions and feeds on mammalian blood [34]; T. platensis is associated with bird nests and feeds preferentially on the blood of birds [35]). The second mechanism is associated with structural incompatibility between male and female genitalia and happens with a certain frequency in only one direction of the crosses, such as, for example, at the crossing of T. platensis females with T. delpontei Romaña & Abalos, 1947 males [36].
Interspecific genomic compatibility between P. tertius and P. coreodes was confirmed by the hatching of the F1 (Table 1). However, these hybrids showed a high mortality rate (Table 1), which suggests a post-zygotic barrier resulting in hybrid unviability (confirming the species status of P. tertius and P. coreodes). Recently, this reproductive barrier was observed for crossings between T. sordida (Stål, 1859) and T. rosai Alevi et al., 2020 [4], contributing to the description of T. rosai by integrative taxonomy.
The few F1 hybrids that reached adulthood presented the dominant phenotypic segregation pattern for P. tertius in both directions (anteocular region measuring at least twofold greater than the post-ocular region) (Fig. 2). This is the first study on the segregation of phenotypic characters in the Rhodniini tribe. However, similar analyses have already been carried out in the Triatomini tribe: hybrids of the T. brasiliensis complex, for example, presented intermediate characteristics or a specific segregation pattern depending on the crossed species [21, 37, 38]. In addition, Mexican triatomine F1 hybrids also resulted in F1 offspring that were morphologically indistinguishable from one of the parental lines [39] and in second-generation hybrids (F2) with phenotypic characteristics either specific to those of one of the parents or with intermediate characteristics [40, 41]. Considering that some factors can result in an increased risk of T. cruzi transmission to humans and animals (such as vigor [42] and hybrid fitness [43]) and that recently it has been reported that hybrids of the T. phyllosoma subcomplex show greater potential to acquire and transmit T. cruzi than parental species [44, 45], the study of segregation of external morphology can also be of epidemiological importance [37, 38].
Morphometric analysis of the heads of adult male hybrids of the experimental cross between P. coreodes and P. tertius. a P. coreodes ♀ × P. tertius ♂ (anteocular region = 1.005 mm; post-ocular region = 0.385 mm; anteocular region is 2.6-fold greater than the post-ocular region), b P. tertius ♀ × P. coreodes ♂ (anteocular region = 0.996 mm; post-ocular region = 0.431; anteocular region is 2.3-fold greater than the post-ocular region)
The F1 hybrids used in the cross between P. tertius ♀ x P. coreodes ♂ died before oviposition (confirming the phenomenon of hybrid unviability). This phenomenon has already been characterized for the Triatomini tribe: Martínez-Ibarra et al. [46] observed total mortality of nymphs resulting from crossing species of the T. phyllosoma subcomplex with T. mexicana (Herrich-Schaeffer, 1848). In addition, Martínez-Ibarra et al. [47,48,49] observed a lack of hybrid fitness resulting from the crossing between species of the T. phyllosoma subcomplex, which resulted in the mortality of nymphs due to not feeding (in the case of the initial stages) and problems during molting (in the older nymphs).
The crossing between the F1 hybrids of P. coreodes ♀ × P. tertius ♂ oviposited and the F2 hybrids hatched (Table 1). However, all specimens died after hatching, still in first-generation nymph (N1) stage (Table 1). The low adaptive value of hybrids from F2 (which resulted in insect mortality) characterizes the collapse of the hybrid. This phenomenon has already been observed in Triatoma Laporte, 1832 hybrids [21, 50] and has been used to confirm the species status of species of the T. brasiliensis complex [21, 50].
Morphological analyses of male gonads from F1 hybrids showed that these hybrids did not have gonadal dysgenesis (Fig. 3). Cytogenetic analyses of these triatomines showed that there were metaphases with 100% pairing between homeologous chromosomes (Fig. 4a) (which justifies hatching of F2 hybrids) and metaphases with pairing errors (Fig. 4b). These results are important from a taxonomic point of view, since according to Riley [51], two species possess distinct genomes when their chromosomes are different in structure and genetic content, so that there is no pairing between one or more pairs of homeologous chromosomes during hybrid meiosis. This behavior leads to sterility and, consequently, to genetic isolation between species.
Metaphase during meiosis from the experimental crossing between P. coreodes and P. tertius. a Metaphase with correct pairing of the homeologous chromosomes of P. coreodes ♀ × P. tertius ♂, b metaphase with chromosome pairing error of P. tertius ♀ × P. coreodes ♂ as indicated by the arrow. Asterisk indicates the sex chromosomes X and Y). Bar: 10 μm
Conclusion
The results of our study demonstrated that Psammolestes spp. have intrageneric genomic compatibility and that there are post-zygotic barriers (hybrid unviability and hybrid collapse) resulting in the breakdown of the hybrids of P. tertius and P. coreodes. These results confirm the species status of species based on the biological concept of species.
Availability of data and materials
The data supporting the conclusions of this article are included within the article.
References
Centers for Disease Control and Prevention. Parasites—American trypanosomiasis (also known as Chagas disease). 2021. https://www.cdc.gov/parasites/chagas/gen_info/detailed.html. Accessed 12 Mar 2021.
World Health Organization. Chagas disease (American trypanosomiasis). 2021. http://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis). Accessed 12 Mar 2021.
Galvão C. Taxonomia dos vetores da doença de Chagas da forma à molécula, quase três séculos de história. In: Oliveira J, Alevi KCC, Camargo LMA, Meneguetti DUO, editors. Atualidades em medicina tropical no Brasil: vetores. São Paulo: Strictu Sensu Editora; 2020. p. 9–37.
Alevi KCC, Oliveira J, Garcia ACC, Cristal DC, Delgado LMG, Bittinelli IF, et al. Triatoma rosai sp. Nov. (Hemiptera, Triatominae): a new species of Argentinian Chagas disease vector described based on integrative taxonomy. Insects. 2020;11:830.
Zhao Y, Galvão C, Cai W. Rhodnius micki, a new species of Triatominae (Hemiptera, Reduviidae) from Bolivia. ZooKeys. 2021;1012:71–93.
Justi SA, Galvão C. The evolutionary origin of diversity in Chagas disease vectors. Trends Parasitol. 2017;33:42–52.
Hernández C, Rosa JA, Vallejo GA, Guhl F, Ramírez JD. Taxonomy, evolution, and biogeography of the Rhodniini tribe (Hemiptera: Reduviidae). Diversity. 2020;12:97.
Lent H, Wygodzinsky P. Revision of the Triatominae (Hemiptera: Reduviidae) and their significance as vector of Chagas’s disease. Bull Am Mus Nat Hist. 1979;163:123–520.
Soares RPP, Barbosa SE, Borges EC, Melo Júnior TA, Romanha AJ, Dujardin JP, et al. Genetic studies of Psammolestes tertius (Hemiptera: Reduviidae: Triatominae) using male genital morphology, morphometry, isoenzymes, and random amplified polymorphic DNA. Biochem Genet. 2001;39:1–13.
Justi SA, Galvão C, Schrago CG. Geological changes of the Americas and their influence on the diversification of the Neotropical kissing bugs (Hemiptera: Reduviidae: Triatominae). PLoS Negl Trop Dis. 2016;10:4.
Hypsa V, Tietz D, Zrzavy J, Rego RO, Galvão C, Jurberg J. Phylogeny and biogeography of Triatominae (Hemiptera, Reduviidae): molecular evidence of a new world origin of the asiatic clade. Mol Phylogenet Evol. 2012;23:447–57.
Galvão C, Carcavallo R, Rocha DS, Jurberg J. A checklist of the current valid species of the subfamily Triatominae Jeannel, 1919 (Hemiptera, Reduviidae) and their geographical distribution, with nomenclatural and taxonomic notes. Zootaxa. 2003;202:1–36.
Cabrera R. Notas breves sobre Psammolestes tertius Bergroth, 1911 (Reduviidae: Hemiptera): un triatomino silvestre. An Fac Med Lima. 2006;67:345–65.
Monteiro FA, Wesson DM, Dotson EM, Schofield CJ, Beard CB. Phylogeny and molecular taxonomy of the Rhodniini derived from mitochondrial and nuclear DNA sequences. Am J Trop Med Hyg. 2000;62:460–5.
Oliveira J, Alevi KCC, Ravazi A, Miraglia Herrera H, Martins Santos F, Azeredo-Oliveira MTV, et al. New evidence of the monophyletic relationship of the genus Psammolestes Bergroth, 1911 (Hemiptera, Triatominae). Am J Trop Med Hyg. 2018;99:1485–8.
de Paula AS, Barreto C, Telmo MCM, Diotaiuti L, Galvão C. Historical biogeography and the evolution of hematophagy in Rhodniini (Heteroptera: Reduviidae: Triatominae). Front Ecol Evol. 2021;9:660151.
Schofield CJ, Dujardin JP. Theories on the evolution of Rhodnius. Actual Biol. 1999;21:183–97.
Dias FBS, Jaramillo N, Diotaiuti L. Description and characterization of the melanic morphotype of Rhodnius nasutus Stål, 1859 (Hemiptera: Reduviidae: Triatominae). Rev Soc Bras Med Trop. 2014;47:637–41.
Arnold ML. Natural hybridization and evolution. Oxford: Oxford Univ Press; 1997.
Pérez R, Hernández M, Quintero O, Scovortzoff E, Canale D, Méndez L, et al. Cytogenetic analysis of experimental hybrids in species of Triatominae (Hemiptera- Reduviidae). Genetica. 2005;125:261–70.
Mendonça VJ, Alevi KCC, Medeiros LM, Nascimento JD, Azeredo-Oliveira MTV, Rosa JA. Cytogenetic and morphologic approaches of hybrids from experimental crosses between Triatoma lenti Sherlock and Serafim, 1967 and T. sherlocki Papa et al., 2002 (Hemiptera: Reduviidae). Infect Genet Evol. 2014;26:123–31.
Neves SJM, Sousa PS, Oliveira J, Ravazi A, Madeira FF, Reis YV, et al. Prezygotic isolation confirms the exclusion of Triatoma melanocephala, T. vitticeps and T. tibiamaculata of the T. brasiliensis subcomplex (Hemiptera, Triatominae). Infect Genet Evol. 2020;79:104149.
Rosa JA, Barata JMS, Barelli N, Santos JLF, Belda Neto FM. Sexual distinction between 5th instar nymphs of six species (Hemiptera: Reduviidae). Mem Inst Oswaldo Cruz. 1992;87:257–64.
Alevi KCC, Mendonça PP, Pereira NP, Rosa JA, Azeredo-Oliveira MTV. Karyotype of Triatoma melanocephala Neiva & Pinto (1923). Does this species fit in the Brasiliensis subcomplex? Infect Genet Evol. 2012;12:1652–3.
De Vaio ES, Grucci B, Castagnino AM, Franca ME, Martinez ME. Meiotic differences between three triatomine species (Hemiptera:Reduviidae). Genetica. 1985;67:185–91.
Almeida LM, Carareto CMA. Gonadal hybrid dysgenesis in Drosophila sturtevanti (Diptera, Drosophilidae). Ilheringia. 2002;92:71–9.
Bargues MD, Marcilla A, Ramsey J, Dujardin JP, Schofield CJ, Mas-Coma S. Nuclear rDNA-based molecular clock of the evolution of Triatominae (Hemiptera: Reduviidae), vectors of Chagas disease. Mem Inst Oswaldo Cruz. 2000;95:567–73.
de Paula AS, Diotaiuti L, Galvão C. Systematics and biogeography of Rhodniini (Heteroptera: Reduviidae: Triatominae) based on 16S mitochondrial rDNA sequences. J Biogeogr. 2007;34:699–712.
Abad-Franch F, Monteiro FA, Jaramillo NO, Gurgel-Gonçalves R, Dias FBS, Diotaiuti L. Ecology, evolution, and the long-term surveillance of vector-borne Chagas disease: a multi-scale appraisal of the tribe Rhodniini (Triatominae). Acta Trop. 2009;112:159–77.
Carvalheiro JR, Barreto MP. Estudos sobre reservatórios e vectores silvestres do Trypanosoma cruzi. LX–tentativas de cruzamento de Rhodnius prolixus Stal, 1859 com Rhodnius neglectus Lent, 1954 (Hemiptera, Reduviidae). Rev Soc Bras Med Trop. 1976;18:17–23.
Galíndez GI, Barazarte R, Márquez J, Oviedo M, Márquez Y, Morón L, et al. Relaciones reproductivas entre Rhodnius prolixus Stal y Rhodnius robustus Larrousse (Hemiptera, Reduviidae, Triatominae) bajo condiciones de laboratorio. Entomol Vect. 1994;1:3–13.
Díaz S, Panzera F, Jaramillo-O N, Pérez R, Fernández R, Vallejo G, et al. Genetic, cytogenetic and morphological trends in the evolution of the Rhodnius (Triatominae: Rhodniini) Trans-Andean group. PLoS ONE. 2014;9:e87493.
Mayr E. Populações, Espécies e Evolução. 1st ed. São Paulo: Editora Nacional; 1963.
Galvão C. Vetores da doença de Chagas no Brasil. 1st ed. Curitiba: Sociedade Brasileira de Zoologia; 2014.
Abalos JW, Wygodzinsky P. Las Triatominae argentinas. Monographia Inst Med Reg Tucuman. 1951;1:1–178.
Usinger RL, Wygodzinsky Ryckman ER. The biosystematics of Triatominae. Annu Rev Entomol. 1966;11:309–29.
Almeida CE, Oliveira HL, Correia N, Dornak LL, Gumiel M, Neiva VL, et al. Dispersion capacity of Triatoma sherlocki, Triatoma juazeirensis and laboratory-bred hybrids. Acta Trop. 2012;122:71–9.
Pinotti H, Alevi KCC, Oliveira J, Ravazi A, Madeira FF, Reis YV, et al. Segregation of phenotypic characteristics in hybrids of Triatoma brasiliensis species complex (Hemiptera, Reduviidae, Triatominae). Inf Gen Evol. 2021;91:104798.
Martínez-Ibarra JA, Ventura-Rodríguez LV, Meillon K, Barajas-Martínez HM, Alejandre-Aguilar R, Lupercio-Coronel P, et al. Biological and genetic aspects of crosses between species of the Phyllosoma complex (Hemiptera: Reduviidae: Triatominae). Mem Inst Oswaldo Cruz. 2008;103:236–43.
Martínez-Ibarra JA, Salazar-Schettino PM, Nogueda-Torres B, Vences MO, Tapia-González JM, Espinoza-Gutiérrez B. Occurrence of hybrids and laboratory evidence of fertility among three species of the Phyllosoma complex (Hemiptera: Reduviidae) in Mexico. Mem Inst Oswaldo Cruz. 2009;104:1125–31.
Martínez-Ibarra JA, Grant-Guillén Y, Ventura-Rodríguez LV, Osorio-Pelayo PD, Macías-Amezcua MD, Meillón-Isáis K, et al. Biological and genetic aspects of crosses between species of the genus Meccus (Hemiptera: Reduviidae Triatominae). Mem Inst Oswaldo Cruz. 2011;106:293–300.
Martínez-Ibarra JA, Nogueda-Torres B, Montañez-Valdez OD, Michel-Parra JG, Valenzuela-Campos R. Biological parameters of two Triatoma rubida subspecies (Hemiptera: Reduviidae) and their laboratory hybrids. J Med Entomol. 2020;57:1390–8.
Martínez-Ibarra JA, Nogueda-Torres B, Salazar-Montaño LF, García-Lino JC, Arroyo-Reyes D, Hernández-Navarro JA. Comparison of biological fitness in crosses between subspecies of Meccus phyllosomus (Hemiptera: Reduviidae: Triatominae) in southern Mexico. Ins Sci. 2017;24:114–21.
Martínez-Ibarra JA, Nogueda-Torres B, Salazar-Schettino PM, Cabrera-Bravo M, Vences-Blanco MO, Rocha-Chavez G. Transmission capacity of Trypanosoma cruzi (Trypanosomatida: Trypanosomatidae) by three subspecies of Meccus phyllosomus (Heteroptera: Reduviidae) and their hybrids. Med Vet Entomol. 2016;53:928–34.
Martínez-Ibarra JA, Nogueda-Torres B, García-Lin JC, Arroyo-Reys D, Salazar- Montaño LF, Hernández-Navarro JA, et al. Importance of hybrids of Meccus phyllosomus mazzottii, and M. p. pallidipennis, and M. p. phyllosomus to the transmission of Trypanosoma cruzi in Mexico. Jpn J Infect Dis. 2016;69:202–6.
Martínez-Ibarra JA, Grant-Guillén Y, Delgadillo-Aceves IN, Zumaya-Estrada FA, Rocha-Chávez G, Salazar-Schettino PM, et al. Biological and genetic aspects of crosses between phylogenetically close species of Mexican Triatomines (Hemiptera: Reduviidae). J Med Entomol. 2011;48:705–7.
Martínez-Ibarra JA, Nogueda-Torres B, García-Benavídez G, Vargas-Llamas V, Bustos-Saldaña R, Montañez-Valdez OD. Bionomics of populations of Meccus pallidipennis (Stal) 1872 (Hemiptera: Reduviidae) from Mexico. J Vect Ecol. 2012;37:474–7.
Martínez-Ibarra JA, Nogueda-Torres B, Licón-Trillo Á, Villagrán-Herrera ME, Diego-Cabrera JA, Montañez-Valdez OD, et al. Comparative bionomics of four populations of Meccus longipennis (Hemiptera:Reduviidae: Triatominae) under laboratory conditions. Mem Inst Oswaldo Cruz. 2013;108:225–8.
Martínez-Ibarra JA, Nogueda-Torres B, Cárdenas-De la Cruz MÁ, Villagrán ME, Diego-Cabrera JA, Bustos-Saldaña R. Biological parameters of interbreeding subspecies of Meccus phyllosomus (Hemiptera: Reduviidae: Triatominae) in western Mexico. Bull Entomol Res. 2015;105:763–70.
Alevi KCC, Pinotti H, Araújo RF, Azeredo-Oliveira MTV, Rosa JA, Mendonça VJ. Hybrid colapse confirm the specific status of Triatoma bahiensis Sherlock and Serafim, 1967 (Hemiptera, Triatominae). Am J Trop Med Hyg. 2018;98:475–7.
Riley R. The secondary pairing of bivalents with genetically similar chromosomes. Nature. 1966;185:751–2.
Acknowledgments
We thank the Heitor Miraglia Herrera and Filipe Martins Santos for their support and logistics help in collecting P. coreodes in the state of Mato Grosso do Sul, Brazil, and Carlos Gustavo Silva Santos, Eduardo Oyama and Orlando Marcos Farias De Sousa for all their support in collecting P. tertius in the state of Bahia, Brazil. Wealso express our appreciation to the Pesquisa do Estado de São Paulo (FAPESP, Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil)—Finance Code 001 and to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), for financial support.
Funding
The study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil)–Finance Code 001, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil).
Author information
Authors and Affiliations
Contributions
AR: conceptualization, methodology, investigation, preparation and writing—original draft, review and editing, JdO: conceptualization, methodology, investigation, data curation and writing—review and editing. FFC: methodology, investigation and data curation. FFM: methodology, investigation and data curation. YVdR: methodology, investigation and data curation. ABBdO: methodology, investigation and data curation. MTVdA-O: conceptualization, funding acquisition and writing—review and editing. JAdR: conceptualization, resources and writing—review and editing, CG: conceptualization, funding acquisition and writing—review and editing. KCCA: conceptualization, methodology, investigation, writing—original draft preparation and review and editing, supervision, project administration and funding acquisition. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ravazi, A., de Oliveira, J., Campos, F.F. et al. Trends in evolution of the Rhodniini tribe (Hemiptera, Triatominae): experimental crosses between Psammolestes tertius Lent & Jurberg, 1965 and P. coreodes Bergroth, 1911 and analysis of the reproductive isolating mechanisms. Parasites Vectors 14, 350 (2021). https://doi.org/10.1186/s13071-021-04854-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-021-04854-8