Abstract
Queens play an essential role in the colonies of stingless bees. Typically, only one queen occurs in a colony at any time, and she dominates the egg laying. Their presence maintains colony cohesion and ensures the development and survivorship of these matriarchal societies. Yet there remain significant gaps in our knowledge of queen life cycles as compared to their daughters, the workers. In this review, we follow chronologically the life of queens from pre-emergence inside brood cells (caste determination), to their interaction with workers (queen selection) and males (sexual selection), and up to adulthood. Stingless bee queens can be determined either trophically or genetically. After emergence, the virgin queens undergo a selection process whereby many are executed by workers. The body size, pheromones, age and behaviour of virgin queens may play a role in queen selection. Queens then leave the nest on a nuptial flight during which they mate just once. After mating, queens are still susceptible to workers’ harassment. For example, if they produce diploid males they are killed by workers. Previous studies have successfully in vitro reared and mated virgin queens under laboratory conditions, which have revealed new insights of queen development time, the threshold of minimum and maximum provided food to larvae developing into queens, and lethal and sublethal effects of agrochemical substances. These new techniques have also provided new information about queen physiology. However, the daily routine of queens inside colonies demands further well-designed experiments to capture other patterns of behaviour which remain poorly understood, including their interactions with other queens, workers and even males.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Stingless bees
The stingless bees (Hymenoptera: Apoidea: Meliponini) are a group of highly eusocial bees native to the world’s tropical and subtropical regions (Michener 1974). They are the most diverse group of social bees, with an estimated 550 species belonging to ~ 58 genera (Grüter 2020). These are divided between three monophyletic clades—Neotropical, African and Indo-Malayan-Australasian—that diverged from each other 50–70 million years ago (Rasmussen and Cameron 2010), with the Neotropics harbouring most of the species diversity (> 400 species). In all stingless bees, the queen is the sole reproductive female in the colony capable of laying female (diploid) eggs, whereas the female workers forage for food, protect the colony against predators and care for the queen’s brood (Michener 1974).
Stingless bees are common flower visitors in tropical ecosystems due to the high number of species, the fact that each colony contains thousands to tens of thousands of workers, differences in morphology and foraging strategies (Michener 1974; Grüter 2020). Many stingless bee species are already propagated in hives for use in crop pollination, honey production and by hobby, which is called meliponiculture (Heard 1999; Cortopassi-Laurino et al. 2006; Slaa et al. 2006; Jaffé et al. 2015). Hence, the ability to rear and mate stingless bee queens underpins the efforts to accelerate the propagation of managed colonies and be used in conservation (Menezes et al. 2013; Jaffé et al. 2015). Although queens are produced in low numbers throughout the year in most stingless bees, queen availability can still be a limiting factor during colony propagation (Imperatriz-Fonseca and Zucchi 1995; Menezes et al. 2013; Jaffé et al. 2015). The stingless bee queens’ basic biology remains poorly understood due to their habits. When unmated, many species hide inside the nest avoiding execution from the workers, once accepted they only leave the nest to mate and once mated they are constantly guarded by workers (Imperatriz-Fonseca and Zucchi 1995).
In this review, we synthesise what is currently understood about the life history of stingless bee queens, including larval development, sexual maturation, social interactions, mating and pheromone production. We highlight recent advances but also point to key gaps that remain in our knowledge of queen biology.
To identify relevant studies for our review, we first revised all articles citing Imperatriz-Fonseca and Zucchi (1995), which summarized many aspects of the stingless bee queens. After that, we looked for more recent data so far by searching keywords as “stingless bees AND queens”, “Stingless bees AND reproduction” and “stingless bee gynes” in databases including ISI Web of Science (https://www.webofknowledge.com), Google Scholar (https://scholar.google.com/), and Scielo (https://scielo.org). The assessed studies were used to build up this review and additionally, all authors contributed with information based on their knowledge of the stingless bee literature.
Virgin queen (gynes)
Queen production
In stingless bees, new queens may be determined genetically, as in the Melipona genus (although there is a slightly plastic ratio, which is affected by nutritional conditions) (Kerr 1950; Hartfelder et al. 2006; Brito et al. 2015), or trophically, whereby any female larvae that are reared in larger brood cells and, consequently, receive more larval food than the larvae in regular brood cells, will develop as queens (Fig. 1a–e). Additionally, in a few stingless bee species (e.g. Frieseomelitta varia), larvae reared in workers’ cells may perforate auxiliary cells and eat extra food there, also then developing as queens (Terada 1974; Faustino et al. 2002; Nunes et al. 2015; Luz et al. 2017) (Fig. 1F). Commonly, queen and worker castes in stingless bees are well-defined physiologically, morphologically and behaviourally. However, it has been observed that adult female bees can sometimes possess both corbiculae and spermatheca, making them intercastes (Hartfelder and Engels 1992; dos Santos et al. 2016a). The process that triggers the intercaste condition is unclear.
Royal brood cells (white ellipses) of some stingless bee species on: horizontal (disc-like) brood combs of A Tetragonisca angustula, B Scaptotrigona aff. depilis and C Paratrigona subnuda; Spiral brood comb of D Tetragona clavipes and clustered brood cells of E Plebeia minima and F Leurotrigona muelleri. Note: commonly the royal brood cells are built in the edge of combs as in A, C, D, except B. However, some species that build clustered brood cells may construct the royal cells randomly distributed, depending on whether the larvae are consuming extra larval food adjacent to auxiliary cells as in F. Image source: A—Charles F. dos Santos; B-F retrieved from Fototeca Cristiano Menezes http://splink.cria.org.br/manager/detail?setlang=pt&resource=FCM
In some species, as Nannotrigona testaceicornis, Plebeia remota and Schwarziana quadripunctata, alternative queen phenotypes, known as dwarf or miniature queens, may also occur and head new colonies (Camargo 1974; Imperatriz-Fonseca et al. 1997; Wenseleers et al. 2005; Ribeiro et al. 2006; Nogueira-Ferreira et al. 2009). Miniature queens are consistent with the theory that under some conditions, female larvae in social insects should self-determine their own developmental fate (Nonacs and Tobin 1992; Bourke and Ratnieks 1999; Ratnieks 2001; Wenseleers et al. 2003). It means that larvae reared in brood cells destined to become workers might adopt a selfish strategy by evading the worker fate and developing into miniature queens (Wenseleers et al. 2005; Ribeiro et al. 2006).
A substance consumed by female larvae has been known to be a factor affecting caste determination of Melipona genus, in which queens are genetically determined (Kerr 1950; Brito et al. 2015). It was suggested that a cohort of adult bees (nurse workers) could apparently coerce female larva to become queens by adding geraniol to larval food (Jarau et al. 2010). Geraniol can also be found in pollen from different plant species, and it has been hypothesized by van Veen (2018) that the availability of fresh pollen, which in Melipona beecheii is collected in short periods in relatively large quantities, coinciding with gyne production in the colonies, may function as a natural regulatory mechanism which limits the excess production of virgin queens to periods of swarming and supersedure. Contrary, recent research re-examined these hypotheses by artificially increasing the levels of geraniol on female larvae food and revealed no effect of the substance on the rearing of new queens (Oliveira et al. 2022).
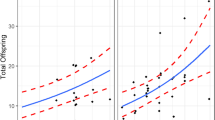
In the majority of stingless bee species in which the amount of food is important for caste determination (non-Melipona genus) (Hartfelder et al. 2006; Grüter 2020). However, could female larvae evade their caste fate? The development of protocols to rear stingless bee queens in vitro has helped to answer this question (Prato 2010; Baptistella et al. 2012; Menezes et al. 2013; dos Santos et al. 2016a). These studies have revealed that there is a threshold for the amount of larval food offered to female larvae, below which the larva becomes a worker. Conversely, volumes of the same larval food above this threshold, and within a larger brood cell, the royal cell, lead to all female larvae developing into queens (Prato 2010; Baptistella et al. 2012; Menezes et al. 2013; dos Santos et al. 2016a). In general, the quantity of larval food provided to potential queens is, at least, twice as much as that given to potential workers (Table 1). This appears to guarantee the emergence only of queens, since to date no “giant worker” has been observed emerging from royal cells, except giant males (references in Table 1).
In some stingless bee species with trophically determined castes, there is an alternative pathway by which eggs laid into worker cells can develop as standard-sized queens. Female larvae may access either auxiliary cells (lacking larva) or neighbouring brood cells (containing eggs, or young larvae) and ingest the additional food stored there, consequently developing as new queens (Frieseomelitta varia, Leurotrigona muelleri, Plebeia lucii and Tetragonula carbonaria) (Terada 1974; Faustino et al. 2002; Nunes et al. 2015; Luz et al. 2017). When this occurs in colonies that have been orphaned, such a process can be considered a mechanism for emergency queen production. For example, queenright colonies of Tetragonula carbonaria build royal cells in which to produce their new queens, and build auxiliary cells for queen production only when queenless (Nunes et al. 2015). On the other hand, in Leurotrigona muelleri, larvae can themselves perforate neighbouring brood cells (containing eggs, or young larvae) and consume their food content, turning into a new queen (Terada 1974).
This special case of queen emergence has two features in common to all species where it has to date been observed: (i) fusion of two neighbouring cells and (ii) no occurrence of males emerging from those fused cells (Terada 1974; Faustino et al. 2002; Nunes et al. 2015; Luz et al. 2017). When workers build auxiliary cells to raise emergency queens, they may recognise brood cells containing female larvae and ignore those with male larvae. However, to date there is no information on whether particular larvae are chosen at the expense of another or even if such larvae emit any type of signal to be chosen (Grüter 2020). Likewise, when larvae perforate adjacent cells themselves, male larvae might refrain from doing so because presumably there is no fitness advantage to being a “giant male”. Those oversized males do occur in several species, as rare events in some (Tetragonula carbonaria; Gloag et al. 2007) and common in others (Friesella schrottkyi, Paratrigona subnuda, Plebeia droryana, P. emerina, P. remota, Scaptotrigona postica, Schwarziana quadripunctata) (Camillo 1971; Bego and Camargo 1984; Imperatriz-Fonseca 1976; Santos-Filho et al. 2006; Alves et al. 2009). These males take longer to develop and produce more sperm cells (Camillo 1971), though it is yet to be determined if they are capable of fertilizing queens (Grüter 2020).
Queen selection by workers
Since stingless bee colonies are usually monogynic and monandric (i.e. contain only a single mated queen), any excess of virgin queens emerging in colonies are commonly killed by the workers or expelled from the colony shortly after emergence (e.g. Melipona genus) (Silva et al. 1972; Imperatriz-Fonseca and Zucchi 1995; Sommeijer et al. 2003; Jarau et al. 2009). Koedam et al. (1995) supposed that the competition between mated and unmated queens could be a mechanism by which the quality of the egg-laying queen is continuously tested by workers. Under queenless conditions, the enlargement (inflation) of the abdomen of gynes was found to be crucial for acceptance (van Veen et al. 1999; Kleinert 2005; Kärcher et al. 2013). Melipona beecheii gynes that are not able to escape from worker aggression through hiding inside empty food pots or “feigning death” are killed by workers shortly after emergence (Koedam et al. 1995; van Veen et al. 1999; Kleinert 2005; Kärcher et al. 2013). Only 19.6% of gynes survived the first 12 h after emergence, all survivors had enlarged abdomens, indicating that this condition in queens might be more accepted or tolerated by the workers (van Veen et al. 1999. Similarly, gynes of Melipona favosa with an enlarged abdomen have an increased number of workers in the court around them (Koedam et al. 1995). Abdominal inflation is likely associated with the activation of the abdominal gland, indicating pheromone production and therefore acceptance by the workers (van Veen et al. 1999). Thus, the success of gynes with inflated abdomens suggests that queen pheromones play an important role in the acceptance of virgin queens by workers.
In honeybees, queens have a functional stinger that can be used multiple times, and when new queens emerge in queenless colonies they will typically kill all the rival queens (Butz and Dietz 1994). In contrast, the constant supply of new queens in stingless bees may give more power over queen selection to workers. Workers rear the brood and so control queen production. Workers outnumber the virgin queens and so can select the best one by killing the others (Koedam et al. 1995; Wenseleers et al. 2004; Jarau et al. 2009; Kärcher et al. 2013). This strategy comes with the obvious risk that if all new queens fail, the colony will be left without an egg-laying female and unable to produce new worker brood, leading the colony to die (Beekman and Ratnieks 2003).
Most stingless bee species are believed to retain multiple virgin queens at all times as a way of insurance against death of the mated queen (Kerr et al. 1962; Nogueira-Ferreira et al. 2009; Inoue et al. 1984; Engels and Imperatriz-Fonseca 1990; Bueno et al. 2020). Up to 44 “spare queens” have been observed in Schwarziana quadripunctata (Nogueira-Ferreira et al. 2009). This strategy enables a rapid and active queen replacement in the event that a queen is producing diploid male offspring (Vollet-Neto et al. 2019). For example, stingless bee queens producing diploid males tend to be killed by their workers (Camargo 1979; Alves et al. 2011; Vollet-Neto et al. 2017, 2019).
In Melipona scutellaris, the life expectancy of queens that produced diploid males was half of those producing haploid males (Alves et al. 2011). Similarly, in Melipona quadrifasciata and Scaptotrigona depilis, the emergence of diploid males triggers queen execution, usually within 6–30 days after the first diploid males begin to emerge (Camargo 1979; Vollet-Neto et al. 2017). This timing is related to the moment when the cuticular hydrocarbons of haploid and diploid males are different, suggesting that the queen’s execution might be triggered by these chemical differences (Vollet-Neto et al. 2017). Workers may decide to execute queens for other motives too, though which other cues might trigger regicide in stingless bees remains understudied.
Under queenright situations, most virgin queens are killed by workers soon after emergence or whenever they become sexually attractive (Imperatriz-Fonseca and Zucchi 1995; Wenseleers et al. 2004; Jarau et al. 2009; Kärcher et al. 2013; de Souza et al. 2017).
In Melipona species, it is common to find several virgin queens fallen in front of their colonies. However, there is no consensus if such individuals are trying to escape from workers’ harassments or whether they are expelled by them (Sommeijer et al. 2003; Wenseleers et al. 2011).
Once the resident queen is removed or dies, the virgin queen(s) are quick to appear on the brood comb; sometimes even within 30 min (Tetragonula carbonaria) (Bueno 2021). From the body posture of the virgin queens, and the trophallaxis that occurs, it can be assumed that there is a hierarchy among them (Silva 1972; Imperatriz-Fonseca and Zucchi 1995). The selection and acceptance of a new queen may depend on the interaction of several factors.
a) Body size
Body size and mass seem to play a role in queen selection and attractiveness in some stingless bee species. For example, in Schwarziana quadripunctata, a species that sometimes produces miniature queens, workers prefer large queens (Nogueira-Ferreira et al. 2000; Ribeiro and Alves 2001; Wenseleers et al. 2005). Indeed, workers in this species kill most miniature queens and cage many large queens in imprisonment chambers (or royal chambers) to keep them as insurance against queen loss (Engels and Imperatriz-Fonseca 1990; Ribeiro et al. 2006). Workers might respond to fertility indicators shown by morphological, pheromonal or behavioural patterns in the miniature queens (Ribeiro et al. 2006). However, in Melipona quadrifasciata, body mass does not seem to determine the acceptance of the queens (Kärcher et al. 2013).
b) Chemical profile
Stingless bee workers recognise virgin queens by the chemical profile of hydrocarbons on their cuticle, which can indicate age, caste and sex (Leonhardt 2017). This cuticular hydrocarbon profile differs between castes (Abdalla et al. 2003; Kerr et al. 2004; Grajales-Conesa et al. 2007; Ferreira-Caliman et al. 2013) and they confer information about the reproductive status and fertility of the queens (Jarau et al. 2009; Veiga et al. 2017), as well as signalling their presence and suppressing ovary activation in workers (Nunes et al. 2014).
At the peak of attractiveness, the virgin queens from several stingless bee species inflate their abdomens constantly, move very quickly around the nest requesting food from the workers and exposing their tergite pockets as they walk around, presumably to release a pheromone (Imperatriz-Fonseca and Zucchi 1995; Jarau et al. 2009; Kärcher et al. 2013). These abdominal inflations seem crucial during the interactions with workers indicating a complex interaction between pheromones emitted by different parts of the body from the queens (Alves et al., unpublished data). This behaviour stimulates the worker’s courtship behaviour towards her (Kärcher et al. 2013), but it may also trigger persecution and execution (Jarau et al. 2009).
c) Sexual maturation
Age might be related to the secretion of specific chemical compounds that dictate the workers’ preference for some virgin queens over the other ones (Silva et al. 1972). In an experimental set-up with the Australian species Tetragonula carbonaria, when three queens were introduced to small queenless colonies, the workers preferred the first one to be introduced (also the oldest one). The subsequent queens (introduced into the nest box one day later and also one day younger than the queen introduced one day before) remained secluded in the trash deposit of the nest box. These younger queens were ignored by the workers and were finally killed (Bueno 2021; Bueno et al. 2022b). Similarly, another study found that newly emerged virgin queens have higher chances of becoming the new resident queen if they emerge shortly after the removal of the physogastric queen (24-48 h) and if they are successful in hiding and avoiding worker attacks until queen choice takes place (Kärcher et al. 2013). The age of workers interacting with new queens is also important, since virgin queens introduced into small colonies containing both callow (younger) and mature (older) workers are often rejected, while those introduced into colonies with only callow workers presented a rate of acceptance above 75% (dos Santos et al. 2015).
d) Behaviour
It has been assumed that queen selection is related to a virgin queen’s dominance over the workers, as well as other virgin queens. This dominance is established by the queen’s aggression towards the workers. Some studies have observed unmated queens of stingless bees grouped in areas like the involucrum (Kleinert and Imperatriz-Fonseca 1994) and establishing dominance hierarchies (Engels and Imperatriz-Fonseca 1990) without aggressive interaction among them (Wenseleers et al. 2004; Kärcher et al. 2013). In Melipona quadrifasciata, aggression among virgin queens has rarely been observed, but fights may occur between the resident queen and one or more virgin queens (Kärcher et al. 2013). This lack of aggression between gynes is interesting given the large number of young queens attempting to become the resident queen in some stingless bee species (i.e. Melipona). Given their lack of a stinger, stingless bee virgin queens could kill another queen only through biting, which might endanger both virgin queens and risk leaving no unharmed winner, jeopardizing the survival of the entire colony. Presumably for the same reason, virgin queens are no threat to a physogastric queen and instead are continuously executed by workers (Peters et al. 1999). Nevertheless, queen aggression may occur in some species. Two or more Tetragonula carbonaria virgin queens in a queenless colony were seen chasing one another, exchanging brief aggressive interactions (Bueno et al. 2022a), and Plebeia remota virgin queens may also attack one another (Ribeiro et al. 2003).
Some behaviours possibly linked to the queen’s survival are resting motionless, running and performing rapid turns to avoid being attacked by workers, and hiding around inside empty food storage pots (Engels and Imperatriz-Fonseca 1990; van Veen et al. 1999; Kärcher et al. 2013). In Melipona quadrifasciata however, behaviours such as running, walking, antennating or trophallaxis played little or no role, as did the factors body mass or nestmate (Kärcher et al. 2013). These behaviours may vary between species, and further studies are needed to understand queen succession, and the role of such behaviours in queen selection and survival.
e) Relatedness
Current evidence suggests that genetic relatedness does not play a strong role in queen selection, since foreign virgin queens are accepted by colonies at similar rates than their nestmates. In Melipona quadrifasciata, queen acceptance rates were not affected by whether a virgin queen was a nestmate or was introduced from another colony (Kärcher et al. 2013). Beekeeping practices in many species include transplanting brood between colonies, whereby queens that hatch from the donor brood cells are readily accepted (Nogueira-Neto 1997).
The willingness of stingless bees eventually to accept unrelated queens helps explain why some species are susceptible to infiltration by unrelated, foreign queens (Reis et al. 2011; Wenseleers et al. 2011; van Oystaeyen et al. 2013; Jaffé et al. 2014), and even the queens of other stingless bee species (Cunningham et al. 2014). However, in this latter situation, it differs from queen parasitism in Melipona, since colonies are taken by force and many adult workers are killed and only callow workers and brood are enslaved and accept the incoming queen (Cunningham et al. 2014). In Melipona scutellaris the invading queen’s strategy to successfully infiltrate an unrelated and queenless colony is associated with her time of arrival at the nest, with success more likely at late hours of the day (after 6:00 p.m.) when guards have lower levels of activity and are less efficient (van Oystaeyen et al. 2013). It remains a question for further studies to understand more about the recognition abilities of non-nestmate virgin queens within a colony (Monteiro and Kerr 1990); specifically, how they can outmanoeuvre the guards, given that guards are very good at recognising non-nestmates (Breed and Page Jr 1991; Grüter 2020).
Acceptance inside the nest
Ultimately, the “accepted” virgin queens are those that are active in the area where the new brood cells are built (Silva et al. 1972), indicating a strong relationship between dominance and territory (Imperatriz-Fonseca 1977). Accepted queens (mated or not yet mated) are fed by workers via trophallaxis (Silva et al. 1972; Engels and Imperatriz-Fonseca 1990) and may perform “dominance dances” in which the queen shakes her inflated abdomen, exposed their pheromone glands and touches workers with it (Engels and Imperatriz-Fonseca 1990; Imperatriz-Fonseca and Zucchi 1995).
Queen-male interaction, mating and fertilization
Queen mating under natural conditions
In stingless bees, males engage in neither courtship nor territory defense (Engels et al. 1990; Roubik 1990). Instead, mate selection occurs via indirect male-male competition. Males aggregate in large numbers near colonies where there are new virgin queens to be fertilized (Cortopassi-Laurino 2007; dos Santos et al. 2014). Similarly, these males can aggregate near new nests that have been founded by workers (van Veen and Sommeijer 2000a; dos Santos et al. 2016b). In both cases, the males typically wait several days for the nuptial flight of virgin queens (Van Veen and Sommeijer 2000b; Cortopassi-Laurino 2007; dos Santos et al. 2014). Even though males commonly aggregate in front or near the nests, virgin queens may fly far from their nests, moment in which they are pursued by several males waiting for her flight (Kerr et al. 1962; Silva et al. 1972; Roubik 1990). The exact distance of the queen’s nuptial flight is unknown, but from the intertegular distance, it was estimated to be between 420 to 966 m, for Plebeia droryana and Scaptotrigona depilis, respectively (Table 2). The reproductive system, the chemical profile and the size of the glands are all less complex and activated in virgin than in physogastric queens (Cruz-Landim et al. 2005; Abdalla 2006) (Fig. 2A-D). However, after copula, the newly mated queen will store the semen of the successful male in her spermathecae (Fig. 2E) and the abdomen will enlarge considerably due to ovary activation and the intensification of oogenesis in the ovaries (Melo et al. 2001; de Souza et al. 2007; Tanaka et al. 2009; Bueno et al. 2020).
Reproductive systems of virgin (A-B) and mated queen (C-D) of Tetragonisca angustula. A—The entire reproductive apparatus of a virgin queen; B—Details of the spermatheca and Dufour´s gland from A; C—The entire reproductive apparatus of a mated, physogastric queen; D—Details of the spermatheca and Dufour’s gland from C; E—Isolated spermatheca of mated, egg-laying queen of Scaptotrigona aff. depilis. Notes: The ovariole is the basic unit of egg production, i.e. the functional unit of the ovary (hatched yellow circles); the spermatheca is a sperm reservoir that stocks sperm from the male; a large number of mature eggs (hatched red circles in C) and other developing eggs can be seen in the activated ovaries of mated queens; the hatched blue circle (A) shows a fragmented fat body (adipocytes) that is a tissue with the functions as storing and releasing of nutrients for growth and reproduction of insects
Physogastric queens of some stingless bee species: A Melipona melanoventer, B Frieseomelitta flavicornis C, D Melipona flavolineata, E Friesella schrottkyi and F Leurotrigona muelleri. Note: A Distended abdomen of physogastric queen as a result of over production of eggs, as shown in Fig. 3C. B Young physogastric queen with their hindwings still worn. C The stingless bee queens do not have corbiculae. D The red arrow points towards a brood cell being inspected by a queen before oviposition. Images retrieved from Fototeca Cristiano Menezes http://splink.cria.org.br/manager/detail?setlang=pt&resource=FCM
How do males locate colonies with virgin queens ready to mate? Presumably, queenless colonies emit a chemical signal into the environment that lures the males to the nest, but the chemical identity and source of this signal has not been identified (Bueno et al. 2022a). Three hypotheses have been suggested to explain male attraction to nests that are re-queening: (i) all workers inside the queenless nest produce a pheromone that wafts out from nest entrances (i.e. overall nest odour changes when queenless (Engels et al. 1993)), (ii) workers that leave the nest (e.g. foragers) spread a pheromone outside of the nest (Engels et al. 1990; Roubik 1990) or (iii) virgin queens inside colonies produce a pheromone that wafts out from nest entrances (Engels 1987; Fierro et al. 2011; Verdugo-Dardon et al. 2011). To date, this third scenario has been most studied, via efforts to identify unique chemical compounds produced by virgin queens.
Virgin queens do release highly volatile chemical compounds that can attract males (Engels et al. 1997; Grajales-Conesa et al. 2007; Fierro et al. 2011; Verdugo-Dardon et al. 2011; Araújo et al. 2017). Such volatile compounds may be present in the cephalic glands of Scaptotrigona species (Engels et al. 1997; Grajales-Conesa et al. 2007; Verdugo-Dardon et al. 2011) or in abdominal glands such as Dufour’s (Fig. 2D, E) and tergal glands of Tetragonisca angustula (Fierro et al. 2011; Araújo et al. 2017).
In Tetragonisca angustula, the candidates for sexual pheromones are the isopropyl hexanoate, butyl hexanoate and hexyl hexanoate present in abdominal glands (Fierro et al. 2011; Araújo et al. 2017). In contrast, in Scaptotrigona species it is the 2-alcohols present in the cephalic glands of virgin queens that appear to attract males to copulate (Engels 1987; Engels et al. 1997; Verdugo-Dardon et al. 2011).
Male attraction to mated queens
It is well established that virgin queens in stingless bees copulate only once and with a single male (Kerr et al. 1962; Peters et al. 1999; Palmer et al. 2002; Vollet-Neto et al. 2018). Therefore, we could expect that mated and physogastric queens would lose their sexual attractiveness after mating. However, at least for Neotropical species, it has long been known that if colonies are artificially disturbed, the egg-laying queens may be harassed by males attempting copulation inside nests (Nogueira-Neto 1957; Sakagami and Laroca 1963; Imperatriz-Fonseca 1973; Cortopassi-Laurino 1979). Interestingly, some of the same volatile compounds produced by virgin queens are also produced by physogastric queens as observed for Tetragonisca angustula (Fierro et al. 2011; Araújo et al. 2017). Recently, it has been also observed that physogastric queens outside nests are as sexually attractive to aggregated males as virgin queens (Fierro et al. 2011; Araújo et al. 2017).
To date, there is no work experimentally demonstrating whether those molecules released by physogastric queens outside nests, as aforementioned, are the same released when these queens are inside nests. However, it demonstrates that the power to sexually attract males to copula remains latent in egg-laying queens from at least seven species of stingless bees. For example, it was recorded and depicted during the opening of a natural nest of Lestrimelitta ehrhardti (Sakagami and Laroca 1963). Later, when a physogastric queen of Schwarziana quadripunctata egg-laid inside a brood cell, some nearby males attempted to copulate with her (Imperatriz-Fonseca 1973). After that, other cases have been rarely but continuously observed inside and outside nests or in confinement conditions as for Paratrigona subnuda (Imperatriz-Fonseca 1977), Melipona quadrifasciata (Campos and Melo 1990), Scaptotrigona postica (Engels and Engels 1988), S. depilis (Menezes, unpublished data) and Tetragonisca angustula (Fierro et al. 2011; Araújo et al. 2017). Outside the nest, physogastric queens perform a persistent and erratic behaviour in which they vibrate their wings, swell their abdomens and visually expose their abdominal glands (Araújo et al. 2017). As suggested by Araújo et al. (2017), even though T. angustula physogastric queens release chemical compounds sexually attractive to males outside nests, inside the nests such molecules may have other biological meaning to workers.
The male attempts at copulating (non-natural conditions) with physogastric queens in stingless bees is most likely a non-adaptive behaviour and costly to both physogastric queens and males. The evidence for this: (i) physogastric queens that are sexually harassed by males try to deter them with vigorous abdominal movements (Imperatriz-Fonseca 1973; Cortopassi-Laurino 1979), (ii) if mating occurs, then the physogastric queen may be seriously injured and die shortly afterwards (Campos and Melo 1990), (iii) workers assault the males involved, injuring their antennae and legs (Imperatriz-Fonseca 1977; Cortopassi-Laurino 1979), (iv) physogastric queens are able to use the spermatozoa from a second male (under laboratory conditions) even mated for a long time (Campos and Melo 1990; Lopes et al. 2003) (Campos and Melo 1990; Lopes et al. 2003). Moreover, such behaviour has not been selected through evolutionary history of stingless bee queens. The mating (v) with a highly related partner (matched mating) may make that queens producing diploid (infertile) males and they are killed by their workers (Camargo 1979; Vollet-Neto et al. 2017, 2019). If hypothetically successful in a inbreeding mating (vi) such males would waste the opportunity to fertilize unrelated queens, because after mating they lose their genitalia and die (Kerr and Krause 1950; Kerr et al. 1962).
The points raised above suggest that, when performing any management in queenright hives, the physogastric queens should be immediately removed or isolated from colonies to avoid any additional and unnecessary attempts of copulation by nearby males.
Queen mating under laboratory conditions
Queen-rearing under laboratory conditions is used to produce stingless bee queens on a large scale (Hartfelder and Engels 1992; Baptistella et al. 2012; Menezes et al. 2013; dos Santos et al. 2015; Bernardes et al. 2018). The controlled copulation or instrumental insemination of these queens after their emergence remains a challenge. Nuptial flights of lab reared queens are rare or risky (Menezes et al. 2013)—“the gyne waste”. Yet under laboratory conditions, the males may ignore the gynes or they may evert their genitalia without copulating with them (Veiga et al. 2017)—“the male waste”.
Even though mating of stingless bees is believed to usually occur naturally in flight and outside nests, some experiments have shown that sexually attractive virgin queens confined with a small number of males inside boxes (Melipona quadrifasciata, M. flavolineata, Scaptotrigona postica) may copulate under laboratory conditions (Camargo 1972; Engels and Engels 1988; Veiga et al. 2017). The age and glandular development of the queens and the social context (e.g. number and age of workers where virgin queens are sheltered) seem to be important factors determining their sexual attractiveness to males (Engels and Engels 1988; Araújo et al. 2017; Veiga et al. 2017). Controlled mating procedures require further investigation.
Although using mating boxes allows some control over which individuals (virgin queens and males) will mate, this procedure still depends on male and virgin queen behaviour. As such, artificial insemination could give a greater control over the semen donor to a particular queen. Protocols to assess the sperm collection and quality already exist for Scaptotrigona aff. depilis (Meneses et al. 2014; Koffler et al. 2021) and could be adapted to other stingless bee species. However, its use to manually inseminate virgin queens under laboratory conditions remains to be tested. The artificial insemination technique was attempted 50 years ago with Melipona bicolor queens; however, they were inseminated with Apis mellifera sperm (Silva et al. 1972) and to date there is still no procedure available for any stingless bee species.
Exposure to agrochemicals
It is well known that (bio)pesticides can alter physiological aspects of non-target insects, such as stingless bees, consequently causing changes in their development, behaviour, and health (Barbosa et al. 2015; Rosa et al. 2016; Araujo et al. 2019; Dorneles et al. 2021; Almeida et al. 2022). In most social insects like stingless bees, the queens are required to fulfil some behaviours early in life as a prerequisite to be selected by workers as the new reproductive queen of the colony (Silva et al. 1972; Jarau et al. 2009; Veiga et al. 2017). If the queens do not have the optimal characteristics needed within this short and risky period, they are more likely to be killed by the workers than selected by the colony (Silva et al. 1972; Jarau et al. 2009; Veiga et al. 2017).
Development
Some studies have shown that larvae can have their caste fate changed by the action of pesticides. Furthermore, larval queens poisoned with residual doses of insecticides have shown delayed development, while emerged virgin queens presented altered behaviour and malformations (dos Santos et al. 2016a; Bernardes et al. 2018; Otesbelgue et al. 2018). For example, an organophosphorous insecticide changed larvae of P. droryana that were destined to become queens into workers (dos Santos et al. 2016a). Even the low-risk insecticide azadirachtin caused alterations in the development time, malformations and reduced reproductive organs in Partamona helleri queens (Bernardes et al. 2018). A reduction of 36% in the area of the reproductive system was found in exposed queens of Partamona helleri (Bernardes et al. 2018). Additionally, the development larval period of queens exposed to agrochemicals increased from 4.5% to 20.5% depending on species (Table 3).
In other cases, exogenous substances may induce an elevated queen production in stingless bees. The insecticides that mimic juvenile hormones (JH) in young insects (i.e. insect growth regulators) applied during the cocoon-spinning phase (predefaecating larvae) in some stingless bee species can induce most female larvae to develop into queens (Campos and Coelho 1993; Bonetti et al. 1994; Pinto et al. 2002). For example, the insecticide pyriproxyfen, an analogue of juvenile hormone, disturbed caste differentiation in Melipona quadrifasciata when more than 80% of the female larvae exposed to this substance became queens compared to 0% in the control (Pinto et al. 2002). Under natural conditions, many queens may compromise the maintenance and survival of colonies in the long-term since they usually do no work and are killed.
Behaviour
Queens exposed to the neonicotinoid imidacloprid when larvae, presented less wing vibrating (78%) and made less trophallaxis (83%) (Otesbelgue et al. 2018), which are important behaviours for their acceptance by workers (Silva et al. 1972; Imperatriz-Fonseca and Zucchi 1995; Araújo et al. 2017). Additionally, imidacloprid-treated queens suffered more harassment (increased by 219%) by workers than non-exposed queens and, over a period of days, such exposed queens reduced (79%) her defensive responses against worker attacks compared to non-exposed virgin queens (Otesbelgue et al. 2018). These variations in behavioural repertoire caused by residual substances can raise the number of queen executions, contributing to a lower number of physogastric queens and new nests, which are harmful for reproduction and colony swarming.
Stingless bees can contaminate each other (and even the queen) by horizontal poisoning (Boff et al. 2018). It means that if a queen is poisoned, probably the whole colony is affected, given that the workers are more exposed to agrochemicals when foraging and are thus the first ones to be in contact with the contaminated food, bringing it back into the colony. When exposed to pesticides, it is known that workers can change not only individual behaviours, but also their collective responses (Tomé et al. 2015; Boff et al. 2018), which surely affect the egg-laying queens; e.g. workers of Melipona quadrifasciata, when exposed to a pesticide composed by acetamiprid and alpha-cypermethrin, presented less trophallaxis and antennation (Boff et al. 2018). Even though the effects in the interactions with the queen were not studied, it is possible that the feeding and communication of the queen would be negatively affected, as it is directly dependent on the workers’ behaviours that presented changes caused by agrochemicals in previous studies. Thus, we can predict that there are several ways, yet unstudied, in which pesticides may indirectly interfere in the social interactions of stingless bee queens. Besides that, possible effects of pesticides in other aspects, such as queens’ oviposition rate, longevity and eggs sizes need to be further studied in stingless bees.
Concluding remarks
The queens of stingless bees are essential for the growth, maintenance and survivorship of their colonies, but they face many challenges from larval development to their adulthood as an egg-laying queen, in which, little seems to be under their control. Although female larvae are postulated to be able to sometimes evade their fates as a future worker, in many cases, it is the workers that control when and how many new queens will emerge. Similarly, when they are just young, unmated queens, they need to display a range of behavioural repertoire to be accepted by workers. Likewise, their mates appear to be selected via indirect competition between males in an aggregation, since we do not have evidence for a direct mate choice by the virgin queens during the nuptial flight. Finally, physogastric queens may be exposed to multiple challenges to sustaining a high rate of oviposition. Agrochemicals and their sublethal effects may put still more pressure on the viability and survival of the queen caste.
Investigation of the life of stingless bee queens is challenging since colonies are dark and stingless bee nests are complex structures obstructing the observation of their behaviour inside of the brood area. Only well-designed experiments can capture their natural routine and their patterns of behaviour towards other queens, workers and even males. Therefore, even though recent work has brought new insights into the lives of queens of stingless bees, further studies on their physiology, genetics, chemical ecology and behaviour are needed. Such studies would ideally be conducted across a diverse range of stingless bee species.
Data availability statement
There is no dataset.
Change history
26 February 2023
Funding update
References
Abdalla FC (2006) Morphological, chemical and developmental aspects of the Dufour gland in some eusocial bees (Hymenoptera, Apidae): a review. Revista Brasileira de Entomologia 50:153–162. https://doi.org/10.1590/S0085-56262006000200002
Abdalla FC, Jones GR, Morgan ED, Cruz-Landim C (2003) Comparative study of the cuticular hydrocarbon composition of Melipona bicolor Lepeletier, 1836 (Hymenoptera, Meliponini) workers and queens. Genet Mol Res 2:191–199
Almeida FCR, Magalhães DM, Favaris AP, Rodríguez J, Azevedo KEX, Bento JMS, Alves DA (2022) Side effects of a fungus-based biopesticide on stingless bee guarding behaviour. Chemosphere 287:132147. https://doi.org/10.1016/j.chemosphere.2021.132147
Alves DA, Imperatriz-Fonseca VL, Francoy TM, Santos PS, Billen J, Wenseleers T (2011) Successful maintenance of a stingless bee population despite a severe genetic bottleneck. Conserv Genet 12:647–658. https://doi.org/10.1007/s10592-010-0171-z
Alves DA, Imperatriz-Fonseca VL, Santos-Filho PS (2009) Production of workers, queens and males in Plebeia remota colonies (Hymenoptera, Apidae, Meliponini), a stingless bee with reproductive diapause. Genet Mol Res 8:672–683
Araújo FDS, dos Santos CF, Imperatriz-Fonseca VL, Marsaioli AJ (2017) Cuticular hydrocarbon profiles and putative sources of sex pheromones in queens of Tetragonisca angustula (Hymenoptera: Apidae: Meliponini). Trends in Entomology 13:79–93
Araujo RS, Bernardes RC, Fernandes KM, Lima MAP, Martins GF, Tavares MG (2019) Spinosad-mediated effects in the post-embryonic development of Partamona helleri (Hymenoptera: Apidae: Meliponini). Environ Pollut 253:11–18. https://doi.org/10.1016/j.envpol.2019.06.087
Baptistella AR, Souza CCM, Santana WC, Soares AEE (2012) Techniques for the in vitro production of queens in stingless bees (Apidae, Meliponini). Sociobiology 59:297–310. https://doi.org/10.13102/sociobiology.v59i1.685
Barbosa WF, Smagghe G, Guedes RNC (2015) Pesticides and reduced-risk insecticides, native bees and pantropical stingless bees: pitfalls and perspectives. Pest Manag Sci 71:1049–1053. https://doi.org/10.1002/ps.4025
Beekman M, Ratnieks FLW (2003) Power over reproduction in social hymenoptera. Philos Trans R Soc Lond B 358:1741–1753. https://doi.org/10.1098/rstb.2002.1262
Bego LR, Camargo CA (1984) On the occurrence of giant males in Nannotrigona (Scaptotrigona) postica Latreille (Hymenoptera, Apidae, Meliponinae). Boletim de Zoologia 8:11–16
Bernardes RC, Barbosa WF, Martins GF, Lima MAP (2018) The reduced-risk insecticide azadirachtin poses a toxicological hazard to stingless bee Partamona helleri (Friese, 1900) queens. Chemosphere 201:550–556. https://doi.org/10.1016/j.chemosphere.2018.03.030
Boff S, Friedel A, Mussury RM, Lenis PR, Raizer J (2018) Changes in social behavior are induced by pesticide ingestion in a Neotropical stingless bee. Ecotoxicol Environ Saf 164:548–553. https://doi.org/10.1016/j.ecoenv.2018.08.061
Bonetti AM, Cruz-Landim C, Kerr WE (1994) Sex determination in bees. XXX. Effects of juvenile hormone on the development of tergal glands in Melipona. J Apic Res 33:11–14. https://doi.org/10.1080/00218839.1994.11100843
Bourke AFG, Ratnieks FLW (1999) Kin conflict over caste determination in social Hymenoptera. Behav Ecol Sociobiol 46:287–297. https://doi.org/10.1007/s002650050622
Breed MD, Page RE Jr (1991) Intra- and interspecific nestmate recognition in Melipona workers (Hymenoptera: Apidae). Journal of Insect Behavior 4:463–469. https://doi.org/10.1007/BF01049331
Brito DV, Silva CGN, Hasselmann M, Viana LS, Astolfi-Filho S, Carvalho-Zilse GA (2015) Molecular characterization of the gene feminizer in the stingless bee Melipona interrupta (Hymenoptera: Apidae) reveals association to sex and caste development. Insect Biochem Mol Biol 66:24–30. https://doi.org/10.1016/j.ibmb.2015.09.008
Bueno FGB (2021) Native bees as alternative crop pollinators: Reproductive behaviour of Tetragonula carbonaria. PhD Thesis, University of Sydney.
Bueno FGB, Gloag R, Latty T, Ronai I (2020) Irreversible sterility of workers and high-volume egg production by queens in the stingless bee Tetragonula carbonaria. J Exp Biol 223:230599. https://doi.org/10.1242/jeb.230599
Bueno FGB, Bueno BGB, Latty T, Oldroyd BP, Hosoi AE, Buchmann G, Heard TA, Gloag R (2022a) Males are capable of long-distance dispersal in a social bee. Front Ecol Evol 10:843156. https://doi.org/10.3389/fevo.2022.843156
Bueno FGB, Hajjar R, Colin T, Buchmann G, Latty T, Gloag R (2022b) Virgin queen behaviour and controlled mating in the stingless bee Tetragonula carbonaria (Meliponini). Insectes Soc. https://doi.org/10.1007/s00040-022-00887-z
Butz VM, Dietz A (1994) The mechanism of queen elimination in two-queen honey bee (Apis mellifera L.) colonies. J Apic Res 33:87–94. https://doi.org/10.1080/00218839.1994.11100855
Camargo CA (1972) Mating of the social bee Melipona quadrifasciata under controlled conditions (Hymenoptera, Apidae). J Kansas Entomol Soc 45:520–523
Camargo CA (1979) Sex determination in bees. XI Production of diploid males and sex determination in Melipona quadrifasciata. J Apic Res 18:77–84. https://doi.org/10.1080/00218839.1979.11099950
Camargo JMF (1974) Notas sobre a morfologia e biologia de Plebeia (Schwarziana) quadripunctata quadripunctata (Hymenoptera, Apidae, Meliponinae). Studia Entomologica 17:433–470
Camillo C (1971) Estudos adicionais sobre zangoes de Trigona (Friesella) schrottkyi Friese (Hymenoptera, Apidae). Ciência e Cultura 23:273
Campos LAO, Coelho CDP (1993) Determinação de sexo em abelhas. XXX. Influência da quantidade de alimento e do hormônio juvenil na determinação das castas em Partamona cupira helleri (Hymenoptera, Apidae, Meliponinae). Revista Brasileira de Zoologia 10:449–452. https://doi.org/10.1590/S0101-81751993000300012
Campos LAO, Melo GAR (1990) Physogastric queen mating in Melipona quadrifasciata Lep (Hymenoptera, Apidae). Revista Brasileira de Genetica 13:491–500
Cortopassi-Laurino M (1979) Observaçoes sobre atividades de machos de Plebeia droryana Friese (Hymenoptera, Apidae, Meliponinae). Rev Bras Biol 23:177–191
Cortopassi-Laurino M (2007) Drone congregations in Meliponini: what do they tell us? Biosci J 23:153–160
Cortopassi-Laurino M, Imperatriz-Fonseca VL, Roubik DW, Dollin A, Heard T, Aguilar I, Venturieri GC, Eardley C, Nogueira-Neto P (2006) Global meliponiculture: Challenges and opportunities. Apidologie 37:275–292. https://doi.org/10.1051/apido:2006027
Cruz-Landim C, Abdalla FC, Gracioli-Vitti LF (2005) Morphological and functional aspects of volatile-producing glands in bees (Hymenoptera: Apidae). Insect Science 12:467–480. https://doi.org/10.1111/j.1744-7917.2005.00059.x
Cunningham JP, Hereward JP, Heard TA, Barro PJ, West SA (2014) Bees at war: interspecific battles and nest usurpation in stingless bees. Am Nat 184:777–786. https://doi.org/10.1086/678399
de Souza EA, Neves CA, Campos LAD, Zanuncio JC, Serrão JE (2007) Effect of mating delay on the ovary of Melipona quadrifasciata anthidioides (Hymenoptera: Apidae) queens. Micron 38:471–477. https://doi.org/10.1016/j.micron.2006.08.005
de Souza EA, Trigo JR, Santos DE, Vieira CU, Serrão JE (2017) The relationship between queen execution and cuticular hydrocarbons in stingless bee Melipona scutellaris (Hymenoptera: Meliponini). Chemoecology 27:25–32. https://doi.org/10.1007/s00049-016-0226-9
Dorneles AL, Rosa-Fontana AS, Santos CF, Blochtein B (2021) Larvae of stingless bee Scaptotrigona bipunctata exposed to organophosphorus pesticide develop into lighter, smaller and deformed adult workers. Environ Pollut 272:116414. https://doi.org/10.1016/j.envpol.2020.116414
dos Santos CF, Acosta AL, Dorneles AL, Santos PDS, Blochtein B (2016) Queens become workers: pesticides alter caste differentiation in bees. Sci Rep 6:31605. https://doi.org/10.1038/srep31605
dos Santos CF, Francisco FO, Imperatriz-Fonseca VL, Arias MC (2016) Eusocial bee male aggregations: Spatially and temporally separated but genetically homogenous. Entomol Exp Appl 158:320–326. https://doi.org/10.1111/eea.12407
dos Santos CF, Menezes C, Vollet-Neto A, Imperatriz-Fonseca VL (2014) Congregation sites and sleeping roost of male stingless bees (Hymenoptera: Apidae: Meliponini). Sociobiology 61:115–118. https://doi.org/10.13102/sociobiology.v61i1.115-118
dos Santos CF, Santos PS, Blochtein B (2015) In vitro rearing of stingless bee queens and their acceptance rates into colonies. Apidologie 47:539–547. https://doi.org/10.1007/s13592-015-0398-2
Engels E, Engels W (1988) Age-dependent queen attractiveness for drones and mating in the stingless bee, Scaptotrigona postica. J Apic Res 27:3–8. https://doi.org/10.1080/00218839.1988.11100773
Engels E, Engels W, Lübke G, Schröder W, Francke W (1993) Age-related patterns of volatile cephalic constituents in queens of the neotropical stingless bee Scaptotrigona postica Latr (Hymenoptera, Apidae). Apidologie 24:539–548. https://doi.org/10.1051/apido:19930601
Engels W (1987) Pheromones and reproduction in Brazilian stingless bees. Mem Inst Oswaldo Cruz 82:35–45. https://doi.org/10.1590/S0074-02761987000700009
Engels W, Engels E, Francke W (1997) Ontogeny of cephalic volatile patterns in queens and mating biology of the neotropical stingless bee, Scaptotrigona postica. Invertebr Reprod Dev 31:251–256. https://doi.org/10.1080/07924259.1997.9672583
Engels W, Engels E, Lübke G, Schroöder W, Francke W (1990) Volatile cephalic secretions of drones, queens and workers in relation to reproduction in the stingless bee, Scaptotrigona postica (Hymenoptera: Apidae: Trigonini). Entomologia Generalis 15:91–101. https://doi.org/10.1127/entom.gen/15/1990/91
Engels W, Imperatriz-Fonseca VL (1990) Caste development, reproductive strategies, and control of fertility in honey bees and stingless bees. In: Engels W (ed) Social insects: An evolutionary approach to castes and reproduction. Springer-Verlag, Berlin, pp 167–230
Faustino CD, Silva-Matos EV, Mateus S, Zucchi R (2002) First record of emergency queen rearing in stingless bees (Hymenoptera, Apinae, Meliponini). Insectes Soc 49:111–113. https://doi.org/10.1007/s00040-002-8287-x
Ferreira-Caliman MJ, Falcón T, Mateus S, Zucchi R, Nascimento FS (2013) Chemical identity of recently emerged workers, males, and queens in the stingless bee Melipona marginata. Apidologie 44:657–665. https://doi.org/10.1007/s13592-013-0214-9
Fierro MM, Cruz-López L, Sánchez D, Villanueva-Gutiérrez R, Vandame R (2011) Queen volatiles as a modulator of Tetragonisca angustula drone behavior. J Chem Ecol 37:1255–1262. https://doi.org/10.1007/s10886-011-0034-1
Gloag RS, Beekman M, Heard TA, Oldroyd BP (2007) No worker reproduction in the Australian stingless bee Trigona carbonaria Smith, (Hymenoptera, Apidae). Insectes Soc 54:412–417. https://doi.org/10.1007/s00040-007-0961-6
Grajales-Conesa J, Rojas JC, Guzman-Diaz M, Rincon-Rabanales M, Cruz-Lopez L (2007) Cephalic and Dufour gland secretions of Scaptotrigona mexicana queens: Chemical composition and biological activity. Apidologie 38:38–46. https://doi.org/10.1051/apido:2006052
Grüter C (2020) Stingless bees: their behaviour, ecology and evolution. Springer, Cham
Hartfelder K, Engels W (1992) Allometric and multivariate analysis of sex and caste polymorphism in the neotropical stingless bee, Scaptotrigona postica. Insectes Soc 39:251–266. https://doi.org/10.1007/BF01323946
Hartfelder K, Makert GR, Judice CC, Pereira GAG, Santana WC, Dallacqua R, Bitondi MMG (2006) Physiological and genetic mechanisms underlying caste development, reproduction and division of labor in stingless bees. Apidologie 37:144–163. https://doi.org/10.1051/apido:2006013
Heard TA (1999) The role of stingless bees in crop pollination. Annu Rev Entomol 44:183–206. https://doi.org/10.1146/annurev.ento.44.1.183
Imperatriz-Fonseca VL (1973) Miscellaneous observations on the behaviour of Schwarziana quadripunctata (Hymenoptera, Apidae, Meliponinae). Boletim de Zoologia e Biologia Marinha 30:633–640
Imperatriz-Fonseca VL (1976) Studies on Paratrigona subnuda (Moure) Hymenoptera, Apidae, Meliponinae). I. Members of the colony. Revista Brasileira de Entomologia 20:101–112
Imperatriz-Fonseca VL (1977) Studies on Paratrigona subnuda (Moure) Hymenoptera, Apidae, Meliponinae. II. Behaviour of the virgin queen. Boletim de Zoologia 2:169–182. https://doi.org/10.11606/issn.2526-3358.bolzoo.1977.121695
Imperatriz-Fonseca VL, Cruz-Landim C, Moraes RLMS (1997) Dwarf gynes in Nannotrigona testaceicornis (Apidae, Meliponinae, Trigonini). Behaviour, exocrine gland morphology and reproductive status. Apidologie 28:113–122. https://doi.org/10.1051/apido:19970302
Imperatriz-Fonseca VL, Zucchi R (1995) Virgin queens in stingless bee (Apidae, Meliponinae) colonies: a review. Apidologie 26:231–244. https://doi.org/10.1051/apido:19950305
Inoue T, Sakagami SF, Salmah S, Yamane S (1984) The process of colony multiplication in the Sumatran stingless bee Trigona (Tetragonula) laeviceps. Biotropica 16:100–111. https://doi.org/10.2307/2387841
Jaffé R, Pioker-Hara FC, Santos CF, Santiago LR, Alves DA, Kleinert AMP, Francoy TM, Arias MC, Imperatriz-Fonseca VL (2014) Monogamy in large bee societies: a stingless paradox. Naturwissenschaften 101:261–264. https://doi.org/10.1007/s00114-014-1149-3
Jaffé R, Pope N, Carvalho AT, Maia UM, Blochtein B, Carvalho CAL, Carvalho-Zilse GA, Freitas BM, Menezes C, Ribeiro MF et al (2015) Bees for development: Brazilian survey reveals how to optimize stingless beekeeping. PLoS ONE 10:1–21. https://doi.org/10.1371/journal.pone.0121157
Jarau S, Van Veen JW, Aguilar I, Ayasse M (2009) Virgin queen execution in the stingless bee Melipona beecheii: The sign stimulus for worker attacks. Apidologie 40:496–507. https://doi.org/10.1051/apido/2009022
Jarau S, van Veen JW, Twele R, Reichle C, Gonzales EH, Aguilar I, Francke W, Ayasse M (2010) Workers make the queens in Melipona bees: identification of geraniol as a caste determining compound from labial glands of nurse bees. J Chem Ecol 36:565–569. https://doi.org/10.1007/s10886-010-9793-3
Kärcher MH, Menezes C, Alves DA, Beveridge OS, Imperatriz-Fonseca VL, Ratnieks FLW (2013) Factors influencing survival duration and choice of virgin queens in the stingless bee Melipona quadrifasciata. Naturwissenschaften 100:571–580. https://doi.org/10.1007/s00114-013-1053-2
Kendall LK, Rader R, Gagic V, Cariveau DP, Albrecht M, Baldock KC, Freitas BM, Hall M, Holzschuh A, Molina FP (2019) Pollinator size and its consequences: robust estimates of body size in pollinating insects. Ecol Evol 9:1702–1714. https://doi.org/10.1002/ece3.4835
Kerr WE (1950) Genetic determination of castes in the genus Melipona. Genetics 35:143–152. https://doi.org/10.1093/genetics/35.2.143
Kerr WE, Jungnickel H, Morgan ED (2004) Workers of the stingless bee Melipona scutellaris are more similar to males than to queens in their cuticular compounds. Apidologie 35:611–618. https://doi.org/10.1051/apido:2004052
Kerr WE, Krause W (1950) Contribuição para o conhecimento da bionomia de Melipona quadrifasciata Lep. (Hymenoptera, Apoidea). Dusenia 1:275–282
Kerr WE, Zucchi R, Nakadaira JT, Butolo JE (1962) Reproduction in the social bees (Hymenoptera: Apidae). J N Y Entomol Soc 70:265–276
Kleinert AMP (2005) Colony strength and queen replacement in Melipona marginata (Apidae: Meliponini). Braz J Biol 65:469–476. https://doi.org/10.1590/S1519-69842005000300012
Kleinert AMP, Imperatriz-Fonseca VL (1994) Virgin queen refuges in colonies of Melipona marginata (Apidae, Meliponinae). Rev Bras Biol 54:247–251
Koedam D, Monge IA, Sommeijer MJ (1995) Social interactions of gynes and their longevity in queenright colonies of Melipona favosa (Apidae: Meliponinae). Netherlands J Zool 45:480–494
Koffler S, Hoppe A, Bienefeld K, Kleinert AdMP, Jaffé R (2021) Long-term storage shapes ejaculate traits in a monogamous stingless bee (Scaptotrigona aff. depilis). Apidologie 52:242–251. https://doi.org/10.1007/s13592-020-00813-x
Leonhardt SD (2017) Chemical ecology of stingless bees. J Chem Ecol 43:385–402. https://doi.org/10.1007/s10886-017-0837-9
Lopes DM, Tavares MG, Campos LAO (2003) Sperm utilisation by Melipona quadrifasciata Lepeletier (Hymenoptera, Apidae) queens subjected to multiple mating. Insectes Soc 50:387–389. https://doi.org/10.1007/s00040-003-0694-0
Luz GF, Campos LAO, Zanuncio JC, Serrão JE (2017) Auxiliary brood cell construction in nests of the stingless bee Plebeia lucii (Apidae: Meliponini). Apidologie 48:681–691. https://doi.org/10.1007/s13592-017-0513-7
Melo GAR, Buschini MLT, Campos LAO (2001) Ovarian activation in Melipona quadrifasciata queens triggered by mating plug stimulation (Hymenoptera, Apidae). Apidologie 32:355. https://doi.org/10.1051/apido:2001135
Meneses HM, Koffler S, Freitas BM, Imperatriz-Fonseca VL, Jaffé R (2014) Assessing sperm quality in stingless bees. Sociobiology 61:517–522. https://doi.org/10.13102/sociobiology.v61i4.517-522
Menezes C, Vollet-Neto A, Imperatriz-Fonseca VL (2013) An advance in the in vitro rearing of stingless bee queens. Apidologie 44:491–500. https://doi.org/10.1007/s13592-013-0197-6
Michener CD (1974) The social behavior of the bees: a comparative study. Belknap Press of Harvard University Press, Massachusetts
Monteiro CA, Kerr WE (1990) Experimental exchange of queens between colonies of Melipona compressipes (Apidae, Meliponini). Rev Bras Biol 50:975–981
Nogueira-Ferreira FH, Baio MV, Noll FB, Tidon-Sklorz R, Zucchi R (2000) Morphometric study in Schwarziana quadripunctata with emphasis on virgin queens (Hymenoptera, Apidae, Meliponinae). Sociobiology 35:99–108
Nogueira-Ferreira FH, Silva-Matos EV, Zucchi R (2009) Interaction and behavior of virgin and physogastric queens in three Meliponini species (Hymenoptera, Apidae). Genet Mol Res 8:703–708. https://doi.org/10.4238/vol8-2kerr008
Nogueira-Neto P (1957) Pesquisas em andamento Chácaras e Quintais 96:880
Nogueira-Neto P (1997) Vida e criação de abelhas indígenas sem ferrão. Editora Nogueirapis, São Paulo.
Nonacs P, Tobin JE (1992) Selfish larvae: Development and the evolution of parasitic behavior in the Hymenoptera. Evolution 46:1605–1620. https://doi.org/10.2307/2410019
Nunes TM, Heard TA, Venturieri GC, Oldroyd BP (2015) Emergency queens in Tetragonula carbonaria (Smith, 1854) (Hymenoptera: Apidae: Meliponini). Austral Entomology 54:154–158. https://doi.org/10.1111/aen.12104
Nunes TM, Mateus S, Favaris AP, Amaral MFZJ, von Zuben LG, Clososki GC, Bento JMS, Oldroyd BP, Silva R, Zucchi R (2014) Queen signals in a stingless bee: suppression of worker ovary activation and spatial distribution of active compounds. Sci Rep 4:7449. https://doi.org/10.1038/srep07449
Oliveira RC, Di Pietro V, Quezada-Euán JJG, Pech JR, Moo-Valle H, Wenseleers T (2022) Tragedy of the commons in Melipona bees revisited. Biol Let 18:20210498. https://doi.org/10.1098/rsbl.2021.0498
Otesbelgue A, dos Santos CF, Blochtein B (2018) Queen bee acceptance under threat: Neurotoxic insecticides provoke deep damage in queen-worker relationships. Ecotoxicol Environ Saf 166:42–47. https://doi.org/10.1016/j.ecoenv.2018.09.048
Palmer KA, Oldroyd BP, Quezada-Euán JJG, Paxton RJ, May-Itzá WD (2002) Paternity frequency and maternity of males in some stingless bee species. Mol Ecol 11:2107–2113. https://doi.org/10.1046/j.1365-294X.2002.01589.x
Peters JM, Queller DC, Imperatriz-Fonseca VL, Roubik DW, Strassmann JE (1999) Mate number, kin selection and social conflicts in stingless bees and honeybees. Proc R Soc Lond B 266:379–384. https://doi.org/10.1098/rspb.1999.0648
Pinto LZ, Hartfelder K, Bitondi MMG, Simões ZLP (2002) Ecdysteroid titers in pupae of highly social bees relate to distinct modes of caste development. J Insect Physiol 48:783–790. https://doi.org/10.1016/S0022-1910(02)00103-8
Prato M (2010). Ocorrência natural de sexuados, produção in vitro de rainhas e multiplicação de colônias de Tetragonisca angustula (Hymenoptera, Apidae, Meliponini). Dissertation, University of São Paulo.
Rasmussen C, Cameron SA (2010) Global stingless bee phylogeny supports ancient divergence, vicariance, and long distance dispersal. Biol J Lin Soc 99:206–232. https://doi.org/10.1111/j.1095-8312.2009.01341.x
Ratnieks FLW (2001) Heirs and spares: caste conflict and excess queen production in Melipona bees. Behav Ecol Sociobiol 50:467–473. https://doi.org/10.1007/s002650100388
Reis EP, Campos LAO, Tavares MG (2011) Prediction of social structure and genetic relatedness in colonies of the facultative polygynous stingless bee Melipona bicolor (Hymenoptera, Apidae). Genet Mol Biol 34:338–344. https://doi.org/10.1590/S1415-47572011005000008
Ribeiro MF, Alves DA (2001) Size variation in Schwarziana quadripunctata queens (Hymenoptera, Apidae, Meliponini). Revista de Etologia 3:59–65
Ribeiro MF, Imperatriz-Fonseca VL, Santos-Filho PS (2003) Exceptional high queen production in the Brazilian stingless bee Plebeia remota. Stud Neotrop Fauna Environ 38:111–114
Ribeiro MF, Wenseleers T, Santos-Filho PS, Alves DA (2006) Miniature queens in stingless bees: Basic facts and evolutionary hypotheses. Apidologie 37:191–206. https://doi.org/10.1051/apido:2006023
Rosa AS, Teixeira JSG, Vollet-Neto A, Queiroz EP, Blochtein B, Pires CSS, Imperatriz-Fonseca VL (2016) Consumption of the neonicotinoid thiamethoxam during the larval stage affects the survival and development of the stingless bee. Scaptotrigona aff depilis Apidologie 47:729–738. https://doi.org/10.1007/s13592-015-0424-4
Roubik DW (1990) Mate location and mate competition in males of stingless bees (Hymenoptera, Apidae, Meliponinae). Entomol Gener 15:115–120. https://doi.org/10.1127/entom.gen/15/1990/115
Sakagami SF, Laroca S (1963) Additional observations on the habits of the cleptobiotic stingless bees, the genus Lestrimelitta Friese (Hymenoptera, Apoidea). J Faculty Sci Hokkaido Univ 15:319–339
Santos-Filho PS, Alves DA, Eterovic A, Imperatiz-Fonseca VL, Kleinert AMP (2006) Numerical investment in sex and caste by stingless bees (Apidae: Meliponini): a comparative analysis. Apidologie 37:207–221. https://doi.org/10.1051/apido:2006015
Silva DLN (1972) Considerações em torno de um caso de substituição de rainha em Plebeia (Plebeia) droryana, in: Cruz-Landim C, Heblin NJ, Lello E, and Takahashi CS (eds.), Homenagem a W. E. Kerr, Editora UNESP, Rio Claro
Silva DLN, Zucchi R, Kerr WE (1972) Biological and behavioural aspects of the reproduction in some species of Melipona (Hymenoptera, Apidae, Meliponinae). Anim Behav 20:123–132
Slaa EJ, Sánchez-Chaves LA, Malagodi-Braga KS, Hofstede FE (2006) Stingless bees in applied pollination: practice and perspectives. Apidologie 37:293–315. https://doi.org/10.1051/apido:2006022
Sommeijer MJ, Bruijn LLM, Meeuwsen F, Slaa EJ (2003) Reproductive behaviour of stingless bees: nest departures of non-accepted gynes and nuptial flights in Melipona favosa (Hymenoptera: Apidae, Meliponini). Entomologische Berichten 63:7–13
Tanaka ED, Santana WC, Hartfelder K (2009) Ovariole structure and oogenesis in queens and workers of the stingless bee Melipona quadrifasciata (Hymenoptera: Apidae, Meliponini) kept under different social conditions. Apidologie 40:163–177. https://doi.org/10.1051/apido/2008071
Terada Y (1974). Contribuição ao estudo da regulaçao social em Leurotrigona muelleri e Frieseomelitta varia. Dissertation, University of São Paulo.
Tomé HVV, Barbosa WF, Corrêa AS, Gontijo LM, Martins GF, Guedes RNC (2015) Reduced-risk insecticides in Neotropical stingless bee species: impact on survival and activity. Ann Appl Biol 167:186–196. https://doi.org/10.1111/aab.12217
van Oystaeyen A, Alves DA, Oliveira RC, Nascimento DL, Nascimento FS, Billen J, Wenseleers T (2013) Sneaky queens in Melipona bees selectively detect and infiltrate queenless colonies. Anim Behav 86:603–609. https://doi.org/10.1016/j.anbehav.2013.07.001
van Veen JW (2018) Impacto del cambio climático sobre las abejas sin aguijón Melipona beecheii. XI Congreso Mesoamericano sobre Abejas Nativas, La Antigua.
van Veen JW, Sommeijer MJ (2000) Colony reproduction in Tetragonisca angustula (Apidae, Meliponini). Insectes Soc 47:70–75. https://doi.org/10.1007/s000400050011
Van Veen JW, Sommeijer MJ (2000) Observations on gynes and drones around nuptial flights in the stingless bees Tetragonisca angustula and Melipona beecheii (Hymenoptera, Apidae, Meliponinae). Apidologie 31:47–54. https://doi.org/10.1051/apido:2000105
van Veen JW, Sommeijer MJ, Aguilar Monge I (1999) Behavioural development and abdomen inflation of gynes and newly mated queens of Melipona beecheii (Apidae, Meliponinae). Insectes Soc 46:361–365. https://doi.org/10.1007/s000400050157
Veiga JC, Menezes C, Contrera FAL (2017) Insights into the role of age and social interactions on the sexual attractiveness of queens in an eusocial bee, Melipona flavolineata (Apidae, Meliponini). Sci Nat 104:31. https://doi.org/10.1007/s00114-017-1450-z
Verdugo-Dardon M, Cruz-Lopez L, Malo EA, Rojas JC, Guzman-Diaz M (2011) Olfactory attraction of Scaptotrigona mexicana drones to their virgin queen volatiles. Apidologie 42:543–550. https://doi.org/10.1007/s13592-011-0042-8
Vollet-Neto A, Imperatriz-Fonseca VL, Ratnieks FL (2019) Queen execution, diploid males, and selection for and against polyandry in the Brazilian stingless bee Scaptotrigona depilis. Am Nat 194:725–735. https://doi.org/10.1086/705393
Vollet-Neto A, Koffler S, dos Santos CF, Menezes C, Nunes FMF, Hartfelder K, Imperatriz-Fonseca VL, Alves DA (2018) Recent advances in reproductive biology of stingless bees. Insectes Soc 65:201–212. https://doi.org/10.1007/s00040-018-0607-x
Vollet-Neto A, Oliveira RC, Schillewaert S, Alves DA, Wenseleers T, Nascimento FS, Imperatriz-Fonseca VL, Ratnieks FLW (2017) Diploid male production results in queen death in the stingless bee Scaptotrigona depilis. J Chem Ecol 43:403–410. https://doi.org/10.1007/s10886-017-0839-7
Wenseleers T, Alves DA, Francoy TM, Billen J, Imperatriz-Fonseca VL (2011) Intraspecific queen parasitism in a highly eusocial bee. Biol Let 7:173–176. https://doi.org/10.1098/rsbl.2010.0819
Wenseleers T, Hart AG, Ratnieks FLW, Quezada-Euan JJG (2004) Queen execution and caste conflict in the stingless bee Melipona beecheii. Ethology 110:725–736. https://doi.org/10.1111/J.1439-0310.2004.01008.X
Wenseleers T, Ratnieks FLW, Billen J (2003) Caste fate conflict in swarm-founding social Hymenoptera: An inclusive fitness analysis. J Evol Biol 16:647–658. https://doi.org/10.1046/j.1420-9101.2003.00574.x
Wenseleers T, Ratnieks FLW, Ribeiro MF, Alves DA, Imperatriz-Fonseca VL (2005) Working-class royalty: Bees beat the caste system. Biol Let 1:125–128. https://doi.org/10.1098/rsbl.2004.0281
Acknowledgements
We thank Conselho Nacional de Desenvolvimento Científico e Tecnológico [CNPq; grant 309542/2020-0 linked to FAPERGS/CNPq/SEBRAE 08/2019 (Programa Doutor Empreendedor—PDEmp, grant 20/2551-0000203-9) to CFS; grant 380653/2021-4 to DAA] and PUCRS/BPA (01/2020 to AO) for financial support.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the study conception and design. Material preparation, data collection and the first draft of the manuscript was written by FGBB, CFS, AO and JV. Comments and improvements on next versions of manuscript were done by DAA, CM, BB, RG, TH and VLIF. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bueno, F.G.B., dos Santos, C.F., Otesbelgue, A. et al. The queens of the stingless bees: from egg to adult. Insect. Soc. 70, 43–57 (2023). https://doi.org/10.1007/s00040-022-00894-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-022-00894-0