Abstract
Background
Rhipicephalus microplus, an invasive tick species of Asian origin and the main vector of Babesia species, is considered one of the most widespread ectoparasites of livestock. The tick has spread from its native habitats on translocated livestock to large parts of the tropical world, where it has replaced some of the local populations of Rhipicephalus decoloratus ticks. Although the tick was reported in Uganda 70 years ago, it has not been found in any subsequent surveys. This study was carried out to update the national tick species distribution on livestock in Uganda as a basis for tick and tick-borne disease control, with particular reference to R. microplus.
Methods
The study was carried out in Kadungulu, Serere district, south-eastern Uganda, which is dominated by small scale livestock producers. All the ticks collected from 240 cattle from six villages were identified microscopically. Five R. microplus specimens were further processed for phylogenetic analysis and species confirmation.
Results
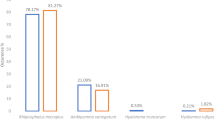
The predominant tick species found on cattle was Rhipicephalus appendiculatus (86.9 %; n = 16,509). Other species found were Amblyomma variegatum (7.2 %; n = 1377), Rhipicephalus evertsi (2.3 %; n = 434) and R. microplus (3.6 %; n = 687). Phylogenetic analysis of the 12S rRNA, 16S rRNA and ITS2 gene sequences of R. microplus confirmed the morphological identification.
Conclusions
It is concluded that R. microplus has replaced R. decoloratus in the sampled villages in Kadungulu sub-county, since the latter was not any longer found in this area. There is currently no livestock movement policy in force in Uganda, which could possibly limit the further spread of R. microplus ticks. Future surveys, but also retrospective surveys of museum specimens, will reveal the extent of distribution of R. microplus in Uganda and also for how long this tick has been present on livestock without being noticed.

Similar content being viewed by others
Background
The Asian blue tick, Rhipicephalus (Boophilus) microplus (Canestrini, 1888), is one of the most important tick species infesting livestock in many parts of the world [1]. Rhipicephalus microplus has extended its distribution through the translocation of tick-infested cattle. In some regions in Africa, R. microplus has successfully competed and replaced the close related African blue tick, Rhipicephalus decoloratus [2,3,4]. Rhipicephalus microplus is vector of Babesia bovis and Babesia bigemina causing extensive production losses [5,6,7].
Rhipicephalus microplus has been introduced from Asia on cattle exported to East and South Africa via Madagascar [3]. Similarly, R. microplus was introduced into Ivory Coast and Benin from Brazil 10 years ago [8]. Since then, it has spread to Burkina Faso, Mali, Togo and very recently into Nigeria and Cameroon [3, 8,9,10,11,12]. This species is now well established in the southern and eastern fringes of South Africa [2, 7]. Displacement of local R. decoloratus populations in the countries where R. microplus was introduced could have resulted from the faster life-cycle of R. microplus, sterile off-spring of interspecific mating [2] or because of the higher degree of acaricide resistance of R. microplus [2].
There are a few isolated reports of R. microplus in East Africa, notably Tanzania and South Sudan [13, 14]. Some of these reports are over 30 years old [15, 16]; hence, this tick species could have spread to several parts of East Africa. Given that R. microplus is an invasive tick species, such isolated reports are likely to be due to a lack of regional or country-wide tick surveys and the distribution may be wider than reported. Furthermore, the differential diagnosis of R. microplus and R. decoloratus in East Africa and R. decoloratus, Rhipicephalus annulatus and Rhipicephalus geigyi in West Africa is difficult because of similarities in morphology and their small size [4].
There are no up-to-date Ugandan or East African tick surveys. As a result, despite records of R. microplus 70 years ago in the Uganda [17], this has never been confirmed. This study was carried out in one of the high tick density districts of south-eastern Uganda to update national tick species data as a basis for a national tick and tick-borne disease control strategy.
Methods
Study area
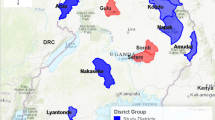
The study was carried out in Serere district, south-eastern Uganda, in 2017. The district is made up of two rural counties (Kasilo and Serere), eight sub-counties (Bugondo, Kadungulu, Pingire, Labor, Atiira, Kateta, Chere and Serere/Olio) encompassing 254 cattle-owning villages. Tick collections were conducted in Kadungulu sub-county. Serere district was selected because it has a large number of small scale livestock producers (1–50 cattle per herd) whose potential to commercialise livestock production is primarily constrained by ticks and tick-borne diseases. Six of the 254 villages were randomly selected for this study (Fig. 1).
Cattle herd and individual animal selection
Farmers in Serere district predominantly keep short-horn East-African Zebu cattle in communal village herds. Given that any animal sampled from each of these villages is likely to be infested with ticks, the number of villages (n = 6) and animals selected (n = 240) need not to be based on any rigorous statistical methods. However, to estimate the chance of finding R. decoloratus ticks, we conducted a power calculation assuming a prevalence of 5% at P = 0.01; we expected to find 18 R. decoloratus ticks in a sample size of 18,000 ticks [18]. In this study we sampled 19,007 ticks. Cattle were included in this study if they had not been sprayed against ticks for the past two weeks, were young (1–2 years old) and non-fractious. Young non-fractious animals were preferred for inclusion because they were easier to restrain and pose very low risk of injury to themselves or personnel. An average of 40 cattle was sampled from each of the six selected villages. All cattle sampling sites were geo-referenced prior to tick collection.
Tick collection and identification
Selected cattle were physically restrained before half-body tick collections were carried out. Each of the collected ticks was morphologically identified to the genus level before they were preserved in 70% ethanol. The tick samples were then transferred to Makerere University for further species identification using taxonomic keys [19]. Five representative R. microplus specimens were selected for molecular species confirmation based on 12S ribosomal RNA (12S rRNA), 16S ribosomal RNA (16S rRNA) and the internal transcribed spacer 2 (ITS2) gene sequences [4, 20]. A taxonomically and molecularly confirmed R. microplus specimen was photographed under a stereomicroscope (Olympus model SZX7, Tokyo, Japan).
DNA extraction
Prior to tick DNA extraction, each tick was cleaned in five one-minute steps, each step involving centrifugation at 10,000× rpm in freshly prepared 1.5 ml of phosphate-buffered saline (PBS). Individual clean ticks were immersed under liquid nitrogen for 5 min and thereafter crushed with a sterile mortar and pestle to create a tick homogenate. DNA was then extracted from each tick homogenate using DNeasy Blood and Tissue Kit (Qiagen, Germantown, MD, USA) following the manufacturers’ instructions. The presence and quality of DNA were checked by resolving 5 µl of the extracted DNA on a 1% agarose gel and viewing them under an ultraviolet transilluminator (Wagtech International, Thatcham, UK). The remaining DNA was stored at − 20 °C until use in the downward amplification steps.
DNA amplification
PCR amplification was performed on 12S rRNA and 16S rRNA genes and the ITS 2 spacer using primers (Table 1) and thermocycling conditions as previously described [4, 19]. Each reaction was prepared into a final volume of 50 µl containing; 1×-reaction buffer (670 mM Tris-HC, pH 8.8, 166 μM (NH4)2SO4, 4.5 % Triton X-100, 2 mg/ml gelatin) (Bioline, Humber Road, London, UK), 0.25 mM of each dNTP, 0.25 mM each of forward and reverse primers, 1.56 U BioTaq DNA polymerase (Bioline, London, UK), 1.25 mM MgCl2, 32.2 µl of PCR grade water and finally 5 µl of the template DNA.
The 16S ribosomal RNA gene was amplified in a thermocycler (Personal Thermocycler, Biometra, Göttingen, Germany) with initial denaturation of 94 °C for 5 min followed by 30 cycles at 94 °C for 30 s, 48 °C for 45 s, 72 °C for 45 s and a final extension at 72 °C for 7 min. Amplification of the ITS2 and 12S ribosomal RNA was performed using similar thermocycling conditions to those of 16S at annealing temperature of 55 °C and 52 °C, respectively. PCR products were resolved on 2% agarose gels. The resultant PCR products were sized against a 1 kb DNA molecular ladder (Bioline, London, UK). The expected PCR product sizes ranged between 300–1200 bp. PCR products were purified using QIAquick PCR Purification Kit (Qiagen, Germantown, MD, USA) and commercially Sanger-sequenced (Inqaba Biotec, Muckleneuk, Pretoria, South Africa).
Gene sequence analysis
Each of the 12S rRNA, 16S rRNA and ITS2 tick sequences from this study were queried in a BLASTn search with default settings (NCBI BLASTn software version 2.6.10) [21] to reveal their identity. The query sequence identity was assigned/matched based on the hits (tick species sequences returned) with the highest identity scores (≥ 80%) and most significant E-values (closest to 0.0). The identified query sequences from this study were annotated and submitted to the GenBank database under the accession numbers MK332390, MK332391, KY688455, KY688459, KY688461 and KY688467.
Annotated sequences from this study were each analysed in a multiple sequence alignment (MSA) with their corresponding reference gene sequences downloaded from GenBank using the ClustalW algorithm in MEGA version 10 [22]. The MSA files were used to infer nucleotide similarity between sequences from this study and their corresponding nucleotide reference sequences from GenBank. Each of the data sequence sets were analysed in MSA using the ClustalW algorithm and trimmed in MEGA software version 10 [23, 24]. Phylogenetic analysis for each nucleotide sequence set was performed using the maximum likelihood method utilising the Tamura 3-parameter with Gamma distribution with 1000 bootstrap replicates [23] as the best-fit model to infer phylogenetic relatedness among the gene sets.
To evaluate the 12S rRNA, 16S rRNA and ITS2 sequence divergence of newly typed Ugandan R. microplus ticks and their corresponding reference sequences from GenBank, pairwise genetic distances were calculated in MEGA software version 10 [23] using default settings for each sequence.
Results
Tick collections
Adult ticks (n = 19,007) were collected upon completion of half-body counts from 240 cattle. The majority (86.9 %; n = 16,509) of these ticks were Rhipicephalus appendiculatus. Other tick species identified were Amblyomma variegatum (7.2 %; n = 1377), R. microplus (3.6 %; n = 687) and Rhipicephalus evertsi (2.3 %; n = 434). The mean adult tick density was 79 ticks per animal. On average, the numbers of adult R. appendiculatus, A. variegatum, R. microplus and R. evertsi per animal were 138, 11, 6 and 4, respectively.
A taxonomically and molecularly confirmed R. microplus specimen was photographed under a stereomicroscope as shown in Fig. 2. Female R. microplus was characterized by hypostome teeth in a typical 4 + 4 column arrangement and internal margin palpal article 1 lacking protuberance and distinctly concave. Male R. microplus carried typical indistinct spurs on the ventral plates.
Morphological identification of R. microplus. a Female, ventral view (1, hypostomal teeth in a typical 4 + 4 column arrangement; 2, short palp of internal margin article 1 (lacks protuberance and is distinctly concave); 3, cornua). b Male, ventral view (4, distinctly small adenal plates; 5, ventral plate spurs (small accessory adanal plates); 6, caudal appendage; 7, genital aperture with a broad U-shape)
Molecular confirmation of R. microplus
Five of the 687 ticks that were identified as R. microplus using standard taxonomic keys were further analysed and all confirmed R. microplus by assessing sequence variation of their 12S rRNA, 16S rRNA and ITS2 regions. The genetic diversity of R. microplus ticks recovered from Uganda and those from elsewhere ranged between 0–0.075 (Table 2). Phylogenetic analysis of the 12S rRNA (Fig. 3), 16S rRNA (Fig. 4) and ITS2 (Fig. 5) regions revealed polymorphic sub-grouping with R. microplus collected from other parts of the world. The Ugandan R. microplus isolates were notably similar to those collected in Taiwan, Mozambique, Nigeria, the USA and South Africa.
Phylogenetic analysis based on the tick 12S ribosomal RNA gene. A phylogenetic tree based on 12S rDNA sequences. The tree was generated by the maximum likelihood method based on the Tamura 3-parameter model. The analysis involved 26 nucleotide sequences. Orange circles represent samples sequenced in this study
Phylogenetic analysis based on the tick 16S ribosomal RNA gene. A phylogenetic tree based on 16S rDNA sequences. The tree was generated by the maximum likelihood method based on the Tamura 3-parameter model. The analysis involved 25 nucleotide sequences. Orange circles represent samples sequenced in this study
Phylogenetic analysis based on the ITS2 spacer of the ribosomal RNA gene cluster of ticks. A phylogenetic tree based on ITS2 rDNA sequences. The tree was generated by the maximum likelihood method based on the Tamura 3-parameter model. The analysis involved 32 nucleotide sequences. Orange circles represent samples sequenced in this study
Discussion
The high burden of adult ticks on cattle in Kadungulu sub-county, Serere District, south-eastern Uganda, confirms that ticks and associated diseases (anaplasmosis, babesiosis, theileriosis and heartwater) constitute a major constraint to livestock production in this region [25, 26]. It has been reported previously that R. appendiculatus, vector of Theileria parva, is the predominant tick species in Serere District [26,27,28,29]. Besides the discovery of R. microplus and the complete absence of R. decoloratus, the other tick species were the same as reported before in south-eastern Uganda [25,26,27,28]. However, the Ugandan tick population structure varies greatly between the different regions of the country, due to variation in microclimatic conditions [25,26,27]. For example, Amblyomma lepidum, Hyalomma truncatum, Amblyomma gemma and Rhipicephalus pulchellus thrive under the arid conditions of north-eastern Uganda [27, 30], and were therefore not found in this less arid study area.
In “The ixodid ticks of Uganda” Matthysse & Colbo [16] reported a systematic survey of ticks on livestock conducted between 1965–1966, wherein not a single R. microplus tick was found. Interestingly, before this survey, R. microplus was reported from Uganda by S. G. Wilson, who conducted a limited survey on cattle along the borders of Karamoja district, closer to the border with Kenya [17]. It is unlikely that R. microplus may have been missed during the nation-wide survey conducted by Matthysse & Colbo [16], now more than 50 years ago, although the sample size of 491 cattle was limited. Interestingly, our results clearly indicate that R. microplus has been overlooked for years, since it takes years to replace an indigenous population of R. decoloratus ticks [31]. Given the invasive nature of this tick species, exacerbated by poor animal movement control and communal grazing practices within the East African region, it may be the case that populations of R. microplus are now well established in Uganda.
Molecular phylogenetic analysis may be a useful tool to discern possible relationships between isolates collected from different geographical regions. In this study, the 12S rRNA and ITS2 regions of the tick isolates from Uganda were identical to those previously isolated from Taiwan, Mozambique, Nigeria, USA and South Africa. It is therefore plausible that the R. microplus ticks collected from cattle in south-eastern Uganda were introduced on livestock imported from the southern parts of Africa. In the past 10–15 years, there have been significant importations of dairy cattle into Uganda from South Africa to improve the Ugandan dairy herd through cross-breeding [32]. However, a more extensive genotyping of ticks collected from different geographical areas is required to confirm this [26, 33].
Unprecedented levels of acaricide-resistant tick populations have recently been reported in Uganda [33]. The cause of this problem is due to farmer-related factors (acaricide overuse and misuse) potentiated by lack of national acaricide and animal movement control policies [33,34,35]. Under such favourable conditions, R. microplus tick populations are known to rapidly become acaricide-resistant as a result of target specific mutations and metabolic adaptations [36]. The introduction of R. microplus into Uganda is likely to exacerbate the already existing problem of ticks and tick-borne diseases in three ways. These include: (i) complete replacement of R. decoloratus by R. microplus, resulting in a national and probably regional upsurge of R. microplus populations; (ii) emergence of acaricide-resistant R. microplus populations; and (iii) a proportional increase of bovine babesiosis given that R. microplus is an efficient vector of B. bovis [11, 26, 32]. Unless effective national acaricide and animal movement control policies are instituted, the Ugandan livestock sector will suffer severe losses due to the direct effects of R. microplus infestation and bovine babesiosis.
Conclusions
It was expected to find R. decoloratus among other tick species on cattle during a survey conducted in south-eastern Uganda. Instead, we discovered that R. microplus has completely displaced R. decoloratus in the six villages studied, an indigenous tick species previously known to this region. There is a need to determine the extent of spread of R. microplus throughout Uganda and to put in place effective control measures considering that R. microplus is capable of developing high levels of resistance towards the major classes of acaricides.
Availability of data and materials
Data supporting the conclusions of this article are included within the article. The newly generated sequences were submitted to the GenBank database under the accession numbers MK332390, MK332391, KY688455, KY688459, KY688461 and KY688467. The datasets used and/or analysed during the present study are available from the corresponding author upon reasonable request.
Abbreviations
- 12S rRNA:
-
12S ribosomal RNA gene
- 16S rRNA:
-
16S ribosomal RNA gene
- AAS:
-
African Academy of Science
- BMGF:
-
Bill & Melinda Gates Foundation
- DELTAS:
-
Developing Excellence in Leadership, Training and Science
- MUII:
-
Makerere University-Uganda Virus Research Institute Centre of Excellence for Infection and Immunity Research and Training
- MSA:
-
multiple sequence alignment
- NEPAD:
-
New Partnership for Africa’s Development Planning and Coordinating Agency
- DNA:
-
deoxyribonucleic acid
- RNA:
-
ribonucleic acid
- GPS:
-
geographical positioning system
- ITS 2:
-
internal transcribed spacer 2
- PBS:
-
phosphate-buffered saline
References
Coetzer JAW, Tustin RC. Infectious diseases of livestock, vol. 2. 2nd ed. Cape Town: Oxfor University Press; 2004.
Tønnesen MH, Penzhorn BL, Bryson NR, Stoltsz WH, Masibigiri T. Displacement of Boophilus decoloratus by Boophilus microplus in the Soutpansberg region, Limpopo Province, South Africa. Exp Appl Acarol. 2004;32:199–208.
Madder M, Thys E, Achi L, Touré A, De Deken R. Rhipicephalus (Boophilus) microplus: a most successful invasive tick species in West-Africa. Exp Appl Acarol. 2011;53:139–45.
Low VL, Tay ST, Kho KL, Koh FX, Tan TK, Lim YAL, et al. Molecular characterisation of the tick Rhipicephalus microplus in Malaysia: new insights into the cryptic diversity and distinct genetic assemblages throughout the world. Parasit Vectors. 2011;8:341.
Waldron SJ, Jorgensen WK. Transmission of Babesia spp. by the cattle tick (Boophilus microplus) to cattle treated with injectable or pour-on formulations of ivermectin and moxidectin. Aust Vet J. 1999;77:657–9.
Rodrigues DS, Leite RC. Economic impact of Rhipicephalus (Boophilus) microplus: estimate of decreased milk production on dairy farm. Arq Bras Med Vet Zootec. 2013;65:1570–2.
Jonsson NN. The productivity effects of cattle tick (Boophilus microplus) infestation on cattle, with particular reference to Bos indicus cattle and their crosses. Vet Parasitol. 2006;137:1–10.
Boka OM, Achi L, Adakal H, Azokou A, Yao P, Yapi YG, et al. Review of cattle ticks (Acari, Ixodida) in Ivory Coast and geographic distribution of Rhipicephalus (Boophilus) microplus, an emerging tick in West Africa. Exp Appl Acarol. 2017;71:355–69.
Awa DN, Adakal H, Luogbou NDD, Wachong KH, Leinyuy I, Achukwi MD. Cattle ticks in Cameroon: is Rhipicephalus (Boophilus) microplus absent in Cameroon and the Central African region? Ticks Tick Borne Dis. 2015;6:117–22.
Kamani J, Apanaskevich DA, Gutiérrez R, Nachum-Biala Y, Baneth G, Harrus S. Morphological and molecular identification of Rhipicephalus (Boophilus) microplus in Nigeria, West Africa: a threat to livestock health. Exp Appl Acarol. 2017;73:283–96.
Adehan SB, Biguezoton A, Adakal H, Assogba MN, Zoungrana S, Gbaguidi AM, et al. Acaricide resistance of Rhipicephalus microplus ticks in Benin. Afr J Agr Res. 2016;11:1199–208.
Adakal H, Biguezoton A, Zoungrana S, Courtin F, de Clercq EM, Madder M. Alarming spread of the Asian cattle tick Rhipicephalus microplus in West Africa-another three countries are affected: Burkina Faso. Mali and Togo. Exp Appl Acarol. 2013;61:383–6.
Lynen G, Zeman P, Bakuname C, Di Giulio G, Mtui P, Sanka P, et al. Shifts in the distributional ranges of Boophilus ticks in Tanzania: evidence that a parapatric boundary between Boophilus microplus and B. decoloratus follows climate gradients. Exp Appl Acarol. 2008;44:147–64.
Kivaria FM, Kapaga AM, Mbassa GK, Mtui PF, Wani RJ. Epidemiological perspectives of ticks and tick-borne diseases in south Sudan: cross-sectional survey results. Onderstepoort J Vet Res. 2012;79:E1–10.
Hoogstraal H. Ticks of the Sudan (with special reference to Equatoria Province and with preliminary reviews of the genera Boophilus, Margaropus, and Hyalomma). Cairo, Egypt: United States Naval Medical Research Unit No. 3; 1956.
Matthysee JG, Colbo MH. The Ixodid ticks of Uganda: together with species pertinent to Unganda because of their present known distribution. College Park: Entomological Society of America; 1987.
Wilson SG. Ticks and tick-borne diseases (a)—tick survey. Uganda Vet ment Anim Report. 1950;1949:21.
Overall JE. Sample size required to observe at least k rare events. Psychol Rep. 1967;21:70–2.
Walker AR. Ticks of domestic animals in Africa: a guide to identification of species. Edinburgh: Bioscience Reports; 2003.
Lv J, Wu S, Zhang Y, Chen Y, Feng C, Yuan X, et al. Assessment of four DNA fragments (COI, 16S rDNA, ITS2, 12S rDNA) for species identification of the Ixodida (Acari: Ixodida). Parasit Vectors. 2014;7:93.
Zhang Z, Schwartz S, Wagner L, Miller W. A greedy algorithm for aligning DNA sequences. J Comput Biol. 2000;7:203–14.
Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9.
Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512–26.
Ocaido M, Otim CP, Okuna NM, Erume J, Ssekitto C, Wafula RZO, et al. Socio-economic and livestock disease survey of agropastoral communities in Serere County, Soroti District, Uganda. 2005. In: Livestock Research for Rural Development. http://www.lrrd.org/lrrd17/8/ocai17093.htm. Accessed 17 Jun 2019.
Kabi F, Magona JW, Nasinyama GW, Walubengo J. Sero-prevalences of tick-borne infections among the Nkedi Zebu and Ankole cattle in Soroti district. Uganda. J Protozool Res. 2008;18:61–70.
Okello-Onen J, Tukahirwa EM, Perry BD, Rowlands GJ, Nagda SM, Musisi G, et al. Population dynamics of ticks on indigenous cattle in a pastoral dry to semi-arid rangeland zone of Uganda. Exp Appl Acarol. 1999;23:79–88.
Byaruhanga C, Collins NE, Knobel D, Kabasa W, Oosthuizen MC. Endemic status of tick-borne infections and tick species diversity among transhumant zebu cattle in Karamoja region, Uganda: support for control approaches. Vet Parasitol Reg Stud Reports. 2015;1–2:21–30.
Magona JW, Walubengo J, Olaho-Mukani W, Jonsson NN, Welburn SW, Eisler MC. Spatial variation of tick abundance and seroconversion rates of indigenous cattle to Anaplasma marginale, Babesia bigemina and Theileria parva infections in Uganda. Exp Appl Acarol. 2011;55:203–13.
Balinandi S, Mugisha L, Bbira J, Kabasa W, Nakayiki T, Bakkes DK, et al. General and local morphological anomalies in Amblyomma lepidum (Acari: Ixodidae) and Rhipicephalus decoloratus infesting cattle in Uganda. J Med Entomol. 2018;56:873–7.
Nyangiwe N, Harrison A, Horak I. Displacement of Rhipicephalus decoloratus by Rhipicephalus microplus (Acari: Ixodidae) in the Eastern Cape Province. South Africa. Exp Appl Acarol. 2013;61:371–82.
Muhanguzi D, Matovu E, Waiswa C. Prevalence and characterization of Theileria and Babesia species in cattle under different husbandry systems in western Uganda. Int J Anim Vet Adv. 2010;2:51–8.
Vudriko P, Okwee-Acai J, Tayebwa DS, Byaruhanga J, Kakooza S, Wampande E, et al. Emergence of multi-acaricide resistant Rhipicephalus ticks and its implication on chemical tick control in Uganda. Parasit Vectors. 2016;9:4.
Vudriko P, Okwee-Acai J, Byaruhanga J, Tayebwa DS, Omara R, Muhindo JB, et al. Evidence-based tick acaricide resistance intervention strategy in Uganda: concept and feedback of farmers and stakeholders. Ticks Tick Borne Dis. 2018;9:254–65.
Vudriko P, Okwee-Acai J, Byaruhanga J, Tayebwa DS, Okech SG, Tweyongyere R, et al. Chemical tick control practices in southwestern and northwestern Uganda. Ticks Tick Borne Dis. 2018;9:945–55.
Foil LD, Coleman P, Eisler M, Fragoso-Sanchez H, Garcia-Vazquez Z, Guerrero FD, et al. Factors that influence the prevalence of acaricide resistance and tick-borne diseases. Vet Parasitol. 2004;125:163–81.
Acknowledgements
The authors wish to acknowledge the District Veterinary Officer of Serere District, Dr Amonya Collins for his help during tick collection. We also acknowledge the cattle owners and local council leaders of the six villages where this study was carried out for offering their cattle and helping with their restraint in this study. We are very grateful to Dr Bigirwa Godfrey, Kakooza Stephen, Dr Bbiira Jonson, Dr Omaido Edward, Gloria Grace Akurut and Dr Akech Olivia for their help during tick collection and field tick identification to genus level.
Funding
This study received funding from the Bill & Melinda Gates Foundation (BMGF) (Grant OPP1125367) through ClinVet International (Pty) (project no. CV 16/337). This work was also supported by the Makerere University-Uganda Virus Research Institute Centre of Excellence for Infection and Immunity Research and Training (MUII). MUII is supported through the DELTAS Africa Initiative (Grant no. 107743). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS), Alliance for Accelerating Excellence in Science in Africa (AESA), and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (Grant no. 107743) and the UK government. All funders of this research had no role in the design of the study, collection, analysis, and interpretation of data and in writing of this manuscript. The views expressed in this publication are those of the authors and not BMGF, MUII, African Academy of Science (AAS), NEPAD Agency, Wellcome Trust and the UK government.
Author information
Authors and Affiliations
Contributions
DM, JF, MM, TS, IH, NJ and FJ conceived and designed the study. DM, JB, CN, WA, SO, JN and FJ collected tick samples and performed taxonomic and molecular tick identification. DM, CN, SO, JN, FNM and RT undertook molecular confirmation of R. microplus. DM, FNM, RT, JF, MM, IH and FJ drafted and critically reviewed this manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from cattle owners. Cattle restraint, tick collection, preservation, morphological and molecular analyses followed highest veterinary care and scientific standards as approved (SBLS/REC/16/136) by the Research and Ethics Committee of Makerere University School of Biosecurity, Biotechnical and Laboratory Sciences and the Uganda National Council of Science and Technology (A 513).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Muhanguzi, D., Byaruhanga, J., Amanyire, W. et al. Invasive cattle ticks in East Africa: morphological and molecular confirmation of the presence of Rhipicephalus microplus in south-eastern Uganda. Parasites Vectors 13, 165 (2020). https://doi.org/10.1186/s13071-020-04043-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-020-04043-z