Abstract
Anaplasma phagocytophilum is the agent of tick-borne fever, equine, canine and human granulocytic anaplasmosis. The common route of A. phagocytophilum transmission is through a tick bite, the main vector in Europe being Ixodes ricinus. Despite the apparently ubiquitous presence of the pathogen A. phagocytophilum in ticks and various wild and domestic animals from Europe, up to date published clinical cases of human granulocytic anaplasmosis (HGA) remain rare compared to the worldwide status. It is unclear if this reflects the epidemiological dynamics of the human infection in Europe or if the disease is underdiagnosed or underreported. Epidemiologic studies in Europe have suggested an increased occupational risk of infection for forestry workers, hunters, veterinarians, and farmers with a tick-bite history and living in endemic areas. Although the overall genetic diversity of A. phagocytophilum in Europe is higher than in the USA, the strains responsible for the human infections are related on both continents. However, the study of the genetic variability and assessment of the difference of pathogenicity and infectivity between strains to various hosts has been insufficiently explored to date. Most of the European HGA cases presented as a mild infection, common clinical signs being pyrexia, headache, myalgia and arthralgia. The diagnosis of HGA in the USA was recommended to be based on clinical signs and the patient’s history and later confirmed using specialized laboratory tests. However, in Europe since the majority of cases are presenting as mild infection, laboratory tests may be performed before the treatment in order to avoid antibiotic overuse. The drug of choice for HGA is doxycycline and because of potential for serious complication the treatment should be instituted on clinical suspicion alone.
Similar content being viewed by others
Background
Researchers interest on tick-borne pathogens (TBPs) has increased during the last decades with recognition of new agents, e.g. Neoehrlichia mikurensis and “Candidatus Anaplasma camelii” [1,2,3] and expansion of established tick-borne pathogens, driven by factors such as climatic changes and altered land use [4, 5]. TBPs dynamics, especially occurrence and abundance, are multifactorial, and strongly influenced by ecological interactions of tick species and their vertebrate hosts. The pivotal impact of climate change upon the geographical distribution of ticks, their abundance and host feeding patterns has become increasingly recognised [4,5,6] together with social changes, globalisation and intercontinental traveling of humans and animals influencing both the geographical distribution and abundance of ticks and pathogens [6].
Genus Anaplasma (Rickettsiales: Anaplasmataceae) is comprised of various species capable of causing disease among a variety of vertebrate hosts, including humans. The currently recognized species are Anaplasma bovis, A. centrale, A. marginale, A. phagocytophilum, A. platys, A. ovis and the more recently described A. odocoilei and A. capra [7,8,9]. These small pleomorphic Gram-negative bacteria (0.2–1.5 µm) are obligate intracellular microbes primarily transmitted by ticks [10]. Anaplasma phagocytophilum, the agent of granulocytic anaplasmosis, from a human perspective, is considered one of the most important species as a result of its zoonotic potential [11]. It is the etiological agent of tick-borne fever (TBF) in ruminants and of equine, canine and human granulocytic anaplasmosis (EGA, CGA and HGA, respectively) [7, 12]. Infections with A. phagocytophilum in animals are commonly reported in the northern hemisphere, being among the most widespread TBP in Europe [13]. Moreover, the geographical distribution of the pathogen and its main vector (Ixodes ricinus) are increasing in latitude and altitude [13] covering almost the entire territory of continental and Atlantic Europe.
Genetic diversity is being increasingly recognised amongst European strains of A. phagocytophilum demonstrated through phylogenetical analysis of genes such as groEL (chaperone protein encoding gene) [14,15,16], ankA (cytoplasmic protein antigen with ankyrin repeats encoding gene) [17,18,19,20,21] and msp4 (major surface protein 4 encoding gene) [22]. GroEL gene is one of the two genes belonging to the heat shock operon groESL which encodes for the expression of highly conserved heat-shock proteins [23]. GroEL gene is considered a suitable marker to discriminate between A. phagocytophilum ecotypes distinguishing variants of different pathogenicity or geographical origin better than the 16S RNA locus [16]. The ankA gene encodes a protein which has repeated ankyrin motifs. It might be a virulence factor and it has been hypothesized to be involved in host adaptation underlying diversifying selection [19, 21, 23]. Sequencing ankA distinguishes variants according to their animal hosts, this gene having a higher sequence variability compared to groEL and msp4 [17, 22]. Both msp2 and msp4 belong to the OMP-1/MSP2/P44 superfamily [23]. The msp4 sequence seems to be stable through the A. phagocytophilum life-cycle being a preferable genetic marker for phylogenetic analyses [22]. Sequences analysis showed a high degree of identity at the msp4 locus, similar to the results using the groESL with the exception of roe deer strains, these being more diverse even than using ankA [22]. Different authors published studies of genetic variants using different terminology, such as ecotype (groEL), cluster (ankA) or genotype (msp4) [16, 20, 21]. “Ecotype” refers to hosts specificity of certain genotypes; “cluster” involves a deeper phylogenetic approach, while “genotype” is based on a purely genetic analysis. To refer to all mentioned terms, “genetic group” is used here. Different correlations of genetic variants have been found amongst vertebrate hosts, tick vectors and geographical locations. Infected humans, whether from Europe or America seem to share related strains belonging to the same genetic group [16, 20, 21]. Domestic animals like horses, dogs and cats, wild animals like red deer (Cervus elaphus), wild boars (Sus scrofa), red foxes (Vulpes vulpes) and hedgehogs (Erinaceus spp.) are harbouring strains with zoonotic potential related with human strains, while roe deer (Capreolus capreolus), rodents and birds seem to carry genetically distant strains [16, 19,20,21]. Regarding the strains infecting domestic ruminants, the studies present different results depending on the gene used for the analysis [16, 19,20,21] leading to some uncertainty about their possible involvement in the epidemiology of zoonotic infections. Further studies are necessary to establish which approach is discriminatory enough to discern between hosts with or without relevance to the epidemiology of HGA, especially since new highly discriminatory approaches such as multilocus sequence typing (MLST) and multiple-locus variable-number tandem repeat analysis (MLVA) are currently used [21, 24]. For instance, the MLST analysis on seven housekeeping genes (pheS, glyA, fumC, mdh, sucA, dnaN and atpA) revealed a similar pattern with ankA gene analysis, with strains from humans, dogs, horses, wild boar and hedgehogs belonging to the same clonal complex while other strains belonged to another seven clonal complexes [21]. The MLVA technique developed by Dugat et al. [24] showed the presence of slightly different profiles among the same host species (e.g. cattle) and different profiles between different hosts [24]. Based on this analysis, two epidemiological cycles were suggested for France, one involving red deer as reservoir hosts and domestic ruminants as either accidental or longer-term hosts, and another involving roe deer as reservoir hosts [24]. However, this study was based on a limited number of samples and a low variety of hosts, and further analysis could reveal the presence of multiple epidemiological cycles.
Despite the increasing number of studies on A. phagocytophilum genetic diversity, there are still insufficient data to understand the geographical distribution, host preferences and pathogenicity to humans of each described genetic variant. In this context, it is hard to analyse the relevance of these genetic groups for the public health. Moreover, despite several recent reviews, epidemiological data regarding human infections in Europe are poorly collated consisting of a collection of case reports and seroprevalence studies. The HGA epidemiology in Europe has not been critically reviewed. In this context, the aim of this review was to update epidemiological knowledge on European HGA, comparing this with what is known from the USA and to review diagnostic approaches.
Anaplasma phagocytophilum (Foggie, 1949)
The microorganism and its variability in Europe
Anaplasma phagocytophilum infection has been described under various acronyms according to the main species affected (TBF, EGA, CGA and HGA) [7, 11]. Anaplasma phagocytophilum infects mammalian neutrophils, where it replicates within cell membrane derived cytoplasmatic vacuoles named morulae [25, 26]. Morulae may contain one or more reticulate cells, dense cored cells, or both [26].
Anaplasma phagocytophilum has a single small circular chromosome (1.47 Mb) with abundant repeats (12.7%) that has been suggested to facilitate antigenic variation through recombination [15]. Of its 1369 open reading frames, 462 are unique encoding hypothetical, conserved hypothetical and conserved domain proteins, membrane proteins and lipoproteins [27,28,29,30,31]. Phylogenetic analyses of genes such as groEL [14,15,16], ankA [17,18,19,20,21] and msp4 [22] of the European strains suggest the presence of different genetic variants and a correlation of these with the vertebrate hosts, tick vectors and also a possible correlation with geographical origin.
Regardless the gene used for analysis, infected humans, whether European or American, revealed the same genetic group [16, 20, 21]. Similarly, domestic animals like horses, dogs, and cats, share the same ecotype/cluster (I) with humans based upon phylogenetic analysis of groEL and ankA [16, 19,20,21]. Conversely, using a more discriminatory ankA gene analysis [32], revealed that dogs were infected with three different strains, one being the above-mentioned human variant and two different canine variants. Furthermore, ankA sequence analysis of infections of cattle, sheep and goats disclosed two strains belonging to clusters I and IV [19, 21]. Although A. phagocytophilum was also detected in others domestic animals, like donkeys [33], there are no data regarding the strain involved.
European wild ruminant infection displays yet further diversity of infecting ecotypes, clusters or genotypes including ecotype/cluster I [16, 19, 21]. Anaplasma phagocytophilum was detected in roe deer (Ca. capreolus) [19, 34], red deer (Ce. elaphus) [13, 35] and in Iberian red deer (Ce. elaphus hispanicus) [36]. It was also detected in fallow deer (Dama dama), sika deer (Ce. nippon) and Dybowskiʼs sika deer (Ce. nippon hortulorum), reindeer (Rangifer tarandus), elk (Alces alces), European bison (Bison bonasus), chamois (Rupicapra rupicapra), alpine ibex (Capra ibex), and mouflon (Ovis musimon) with variable prevalence [13, 24, 37, 38]. Among these, the red deer are considered one of the reservoir hosts for the human pathogenic strain, based on groEL sequence analysis belonging to ecotype I [16, 39]. Use of a more discriminatory ankA sequence revealed infection with strains belonging to cluster I and IV, and less in cluster III amongst red deer [19,20,21]. In contrast, roe deer seem to be infected mainly by strains belonging to ankA gene clusters II, III and less so by strains belonging to cluster IV [19,20,21] and by strains belonging to groEL ecotype II and a few strains belonging to ecotype I [16]. AnkA sequences from European bison and chamois mirror the strains found in red deer, belonging to clusters I and IV [19,20,21]. Whereas, mouflon share the same ecotype I with human strains based upon groEL analysis [16].
Limited reports have suggested wild boar (S. scrofa) as a potential reservoir hosts for A. phagocytophilum [40] with ankA, groEL and msp4 gene analysis suggesting an overlap of clusters, ecotypes or genotypes with those of human significance [16, 21, 32].
Similarly to dogs, sequences obtained in wild carnivores, including red fox (V. vulpes), brown bear (Ursus arctos) and one timber wolf (Canis lupus occidentalis) cluster with human strains (cluster/ecotype/genotype I) using ankA, groEL and msp4 sequences [16, 21, 32, 41].
Small mammals have been considered reservoir hosts for A. phagocytophilum [42] with infection reported in mice, voles and shrews [13, 16, 43,44,45,46,47,48,49,50], but their short life-span is likely to reduce their epidemiological importance as reservoir hosts, but this remains hotly debated [48]. Subsequent studies of A. phagocytophilum associated with I. trianguliceps and rodents, suggest distinct enzootic cycles [20, 44,45,46,47]. Infected voles and shrews revealed a distinct cluster V of A. phagocytophilum [20, 21]. There are also several reports regarding the occurrence of A. phagocytophilum infection in European hedgehogs (Erinaceus europaeus) [21, 51, 52], northern white-breasted hedgehog (E. roumanicus) [53] and black rat (Rattus rattus) [54]. Based on both ankA and groEL analysis, these hedgehog strains belonged to cluster I [16, 21]. In addition to these, A. phagocytophilum was also detected in European hares (Lepus europaeus) [55] and large rodents such as the crested porcupine (Hystrix cristata) [56] but phylogenetic data regarding these strains are limited.
A further lack of clarity surrounds infection of avian hosts with A. phagocytophilum. A distinct ecotype (IV) was reported in ticks collected from blackbirds (Turdus merula), suggesting the existence of a separate enzootic cycle for A. phagocytophilum in birds and probably utilizing I. frontalis ticks [16]. Nevertheless, birds are additionally important hosts for immature I. ricinus, which are also the main ticks biting humans [48], thus of public health relevance.
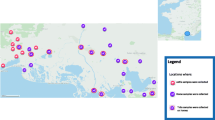
The data on the geographical distribution of the summarized genetic variants are limited. The known geographical distribution of the genetic group containing strains with zoonotic potential is presented in Fig. 1. The hosts harbouring these strains and their geographical origin are presented in Table 1. Based on all these studies, the genetic group including human strains seems to be the most diverse and widespread [16, 17, 19,20,21]. In one of these studies the geographical distribution of each ecotype is presented [16]. In contrast with ecotype I, which seems to be spread in almost all Europe, the remaining ecotypes (II–IV) have a more limited distribution. Although their distribution overlaps with that of ecotype I [16], the limited distribution of the ecotypes II–IV may be influenced by the limited origin of the samples tested. In order to clarify the spread of each genetic group further studies should be performed.
The geographical distribution of HGA cases and genetic groups including strains with zoonotic potential. Notes: 1Andora, Monaco, San Marino, Vatican; *Detected in various hosts (details presented in Table 1) [19, 21]; **Detected in various hosts (details presented in Table 1) [22, 32]. *** Detected in various hosts (details presented in Table 1) [16, 145]
This high diversity may be the result of an adaptation of A. phagocytophilum to different host species. Moreover, the co-infection of vectors with multiple genetic variants as it was suggested before in roe deer [57], may lead to the occurrence of new strains with different host preferences. The impact of strain heterogeneity on public health is not enough explored. However, the possible existence of independent enzootic cycles should decrease the pressure on human health.
In contrast with the heterogeneity of the European strains, American strains of A. phagocytophilum appear more restricted, primarily belonging to two variants (AP-ha and AP-V1), of which only AP-ha was detected in humans [13, 22]. However, a comparison between pathogenic and non-pathogenic strain diversity from the two continents is unsubstantiated since the hypothesis referring to non-pathogenic strains is not based on experimental data but on the lack of detection in humans.
In contrast with the European situation, in the USA human strains are maintained in nature through reservoir hosts such as white footed mice (Peromyscus leucopus), deer mice (P. maniculatus) and other rodents [13, 58, 59]. White-tailed deer (Odocoileus virginianus) are considered major reservoir hosts for variants which were never detected in humans, being suggested as non-pathogenic [13, 22]. Based on different markers (ankA, groEL, gltA and msp4 genes), American and European human strains are grouped in different clades, being phylogenetically distinct [21, 22, 60]. However, based on ankA gene analysis, both European and American strains belong to the same genotype I, suggesting a degree of relatedness [19]. Whether differences in virulence and clinical manifestations observed between the American and European strains reside within their genetic composition or differences are driven by their eco-epidemiology remains to be resolved.
Transmission and vectors
Transmission of A. phagocytophilum commonly occurs through the bite of an infected tick. Ixodes ricinus serves as the main vector in Europe [61, 62]. Transstadial transmission is important in maintaining A. phagocytophilum within its endemic cycles [6, 16, 49]. Although transovarial transmission has been suggested, its efficacy seems to be low [16, 49], necessitating further amplification by feeding upon reservoir species to maintain the bacteria in endemic cycles [49, 62]. Ixodes ricinus may become infected with A. phagocytophilum after feeding on an infected host, depending on various factors such as the percentage of infected neutrophils and the density of ticks feeding on the same host [63]. Co-feeding transmission from infected to uninfected ticks whilst feeding at common sites has not yet been reported for Anaplasma [64].
In addition to its main vectors, A. phagocytophilum has been detected in questing ticks belonging to other members of genus Ixodes including the European I. persulcatus [65], I. trianguliceps [66], I. ventalloi [67] and I. hexagonus [68]. Beyond Ixodes, A. phagocytophilum DNA has been detected in Dermacentor reticulatus [69], Haemaphysalis punctata, H. concinna, and Rhipicephalus bursa [70]. The vectorial capacity or these other European tick species has not been fully elucidated.
Despite regular detection of A. phagocytophilum DNA in I. ricinus in Europe, reports of infected ticks removed from humans are infrequent, being reported in Poland [71], Italy [49, 72], Romania [73] and the UK [74]. Among these, only in the UK study, the presence of A. phagocytophilum in two of the three ticks removed from a patient with non-specific clinical signs was demonstrated [74]. The patient developed clinical signs 3 days after the tick bite and was serologically diagnosed with HGA in accordance with CDC criteria by a 4-fold increase of A. phagocytophilum-specific IgG and IgM in paired serum samples collected at 8 and 28 days after tick removal [74]. The remaining studies only evaluate exposure risk [49, 71,72,73], rather than follow-up of those patients bitten by infected ticks. In the absence of patients’ follow-up, the results are difficult to interpret. However, the difference between high prevalence in ticks (e.g. 23.7% in Poland) and the patients not coming back for a medical consultation, together with the relative low number of reported cases, may be explained by a low transmission rate, asymptomatic cases or undiagnosed mild infection. Another suggested explanation for this discrepancy was the blood meal, which may trigger bacterial reactivation in infected ticks [71].
Beyond tick bite transmission, human infections have followed blood or red cell transfusions in both the USA and Europe [59, 75, 76]. Although only a single infection case of transfusion-acquired HGA has been described in Europe [75], several countries such as Poland and Belgium have reported blood donor seroprevalence to be high (5.4 and 14.5%, respectively), consequently the risk of infection via blood transfusion should be further investigated [77, 78].
Perinatal transmission from mother to child has only been described in the USA [79, 80]. The timing of neonatal infection was consistent with three potential transmission routes (intrauterine/transplacental, during the birth or through breast feeding); however, the transplacental route was suggested as being the most probable [81]. In Europe, transplacental transmission has been demonstrated in both cows and sheep [81, 82], and it was also suggested for dogs infected with a different Anaplasma species (i.e. A. platys) from Europe [83] and Africa [84].
Nosocomial exposure to HGA by direct contact with blood or respiratory secretion from a fatal HGA case was suggested only once in a Chinese hospital [85], but other authors contested the hypothesis, due to insufficient evidence [86]. Moreover, later it was confirmed that all patients had severe fever with thrombocytopenia syndrome virus (SFTSV) infection [87]. This agent, a newly discovered bunyavirus, causes a clinical picture which resembles previously described Chinese HGA cases [85, 87]. Subsequently, the possibilities of SFTSV and HGA co-infection or HGA misdiagnosis were debated in a series of comments and responses [88, 89]. In addition, by comparing the clinical picture of USA HGA cases with Chinese HGA cases and arguing the slight chances for simultaneous infection with both infectious agents, Wormser [90] impugned the accuracy of the diagnostics in reported Chinese cases.
Human granulocytic anaplasmosis in Europe
Geographical distribution and epidemiological indices
HGA was first diagnosed in 1990, in Wisconsin (USA) in a patient with tick bite history and severe febrile illness [11]. In Europe, the first human clinical case was described in Slovenia in 1997, but evidence of human infection pre-dated this back to 1995 in Switzerland and the UK [91,92,93]. Subsequently, HGA has been reported in several European countries (Fig. 1): Austria [94, 95]; Belgium [78, 96]; Croatia [97, 98]; Czech Republic [99, 100]; France [101, 102]; Germany [103]; Italy [22, 104]; Portugal [105]; the Netherlands [106]; Norway [107, 108]; Poland [109, 110]; Slovakia [111, 112]; Spain [113]; and Sweden [114]. The geographical distribution of A. phagocytophilum reported herein being based upon case reports, serological surveys or genetic studies.
The incidence of human HGA cases in Europe is lower (estimated under 300) than reported from the USA, where a steady increase has been reported since 2001, with more than 15,000 accumulated cases until 2015 [59]. This difference cannot be explained by pathogen prevalence in ticks as A. phagocytophilum is reported in some 3% of European I. ricinus, nearly as high as that among ticks in the USA [115]. On a cautionary note, the majority of studies do not provide sufficient data regarding the prevalence of each ecotype/genotype circulating in ticks and humans, potentially masking prevalence of potential zoonotic strains.
Human seroprevalence in Europe is on average ~ 8.3%, reaching up to 31% (Table 2). This incongruence between human seroprevalence and observed clinical cases might arise from incomplete diagnosis, or a high rate of asymptomatic infections [116], or serological cross-reactivity that might lead to an overestimation of seroprevalence rate [115]. This disparity is partially explained by Swedish studies in which more than half of the patients with an ongoing A. phagocytophilum infection (seroconversion or 4-fold increased antibody titre), failed to develop any other associated clinical symptoms upon follow-up interview, being defined as having subclinical infection [116].
Reported seroprevalence appears highly variable, depending on the study, country, year and population included (Table 2). The majority of summarised studies refer to seropositive individuals in accordance with the probable case definition: serological evidence of elevated IgG antibody reactive with A. phagocytophilum antigen by IFA, with a cut-off of 1:64 (CDC case definitions of Anaplasma phagocytophilum infection). In the majority of these studies, serological testing was performed using commercial IFA kits utilising human isolates of A. phagocytophilum (different strains) cultivated in HL60 cells as antigen, with a cut-off value of 1:64. For the studies in which other serological assays or other criteria for interpretation were used, the details are provided.
Bakken et al. [107] compared HGA seroprevalence between Lyme borreliosis (LB) patients (study group) and healthy people (control group) in Norway. A total of 58 patients diagnosed with LB were tested for the presence of antibodies against A. phagocytophilum (at that time known as “Ehrlichia equi”) using Ehrlichia equi infected neutrophils as antigen. Values ≥ 1:80 were considered positive. The study group included patients with a presumed recent I. ricinus bite and serologically-confirmed active LB [107]. The results indicated that 10.34% of the patients were seropositive for both HGA and LB, showing that patients with LB were 5.28 times more likely to have had HGA than the control subjects [107]. Dumler et al. [114] published a similar survey on Koster Islands (Sweden). They tested randomly the population for the presence of HGA using the same protocol as described by Bakken et al. [107], and LB antibodies and found among the 21 HGA seropositive residents, six were seropositive also for LB [114]. Both these studies considered as seropositive patients with elevated antibody titer (≥ 1:80), having lower probability for non-specific reactivity compared to studies using lower titer (≥ 1:64). However, in both studies the results showed the presence of both HGA and LB antibody, without the confirmation of HGA, suggesting not necessarily a high probability of co-infection, but the increased contact risk with both pathogens. Since the vector is the same for both, the results indirectly showed an increase of seropositivity in individuals with high risk to tick exposure. This is also sustained by the results of an extensive study published by Pusterla et al. [117] involving 1515 individuals from Switzerland, stratified into groups according to their risk for tick exposure. Low risk groups included newborns, and randomly chosen blood donors with unknown tick exposure rate and a high-risk group comprised of hunters and those with other tick-borne infections. Serum samples were examined by IFA using a 1:80 cut-off value for antibodies against A. phagocytophilum (bovine leucocytes infected with “Ehrlichia phagocytophila” Swiss strain). Only 0.54% of the newborn samples had positive titres, potentially reflecting maternal antibodies, whereas 1.1% of blood donors were seropositive and for the high-risk group 9% seroprevalence in hunters; those with LB yielded 12.7%; whereas TBE cases revealed 19.5% seropositive for HE [117]. In addition to the studies in Norway and Sweden [107, 114], this study [117] showed high prevalence of HGA antibody in all tested high tick exposure risk groups, all suggesting the high exposure to ticks as a risk factor for HGA. The different seroprevalence between the tick exposed groups (hunters vs LB or TBE patients) may suggest also an increased risk for co-infection with other pathogens transmitted by I. ricinus. This is also sustained by other studies from other countries. In Slovakia, between 2002 and 2005, from 76 patients with a history of tick bite and symptoms resembling LB, 19 (25%) were seropositive, having ≥ 1:64 IgG antibody titer against A. phagocytophilum. Among these positives, 14 were additionally seropositive for LB [111]. In Germany, Kowalski et al. [104] conducted an 8-year (1994–2001) seroprevalence study in Berlin/Brandenburg, north-eastern Germany. They compared 422 sera from patients with a confirmed tick-bite (positive antibodies against B. burgdorferi) with 249 control sera positive for antibodies against a different spirochaete (Treponema pallidum) or against different obligate intracellular bacteria (Chlamydia spp.). As in other studies, among the LB antibody-positive specimens there were significantly more A. phagocytophilum antibody-positive samples (4.5%) than among controls (1.2%) [104]. However, without confirmations of HGA cases these results alone cannot confirm the hypothesis.
In addition to these serological data, three other studies described confirmed co-infections through seroconversion or DNA detection. An Italian study on 79 patients with tick bite history within 6 months and/or who were presented to hospital with a suspected tick-borne infection or aseptic meningitis yielded five cases (6%) with a positive HGA serology [118]. Among these, two were confirmed HGA cases (fever and seroconversion with a 4-fold change in serum antibody titer to A. phagocytophilum), one was a probable HGA case (fever and acute and convalescent serum samples with unchanging IFA titer), two patients had a possible HGA infection (serum samples with a titer of ≥ 1:128 at only the testing point), whilst three individuals had positive serology for LB. Moniuszko et al. [110] published a report on the presence of A. phagocytophilum, Borrelia and Babesia spp. DNA in the blood of 110 TBE (meningitis/encephalitis and positive serology) patients in Poland, comparing the results with a control group of 20 healthy blood donors. A prevalence of 10.9% A. phagocytophilum-TBEv co-infection was recorded and 2.7% for triple co-infection (TBEV–Borrelia sp.–A. phagocytophilum). Similarly, in Czech Republic, among 66 patients with erythema migrans (EM) twelve (all with positive PCR for B. burgdorferi (s.l.)) were seropositive by IFA IgG to HGA and ten (nine with positive PCR for B. burgdorferi (s.l.)) were PCR-positive from blood or skin samples [100]. Among 14 A. phagocytophilum and B. burgdorferi (s.l.) co-infected patients (confirmed by DNA detection), three were pregnant women; one subsequently aborted and the mother’s blood sample was positive for both A. phagocytophilum and B. garinii DNA. The two other women safely delivered, although one had A. phagocytophilum-positive blood and placenta, and the other B. garinii-positive skin, A. phagocytophilum-positive blood and B. garinii-positive placenta [100]. Despite the case confirmation through A. phagocytophilum DNA isolation, both these studies used 16S rRNA gene fragment amplification and provided no data regarding the sequence analysis and the strain involved.
Similarly, high HGA seroprevalence was associated with occupational risks and/or populations living in endemic areas, with multiple cases reported. Tomasiewicz et al. [119] compared the HGA seroprevalence in 63 individuals with occupational exposure to tick bites (forest workers) and with tick bite history, with a blood donor control group (n = 30) from Poland. A seroprevalence of 20.6% was found for among the tick exposed group, with the vast majority (85%) also additionally having anti-B. burgdorferi antibodies. In contrast, none of the blood donors were seropositive. Grzeszczuk et al. [120] tested for HGA antibodies from 450 serum samples originating from north-eastern Poland (known to be endemic for LB and TBE) which were submitted for serological diagnosis of LB. The study included a control group comprised of 50 healthy blood donors. The HGA seroprevalence was 9.1% for people living in the endemic area, compared to 2% in healthy blood donors. A significant difference was found between forest workers (16.7%) and other occupational categories (4.6%). Similar serological findings were reported from other studies in Poland [77, 121, 122]. Cisak et al. [77] and Chmielewska-Badora et al. [122] reported a high seroprevalence (17.7% and 23%, respectively) in forestry workers compared with the control group consisting in healthy blood donors (5.4% in both studies) in Lublin region. Grzeszczuk et al. [121] reported a low seroprevalence (3.9%) in both forestry and office workers in Białystok vicinity [121].
In all Polish studies, a cut-off value of 1:64 was used, increasing the risk for non-specific reactivity, in this case the true seroprevalence being lower. Nevertheless, the differences between exposed and control populations may be still sustained by the obtained data. The low seroprevalence observed by Grzeszczuk et al. [121] compared with other studies [77, 122] may sustain the presence of endemic and non-endemic areas in Poland. However, despite these three studies using the same serology kit and cut-off value, a comparison between them is not possible since in the study by Grzeszczuk et al. [121] a control group was not tested. A low A. phagocytophilum seroprevalence was also reported in a survey on a high risk population (forestry workers, which are in general tick exposed) in north-eastern France, using an anti-A. phagocytophilum recombinant P44 antigen IgG ELISA, and IFA re-tested of doubtful or positive sera [123]. This ELISA technique was previously tested and showed a sensitivity of 87% at a 1:160 cut-off value and a specificity of 98%, being comparable to IFA procedures for the laboratory diagnosis of HGA [124]. From a total of 2908 forestry workers, only 1.7% were seropositive; however, regional variation with a higher seroprevalence (2.6%) was reported from Alsace [123]. This finding was consistent with previous findings according to which Alsace may be a focal endemic area [125]. During a ten-year study in France, involving 141,007 patients with a history of tick bite, sera were tested using a micro-immunofluorescence assay. Titres of ≥ 1:100 for IgG and ≥ 1:50 for IgM in acute phase serum and/or the presence of seroconversion were considered for the positive cases. Only one HGA case was diagnosed from 112,995 tested sera samples from 2000–2008, whereas five new confirmed cases of HGA among the 14,000 tested sera were identified in 2009 [125]. Similarly, from a total of 261 samples tested for A. phagocytophilum DNA using molecular diagnostic assays during 2000–2008, only one HGA case was diagnosed, whereas three new cases of HGA among the 81 samples were identified in 2009 [125]. All PCR confirmed HGA cases originated from Alsace, from where only nine samples were tested in total [125], highlighting the existence of focal endemic areas. Despite the amplification and sequencing of 16S rRNA confirmed the infection, this conserved gene is not useful for genotyping, providing little information regarding the genetic variants involved in these cases.
In contrast with the suggested influence of diverse factors on the HGA seroprevalence, other studies seem to report no difference between the different risk categories. In Belgium, among 148 samples from workers who were professionally exposed to tick bites (veterinarians, farmers, hunters, and gamekeepers), 209 samples from rural blood donors and 193 samples from urban blood donors tested by IFA, a high A. phagocytophilum seroprevalence was observed, suggesting the presence of endemic areas in the country. Seroprevalence of A. phagocytophilum was estimated as 14.2% for the exposed workers, 17.2% for the rural blood donors, and 14.5% for the urban blood donors [78]. Even if a low cut-off value (1:64) was used, this high seroprevalence is sustained by another study from Belgium [96]. Among 1350 patients suspected of a tick-borne infection between 2000 and 2009, 418 (31%) of patients were found positive for either IgG or IgM antibodies, using IFA against A. phagocytophilum, for both IgG and IgM antibody (cut-off value 1:64 and 1:20, respectively) [96]. Among 322 serum samples available for confirmation, 111 fulfilled the case definition, namely history of tick bite, fever, and an at least a 4-fold increase in IgG titre [96, 126]. Similarly, in Norway, among 301 healthy blood donors, 49 (16.2%) were seropositive having an antibody titer higher than 80 [127]. The authors observed no significant difference according to gender, age, geography, self-reported number of tick bites or presence of antibodies to B. burgdorferi (s.l.) [127].
Based on these studies, countries with a greater risk highlighted by a high seroprevalence are Norway, Sweden, Germany, Belgium, Poland and Switzerland. The high HGA prevalence in co-infections with pathogens transmitted by the same tick vectors may be explained by simultaneous exposure. However, based on limited published data, a previous infection cannot be ruled out especially in non-confirmed cases through fever and seroconversion, or a 4-fold change in serum antibody titer to A. phagocytophilum, and/or a positive PCR.
Clinical manifestation
Surprisingly few HGA cases have been reported from Europe, limiting reliable clinical description of these individuals. We reviewed the published data from the 76 patients in Europe for which clinical and laboratory data were available (Table 3) [111, 128, 129].
Age of patients varied between 5–70 years-old with a median of 53.5. Most of them (78.8%), recalled tick bite between 3–30 days (mean 12.7) before the onset of the disease, with most cases occurring between April and October. Determination of the duration and magnitude of bacteraemia in humans with HGA is challenging as laboratory examination is rarely undertaken during the early acute phase of infection. In a Slovenian study, the febrile period of the first five confirmed HGA cases had a mean of 7.5 days [130]. European HGA cases tend to present with mild or even asymptomatic infection, with complete recovery in two weeks, even in the absence of specific treatment [131]. Transient infection may occur in the absence of associated clinical signs; consequently, cases may not always be detected. However, among the patients included in this analysis, 62.8% were hospitalized, 73.1% received specific treatment and only in one report, two patients were asymptomatic. This discrepancy might relate to selective publishing bias with over-reporting of more severe clinical cases. Clinical presentation was usually as an acute non-specific febrile infection. Of those infected, 79.3% presented with pyrexia, 89% headache, 67.6% fatigue or malaise, 63.3% myalgia, 56.6% arthralgia and 39.2% with nausea. However, fever is more often reported. Considering the vast majority of the reports, the frequency of fever varies between 90–100%. One study [111], in which fever was reported in only 26.3% of the serologically confirmed cases can be considered doubtful since the authors refer to serologically confirmed cases but provide no data regarding the confirmation method. However, the authors report other clinical signs consistent with HGA infection and/or HGA and LB co-infections. Nevertheless, it is not clear if the reported cases are in acute or convalescent phase. Other clinical observation were: digestive signs (51.5%, including vomiting, diarrhea, abdominal pain, splenomegaly, hepatomegaly); exanthema/rash (23.8%); conjunctivitis (21.2%); lymphadenopathy (21.2%); cough (17.5%, including two cases of interstitial pneumonia, one atypical pneumonia, one case of ARDS-acute respiratory distress syndrome); neurological signs (15.5%, including vertigo in most of the cases, one case of facial palsy; one case of meningeal signs, one case of aseptic meningitis); and cardiac signs (tachycardia and hypotension in one case; one case of systolic murmur). The presence of erythema/rash was reported mainly in patients with LB or seropositive for B. burgdorferi (s.l.). However, there are reports in which co-infections are not specified [132] or are excluded [133]. In this last study, the patient developed a diffuse rash while acute and convalescent sera were negative for antibodies against B. burgdorferi, Coxiella burnetii, Rickettsia conorii, R. typhi, Mycoplasma pneumoniae, Leptospira, Chlamydia pneumoniae and Ch. psittaci [133]. Fatal infections are rare, but infection can cumulate in multi-system failure [59]. Co-infection with other tick-borne pathogens should be considered. Almost a third of HGA patients were additionally seropositive for B. burgdorferi (s.l.), less for Ehrlichia chaffeensis and two patients had concurrent TBE. The presumed co-infection with E. chaffeensis was not proven by DNA detection in these cases [129], suggesting a cross reaction. However, seroreactivity to E. chaffeensis in the absence of A. phagocytophilum antibody has been occasionally reported in the European human population [98], and one patient in Serbia was recognized to have clinical illness [134].
Cases acquired in Europe share the same clinical picture observed in USA, however, European cases are generally milder and thus far no fatalities have been reported. There is evidence of higher strain heterogeneity in Europe [21] that could correlate with host preference, pathogenesis and resulting virulence in humans [16]. This hypothesis is sustained by experimental infection in lambs with different A. phagocytophilum variants showing different pathogenic traits [135, 136]. In addition, factors related to different species of vectors may influence the virulence in an analogous situation as that between American and European B. burgdorferi strains [137].
Diagnosis
According to the Centre for Disease Control and Prevention (CDC, Atlanta, Georgia, USA), confirmatory criteria for patients with consistent clinical presentations are either detection of A. phagocytophilum DNA in a clinical specimen via PCR amplification of a specific target, demonstration of Anaplasma antigen in a biopsy/autopsy sample by immunohistochemical methods, or isolation of A. phagocytophilum from a clinical specimen in a cell culture system. Serologically, a 4-fold change in antibody titre (IgG) against A. phagocytophilum antigen by IFA in paired (2–4 weeks) serum samples is confirmatory. Although for European HGA, there is no official case definition yet, both the European Centre for Disease Control and Prevention (ECDC) and the European Society of Chlamydia, Coxiella, Anaplasma and Rickettsia, formerly ESCAR: ESCMID Study Group on Coxiella, Anaplasma, Rickettsia and Bartonella (ESCCAR) guidelines are in concordance with the CDC guidelines.
Diagnosis of HGA should be based on clinical signs and patient’s history and can be supported by laboratory confirmatory tests. As described above, the symptoms of HGA may vary from patient to patient and can be difficult to distinguish from other conditions, especially other tick-borne diseases. Information such as recent tick bite, exposure to areas where ticks are likely to be found, or a history of recent travel to areas where HGA is endemic can be helpful in supporting the diagnosis. However, since A. phagocytophilum is endemic throughout Europe, the appropriateness of this latter criterion is limited. Routine blood tests, such as a complete blood cell count or a chemistry panel may be useful since thrombocytopenia, leukopenia or elevated liver enzyme levels are helpful predictors of anaplasmosis but may not be present in all patients. Common laboratory findings were: elevated CRP in 93.03%; elevated liver enzymes in 90% (alanine transaminase level; aspartate transaminase level); thrombocytopenia in 83.7% and leukopenia in 63%. Less commonly, in less than 50% of the cases the levels of lactate dehydrogenase and neopterin were increased, associated with elevated erythrocyte sedimentation rate and increased serum bilirubin [128].
Once clinically suspected, specialised laboratory testing should be undertaken for HGA confirmation. Indirect immunofluorescence using A. phagocytophilum whole antigen is often considered the gold standard serological test for diagnosis of HGA. Use of paired serum samples enables demonstration of a significant rise (4-fold) in antibody titres, using a cut-off value of at least 1:64 [126]. Ideally, the first sample should be collected in the first week of illness (during the acute phase) and the second and/or third between two to four weeks later [126]. IgM antibodies are less specific than IgG antibodies and are more likely to generate false positive results. Moreover, IgM results alone should not be used for laboratory diagnosis due to the low sensitivity [126, 138]. Serological tests based on enzyme immunoassay (EIA) technology are commercially available. IFA is generally used for screening and confirmation of HGA cases. The most commonly used commercial kit for IgG detection in the studies summarized in this review was from Focus Technologies, USA. According to the manufactures, the specificity of this test reaches 100%, and the sensitivity depends on the period between the moment of sampling and the beginning of the clinical signs, which ranges from 66.7% to 100% at a cut-off value of 1:64. Similarly, other IFA IgG kits have an 80–86.6% sensitivity and 92.7% specificity [126]. The ELISA technique was used only in few reports. The performance characteristics were evaluated by Ijdo et al. [124], showing an 87% sensitivity at a 1:160 cut-off value and a specificity of 98%, being comparable to IFA procedures [124]. However, this technique has been used in a limited number of studies providing insufficient support for routine use in diagnostic laboratories.
Acute phase whole blood samples can be tested by PCR targeting various genes such as 16S rRNA or msp2 [139]. This method is most sensitive in the first week of illness, but rapidly decreases in sensitivity following the administration of appropriate antibiotics. The analysis of published HGA cases in Europe (see above) have shown a relative low percent 68.2% of positive PCR results. Similarly, among 46 Slovenian confirmed cases of human anaplasmosis compatible with ESCCAR guidelines, only 28 (60.9%) of them were positive for the presence of A. phagocytophilum DNA [139]. Thus, a positive PCR result may be helpful, but a negative result does not exclude the diagnosis, and treatment should not be withheld due to a negative PCR result. In addition to acute phase sample collection time, the sensitivity of molecular detection also depends on: (i) sample type and quality, full blood or buffy coat being considered the more suitable compared with plasma [139] because of the tropism of A. phagocytophilum for white cells; and (ii) the number of genomic target gene copies and the amplicon length (short sequences being generally preferred to long ones for screening; longer ones being more used for sequencing and phylogenetic analysis) [139]. Most frequently used target genes for Anaplasma spp., include 16S rRNA (rrs), heat-shock protein (groEL), citrate synthase (gltA), and major surface proteins (msp1, msp2, msp4, msp5). For molecular screening, the sensitive multicopy msp2 is particularly useful, whereas for sequence comparison and database crossmatch, conservative or moderately conservative rrs and groEL strategies are regarded as a better choice [139].
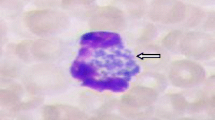
During the first week of illness, a microscopic examination of blood smears may reveal morulae of A. phagocytophilum in the cytoplasm of the neutrophils. However, the percent of patients presenting intracytoplasmic morulae in the acute phase may vary from low values in Europe [129] to high values of 25–60% or even more in the USA [140, 141]. Although sensitivity is limited, this can be improved if the smear is performed from the buffy coat [139]. Gram staining is not suitable to visualize intracellular bacteria because of a lack of contrast against the host cytoplasm. Romanowsky staining is generally used, usually with a quick method such as Diff-Quik. This approach stains the bacteria purple, which allows the visualization of characteristic morulae. Morulae are usually 1.5–2.5 µm in diameter but can be as large as 6 µm [140].
Similar with the DNA detection, in vitro cultivation may be used also in the acute phase of illness. Cultivation of A. phagocytophilum from human blood has been used since 1996, when Goodman et al. [142] successfully isolated the bacterium on HL-60 cells. More recently, cultivation from blood was also successfully achieved from two patients from Czech Republic [100].
Treatment
Chemical prophylaxis is not recommended after a tick bite, even in endemic regions [131]. Doxycycline is considered the drug of choice with good results for HGA in adults as well as in children older than eight years. Treatment should be instituted on clinical suspicion alone to avoid the potential for serious complication, [59]. Doxycycline (100 mg twice daily by IV or PO between 10–14 days) is highly effective and post-therapeutic relapses have not been reported [59, 143]. There is generally a rapid response to treatment with a marked clinical improvement within 24–48 h [59]. A possible alternative for children and patients with a doxycycline allergy or pregnant women is rifampicin with the following dose: for children 20 mg/kg/day, maximum 600 mg in two doses PO and for adults 300 mg, twice 2 times daily PO for 5–7 days in both cases [59, 144]. Other antibiotics, such as quinolones, cephalosporin’s, penicillin’s, and macrolides are ineffective [143]. To prevent infection, precautions should be taken to avoid exposure to ticks.
Gaps remaining
Despite the great efforts of researchers for a better characterisation of HGA and A. phagocytophilum in Europe, there are several gaps remaining. The majority of them are related with the ecology and genetic diversity and their correlation with the pathogenicity.
First of all, it is important to be established how much the terms of different genetic variants (e.g. clusters, ecotype or genotype) are overlapping. The authors used different terms for these variants according to the gene analysed or maybe to their own preferences. However, for a better understanding of the pathogen genetic variability it is necessary to reach a consensus.
Regarding the pathogenicity to humans, it is not clear if strains less related with the human isolates, belonging to different genetic groups (e.g. rodent, bird, or roe deer strains in Europe or AP-V1 in USA) were not detected in humans because they are non-pathogenic, or because they cause asymptomatic infections. One important question is if they cause or not a serological response.
Similarly, the strains belonging to the same genetic group as human strains have zoonotic potential; however, it is not clear whether they have a different pathogenic potential for humans. Regardless of the study, the genetic group including human strains is the most diverse, clustering together strains from a large variety of hosts (Table 1). However, depending on the gene used, some strains (detected in sheep, goats, cows, hedgehogs, wild carnivores etc.) may belong to different groups. Because of this it is important to be established which approach is discriminatory enough to evaluate the risk for human health. Additionally, in order to evaluate the public health risks, the prevalence and geographical distribution of each genetic group should be further evaluated.
Another important but insufficiently clear aspect is the understanding of differences between HGA in USA and Europe. Although, there are clear differences between the ecology of American and European strains (e.g. different vectors, different hosts, apparently different genetic variability), it is not clear if the ecology or the genetic differences alone influence the pathogenicity to humans, or whether this may influence the prevalence of infections and increase the risk for developing more severe forms of diseases. Even more unclear, and therefore an important topic for future research, is tackling the differences in both pathogenicity and ecology between European and Asian strains.
Other gaps are related to the diagnosis and the seroprevalence or prevalence of HGA. Since HGA in Europe is not a disease with a mandatory surveillance and reporting, some cases may be not published. Moreover, clinical suspicion or even serological detection may be not be followed by confirmatory tests but may be treated. In addition, the unspecific clinical picture may lead to underdiagnosing. In this case, the prevalence is estimated based on published data and thus influenced by researchersʼ interest and by the approach they used. In addition, in the absence of mandatory surveillance and an official case definition in Europe, the diagnostic approaches may differ between the laboratories. In this case, interpretation of results interpretation and classification of suspected, probable and confirmed cases should be made with caution. Even following supportive and/or confirmed laboratory criteria published by CDC, (e.g. IFA IgG with a cut-off of ≥ 1:64 as supportive criteria, or detection of A. phagocytophilum DNA in a clinical specimen via amplification of a specific target in a PCR assay as confirmatory criteria), the published cases can be questioned if a single approach is used, especially if low titer of < 640 is obtained or if a single target gene is amplified but sequenced.
Conclusions
Despite the apparently ubiquitous presence of A. phagocytophilum in ticks and various wild and domestic animals from Europe, published clinical cases of HGA remain rare, currently only a few hundred. It is unclear if this reflects the incidence of human infection in Europe or if the disease is underdiagnosed or underreported. Epidemiologic studies in Europe have suggested an increased occupational risk of infection for forestry workers, hunters, veterinarians, and farmers with a tick-bite history and also those living in endemic areas. Another risk factor for HGA seems to be infection with other pathogens transmitted by I. ricinus, mainly B. burgdorferi (s.l.). Although the overall genetic diversity of A. phagocytophilum in Europe seems to be higher than in the USA, the strains responsible for human infections are related on both continents, hence a difference in pathogenicity seems unlikely. However, to date, the study of the genetic variability and assessment of the difference in pathogenicity and infectivity between strains to various hosts has been insufficiently explored.
Availability of data and materials
Not applicable.
Abbreviations
- ankA :
-
gene encoding a cytoplasmic protein antigen with ankyrin repeats
- ARDS:
-
acute respiratory distress syndrome
- CDC:
-
Centers for Disease Control and Prevention
- CGA:
-
canine granulocytic anaplasmosis
- CRP:
-
C-reactive protein
- DNA:
-
deoxyribonucleic acid
- EGA:
-
equine granulocytic anaplasmosis
- EIA:
-
enzyme immunoassay
- ELISA:
-
enzyme-linked immunosorbent assay
- EM:
-
erythema migrans
- ESCAR:
-
ESCMID Study Group on Coxiella, Anaplasma, Rickettsia and Bartonella
- ESCCAR:
-
European Society of Chlamydia, Coxiella, Anaplasma and Rickettsia
- groEL :
-
chaperone protein encoding gene
- HGA:
-
human granulocytic anaplasmosis
- IFA:
-
immunofluorescence assay
- IgG:
-
immunoglobulin G
- IgM:
-
immunoglobulin M
- IV:
-
intravenous
- LB:
-
Lyme borreliosis
- msp1, 2, 3, 4 :
-
genes encoding major surface protein 1, 2, 3, and 4
- MLST:
-
multilocus sequence typing
- MLVA:
-
multiple-locus variable-number tandem repeat analysis
- P44:
-
protein p44
- PCR:
-
polymerase chain reaction
- PO:
-
per os (oral therapy)
- RNA:
-
ribonucleic acid
- rrs :
-
16S rRNA (16S ribosomal RNA gene)
- s.l. :
-
sensu lato
- TBE:
-
tick borne encephalitis
- TBEv:
-
tick borne encephalitis virus
- TBF:
-
tick-borne fever
- TBP:
-
tick-borne pathogen
References
Kawahara M, Rikihisa Y, Isogai E, Takahashi M, Misumi H, Suto C, et al. Ultrastructure and phylogenetic analysis of “Candidatus Neoehrlichia mikurensis” in the family Anaplasmataceae, isolated from wild rats and found in Ixodes ovatus ticks. Int J Syst Evol Microbiol. 2004;54:1837–43.
Bastos AD, Mohammed OB, Bennett NC, Petevinos C, Alagaili AN. Molecular detection of novel Anaplasmataceae closely related to Anaplasma platys and Ehrlichia canis in the dromedary camel (Camelus dromedarius). Vet Microbiol. 2015;179:310–4.
Wass L, Grankvist A, Bell-Sakyi L, Bergström M, Ulfhammer E, Lingblom C, Wennerås C. Cultivation of the causative agent of human neoehrlichiosis from clinical isolates identifies vascular endothelium as a target of infection. Emerg Microb Infect. 2019;8:413–25.
Casimiro E, Calheiros J, Santos FD, Kovats S. National assessment of human health effects of climate change in Portugal: approach and key findings. Environ Health Perspect. 2006;114:1950–6.
Gray JS, Dautel H, Estrada-Peña A, Kahl O, Lindgren E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip Perspect Infect Dis. 2009;2009:593232.
Medlock JM, Hansford KM, Bormane A, Derdakova M, Estrada-Peña A, George JC, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013;6:1.
Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‛HGE agentʼ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–65.
Tate CM, Howerth EW, Mead DG, Dugan VG, Luttrell MP, Sahora AI, et al. Anaplasma odocoilei sp. nov. (Family Anaplasmataceae) from white-tailed deer (Odocoileus virginianus). Ticks Tick Borne Dis. 2013;4:110–9.
Li H, Zheng YC, Ma L, Jia N, Jiang BG, Jiang RR, et al. Human infection with a novel tick-borne Anaplasma species in China: a surveillance study. Lancet Infect Dis. 2015;15:663–70.
Walker DH, Dumler JS. Emergence of the ehrlichioses as human health problems. Emerg Infect Dis. 1996;2:18–29.
Chen SM, Dumler JS, Bakken JS, Walker DH. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–95.
Woldehiwet Z. The natural history of Anaplasma phagocytophilum. Vet Parasitol. 2010;167:108–22.
Stuen S, Granquist EG, Silaghi C. Anaplasma phagocytophilum—a widespread multi-host pathogen with highly adaptive strategies. Front Cell Infect Microbiol. 2013;3:31.
Liz JS, Sumner JW, Pfister K, Brossard M. PCR detection and serological evidence of granulocytic ehrlichial infection in roe deer (Capreolus capreolus) and chamois (Rupicapra rupicapra). J Clin Microbiol. 2002;40:892–7.
Petrovec M, Bidovec A, Sumner JW, Nicholson WL, Childs JE, Avsic-Zupanc T. Infection with Anaplasma phagocytophila in cervids from Slovenia: evidence of two genotypic lineages. Wien Klin Wochenschr. 2002;114:641–7.
Jahfari S, Coipan EC, Fonville M, Van Leeuwen AD, Hengeveld P, Heylen D, et al. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasit Vectors. 2014;7:365.
von Loewenich FD, Baumgarten BU, Schröppel K, Geißdörfer W, Röllinghoff M, Bogdan C. High diversity of ankA sequences of Anaplasma phagocytophilum among Ixodes ricinus ticks in Germany. J Clin Microbiol. 2003;41:5033–40.
Park J, Kim KJ, Choi KS, Grab DJ, Dumler JS. Anaplasma phagocytophilum ankA binds to granulocyte DNA and nuclear proteins. Cell Microbiol. 2004;6:743–51.
Scharf W, Schauer S, Freyburger F, Petrovec M, Schaarschmidt-Kiener D, Liebisch G, et al. Distinct host species correlate with Anaplasma phagocytophilum ankA gene clusters. J Clin Microbiol. 2011;49:790–6.
Majazki J, Wüppenhorst N, Hartelt K, Birtles R, von Loewenich FD. Anaplasma phagocytophilum strains from voles and shrews exhibit specific ankA gene sequences. BMC Vet Res. 2013;9:235.
Huhn C, Winter C, Wolfsperger T, Wüppenhorst N, Smrdel KS, Skuballa J, et al. Analysis of the population structure of Anaplasma phagocytophilum using multilocus sequence typing. PLoS ONE. 2014;9(4):e93725.
De La Fuente J, Massung RF, Wong SJ, Chu FK, Lutz H, Meli M, et al. Sequence analysis of the msp4 gene of Anaplasma phagocytophilum strains. J Clin Microbiol. 2005;43:1309–17.
Rymaszewska A. PCR for detection of tick-borne Anaplasma phagocytophilum pathogens: a review. Vet Med. 2011;56:529–36.
Dugat T, Chastagner A, Lagrée AC, Petit E, Durand B, Thierry S, et al. A new multiple-locus variable-number tandem repeat analysis reveals different clusters for Anaplasma phagocytophilum circulating in domestic and wild ruminants. Parasit Vectors. 2014;7:439.
Rikihisa Y, Zhi N, Wormser GP, Wen B, Horowitz HW, Hechemy KE. Ultrastructural and antigenic characterization of a granulocytic ehrlichiosis agent directly isolated and stably cultivated from a patient in New York state. J Infect Dis. 1997;175:210–3.
Popov VL, Han VC, Chen SM, Dumler JS, Feng HM, Andreadis TG, et al. Ultrastructural differentiation of the genogroups in the genus Ehrlichia. J Med Microbiol. 1998;47:235–51.
Hotopp JCD, Lin M, Madupu R, Crabtree J, Angiuoli SV, Eisen J, et al. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2006;2(e21):0208–23.
Nelson CM, Herron MJ, Felsheim RF, Schloeder BR, Grindle SM, Chavez AO, et al. Whole genome transcription profiling of Anaplasma phagocytophilum in human and tick host cells by tiling array analysis. BMC Genomics. 2008;9:364.
Lin M, Dulk-Ras D, Hooykaas PJ, Rikihisa Y. Anaplasma phagocytophilum AnkA secreted by type IV secretion system is tyrosine phosphorylated by Abl-1 to facilitate infection. Cell Microbiol. 2007;9:2644–57.
Niu H, Kozjak-Pavlovic V, Rudel T, Rikihisa Y. Anaplasma phagocytophilum Ats-1 is imported into host cell mitochondria and interferes with apoptosis induction. PLoS Pathog. 2010;6:e1000774.
Rikihisa Y. Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum. Clin Microbiol Rev. 2011;24:469–89.
Smrdel KS, von Loewenich FD, Petrovec M, Županc TA. Diversity of ankA and msp4 genes of Anaplasma phagocytophilum in Slovenia. Ticks Tick Borne Dis. 2015;6:164–6.
Torina A, Vicente J, Alongi A, Scimeca S, Turlá R, Nicosia S, et al. Observed prevalence of tick-borne pathogens in domestic animals in Sicily, Italy during 2003–2005. Zoonoses Public Health. 2007;54:8–15.
Overzier E, Pfister K, Herb I, Mahling M, Böck G Jr, Silaghi C. Detection of tick-borne pathogens in roe deer (Capreolus capreolus), in questing ticks (Ixodes ricinus), and in ticks infesting roe deer in southern Germany. Ticks Tick Borne Dis. 2013;4:320–8.
Zeman P, Pecha M. Segregation of genetic variants of Anaplasma phagocytophilum circulating among wild ruminants within a Bohemian forest (Czech Republic). Int J Med Microbiol. 2008;298:203–10.
Naranjo V, Ruiz-Fons F, Höfle U, Fernandez De Mera IG, Villanúa D, et al. Molecular epidemiology of human and bovine anaplasmosis in southern Europe. Ann N Y Acad Sci. 2006;1078:95–9.
Hapunik J, Vichova B, Karboviak G, Wita I, Bogdaszewski M, Petko B. Wild and farm breeding cervids infections with Anaplasma phagocytophilum. Ann Agric Environ Med. 2011;18:73–7.
Malmsten J, Widén DG, Rydevik G, Yon L, Hutchings MR, Thulin CG, et al. Temporal and spatial variation in Anaplasma phagocytophilum infection in Swedish moose (Alces alces). Epidemiol Infect. 2014;142:1205–13.
Rymaszewska A. Divergence within the marker region of the groESL operon in Anaplasma phagocytophilum. Eur J Clin Microbiol Infect Dis. 2008;27:1025–36.
Michalik J, Stańczak J, Cieniuch S, Racewicz M, Sikora B, Dabert M. Wild boars as hosts of human-pathogenic Anaplasma phagocytophilum variants. Emerg Infect Dis. 2012;18:998–1001.
Leschnik M, Kirtz G, Virányi Z, Wille-Piazzai W, Duscher G. Acute granulocytic anaplasmosis in a captive timber wolf (Canis lupus occidentalis). J Zoo Wildl Med. 2012;43:645–8.
Liz JS, Anderes L, Sumner JW, Massung RF, Gern L, Rutti B, Brossard M. PCR detection of granulocytic ehrlichiae in Ixodes ricinus ticks and wild small mammals in western Switzerland. J Clin Microbiol. 2000;38:1002–7.
Silaghi C, Woll D, Hamel D, Pfister K, Mahling M, Pfeffer M. Babesia spp. and Anaplasma phagocytophilum in questing ticks, ticks parasitizing rodents and the parasitized rodents-analyzing the host–pathogen-vector interface in a metropolitan area. Parasit Vectors. 2012;5:191.
Bown KJ, Lambin X, Ogden NH, Begon M, Telford G, Woldehiwet Z, Birtles RJ. Delineating Anaplasma phagocytophilum ecotypes in coexisting, discrete enzootic cycles. Emerg Infect Dis. 2009;15:1948–54.
Pangrácová L, Derdáková M, Pekárik L, Hviščová I, Víchová B, Stanko M, et al. Ixodes ricinus abundance and its infection with the tick-borne pathogens in urban and suburban areas of Eastern Slovakia. Parasit Vectors. 2013;6:238.
Blaňarová L, Stanko M, Carpi G, Miklisová D, Víchová B, Mošanský L, et al. Distinct Anaplasma phagocytophilum genotypes associated with Ixodes trianguliceps ticks and rodents in central Europe. Ticks Tick Borne Dis. 2014;5:928–38.
Kallio ER, Begon M, Birtles RJ, Bown KJ, Koskela E, Mappes T, Watts PC. First report of Anaplasma phagocytophilum and Babesia microti in rodents in Finland. Vector Borne Zoonotic Dis. 2014;14:389–93.
Tomassone L, Berriatua E, De Sousa R, Duscher GG, Mihalca AD, Silaghi C, et al. Neglected vector-borne zoonoses in Europe: into the wild. Vet Parasitol. 2018;215:17–26.
Krücken J, Schreiber C, Maaz D, Kohn M, Demeler J, Beck S, et al. A novel high-resolution melt PCR assay discriminates Anaplasma phagocytophilum and “Candidatus Neoehrlichia mikurensis”. J Clin Microbiol. 2013;51:1958–61.
Baráková I, Derdáková M, Carpi G, Rosso F, Collini M, Tagliapietra V, et al. Genetic and ecologic variability among Anaplasma phagocytophilum strains, northern Italy. Emerg Infect Dis. 2014;20:1082–5.
Skuballa J, Petney T, Pfäffle M, Taraschewski H. Molecular detection of Anaplasma phagocytophilum in the European hedgehog (Erinaceus europaeus) and its ticks. Vector Borne Zoonotic Dis. 2010;10:1055–7.
Silaghi C, Skuballa J, Thiel C, Pfister K, Petney T, Pfäffle M, et al. The European hedgehog (Erinaceus europaeus)—a suitable reservoir for variants of Anaplasma phagocytophilum? Ticks Tick Borne Dis. 2012;3:49–54.
Földvári G, Jahfari S, Rigó K, Jablonszky M, Szekeres S, Majoros G, et al. “Candidatus Neoehrlichia mikurensis” and Anaplasma phagocytophilum in urban hedgehogs. Emerg Infect Dis. 2014;20:496–8.
Christova I, Gladnishka T. Prevalence of infection with Francisella tularensis, Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in rodents from an endemic focus of tularemia in Bulgaria. Ann Agric Environ Med. 2005;12:149–52.
Hulínská D, Langrová K, Pejčoch M, Pavlásek I. Detection of Anaplasma phagocytophilum in animals by real-time polymerase chain reaction. APMIS. 2004;112:239–47.
Torina A, Alongi A, Naranjo V, Estrada-Peña A, Vicente J, Scimeca S, et al. Prevalence and genotypes of Anaplasma species and habitat suitability for ticks in a Mediterranean ecosystem. Appl Environ Microbiol. 2008;74:7578–84.
Jouglin M, Chagneau S, Faille F, Verheyden H, Bastian S, Malandrin L. Detecting and characterizing mixed infections with genetic variants of Anaplasma phagocytophilum in roe deer (Capreolus capreolus) by developing an ankA cluster-specific nested PCR. Parasit Vectors. 2017;10:377.
Bakken JS, Dumler S. Human granulocytic anaplasmosis. Infect Dis Clin. 2008;22:433–48.
Bakken JS, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin. 2015;29:341–55.
Shukla SK, Aswani V, Stockwell PJ, Reed KD. Contribution of polymorphisms in ankA, gltA, and groESL in defining genetic variants of Anaplasma phagocytophilum. J Clin Microbiol. 2007;45:2312–5.
Strle F. Human granulocytic ehrlichiosis in Europe. Int J Med Microbiol. 2004;293(Suppl. 37):27–35.
Parola P, Davoust B, Raoult D. Tick- and flea-borne rickettsial emerging zoonoses. Vet Res. 2005;36:469–92.
Ogden NH, Casey ANJ, Woldehiwet Z, French NP. Transmission of Anaplasma phagocytophilum to Ixodes ricinus ticks from sheep in the acute and post-acute phases of infection. Infect Immun. 2003;71:2071–8.
Rar V, Golovljova I. Anaplasma, Ehrlichia, and “Candidatus Neoehrlichia” bacteria: pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect Genet Evol. 2011;11:1842–61.
Alekseev AN, Dubinina HV, Antykova LP, Dzhivanyan TI, Rijpkema SG, De Kruif NV, Cinco M. Tick-borne borrelioses pathogen identification in Ixodes ticks (Acarina, Ixodidae) collected in St. Petersburg and Kaliningrad Baltic regions of Russia. J Med Entomol. 1998;35:136–42.
Ogden NH, Bown K, Horrocks BK, Woldehiwet Z, Bennett M. Granulocytic Ehrlichia infection in ixodid ticks and mammals in woodlands and uplands of the UK. Med Vet Entomol. 1998;12:423–9.
Santos AS, Santos-Silva MM, Almeida VC, Bacellar F, Dumler JS. Detection of Anaplasma phagocytophilum DNA in Ixodes ticks (Acari: Ixodidae) from Madeira Island and Setubal District, mainland Portugal. Emerg Infect Dis. 2004;10:1643–8.
Pfäffle M, Petney T, Skuballa J, Taraschewski H. Comparative population dynamics of a generalist (Ixodes ricinus) and specialist tick (I. hexagonus) species from European hedgehogs. Exp Appl Acarol. 2011;54:151–64.
Karbowiak G, Vichová B, Slivinska K, Werszko J, Didyk J, Peťko B, et al. The infection of questing Dermacentor reticulatus ticks with Babesia canis and Anaplasma phagocytophilum in the Chernobyl exclusion zone. Vet Parasitol. 2014;204:372–5.
Barandika JF, Hurtado A, García-Esteban C, Gil H, Escudero R, Barral M, et al. Tick-borne zoonotic bacteria in wild and domestic small mammals in northern Spain. Appl Environ Microbiol. 2007;73:6166–71.
Grzeszczuk A, Stanczak J. High prevalence of Anaplasma phagocytophilum infection in ticks removed from human skin in north-eastern Poland. Ann Agric Environ Med. 2006;13:45–8.
Otranto D, Dantas-Torres F, Giannelli A, Latrofa MS, Cascio A, Cazzin S, et al. Ticks infesting humans in Italy and associated pathogens. Parasit Vectors. 2014;7:328.
Matei IA, Kalmár Z, Lupşe M, D’Amico G, Ionică AM, Dumitrache MO, et al. The risk of exposure to rickettsial infections and human granulocytic anaplasmosis associated with Ixodes ricinus tick bites in humans in Romania: a multiannual study. Ticks Tick Borne Dis. 2017;8:375–8.
Hagedorn P, Imhoff M, Fischer C, Domingo C, Niedrig M. Human granulocytic anaplasmosis acquired in Scotland, 2013. Emerg Infect Dis. 2014;20:1079–81.
Jereb M, Pecaver B, Tomazic J, Muzlovic I, Avsic-Zupanc T, Premru-Srsen T, et al. Severe human granulocytic anaplasmosis transmitted by blood transfusion. Emerg Infect Dis. 2012;18:1354–7.
Shields K, Cumming M, Rios J, Wong MT, Zwicker JI, Stramer SL, Alonso CD. Transfusion-associated Anaplasma phagocytophilum infection in a pregnant patient with thalassemia trait: a case report. Transfusion. 2015;55:719–25.
Cisak E, Chmielewska-Badora J, Zwolinski J, Wójcik-Fatla A, Polak J, Dutkiewicz J. Risk of tick-borne bacterial diseases among workers of Roztocze National Park (south-eastern Poland). Ann Agric Environ Med. 2005;12:127–32.
de Keukeleire M, Vanwambeke SO, Cochez C, Heyman P, Fretin D, Deneys V, et al. Seroprevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, and Francisella tularensis infections in Belgium: results of three population-based samples. Vector Borne Zoonotic Dis. 2017;17:108–15.
Horowitz HW, Kilchevsky E, Haber S, Aguero-Rosenfeld M, Kranwinkel R, James EK, et al. Perinatal transmission of the agent of human granulocytic ehrlichiosis. N Engl J Med. 1998;339:375–8.
Dhand A, Nadelman RB, Aguero-Rosenfeld M, Haddad FA, Stokes DP, Horowitz HW. Human granulocytic anaplasmosis during pregnancy: case series and literature review. Clin Infect Dis. 2007;45:589–93.
Pusterla N, Braun U, Wolfensberger C, Lutz H. Intrauterine infection with Ehrlichia phagocytophila in a cow. Vet Rec. 1997;141:101–2.
Reppert E, Galindo RC, Breshears MA, Kocan KM, Blouin EF, la Fuente J. Demonstration of transplacental transmission of a human isolate of Anaplasma phagocytophilum in an experimentally infected sheep. Transbound Emerg Dis. 2013;60(Suppl. 2):93–6.
Latrofa MS, Dantas-Torres F, de Caprariis D, Cantacessi C, Capelli G, Lia RP, et al. Vertical transmission of Anaplasma platys and Leishmania infantum in dogs during the first half of gestation. Parasit Vectors. 2016;9:269.
Matei IA, Stuen S, Modrý D, Degan A, D’Amico G, Mihalca AD. Neonatal Anaplasma platys infection in puppies: further evidence for possible vertical transmission. Vet J. 2017;219:40–1.
Zhang L, Liu Y, Ni D, Li Q, Yu Y, Yu XJ, et al. Nosocomial transmission of human granulocytic anaplasmosis in China. JAMA. 2008;300:2263–70.
Krause PJ, Wormser GP. Nosocomial transmission of human granulocytic anaplasmosis? JAMA. 2008;300:2308–9.
Liu Y, Li Q, Hu W, Wu J, Wang Y, Mei L, et al. Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne Zoonotic Dis. 2012;12:156–60.
Wormser GP. Human granulocytic anaplasmosis and Lyme disease. JAMA. 2016;316:98–9.
Liu Y, Yu XJ. Human granulocytic anaplasmosis and Lyme disease—reply. JAMA. 2016;316:99.
Wormser GP. Accuracy of diagnosis of human granulocytic anaplasmosis in China. Emerg Infect Dis. 2016;22:1728.
Petrovec M, Furlan SL, Zupanc TA, Strle F, Brouqui P, Roux V, Dumler JS. Human disease in Europe caused by a granulocytic Ehrlichia species. J Clin Microbiol. 1997;35:1556–9.
Brouqui PH, Dumler JS, Lienhard R, Brossard M, Raoult D. Human granulocytic ehrlichiosis in Europe. Lancet. 1995;346:782–3.
Sumption KJ, Wright DJ, Cutler SJ. Human ehrlichiosis in the UK. Lancet. 1995;346:1487–8.
Walder G, Fuchs D, Sarcletti M, Berek K, Falkensammer B, Huber K, et al. Human granulocytic anaplasmosis in Austria: epidemiological, clinical, and laboratory findings in five consecutive patients from Tyrol, Austria. Int J Med Microbiol. 2006;296:297–301.
Lagler H, Harrison N, Kussmann M, Obermüller M, Burgmann H, Makristathis A, Ramharter M. Direct detection of Anaplasma phagocytophilum by polymerase chain reaction followed by electrospray ionization mass spectrometry from human blood. Int J Infect Dis. 2017;60:61–3.
Cochez C, Ducoffre G, Vandenvelde C, Luyasu V, Heyman P. Human anaplasmosis in Belgium: a 10-year seroepidemiological study. Ticks Tick Borne Dis. 2011;2:156–9.
Misić-Majerus LJ, Bujic N, Madjaric V, Janes-Poje V. First description of the human granulocytic ehrlichiosis in Croatia. Clin Microbiol Infect. 2000;25:194–5.
Topolovec J, Puntaric D, Antolovic-Pozgain A, Vukovic D, Topolovec Z, Milas J, et al. Serologically detected “new” tick-borne zoonoses in eastern Croatia. Croat Med J. 2003;44:626–9.
Hulínská D, Kurzova D, Drevova H, Votýpka J. First detection of ehrlichiosis detected serologically and with the polymerase chain reaction in patients with borreliosis in the Czech Republic. Cas Lek Cesk. 2001;140:181–4.
Hulínská D, Votýpka J, Vaňousová D, Hercogová J, Hulínský V, Dřevová H, et al. Identification of Anaplasma phagocytophilum and Borrelia burgdorferi sensu lato in patients with erythema migrans. Folia Microbiol. 2009;54:246–56.
Remy V, Hansmann Y, De Martino S, Christmann D, Brouqui P. Human anaplasmosis presenting as atypical pneumonitis in France. Clin Infect Dis. 2003;37:846–8.
Koebel C, Kern A, Edouard S, Hoang AT, Celestin N, Hansmann Y, et al. Human granulocytic anaplasmosis in eastern France: clinical presentation and laboratory diagnosis. Diagn Microbiol Infect Dis. 2012;72:214–8.
Kowalski J, Hopfenmüller W, Fingerle V, Malberg H, Eisenblätter M, Wagner J, et al. Seroprevalence of human granulocytic anaplasmosis in Berlin/Brandenburg, Germany: an 8-year survey. Clin Microbiol Infect. 2006;12:924–7.
Ruscio M, Cinco M. Human granulocytic ehrlichiosis in Italy. Ann N Y Acad Sci. 2003;990:350–2.
Santos AS, Bacellar F, Dumler JS. A 4-year study of Anaplasma phagocytophilum in Portugal. Clin Microbiol Infect. 2009;15:46–7.
van Dobbenburgh A, van Dam AP, Fikrig E. Human granulocytic ehrlichiosis in western Europe. N Engl J Med. 1999;340:1214–6.
Bakken JS, Krueth J, Tilden RL, Dumler JS, Kristiansen BE. Serological evidence of human granulocytic ehrlichiosis in Norway. Eur J Clin Microbiol Infect Dis. 1996;15:829–32.
Kristiansen BE, Jenkins A, Tveten Y, Karsten B, Line Ø, Bjöersdorff A. Human granulocytic ehrlichiosis in Norway. Tidsskr Nor Laegeforen. 2001;121:805–6.
Tylewska-Wierzbanowska S, Chmielewski T, Kondrusik M, Hermanowska-Szpakowicz T, Sawicki W, Sułek K. First cases of acute human granulocytic ehrlichiosis in Poland. Eur J Clin Microbiol Infect Dis. 2001;20:196–8.
Moniuszko A, Dunaj J, Święcicka I, Zambrowski G, Chmielewska-Badora J, Żukiewicz-Sobczak W, et al. Co-infections with Borrelia species, Anaplasma phagocytophilum and Babesia spp. in patients with tick-borne encephalitis. Eur J Clin Microbiol Infect Dis. 2014;33:1835–41.
Kocianová E, Košt’anová Z, Štefanidesová K, Špitalská E, Boldiš V, Hučková D, Stanek G. Serologic evidence of Anaplasma phagocytophilum infections in patients with a history of tick bite in central Slovakia. Wien Klin Wochenschr. 2008;120:427–31.
Novakova M, Vichova B, Majlathova V, Lesnakova A, Pochybova M, Petʼko B. First case of human granulocytic anaplasmosis from Slovakia. Ann Agric Environ Med. 2010;17:173–5.
Oteo JA, Blanco JR, de Artola VM, Ibarra V. First report of human granulocytic ehrlichiosis from southern Europe (Spain). Emerg Infect Dis. 2000;6:430–2.
Dumler JS, Dotevall L, Gustafson R, Granstrom M. A population-based seroepidemiologic study of human granulocytic ehrlichiosis and Lyme borreliosis on the west coast of Sweden. J Infect Dis. 1997;175:720–2.
Dumler JS, Choi KS, Garcia-Garcia JC, Barat NS, Scorpio DG, Garyu JW, et al. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis. 2005;11:1828–34.
Nordberg M. Tick-borne infections in humans: aspects of immunopathogenesis, diagnosis and co-infections with Borrelia burgdorferi and Anaplasma phagocytophilum. Ph.D thesis, Linköping University, Sweden; 2012.
Pusterla N, Huder JB, Leutenegger CM, Braun U, Madigan JE, Lutz H. Quantitative real-time PCR for detection of members of the Ehrlichia phagocytophila genogroup in host animals and Ixodes ricinus ticks. J Clin Microbiol. 1999;37:1329–31.
Beltrame A, Ruscio M, Arzese A, Rorato G, Negri C, Londero A, et al. Human granulocytic anaplasmosis in northeastern Italy. Ann N Y Acad Sci. 2006;1078:106–9.
Tomasiewicz K, Buczek A, Stańczak J, Modrzewska R, Maciukajć J. The risk of exposure to Anaplasma phagocytophilum infection in mid-eastern Poland. Ann Agric Environ Med. 2004;11:261–4.
Grzeszczuk A, Puzanowska B, Miegoć H, Prokopowicz D. Incidence and prevalence of infection with Anaplasma phagocytophilum. Prospective study in healthy individuals exposed to ticks. Ann Agric Environ Med. 2004;11:55–7.
Grzeszczuk A. Anaplasma phagocytophilum in Ixodes ricinus ticks and human granulocytic anaplasmosis seroprevalence among forestry rangers in Białystok region. Adv Med Sci. 2006;51:283–6.
Chmielewska-Badora J, Zwolinski J, Cisak E, Wojcik-Fatla A, Buczek A, Dutkiewicz J. Prevalence of Anaplasma phagocytophilum in Ixodes ricinus ticks determined by polymerase chain reaction with two pairs of primers detecting 16S rRNA and ankA genes. Ann Agric Environ Med. 2007;14:281–5.
Rigaud E, Jaulhac B, Garcia-Bonnet N, Hunfeld KP, Femenia F, Huet D, et al. Seroprevalence of seven pathogens transmitted by the Ixodes ricinus tick in forestry workers in France. Clin Microbiol Infect. 2016;22:735.
Ijdo JW, Wu C, Magnarelli LA, Fikrig E. Serodiagnosis of human granulocytic ehrlichiosis by a recombinant HGE-44-based enzyme-linked immunosorbent assay. J Clin Microbiol. 1999;37:3540–4.
Edouard S, Koebel C, Goehringer F, Socolovschi C, Jaulhac B, Raoult D, Brouqui P. Emergence of human granulocytic anaplasmosis in France. Ticks Tick Borne Dis. 2012;3:403–5.
Brouqui P, Bacellar F, Baranton G, Birtles RJ, Bjoersdorff A, Blanco JR, ESCMID Study Group on Coxiella, Anaplasma, Rickettsia and Bartonella, European Network for Surveillance of Tick-Borne Diseases, et al. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin Microbiol Infect. 2004;10:1108–32.
Hjetland R, Henningsson AJ, Vainio K, Dudman SG, Grude N, Ulvestad E. Seroprevalence of antibodies to tick-borne encephalitis virus and Anaplasma phagocytophilum in healthy adults from western Norway. Infect Dis. 2015;47:52–6.
Lotrič-Furlan S, Petrovec M, Avšič-Županc T, Strle F. Human granulocytic ehrlichiosis in Slovenia. Ann N Y Acad Sci. 2003;990:279–84.
Lotrič-Furlan S, Rojko T, Petrovec M, Avšič-Županc T, Strle F. Epidemiological, clinical and laboratory characteristics of patients with human granulocytic anaplasmosis in Slovenia. Wien Klin Wochenschr. 2006;118:708–13.
Lotrič-Furlan S, Avsic-Zupanc T, Petrovec M, Nicholson WL, Sumner JW, Childs JE, Strle F. Clinical and serological follow-up of patients with human granulocytic ehrlichiosis in Slovenia. Clin Diagn Lab Immunol. 2001;8:899–903.
Bakken JS, Dumler JS. Clinical diagnosis and treatment of human granulocytotropic anaplasmosis. Ann N Y Acad Sci. 2006;1078:236–47.
Eliasson I. Ehrlichiosis, human granulocytic—Sweden. Reviewed in Blanco JR, Oteo JA. Human granulocytic ehrlichiosis in Europe. Clin Microbiol Infect. 2002;8:763–72.
Karlsson U, Bjöersdorff A, Massung RF, Christensson B. Human granulocytic ehrlichiosis—a clinical case in Scandinavia. Scand J Infect Dis. 2001;33:73–4.
Arsić B, Gligić A, Ristanović E, Lako B, Potkonjak A, Peruničić M, Pavlović M. A case of human monocytic ehrlichiosis in Serbia. Srp Arh Celok Lek. 2014;142:79–82.
Stuen S, Bergstrom K, Petrovec M, Van de Pol I, Schouls LM. Differences in clinical manifestations and hematological and serological responses after experimental infection with genetic variants of Anaplasma phagocytophilum in sheep. Clin Diagn Lab Immunol. 2003;10:692–5.
Granquist EG, Bårdsen K, Bergström K, Stuen S. Variant- and individual-dependent nature of persistent Anaplasma phagocytophilum infection. Acta Vet Scand. 2010;52:25.
Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest. 2004;1138:1093–101.
Brouqui P, Salvo E, Dumler JS, Raoult D. Diagnosis of granulocytic ehrlichiosis in humans by immunofluorescence assay. Clin Diagn Lab Immunol. 2001;8:199–202.
Silaghi C, Santos AS, Gomes J, Christova I, Matei IA, Walder G, et al. Guidelines for the direct detection of Anaplasma spp. in diagnosis and epidemiological studies. Vector Borne Zoonotic Dis. 2017;17:12–22.
Aguero-Rosenfeld ME, Horowitz HW, Wormser GP, McKenna DF, Nowakowski J, Munoz J, Dumler JS. Human granulocytic ehrlichiosis: a case series from a medical center in New York State. Ann Intern Med. 1996;125:904–8.
Bakken JS, Aguero-Rosenfeld ME, Tilden RL, Wormser GP, Horowitz HW, Raffalli JT, et al. Serial measurements of hematologic counts during the active phase of human granulocytic ehrlichiosis. Clin Infect Dis. 2001;32:862–70.
Goodman JL, Nelson C, Vitale B, Madigan JE, Dumler JS, Kurtti TJ, Munderloh UG. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N Engl J Med. 1996;334:209–15.
Dumler JS, Barat NC, Barat CE, Bakken JS. Human granulocytic anaplasmosis and macrophage activation. Clin Infect Dis. 2007;45:199–204.
Krause PJ, Corrow CL, Bakken JS. Successful treatment of human granulocytic ehrlichiosis in children using rifampin. Pediatrics. 2003;112:e252–3.
Jaarsma RI, Sprong H, Takumi K, Kazimirova M, Silaghi C, Mysterud A, et al. Anaplasma phagocytophilum evolves in geographical and biotic niches of vertebrates and ticks. Parasit Vectors. 2019;12:328.
Walder G, Falkensammer B, Aigner J, Tiwald G, Dierich MP, Würzner R, Lechleitner P. First documented case of human granulocytic ehrlichiosis in Austria. Wien Klin Wochenschr. 2003;115:263.
Arnež M, Petrovec M, Lotrič-Furlan S, Zupanc TA, Strle F. First European pediatric case of human granulocytic ehrlichiosis. J Clin Microbiol. 2001;39:4591–2.
Lotrič-Furlan S, Petrovec M, Zupanc TA, Nicholson WL, Sumner JW, Childs JE, Strle F. Human granulocytic ehrlichiosis in Europe: clinical and laboratory findings for four patients from Slovenia. Clin Infect Dis. 1998;27:424–8.
Laferl H, Hogrefe W, Köck T, Pichler H. A further case of acute human granulocytic ehrlichiosis in Slovenia. Eur J Clin Microbiol Infect Dis. 1999;18:385–6.
Weber R, Pusterla N, Loy M, Lutz H. Fever, leukopenia, and thrombocytopenia in a patient with acute Lyme borreliosis were due to human granulocytic ehrlichiosis. Clin Infect Dis. 1998;26:253–4.
Lotrič-Furlan S, Ruzic-Sabljic E, Strle F. Concomitant human granulocytic anaplasmosis and Lyme neuroborreliosis. Clin Microbiol Infect. 2009;15(Suppl. 2):28–9.
Pokorn M, Županc TA, Strle F. Pediatric human granulocytic anaplasmosis is rare in Europe. Pediatr Infect Dis J. 2016;35:358–9.
Acknowledgements
We would like to thank the authors whose articles have been used in this review.
Funding
This review was prepared as part of the ECDC Project OJ/24/04/2014-PROC/2014/013 “Guidance, data collection and scientific advice on tick-borne diseases”. Its publication was funded by the Ministry of Research and Innovation through Program 1—Development of the National Research and Development System, Subprogram 1.2—Institutional Performance—Projects for Financing the Excellence in CDI, Contract no. 37PFE/06.11.2018. Title of the project: “Increasing the institutional performance through consolidation and development of research directions within the USAMVCN”.
Author information
Authors and Affiliations
Contributions
IAM and SJC wrote the paper. LV-C and AE-P collect part of the data. MV-T, AP, HZ and ADM conceived the paper and provided critical revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Matei, I.A., Estrada-Peña, A., Cutler, S.J. et al. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasites Vectors 12, 599 (2019). https://doi.org/10.1186/s13071-019-3852-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-019-3852-6