Abstract
Background
Dengue is a serious public health problem worldwide, including in Selangor, Malaysia. Being an important vector of dengue virus, Aedes aegypti are subjected to control measures which rely heavily on the usage of insecticides. Evidently, insecticide resistance in Ae. aegypti, which arise from several different point mutations within the voltage-gated sodium channel genes, has been documented in many countries. Thus, this robust study was conducted in all nine districts of Selangor to understand the mechanisms of resistance to various insecticides in Ae. aegypti. Mosquitoes were collected from dengue epidemic and non-dengue outbreak areas in Selangor.
Methods
Using the Center for Disease Control and Prevention (CDC) bottle assays, the insecticide resistance status of nine different Ae. aegypti strains from Selangor was accessed. Synergism tests and biochemical assays were conducted to further understand the metabolic mechanisms of insecticide resistance. Polymerase chain reaction (PCR) amplification and sequencing of the IIP-IIS6 as well as IIIS4-IIIS6 regions of the sodium channel gene were performed to enable comparisons between susceptible and resistant mosquito strains. Additionally, genomic DNA was used for allele-specific PCR (AS-PCR) genotyping of the gene to detect the presence of F1534C, V1016G and S989P mutations.
Results
Adult female Ae. aegypti from various locations were susceptible to malathion and propoxur. However, they exhibited different levels of resistance against dichlorodiphenyltrichloroethane (DDT) and pyrethroids. The results of synergism tests and biochemical assays indicated that the mixed functions of oxidases and glutathione S-transferases contributed to the DDT and pyrethroid resistance observed in the present study. Besides detecting three single kdr mutations, namely F1534C, V1016G and S989P, co-occurrence of homozygous V1016G/S989P (double allele) and F1534C/V1016G/S989P (triple allele) mutations were also found in Ae. aegypti. As per the results, the three kdr mutations had positive correlations with the expressions of resistance to DDT and pyrethroids.
Conclusions
In view of the above outcomes, it is important to seek new tools for vector management instead of merely relying on insecticides. If the latter must be used, regular monitoring of insecticide resistance should also be carried out at all dengue epidemic areas. Since the eggs of Ae. aegypti can be easily transferred from one location to another, it is probable that insecticide-resistant Ae. aegypti can be found at non-dengue outbreak sites as well.
Similar content being viewed by others
Background
Dengue is a mosquito-borne disease which has now become a global problem owing to rapid urbanisation as well as cheapness and ease of travel [1, 2]. Currently, the incidence of dengue is about 390 million [3] in 128 countries [4]. This is a 30-fold increase in dengue cases compared to 50 years ago [5]. Malaysia is no exception as the cases of dengue have increased over the years. In 2018 (until 22nd December), 78,066 dengue cases were reported in Malaysia [6], a 77-fold increase compared to the first epidemic which occurred in 1973 [7]. In Malaysia, the state of Selangor, which is the most developed and densely-populated state, has the highest number of dengue cases (47,711 cases) [6].
The hallmark of the dengue control programmes in most countries are fogging and ultra low-volume (ULV) sprays when cases of dengue are reported [8]. It has been established that ULV is not very effective and that the insecticide droplets only get carried as far as the living rooms, whereas the mosquitoes tend to rest in the bedrooms or bathrooms [9,10,11]. However, due to frequent outbreaks and lack of manpower, ULV sprays must be carried out to cover larger areas.
Owing to the excessive utilization of insecticides in agriculture and public health, mosquitoes are developing resistance to the currently used insecticides [12, 13]. Most countries in Southeast Asia have reported mosquito resistance to the most commonly employed pyrethroids [14,15,16,17,18,19], but these vectors are still susceptible to organophosphates [15, 18, 20]. However, it is difficult to rely on the above results as the standard procedures have not always been followed. There is only a limited number of insecticides in our armamentarium for use in public health [21]. Pyrethroids are a common class of insecticides being used in vector control strategies and it has been shown that there is cross-resistance between pyrethroids and organochlorines [22]. Thus, insecticides should be used judiciously to prevent resistance in vectors.
Bioassays were among the first methods for the detection of resistance in mosquitoes [23]. This method employs a simple procedure, so control programmes can monitor resistance levels with ease. Subsequently, synergists were discovered to be able to improve the efficacy of the insecticides [24] by inhibiting the enzymes that were involved in detoxification of the insecticides.
It is also known that metabolic resistance owing to the detoxification of enzymes like esterases (ESTs), mixed-function oxidisases (MFO), glutathione S-transferases (GST), and acetylcholinesterases (AChE) are associated with insecticide resistance [18, 25, 26]. Generally, EST and AChE play important roles in organophosphate and carbamate resistance, GST and MFO play important role in organochlorine (DDT) and pyrethroid resistance [25]. Voltage-gated sodium channels are integral transmembrane proteins responsible for the rapidly rising phase of action potentials, and they are crucial for electrical signalling in most excitable cells [27]. Sodium channels are thus primary target of DDT and synthetic pyrethroids [27]. Due to intensive use of insecticides, kdr (knockdown resistance) have developed in mosquitoes [19, 28,29,30]. This mechanism has reduced the sodium channel sensitivity to pyrethroids and DDT, via one or more point mutations in the sodium channel protein [27].
Since dengue cases are increasing by the year in Selangor, Malaysia, fogging and ULV are being carried out on a regular basis. Thus, it is highly important for the overseers of control programmes to be aware of the effectiveness of these chemicals against Ae. aegypti. Limited studies have been carried out in Malaysia [16, 19, 31] and thus, this study was conducted in all nine districts of the state to understand the resistance mechanisms to various insecticides in Ae. aegypti. The mosquitoes were collected from dengue-epidemic (dengue reported every year; Aedes mosquito populations high during dengue outbreak season) as well as non-dengue outbreak areas. The present study, according to the authors’ knowledge, represents the first attempt to investigate the biochemical and molecular basis of insecticide resistance mechanisms in Ae. aegypti from dengue epidemic and non-dengue outbreak areas from Selangor. The outcome of the present study will be of importance when selecting the insecticides for application against Ae. aegypti, since use of chemicals are extensively practiced in vector control.
Methods
Study site
Selangor is located at the center of Peninsular Malaysia, and it serves as the main transportation hub of the country. It is also the most populated and well-developed state in Malaysia. Twenty-three percent of the total gross domestic product (GDP) of Malaysia is contributed by Selangor [32]. Collection of Ae. aegypti from all nine districts of the state was performed from September 2015 to April 2016 using ovitraps (Fig. 1). The nine districts are Hulu Selangor (HS), Gombak (G), Hulu Langat (HL), Kuala Langat (KL), Kuala Selangor (KS), Petaling (P), Klang (K), Sabak Bernam (SB) and Sepang (S). Forty ovitraps were set each week for three continuous weeks in each district. The traps were set at a distance of at least 20 m from each other. The traps were checked weekly. The selection of study sites was based on their dengue outbreak and non-dengue outbreak status. The eggs collected from each site were hatched in the laboratory.
All emerged adult mosquitoes were identified and segregated according to species using morphological characteristics [33]. Aedes aegypti colonies were maintained in standard insectary conditions (27 ± 2 °C, 75 ± 5% relative humidity, 10:14 h light:dark photocycle). Ten percent sucrose solution with vitamin B complex was provided as food to the mosquitoes. Five-to-seven-day-old adult female mosquitoes were provided with blood meals (using live white mice) for breeding purposes. Each field colony was established from about 500–1000 mosquitoes. F1 or F2 generation were used for all studies. Aedes aegypti of Bora-bora strain served as the reference susceptible strain. The Bora-bora strain has been maintained in the insectary for 134 generations without any exposure to insecticides.
Tested insecticides
The present study employed all four major classes of neurotoxic insecticides, namely pyrethroids (cyfluthrin 99.8%, deltamethrin 99.6%, etofenprox 97.7%, lambda cyhalothrin 97.8% and permethrin 98.1%), organophosphates (malathion 98.7%), carbamates (propoxur 99.8%) and organochlorines (dichlorodiphenyltrichloroethane; DDT 98%). All insecticides were purchased from Sigma-Aldrich (Darmstadt, Germany).
CDC adult bioassays
The CDC bottle bioassays were conducted as described by Brogdon and Chan [34]. To determine the diagnostic dosage and time for each insecticide, Bora-Bora strain was used as reference. The diagnostic dosage and time were used to evaluate the resistance thresholds against all field strains. Each test consisted of three insecticide-treated bottles and one ethanol-treated bottle as a control. These tests were conducted for three consecutive days (9 replicates in total). Each bottle was prepared according to Brogdon & Chan [34]. Briefly, 20–25 three-to-five-day-old sucrose-fed female Ae. aegypti were introduced into each 250 ml bottle coated with the diagnostic dosage of each test insecticide. The number of dead mosquitoes was recorded at one-minute intervals for a maximum of 2 h. Mosquitoes that were incapable of flying or maintaining an upright posture were considered dead. Live mosquitoes were further transferred to a paper cup with netting and 10% sucrose solution was provided. The final mortality was recorded 24 h after treatment. The diagnostic dosage was determined according to rapid end-point assays to determine the doses which killed 100% of susceptible mosquitoes within 30 min to 1 h. Table 1 shows the diagnostic dosages and resistance thresholds (time). After 24 h, dead mosquitoes, including those that were alive without the capability of coordinated movement, were labelled as susceptible (S). The survivors were labelled as resistant (R). Thirty samples of mosquitoes each susceptible and resistant to DDT and pyrethroids were randomly selected for kdr mutation detection using allele-specific polymerase chain reaction (AS-PCR).
Synergism tests
In order to evaluate the capability of Ae. aegypti to detoxify insecticides, synergism tests were performed against all field strains. The synergism tests were performed as described by Brogdon & Chan [34]. Three synergists, piperonyl butoxide 99% (PBO), S.S.S-tributyl phosphorotrithioate 97.2% (DEF), and ethacrynic acid 99% (EA) were purchased from Sigma-Aldrich for use in this study. The maximum sublethal concentration of each synergist was determined by a trial-and-error series of sublethal dosages which were administered on the reference strain. The sublethal dosage of adult synergism tests were 160 µg/bottle, 37.5 µg/bottle, and 16 µg/bottle for PBO, DEF and EA, respectively. The adult synergism tests were conducted in a manner similar to that of the CDC bottle assays, except that the female mosquitoes were exposed to the synergist-coated bottle for 1 h before being exposed to the insecticide-coated bottle; while the control was performed using ethanol-coated bottle. Each synergist was used in combination with all insecticides.
Biochemical assays
Biochemical assays were performed to determine if the observed insecticide resistances in the Selangor Ae. aegypti population were due to elevated enzymatic activities. To determine the differences in the enzyme levels of individual adult female Ae. aegypti, biochemical assays of the susceptible strain (Bora-Bora) and field strain were performed as described by Hemingway & Brogdon [35] with minor modifications. The adult mosquitoes from the nine different districts in Selangor were individually assayed for α-EST, β-EST, AChE, GST and MFO enzymatic activities. Briefly, three-to-five-day-old female mosquitoes were individually homogenized in 200 µl of distilled water (on ice). Then, 25 µl of homogenate was pipetted for AChE assay. The remaining homogenate was centrifuged at 14,000× rpm at 4 °C for one minute, and the supernatant used as an enzyme source for all other enzyme assays. In total, 94 female mosquitoes from each site were assayed. All assays were conducted in duplicates using 96-well microplates. The absorbances [optical density (OD) values] were measured using the Infinite M200Pro microtitre plate reader (Tecan Trading AG, Männedorf, Switzerland). The assay for each enzyme and the enzymatic activities were calculated as described below.
AChE assay
Some 145 µl of Triton phosphate buffer and 10 µl of 0.01 M dithiobis 2-nitrobenzoic acid solution were added to 25 µl of mosquito homogenate. This was followed by the addition of 25 µl of 0.01 M acetylthiocholine iodide to initiate the reaction. One reaction was inhibited via the addition of 0.05 µl of 0.1 M propoxur while the other was allowed to progress. After 1 h of incubation at room temperature, the reactions were measured at 405 nm absorbance. The AChE activity was calculated with respect to the percentage of insensitivity to AChE activity after propoxur inhibition [36].
Non-specific esterase assay
Twenty µl of supernatant from the mosquito homogenates was added in duplicates to each well. To one set of samples, 200 µl of 30 mM α-naphthyl acetate was added, while to the other, 200 µl of 30 mM β-naphthyl acetate was added. The plate was incubated for 15 min at room temperature. After incubation, 50 µl of fast-blue stain was added to each well. The mixture was allowed to incubate for another 15 min, following which the OD values were measured at 570 nm. The EST activity against each substrate was calculated based on the standard curves of absorbance for known concentrations of α-naphthol or β-naphthol. The enzymatic activities were expressed as nmol of α-naphthol or β-naphthol/min/mg protein.
GST assay
First, 10 µl of supernatant from the mosquito homogenates was added to a mixture of 200 µl 63 mM 1-chloro-2,4-dinitrobenzene (CDNB) and 10 mM reduced glutathione. The plate was allowed to incubate for 20 min at room temperature before the OD values were measured at 340 nm absorbance. Beer’s Law (A = €cl) was employed in the calculation of GST activity, which was expressed as CDNB/min/mg protein. The OD value (A) was transformed into µmol of CDNB conjugates using the extinction coefficient (€) of 4.39 mM−1. The path length (i.e. the depth of the buffer solution in the microplate well) was 0.6 cm.
MFO assay
A total of 2 µl of supernatant was added to the duplicate wells. To initiate the assay, 80 µl of 0.625 M potassium phosphate buffer at pH 7.2 was added to each well, followed by 200 µl of 3,3,5,5-tetramethylbenzidine (TMBZ) (with methanol as the solvent) and 25 µl of 3% hydrogen peroxide. The mixture was incubated at room temperature for 2 h, after which the OD value was measured at 650 nm absorbance. The MFO activity was calculated from the standard curve of absorbance for known concentrations of cytochrome C [37]. The enzymatic activity was expressed as equivalent units of cytochrome P450/min/mg protein.
Protein assay
Owing to size variances between individual mosquitoes, the analyses of all enzyme activities were corrected using the protein concentration as a standard correction factor. The bovine serum albumin standard curve was obtained using a commercial protein assay kit (Bio-Rad, Hercules, California, USA). Subsequently, the protein concentration was transformed and calculated based on the same. The protein assay was conducted by mixing 300 µl of Bio-Rad dye reagent with 10 µl of mosquito homogenate, after which the mixture was allowed to incubate for 5 min at room temperature. The plate was read at an OD of 570 nm.
DNA extraction
Thirty adult Ae. aegypti each resistant and susceptible to DDT and pyrethroids from each site were subjected to molecular analysis. DNA was extracted from each specimen using the DNeasy® Blood & Tissue Kit (Qiagen, Düsseldorf, Germany). All isolation steps were conducted according to the instructions of the manufacturer.
Allele-specific PCR (AS-PCR) detection of V1016G, F1534C, and S989P mutation
There are three kdr point mutations that confer pyrethroids resistance to Ae. aegypti namely, F1534C, V1016G and S989P [38]. These kdr mutations are widespread in Southeast Asia [19, 29, 39, 40]. In Malaysia point mutations of F1534C and V1016G but not S989P mutation have been detected [19]. Therefore, this study aims to detect S989P kdr point mutation in Malaysia.
In order to determine the associations of F1534C, V1016G, and S989P mutations with organochlorine and pyrethroid resistance, 30 randomly-selected mosquitoes from each susceptible and resistant field strain were subjected to AS-PCR. The F1534C AS-PCR was performed according to Yanola et al. [39]. Each reaction was performed in a volume of 10 µl with final concentrations of 1.5 mM MgCl2, 1× PCR buffer (Promega, Madison, Wisconsin, USA), 0.5 µM Phe forward primer (5′-GCG GGC TCT ACT TTG TGT TCT TCA TCA TAT T-3′), 0.165 µM Cys forward primer (5′-GCG GGC AGG GCG GCG GGG GCG GGG CCT CTA CTT TGT GTT CTT CAT CAT GTG-3′), 0.5 µM common reserve primer (5′-TCT GCT CGT TGA AGT TGT CGA T-3′), 200 µM dNTP mixture (Promega), 1 U Taq polymerase (Promega), and 25–100 ng of genomic DNA. The PCR reaction was performed at 95 °C for 2 min (initial denaturation), followed by 35 cycles of the following: 95 °C for 30 s (denaturation), 60 °C for 30 s (annealing) and 72 °C for 30 s (extension). Subsequently, final extension was performed at 72 °C for 2 min. The amplified PCR products were loaded onto a 3% agarose gel pre-stained with SYBR SafeTM DNA stain (Invitrogen, Carlsbad, California, USA). Gel electrophoresis was run at 100 V for 45 min in 0.5× TBE buffer.
The V1016G AS-PCR was performed as per Stenhouse et al. [40]. Each reaction was performed in a final volume of 10 µl with final concentrations of 1.5 mM MgCl2, 1× PCR buffer (Promega), 0.25 µM forward primer (5′-ACC GAC AAA TTG TTT CCC-3′), 0.125 µM of each reverse primer specific for either Gly (5′-GCG GGC AGG GCG GCG GGG GCG GGG CCA GCA AGG CTA AGA AAA GGT TAA CTC-3′) or Val (5′-GCG GGC AGC AAG GCT AAG AAA AGG TTA ATT A-3′), 200 µM dNTP mixture (Promega), 1 U Taq polymerase (Promega), and 25–100 ng of genomic DNA.
PCR was carried out on a Bio-rad MyCyclerTM Thermal Cycle (Hercules, California, USA). The PCR conditions included an initial denaturation of 94 °C for 2 min, followed by 35 cycles of the following: 94 °C for 30 s (denaturation), 55 °C for 30 s (annealing) and 72 °C for 30 s (extension). Subsequently, final extension was performed at 72 °C for 2 min. Since the primers used in this study had GC-rich tails of varying lengths, the amplified products could be differentiated by size (i.e. 60 bp for Val and 80 bp for Gly). The amplified PCR products were loaded onto a 5% agarose gel pre-stained with SYBR SafeTM DNA stain (Invitrogen). Gel electrophoresis was run at 100 V for 50 min in 0.5× TBE buffer.
A modified S989P AS-PCR was performed in accordance with the protocol of Li et al. [41]. Each reaction was performed in a final volume of 10 µl with final concentrations of 1.5 mM MgCl2, 1× PCR buffer (Promega), 0.4 µM M1-F common forward primer (5′-AAT GAT ATT AAC AAA ATT GCG C-3′), 0.2 µM M1-S specific forward primer (5′-GCG GCG AGT GGA TCG AAT-3′) or 0.2 µM M1-P specific forward primer (5′-GCG GCG AGT GGA TCG AAC-3′), with 0.6 µM M2-Rev common reverse primer (5′-GCA CGC CTC TAA TAT TGA TGC-3′), 200 µM dNTP mixture (Promega), 1 U Taq polymerase (Promega), and 25–100 ng of genomic DNA. The PCR reaction was performed at 94 °C for 3 min (initial denaturation) and followed by 35 cycles of the following: 94 °C for 30 s (denaturation), 60 °C for 30 s (annealing) and 72 °C for 1 min (extension). Subsequently, final extension was performed at 72 °C for 7 min. The amplified PCR products were loaded onto a 1.5% agarose gel pre-stained with SYBR SafeTM DNA stain (Invitrogen). Gel electrophoresis was run at 100 V for 45 min in 0.5× TBE buffer.
Amplification and DNA sequencing of a fragment of Ae. aegypti voltage-gated sodium channel gene
To confirm the AS-PCR results, amplification of DNA was conducted as per Yanola et al. [39]. The primer IIP_F (5′-GGT GGA ACT TCA CCG ACT TC-3′) was used with IIS_R (5′-GGA CGC AAT CTG GCT TGT TA-3′) to encompass the region with V1016G and S989P mutations in the IIP-IIS6 region within exons 16 to 17. The amplified product size was 581 bp. On the other hand, the F1534C mutation was sequenced using the primers GE-IIIS6_F (5′-GCT GTC GCA CGA GAT CAT T-3′) with IIIS6_R (5′-GTT GAA CCC GAT GAA CAA CA-3′) which amplified the IIIS4-IIIS6 region within the exons 24–26. The amplified product’s size was 635 bp.
PCR was carried out in a reaction volume of 50 µl, which contained 1.5 mM MgCl2, 1× PCR buffer (Promega), 0.5 µM forward and reverse primers, 200 µM dNTP mixture (Promega), 1 U Taq polymerase (Promega), and 25–100 ng of genomic DNA. The amplification consisted of an initial heat-activation step of 95 °C for 2 min, followed by 35 cycles of the following: 95 °C for 30 s, 63 °C for 30 s and 72 °C for 30 s. Final extension was done at 72 °C for 2 min.
The amplified PCR products were loaded onto a 1.5% agarose gel pre-stained with SYBR SafeTM DNA stain (Invitrogen, USA), after which gel electrophoresis was run at 100 V for 45 min in TBE buffer. The gel was viewed under UV-light, after which the designated band was cut out, placed inside a 1.5 ml microcentrifuge tube, and stored in − 20 °C until required for sequencing. DNA sequencing of the PCR products was performed using the service provided by Genomics BioScience and Technology Co. Ltd. (New Taipei City, Taiwan), which employed a BigDye® Terminator v3.1 in ABI PRISM® 3730xl DNA Analyzer (Applied Biosystems, Foster City, California, USA). Forward and reverse sequencing reactions were done using the forward and reverse PCR primers as mentioned above. All sequence analyses and editing were performed using the BioEdit Sequence Alignment Editor v7.2.3. Both forward and reverse nucleotide sequences were aligned, and a consensus sequence was formed for each sample. Only sequences of good quality were trimmed for further analysis. The trimmed sequences were then aligned using Clustal W, along with other similar sequences available in GenBank. All sequences generated in the present study were deposited in the GenBank database under the accession numbers MK005552-MK005584. For the 1016 and 989 mutation-point analyses, the included sequences were: MF794972 (V1016V, F1534F homozygous allele); MF794974 (V1016V, F1534C homozygous allele); MF794978 (V1016V/G heterozygous, F1534F homozygous allele), MF794984 (G1016G, F1534F homozygous allele) [42]; KY057038 (V1016G homozygous allele), KY057037 (S989P homozygous allele) [28]; and AB914689 (V1016G, S989P homozygous allele) [43]. As for the F1534C mutation analysis, sequences AB914688 (F1534F homozygous allele), AB914687 (F1534C homozygous allele) [43], EU259810 (DDT-resistant, F1534F homozygous allele), EU259811 (DDT- and permethrin-resistant, F1534C homozygous allele) [39], and MF794990 (F1534F/C heterozygous allele) [42] were included.
Statistical analysis
The mortality rate (%) was used to describe the susceptibility statuses of Ae. aegypti and was used to evaluate the effectiveness of the synergists against the toxicities of the insecticides. Mortality rates as derived from the CDC bottle bioassays were used to determine the susceptibility statuses of the field strains of Ae. aegypti vis-à-vis the diagnostic dosages and times of the reference strain (Bora-Bora). A mortality rate of 98–100 indicates susceptibility; 90–97 indicates tolerance/ intermediate resistance; and < 90 indicates resistance [23, 34]. The data of the CDC bottle assays which were within 5–95% were subjected to probit analyses of Finney [44] to obtain the knockdown rates, KT50 and KT99, for each insecticide. The said data were then pooled for analysis. Resistance ratios (RR50) were calculated by dividing the KT50 values for the field strain with those for the reference strain, based on the CDC bottle bioassays. The RR50 was used to determine the correlation between the different insecticides [45]. If the control’s mortality was between 5–20%, the percentage mortalities would be corrected by Abbott’s formula [46].
Levene’s and Kolmogorov-Smirnov tests were performed to check the normality of the knockdown rates, mortality rates and enzymatic activities. To stabilize the variances between the data, an arcsine log function was performed on data that were not normally distributed. The differences between the Bora-Bora and field strains were determined using the Mann-Whitney non-parametric test or two-sample t-test. Cross-resistances between insecticides were determined using the Spearmanʼs rank-order correlation. Independent chi-squared test was carried out to compare the differences in Ae. aegypti possessing V1016G, F1534C, and V1016G and S989P mutations. Statistical significance was set at P < 0.05. The Statistical Package for Social Sciences (SPSS; IBM SPSS Statistics 19) software was used for data analyses and interpretations, while Microsoft Excel version 2016 (Microsoft Inc.) for generating graphs.
Results
Bioassays
The diagnostic dosages and diagnostic times of the different insecticides (based on Bora-Bora strain) are shown in Table 1. Female Ae. aegypti from all sites showed 100% mortality to malathion and propoxur within 2 h of exposure, except for the Petaling strain which recorded 92.22% mortality 24 h post-treatment with propoxur (Fig. 2) (Additional file 1: Table S1). The Klang strain was susceptible to all test insecticides, with 100% mortality achieved within the diagnostic time. As for DDT, all field strains exhibited resistance to it with mortality rates of < 90%, except for Sabak Bernam and Klang (100%).
Most of the female Ae. aegypti field strains were resistant to all five pyrethroid insecticides, except for Klang and Sabak Bernam strains which recorded 100% mortality 24 h post-treatment. However, the Gombak strain had 100% mortality to cyfluthrin while the Kuala Langat strain showed 100% mortality to cyfluthrin and deltamethrin. All other strains (Hulu Langat, Hulu Selangor, Kuala Selangor and Petaling) exhibited different degrees of resistance to pyrethroids as shown in Fig. 2. The KT50 (Fig. 3) and KT99 also varied among the different field strains (Additional file 1: Table S2). Furthermore, the Spearmanʼs rank-order correlation test indicated a significant correlation between the resistance ratios of DDT and lambdacyhalothrin (r(8) = 0.767, P = 0.016), cyfluthrin and permethrin (r(8) = 0.800, P = 0.010), cyfluthrin and lambdacyhalothrin (r(8) = 0.833, P = 0.005), cyfluthrin and deltamethrin (r(8) = 0.867, P = 0.002), cyfluthrin and etofenprox (r(8) = 0.800, P = 0.010), deltamethrin and permethrin (r(8) = 0.750, P = 0.020), deltamethrin and lambdacyhalothrin (r(8) = 0.767, P = 0.016), etofenprox and permethrin (r(8) = 0.867; P = 0.002), as well as etofenprox and lambdacyhalothrin (r(8) = 0.667, P = 0.050). There were no significant correlations between other insecticides (Fig. 4).
Correlation between resistance ratios of DDT and lambda cyhalothrin (a), cyfluthrin and deltamethrin (b), deltamethrin and lambda cyhalothrin (c), cyfluthrin and permethrin (d), cyfluthrin and etofenprox (e), deltamethrin and permethrin (f), etofenprox and permethrin (g), cyfluthrin and lambda cyhalothrin (h), and etofenprox and lambda cyhalothrin (i)
Effectiveness of synergists
Synergists (DEF, EA and PBO) were investigated for their efficiency in improving the effectiveness of insecticides against mosquitoes. The mortality rates of the field strains of Ae. aegypti which were treated with different combinations of insecticides and synergists are shown in Fig. 2. In summary, synergists improved the efficiencies of the insecticides, but only Sabak Bernam and Sepang female Ae. aegypti strains exhibited 100% mortality following treatment with a combination of insecticides and synergists. The results showed that synergists increased the mortality rates of all strains of female Ae. aegypti against all the tested insecticides. However, most of the field female Ae. aegypti strains still showed resistance (i.e. < 90% mortality rate) against DDT and pyrethroids even when these have been used in combination with synergists.
Biochemical assays
All data were pooled and analyzed. Four strains of Ae. aegypti (Kuala Selangor, Kuala Langat, Hulu Selangor and Gombak) exhibited elevated levels of GST activity using Mann-Whitney U-test (Gombak U(273) = 579.00, Z = − 12.40, P < 0.0001; Hulu Selangor U(266) = 5677.50, Z = − 3.57, P < 0.0001; Kuala Langat U(268) = 2126.50, Z = − 9.67, P < 0.0001; Kuala Selangor U(272) = 1017.50, Z = − 11.61, P < 0.0001) when compared with the Bora-Bora strain as shown in Fig. 5. These four strains, along with the Hulu Langat strain, also exhibited a significant increase in MFO activity (Gombak U(372) = 3954.50, Z = − 12.94, P < 0.0001; Hulu Langat U(372) = 798.00, Z = − 15.95, P < 0.0001; Hulu Selangor U(372) = 472.00, Z = − 16.27, P < 0.0001; Kuala Langat U(369) = 268.50, Z = − 16.46, P < 0.0001; Kuala Selangor U(372) = 12548.00, Z = − 4.66, P < 0.0001). Although significantly increased activities of GST and MFO have been observed in some field strains of Ae. aegypti, the Spearmanʼs rank-order correlation test did not reveal any significant correlation with other insecticides or enzymes.
Mean (± SE) levels of insensitive acetylcholinesterase (AChE), glutathione-S-transferase (GST), non-specific esterase (α- and β-EST) and mono-oxygenase (MFO) activities of Ae. aegypti in Selangor. Asterisks indicate significantly higher values when compared with Bora-Bora Strain (P < 0.05, Mann-Whitney test)
Kdr screening
In the present study, a cheap, reliable, and rapid AS-PCR was used to detect the kdr mutation as it provided results after gel electrophoresis (Additional file 2: Figure S1). The genotype and allele frequencies (Fig. 6, Additional file 1: Tables S3–S5) were derived from 270 susceptible and 210 resistant randomly-selected Ae. aegypti with 30 Bora-Bora strain of Ae. aegypti. The results show that the frequency of Ae. aegypti possessing the homozygous F1534C mutation with heterozygous V1016G was significantly higher than the Ae. aegypti possessing the homozygous V1016G with heterozygous F1534C (χ2 = 113, df = 2, P < 0.001). On the other hand, frequency of single homozygous mutation of F1534C (χ2 = 116, df = 2, P < 0.001) and V1016G (χ2 = 100, df = 2, P < 0.001) were significantly higher compared to co-occurrence of homozygous V1016G and S989P.
V1016G, F1534C, and S989P point mutations in Aedes aegypti collected from Selangor. Abbreviations: VV, homozygous wild type of V1016G; VG, heterozygous of V1016G; GG, homozygous mutant of V1016G; FF, homozygous wild type of F1534C; FC, heterozygous of F1534C; CC, homozygous mutant of F1534C; SS, homozygous wild type of S989P; SP, heterozygous of S989P; PP, homozygous mutant of S989P
All resistant and susceptible mosquitoes had the three mutations genotyped for DDT and pyrethroids resistance/susceptibility (Table 2). The 1534C-mutated allele was significantly associated with DDT (r(8) = 0.711, P = 0.032), cyfluthrin (r(8) = 0.812, P = 0.008), deltamethrin (r(8) = 0.845, P = 0.004), etofenprox (r(8) = 0.742, P = 0.021) and lambdacyhalothrin (r(8) = 0.879, P = 0.002) resistance. The 1016G-mutated allele significantly correlated with cyfluthrin (r(8) = 0.783, P = 0.013), deltamethrin (r(8) = 0.833, P = 0.005), etofenprox (r(8) = 0.850, P = 0.004), lambdacyhalothrin (r(8) = 0.817, P = 0.007) and permethrin (r(8) = 0.717, P = 0.030) resistance. On the other hand, only permethrin resistance (r(8) = 0.700, P = 0.036) was associated with the S989P-mutated allele.
The results of the present study showed that triple and double homozygous mutations were detected in a single Ae. aegypti. Three samples had triple homozygous mutations (Gombak-02, Gombak-11, and Kuala Selangor-08) (Additional file 2: Figures S2–S4). Double homozygous mutations of V1016G and S989P were observed in the three strains: Hulu Selangor (Hulu Selangor-02 and Hulu Selangor-14); Petaling (Petaling-02 and Petaling-24); and Kuala Selangor (Kuala Selangor-07) (Additional file 2: Figures S2, S3). In order to further confirm the presence of triple and double homozygous mutations, the IIP-IIS6 and/or IIIS4-IIIS6 regions of all samples that exhibited these mutations were sequenced.
After examining the DNA sequence chromatograms, 16 of the 20 sample nucleotide sequences for the 1016 and 989 mutation point analyses (IIP-IIS6 region), and 17 out of 20 sequences for the F1534C mutation analysis (IIIS4-IIIS6 region) exhibited clear, singular peaks, indicating good quality sequencing and no contamination. No mutations were observed in the Bora-Bora-01, Bora-Bora-02, Klang-05, Klang-09 and Klang-18 sequences, thereby supporting the AS-PCR results. Sequencing further confirmed the results of AS-PCR, whereby Kuala Selangor-01, Kuala Selangor-15, and Gombak-22 were shown to have the F1534C mutation but not S989P and V1016G. Gombak-02, Gombak-11, Hulu Selangor-02, Hulu Selangor-14, Petaling-05, Kuala Selangor-07, Kuala Selangor-08 and Petaling-24 were all homozygous for the S989P and V1016G mutations (Additional file 2: Figures S2–S4). On the other hand, Hulu Selangor-01, Hulu Selangor-02, Hulu Selangor-14 and Petaling-02 exhibited heterozygous F1534F/C mutations, while Gombak-02, Gombak-22, Gombak-11, Kuala Selangor-01, Kuala Selangor-08, Kuala Selangor-14 and Kuala Selangor-15 exhibited homozygous F1534C mutation (Additional file 2: Figure S4).
Discussion
Aedes aegypti from Selangor showed various levels of resistance against organochlorine and pyrethroids. However, they exhibited susceptibility against malathion (organophosphates) and propoxur (carbamate). Organophosphates (especially malathion) have been the insecticide of choice during dengue epidemics, whereby control measures relied heavily on both thermal fogging and ULV to rapidly kill the infected Aedes mosquitoes [47]. However, all field Ae. aegypti were susceptible to the said chemical, with 100% mortality. In Malaysia, propoxur has never been used as an active ingredient in vector control programmes or public health activities. It is noteworthy that propoxur has been used as a household pest control product in the early 1970s, but its utilization was terminated in the 1990s [48]. Therefore, the resistance of Ae. aegypti against propoxur was low, presumably due to infrequent application of the same.
Among all the field strains, Klang and Sabak Bernam strains were most susceptible to all insecticides, with 24 h post-treatment mortalities of > 98%. Evidently, these areas have not been affected by dengue outbreaks in recent years. On the other hand, the remaining field strains of Ae. aegypti were resistant to DDT and pyrethroids. Pyrethroids are a major class of insecticides in the pest control industry and are widely used in dengue and malaria control programmes [49]. Although DDT has never been used for dengue control in Malaysia, it has been utilized from the late 1950s until the 1980s for malaria eradication [50]; its usage was stopped in 1998 [49]. Few studies have shown that the DDT-resistant phenotype was still present in Ae. aegypti (Malaysia) [51], Culex quinquefasciatus (Malaysia) [52], and Anopheles darlingi (Colombia) [53] even though DDT has no longer been used for decades. The reference strain of Culex quinquefasciatus exhibited resistance against DDT after being maintained in an insecticide-free insectary for 117 generations [52]. On the other hand, after 17 years of banning DDT application, An. darlingi in Colombia was still found to be resistant against DDT and also lambda-cyhalothrin [53]. Furthermore, DDT and pyrethroids share the same mode of action in which the voltage-gated sodium channels were targeted. Thus, the observed resistances may have been due to the extensive usage of pyrethroids in pest control and public health activities. Additionally, cross-resistances between DDT and pyrethroids [54] as well as within pyrethroids owing to the same target sites are well-known [55, 56] and these may be similarly observed in the present study. The results of this research showed that the emergence of insecticide resistance is likely to be associated with the frequency of dengue outbreaks owing to the excessive utilization of insecticides in control measures. Therefore, new strategies are urgently required to replace fogging and ULV during dengue outbreaks.
Biochemical assays demonstrated elevated levels of GSTs and MFO in some of the field strains of Ae. aegypti, but this was not the case for ESTs and AChE. These findings corroborated with the bioassay results, in which the field strains of Ae. aegypti were susceptible to malathion and propoxur but resistant to DDT and pyrethroids. Elevated GST levels are responsible for DDT resistance [57], and this was observed in the Gombak, Hulu Selangor, Kuala Langat and Kuala Selangor strains of Ae. aegypti. Since DDT and pyrethroids share the same target site (voltage-gated sodium channels), the observed elevation in GST level could have been due to resistance towards pyrethroids, as a result of the extensive usage of this class of insecticides in vector control programmes [57,58,59].
Nevertheless, the results have shown partial synergistic effects of DEF (the main inhibitor of esterases) in some of the field strains. This was probably attributable to its secondary GST-inhibitor ability [60, 61] since the biochemical assays have detected elevated GST activities in five of the field strains. Esterase (including AChE) activities are well known for conferring organophosphate and carbamate resistance in mosquitoes [48, 62]. However, the low frequency of ESTs and AChE activities in the present study showed that this mechanism was not involved. Furthermore, as per the bioassay results, the susceptibility statuses of all field strains to propoxur and malathion further supported this theory.
Pyrethroid resistance is often related to elevated MFOs levels [63, 64], as detected in Kuala Selangor, Kuala Langat, Hulu Selangor, Hulu Langat and Gombak strains of Ae. aegypti. Many studies have identified PBO as a MFOs inhibitor [57]. Similarly, this study has demonstrated that the addition of PBO to pyrethroids helped increase the mortality, thereby confirming the involvement of MFO in pyrethroid resistance.
Although the employment of synergists has significantly promoted the mortality of field Ae. aegypti as compared to the reference strain, many of the field strains still remained resistant (24 h post-treatment mortality < 90%). In addition, all field strains (except for Sabak Bernam and Klang) exhibited resistance to DDT and pyrethroids. This could also be due to cross-resistance between organochlorine and pyrethroids. Therefore, this further suggests the involvement of more than one mechanism giving rise to insecticide resistance. Additionally, a few studies have suggested that toxicological changes in arthropods were not directly correlated with enzymatic activities [65, 66]. Indeed, the evolution/mutation of multiple strains is not a new phenomenon and is becoming a serious issue worldwide. In Malaysia, evidence of pyrethroid resistance in Ae. aegypti has been reported [67, 68]. However, the mechanisms that conferred resistances toward these insecticides in the mosquitoes were poorly understood. Therefore, the present study has utilized AS-PCR to detect the involvement of target site insensitivity mechanism in DDT and pyrethroid resistance in Ae. aegypti.
This study to our knowledge is the first to describe the S989P mutation in Malaysian Ae. aegypti. The first report on F1534C and V1016G mutations in Ae. aegypti was in 2015 [19]. In the present study, low frequencies of F1534C (13.33%), V1016G (8.75%) and S989P (5.09%) mutations were found in the Selangor Ae. aegypti. Evidently, these mutations have also been documented in Thailand [39, 40], Singapore [29] and Myanmar [43]. The F1534C mutation was found to be significantly associated with DDT and pyrethroid resistance in the present study, in line with the outcomes of other researches [19, 43, 69]. However, other studies have only found F1534C mutation to be significantly associated with type I pyrethroid resistance [39, 40]. The contribution of F1534C to multiple-pyrethroid resistance was possibly due to the additive contribution of the V1016G mutation since the latter has been frequently reported to be responsible for pyrethroid resistance [22, 42, 70, 71] (especially type II pyrethroids) [38, 40]. Indeed, most of the pyrethroid-resistant mosquitoes with F1534C mutation also carry the V1016G mutation. It should be noted that mosquitoes with the V1016G mutation are thought to be protected from deltamethrin [40]. Statistical analyses have shown that S989P mutation is correlated with permethrin resistance only. It cannot be definitively concluded if this is so, as the effect of only S989P mutation on permethrin resistance was not directly investigated in this study. The S989P mutation can be often found co-occurring with the V1016G mutation and also F1534C mutation in the present study. Hirata et al. [70] demonstrated that S989P mutation does not affect permethrin sensitivity whereas other studies [38, 40] have been unable to provide direct evidence to justify the effect of the single S989P mutation in pyrethroid resistance. Therefore, the role of the S989P mutation in permethrin resistance needs additional confirmation as suggested by some researchers [72, 73].
This study found two co-occurrent point mutations, namely S989P/V1016G and F1534C/S989P/V1016G. However, no F1534C/V1016G mutation only was found, in congruence with the study by Ishak et al. [19] in Penang (Malaysia). The S989P mutation has been frequently linked to the V1016G mutation but sometimes, the V1016G mutation has been found in the absence of the S989P mutation [38, 40]. Both studies have reported that the co-occurrence of S989P/V1016G enhances the resistance towards deltamethrin. Similarly, Hirata et al. [70] have found that the combination of V1016G/S989P moderately reduced the sodium channel’s sensitivity to deltamethrin. Furthermore, these authors have also detected a gradual decline in the sensitivities to permethrin and deltamethrin when there was a co-occurrent F1534C/S989P/V1016G triple mutation [70] which points to the synergistic effect of the combination of mutant alleles. In addition, Plernsub et al. [74] have also reported that combinations of single kdr mutations led to a relatively higher level of resistance against pyrethroids. Interestingly, it has been found that the triple heterozygote (F1534C, V1016G and S989P) was resistant against deltamethrin and permethrin though exhibiting intermediate resistance compared to F1534C homozygote which has 2-fold lower resistance and S989P + G1016 homozygote which has 2-fold higher resistance. However, addition of PBO reduced their resistance by 2-fold, suggesting the partial role of oxidase enzymes in resistance [74]. In the present study, this triple heterozygous mutation was found distributed in the susceptible and resistant individuals, therefore, we agree that the oxidase may be contributing to pyrethroid resistance in the resistant triple heterozygotes. One of the limitations of this study was that genetic linkages of resistant trait among all nine study areas could not be established as sequencing was not performed on all the samples from all the nine study areas. Furthermore, there could be other point mutations on the kdr gene such as the G923V and D1794Y mutations, outside of the sequencing regions being studied here, that could have contributed to the variability observed [39].
In Southeast Asia, these three mutations have been reported in Ae. aegypti populations. However, this is the first report on triple homozygous mutations in Malaysia, even though only three samples (0.63%) were found to have the same. Similarly, studies in Myanmar [43] and Malaysia [19] have also detected a low occurrence of multiple homozygous mutations. Both studies have reported a higher resistance to pyrethroids when combinations of single kdr mutations were present. Moreover, an outdoor thermal fogging study, which employed a combination of deltamethrin, S-bioallethrin and PBO, has found that S989P/V1016G homozygous Ae. aegypti survived the spray. On the contrary, most of the F1534C homozygous Ae. aegypti were killed [71]. The efficiency of thermal fogging spray was most likely to be even less effective in natural situations. Hence, the present study highlights the significant impact of multiple homozygous mutations of Ae. aegypti on vector control programmes which utilize pyrethroid-based approaches. Notably, when this triple homozygous mutation occurs naturally in highly-resistant Ae. aegypti, it is timely to consider other methods for control. However, the current low occurrence of this triple homozygous mutation was most probably attributable to its low fitness as proposed by Stenhouse et al. [40] and Hirata et al. [70]. Yet, the possibility of compensatory mutations that restore fitness might enable this genotype to become more widespread, which will lead to the ineffectiveness of pyrethroids against this dengue vector. Owing to emergence of insecticide resistance in many dengue-prone countries, new strategies should be considered to prevent outbreaks [75]. It has been shown that asymptomatic persons are more infectious to Aedes mosquitoes [76], so the current control measures (which are instituted only after dengue cases have been reported), might perhaps be too late. Thus, early detection of dengue outbreaks, in addition to the prudent management and use of insecticides, is required to avoid an increase in dengue cases.
Conclusions
Generally, Ae. aegypti from dengue outbreak areas had higher resistance to insecticides than those from non-dengue outbreak areas. The results show that organophosphates and carbamates are still suitable for use in vector control programmes. When pyrethroids are the major class of insecticides in vector control programmes, the kdr mutations in Malaysian Ae. aegypti populations contributed significantly to pyrethroid resistance, while MFO and GST enzymes had a partial role. Therefore, the development of new insecticides with novel modes of action is required to replace the conventional ones. To ensure the success of vector control, new tools for countering resistance are required. Also, innovative strategies should be constructed to inhibit the spread and evolution of resistance. It should be noted that Ae. aegypti eggs can easily be spread from one location to another and thus, it is postulated that the occurrence of insecticide-resistant Ae. aegypti might also occur in non-dengue outbreak areas in the future.
Abbreviations
- AS-PCR:
-
Allele specific polymerase chain reaction
- MFO:
-
mixed function oxidase
- EST:
-
esterase
- GST:
-
glutathione S-transferase
- AcHE:
-
acetylcholinesterase
- ULV:
-
ultra-low volume
- CDC:
-
Centre for Diseases Control and Prevention
- Kdr :
-
knock-down resistance
- DDT:
-
dichlorodiphenyltrichloroethane
- GDP:
-
gross domestic product
- EA:
-
ethacrynic acid
- PBO:
-
piperonyl butoxide
- DEF:
-
S.S.S-tributyl phosphorotrithioate
- OD:
-
optical density
- CDNB:
-
1-chloro-2,4-dinitrobenzene
- TMBZ:
-
tetramethylbenzidine
- RR50 :
-
resistance ratio 50%
References
Murray NEA, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013;5:299–309.
Gubler DJ. Dengue, urbanization and globalization the unholy trinity of the 21st century. Trop Med Health. 2011;39:3–11.
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7.
Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6:e1760.
Achee NL, Gould F, Perkins TA, Reiner RC Jr, Morrison AC, Ritchie SA, et al. A critical assessment of vector control for dengue prevention. PLoS Negl Trop Dis. 2015;9:e0003655.
IDengue. Ministry of Health. Malaysia; 2018. http://idengue.remotesensing.gov.my/idengue. Accessed 23 Dec 2018.
Wallace H, Lim T, Rudnick A, Knudsen A, Cheong W, Chew V. Dengue hemorrhagic fever in Malaysia: the 1973 epidemic. Southeast Asian J Trop Med Public Health. 1980;11:1–13.
Chang MS, Christophel EM, Gopinath D, Abdur RM. Challenges and future perspective for dengue vector control in the Western Pacific Region. Western Pac Surveill Response. 2011;2:9–16.
Reiter P, Gubler DJ, Gubler D, Kuno G. Surveillance and control of urban dengue vectors. Wallinford: CAB International; 1997.
Perich M, Davila G, Turner A, Garcia A, Nelson M. Behavior of resting Aedes aegypti (Culicidae: Diptera) and its relation to ultra-low volume adulticide efficacy in Panama City, Panama. J Med Entomol. 2000;37:541–6.
Tanrang Y, Vythilingam I. Field trial to determine the efficacy of pyrethroid Fendona 10 SC@ application using ultra- low- volume for the eontrol of Aedes mosquitoes. Trop BioMed. 2004;21:57–65.
Reid MC, McKenzie FE. The contribution of agricultural insecticide use to increasing insecticide resistance in African malaria vectors. Malar J. 2016;15:107.
Cuervo-Parra JA, Cortes TR, Ramirez-Lepe M. Mosquito-borne diseases, pesticides used for mosquito control, and development of resistance to insecticides. In: Trdan S, editor. Insecticides resistance. Rijeka: InTechOpen; 2016. p. 111–34.
Ponlawat A, Scott JG, Harrington LC. Insecticide susceptibility of Aedes aegypti and Aedes albopictus across Thailand. J Med Entomol. 2005;42:821–5.
Jirakanjanakit N, Rongnoparut P, Saengtharatip S, Chareonviriyaphap T, Duchon S, Bellec C, et al. Insecticide susceptible/resistance status in Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Thailand during 2003–2005. J Eco Entomol. 2007;100:545–50.
Wan-Norafikah O, Nazni WA, Lee HL, Zainol-Ariffin P, Sofian-Azirun M. Permethrin resistance in Aedes aegypti (Linnaeus) collected from Kuala Lumpur, Malaysia. J Asia-Pac Entomol. 2010;13:175–82.
Koou SY, Chong CS, Vythilingam I, Lee CY, Ng LC. Insecticide resistance and its underlying mechanisms in field populations of Aedes aegypti adults (Diptera: Culicidae) in Singapore. Parasite Vectors. 2014;7:471.
Koou S-Y, Chong CS, Vythilingam I, Ng LC, Lee CY. Pyrethroid resistance in Aedes aegypti larvae (Diptera: Culicidae) from Singapore. J Med Entomol. 2014;51:170–81.
Ishak IH, Jaal Z, Ranson H, Wondji CS. Contrasting patterns of insecticide resistance and knockdown resistance (kdr) in the dengue vectors Aedes aegypti and Aedes albopictus from Malaysia. Parasite Vectors. 2015;8:181.
Huong VD, Thi Bach Ngoc N. Susceptibility of Aedes aegypti to insecticides in South Vietnam. Den Bull. 1999;23:85–8.
WHO. Guidelines on public health pesticide management policy. Geneva: World Health Organization; 2010.
Brengues C, Hawkes NJ, Chandre F, McCarroll L, Duchon S, Guillet P, et al. Pyrethroid and DDT cross-resistance in Aedes aegypti is correlated with novel mutations in the voltage-gated sodium channel gene. Med Vet Entomol. 2003;17:87–94.
WHO. Monitoring and managing insecticide resistance in Aedes mosquito populations: Interim guidance for entomologists. Geneva: World Health Organisation; 2016.
Serrano RM. Susceptibility status of Aedes aegypti to insecticides in Colombia. In: Perveen F, editor. Insecticides-pest engineering. Rijeka: InTechOpen; 2012. p. 164–200.
Nauen R. Insecticide resistance in disease vectors of public health importance. Pest Man Sci. 2007;63:628–33.
Lumjuan N, McCarroll L, Prapanthadara L-A, Hemingway J, Ranson H. Elevated activity of an Epsilon class glutathione transferase confers DDT resistance in the dengue vector, Aedes aegypti. Inst Biochem Mol Biol. 2005;35:861–71.
Dong K. Insect sodium channels and insecticide resistance. Invert Neurosci. 2018;7:17–30.
Hamid PH, Prastowo J, Widyasari A, Taubert A, Hermosilla C. Knockdown resistance (kdr) of the voltage-gated sodium channel gene of Aedes aegypti population in Denpasar, Bali, Indonesia. Parasite Vectors. 2017;10:283.
Pang S, Chiang L, Tan C, Vythilingam I, Lam-Phua S, Ng L. Low efficacy of delthamethrin-treated net against Singapore Aedes aegypti is associated with kdr-type resistance. Trop Biomed. 2015;32:140–50.
Kushwah RBS, Dykes CL, Kapoor N, Adak T, Singh OP. Pyrethroid-resistance and presence of two knockdown resistance (kdr) mutations, F1534C and a novel mutation T1520I, in Indian Aedes aegypti. PLoS Negl Trop Dis. 2015;9:e3332.
Loke SR, Tan AWA, Ahmad NW, Lee HL, Sofian-Azirun M. Insecticide susceptibility status of field-collected Aedes (Stegomyia) aegypti (L.) at a dengue endemic site in Shah Alam, Selangor, Malaysia. Southeast Asian J Trop Med Public Health. 2012;43:34–47.
Department of Statistics Malaysia Official Portal. 2018. https://www.dosm.gov.my/v1. Accessed 30 Sept 2018
WHO. Guidelines for dengue surveillance and mosquito control. 2nd ed. Geneva: World Health Organisation; 2003.
Brogdon W, Chan A. Guidelines for evaluating insecticide resistance in vectors using the CDC bottle bioassay/methods in Anopheles research. CDC Atlanta USA: CDC technical report 2010. https://www.cdc.gov/malaria/resources/pdf/fsp/ir_manual/ir_cdc_bioassay_en.pdf. Accessed 22 Feb 2015.
Hemingway J, Brogdon W. Techniques to detect insecticide resistance mechanisms. Geneva: World Health Organization; 1998.
Saelim V, Brogdon WG, Rojanapremsuk J, Suvannadabba S. Bottle and biochemical assays on temephos resistance in Aedes aegypti in Thailand. Southeast Asian J Trop Med Public Health. 2005;36:417.
Brogdon W, Janet C. Heme peroxidase activity measured in single mosquitoes identifies individuals expressing an elevated oxidase for insecticide resistance. J Am Mosq Control Assoc. 1997;13:233–7.
Srisawat R, Komalamisra N, Eshita Y, Zheng M, Ono K, Itoh TQ, et al. Point mutations in domain II of the voltage-gated sodium channel gene in deltamethrin-resistant Aedes aegypti (Diptera: Culicidae). App Entomol Zool. 2010;45:275–82.
Yanola J, Somboon P, Walton C, Nachaiwieng W, Somwang P, Prapanthadara L. High-throughput assays for detection of the F1534C mutation in the voltage-gated sodium channel gene in permethrin-resistant Aedes aegypti and the distribution of this mutation throughout Thailand. Trop Med Intl Health. 2011;16:501–9.
Stenhouse SA, Plernsub S, Yanola J, Lumjuan N, Dantrakool A, Choochote W, et al. Detection of the V1016G mutation in the voltage-gated sodium channel gene of Aedes aegypti (Diptera: Culicidae) by allele-specific PCR assay, and its distribution and effect on deltamethrin resistance in Thailand. Parasite Vectors. 2013;6:1.
Li C-X, Kaufman PE, Xue R-D, Zhao M-H, Wang G, Yan T, et al. Relationship between insecticide resistance and kdr mutations in the dengue vector Aedes aegypti in Southern China. Parasite Vectors. 2015;8:325.
Saingamsook J, Saeung A, Yanola J, Lumjuan N, Walton C, Somboon P. A multiplex PCR for detection of knockdown resistance mutations, V1016G and F1534C, in pyrethroid-resistant Aedes aegypti. Parasite Vectors. 2017;10:465.
Kawada H, Oo SZM, Thaung S, Kawashima E, Maung YNM, Thu HM, et al. Co-occurrence of point mutations in the voltage-gated sodium channel of pyrethroid-resistant Aedes aegypti populations in Myanmar. PLoS Negl Trop Dis. 2014;8:e3032.
Finney DJ. Probit analysis; a statistical treatment of the sigmoid response curve. Cambridge: Cambridge University Press; 1947.
Brown AWA. Insecticide resistance in arthropods. Geneva: World Health Organization; 1958.
Abbott W. A method of computing the effectiveness of an insecticide. J Am Mosq Control Assoc. 1987;3:302–3.
Lam W, Tham A. A field evaluation of the effectiveness of ULV application of malathion 96% technical grade and sumithion L-40S against Aedes aegypti (Linnaeus) and Aedes albopictus (Skuse) in Ipoh Municipality, Perak. Malaysia. Trop Biomed. 1988;5:81–8.
Low VL, Chen CD, Lee HL, Tan TK, Chen CF, Leong CS, et al. Enzymatic characterization of insecticide resistance mechanisms in field populations of Malaysian Culex quinquefasciatus Say (Diptera: Culicidae). PLoS ONE. 2013;8:e79928.
Yap H, Lee Y, Zairi J. Chemical control of mosquitoes. In: Ng F, Yong H, editors. Mosquitoes and mosquito-borne diseases: biology, surveillance, control, personal and public protection measures. Kuala Lumpur: Academy of Sciences Malaysia; 2000. p. 197–210.
Sandosham AA, Thomas V. Malariology: with special reference to Malaya. Singapore: NUS Press; 1983.
Nazni W, Selvi S, Lee H, Sadiyah I, Azahari H, Derric N, et al. Susceptibility status of transgenic Aedes aegypti (L.) against insecticides. Den Bull. 2009;33:124–9.
Low VL, Chen CD, Lee HL, Lim PE, Leong CS, Sofian-Azirun M. Current susceptibility status of Malaysian Culex quinquefasciatus (Diptera: Culicidae) against DDT, propoxur, malathion and permethrin. J Med Entomol. 2013;50:103–11.
Fonseca-González I, Quiñones ML, McAllister J, Brogdon WG. Mixed-function oxidases and esterases associated with cross-resistance between DDT and lambda-cyhalothrin in Anopheles darlingi Root 1926 populations from Colombia. Mem Ins Oswaldo Cruz. 2009;104:18–26.
Davies T, Field L, Usherwood P, Williamson M. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB life. 2007;59:151–62.
Bernard CB, Philogène BJ. Insecticide synergists: role, importance, and perspectives. J Toxicol Environ Health. 1993;38:199–223.
Fonseca-González I, Quiñones ML, Lenhart A, Brogdon WG. Insecticide resistance status of Aedes aegypti (L.) from Colombia. Pest Manag Sci. 2011;67:430–7.
Aïzoun N, Aïkpon R, Gnanguenon V, Azondekon R, Oke-Agbo F, Padonou GG, et al. Dynamics of insecticide resistance and effect of synergists piperonyl butoxide (PBO), SSS-tributylphosphorotrithioate (DEF) and ethacrynic acid (ETAA or EA) on permethrin, deltamethrin and dichlorodiphenyltrichloroethane (DDT) resistance in two Anopheles gambiae s.l. populations from southern Benin, West Africa. J Parasit Vector Biol. 2014;6:1–10.
Hemingway J, Hawkes NJ, McCarroll L, Ranson H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol. 2004;34:653–65.
Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Ann Rev Entomol. 2000;45:371–91.
Tsagkarakou A, Pasteur N, Cuany A, Chevillon C, Navajas M. Mechanisms of resistance to organophosphates in Tetranychus urticae (Acari: Tetranychidae) from Greece. Insect Biochem Mol Biol. 2002;32:417–24.
Vontas JG, Graham J, Hemingway J. Glutathione S-transferases as antioxidant defence agents confer pyrethroid resistance in Nilaparvata lugens. Biochem J. 2001;357:65–72.
Polson KA, Brogdon WG, Rawlins SC, Chadee DD. Characterization of insecticide resistance in Trinidadian strains of Aedes aegypti mosquitoes. Acta Trop. 2011;117:31–8.
Kasai S, Komagata O, Itokawa K, Shono T, Ng LC, Kobayashi M, et al. Mechanisms of pyrethroid resistance in the dengue mosquito vector, Aedes aegypti: target site insensitivity, penetration, and metabolism. PLoS Negl Trop Dis. 2014;8:e2948.
Smith LB, Kasai S, Scott JG. Pyrethroid resistance in Aedes aegypti and Aedes albopictus: important mosquito vectors of human diseases. Pest Biochem Physiol. 2016;133:1–12.
Scharf ME, Neal JJ, Bennett GW. Changes of insecticide resistance levels and detoxication enzymes following insecticide selection in the German cockroach, Blattella germanica (L.). Pest Biochem Physiol. 1997;59:67–80.
Gong YJ, Wang ZH, Shi BC, Kang ZJ, Zhu L, Jin GH, et al. Correlation between pesticide resistance and enzyme activity in the diamondback moth, Plutella xylostella. J Insect Sci. 2013;13:135.
Chin A, Chen C, Low V, Lee H, Azidah A, Lau K, et al. Comparative efficacy of commercial mosquito coils against Aedes aegypti (Diptera: Culicidae) in Malaysia: a nationwide report. J Eco Entomol. 2017;110:2247–51.
Amelia-Yap ZH, Chen CD, Sofian-Azirun M, Low VL. Pyrethroid resistance in the dengue vector Aedes aegypti in Southeast Asia: present situation and prospects for management. Parasite Vectors. 2018;11:332.
Harris AF, Rajatileka S, Ranson H. Pyrethroid resistance in Aedes aegypti from Grand Cayman. Am J Trop Med Hyg. 2010;83:277–84.
Hirata K, Komagata O, Itokawa K, Yamamoto A, Tomita T, Kasai S. A single crossing-over event in voltage-sensitive Na+ channel genes may cause critical failure of dengue mosquito control by insecticides. PLoS Negl Trop Dis. 2014;8:e3085.
Plernsub S, Saingamsook J, Yanola J, Lumjuan N, Tippawangkosol P, Walton C, et al. Temporal frequency of knockdown resistance mutations, F1534C and V1016G, in Aedes aegypti in Chiang Mai city, Thailand and the impact of the mutations on the efficiency of thermal fogging spray with pyrethroids. Acta Trop. 2016;162:125–32.
Du Y, Nomura Y, Satar G, Hu Z, Nauen R, He SY, et al. Molecular evidence for dual pyrethroid-receptor sites on a mosquito sodium channel. Proc Natl Acad Sci USA. 2013;110:11785–90.
Sayono S, Hidayati APN, Fahri S, Sumanto D, Dharmana E, Hadisaputro S, et al. Distribution of voltage-gated sodium channel (Nav) alleles among the Aedes aegypti populations in central Java Province and its association with resistance to pyrethroid insecticides. PLoS ONE. 2016;11:e0150577.
Plernsub S, Saingamsook J, Yanola J, Lumjuan N, Tippawangkosol P, Sukontason K, et al. Additive effect of knockdown resistance mutations, S989P, V1016G and F1534C, in a heterozygous genotype conferring pyrethroid resistance in Aedes aegypti in Thailand. Parasite Vectors. 2016;9:417.
Lau SM, Chua TH, Sulaiman W-Y, Joanne S, Lim YA-L, Sekaran SD, et al. A new paradigm for Aedes spp. surveillance using gravid ovipositing sticky trap and NS1 antigen test kit. Parasite Vectors. 2017;10:151.
Duong V, Lambrechts L, Paul RE, Ly S, Lay RS, Long KC, et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc Natl Acad Sci USA. 2015;112:14688–93.
Acknowledgements
We would like to thank Dr Lau Sai Meng who helped in the collection of some samples from Selangor and Dr Tan Wing for proof reading the manuscript.
Funding
This study was financially supported by the University of Malaya Student Grant IPP grant (PG004-2015A). It comprises part of the PhD thesis of the first author, University of Malaya, Kuala Lumpur.
Availability of data and materials
The datasets of the present study are available in the article and its additional files. The newly generated sequences were submitted to the GenBank database under the accession numbers MK00552-MK005584.
Authors’ contributions
CSL planned the experiments; conducted the study, analysed the results, drafted the manuscript. IV conceptualised the study, helped in the experiments, corrected the manuscript. JWKL helped with the molecular analysis, preparation of manuscript. MLW helped with some experiments, phylogenetic analysis, drafting of manuscript. WSWY conceptualised the study, collection and colonisation of Ae. aegypti, preparation of manuscript. YLL conceptualised the study, molecular input, preparation of manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This research was regulated by the Institutional Animal Care and Use Committee (IACUC no.: 20150407/PARA/R/MBK) with ethical approval obtained before the commencement of the study to use mice to feed mosquitoes.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1: Table S1.
Knockdown rate and mortality rate of adult female Ae. aegypti against various insecticides and synergists. Table S2. Knockdown times KT50 and KT99 of adult female Ae. aegypti to various insecticides. Table S3. Frequency of the F1534C mutation in the Ae. aegypti voltage-gated sodium channel gene within resistance and susceptible mosquitoes from nine different districts of Selangor determined using AS-PCR. Table S4. Frequency of the V1016G mutation in the Ae. aegypti voltage-gated sodium channel gene within resistance and susceptible mosquitoes from nine different districts of Selangor determined using AS-PCR. Table S5. Frequency of the S989P mutation in the Ae. aegypti voltage-gated sodium channel gene within resistance and susceptible mosquitoes from nine different districts of Selangor determined using AS-PCR.
Additional file 2: Figure S1.
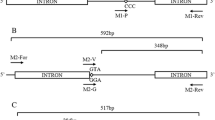
Gel electrophoresis of AS-PCR products corresponding to the Ae. aegypti sodium channel gene mutation. a F1534C mutation: each of the three genotypes is shown. Lane 1: ultra-low range DNA ladder; Lane 2: wild-type homozygous (FF); Lane 3: heterozygous (FC); Lane 4: mutant homozygous (CC); Lane 5: negative control. b V1016G mutation: Lane 1: ultra-low range DNA ladder marker; Lane 2: mutant homozygous (GG); Lane 3: heterozygous (VG); Lane 4: wild-type homozygous (VV); Lane 5: negative control. c S989P mutation: Lane 1: 100 bp DNA ladder marker; Lanes 2, 3: wild-type homozygous (SS); Lanes 4, 5: heterozygous (SP); Lanes 6, 7: mutant homozygous (PP); Lanes 8, 9: negative control. Figure S2. Genotype sequence of V1016G mutation. Figure S3. Genotype sequence of S989P mutation. Figure S4. Genotype sequence of F1534C mutation.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Leong, CS., Vythilingam, I., Liew, J.WK. et al. Enzymatic and molecular characterization of insecticide resistance mechanisms in field populations of Aedes aegypti from Selangor, Malaysia. Parasites Vectors 12, 236 (2019). https://doi.org/10.1186/s13071-019-3472-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-019-3472-1