Abstract
Introduction
Isolated bioactive components of plants or their raw extract are utilized as complementary or alternate remedy in copious illnesses. The current research was aimed at assessing the activity of aloin A isolated from Aloe barbadensis Miller and its formulated ointment against six (6) selected clinical isolates.
Methods
The column chromatography was utilized in isolating aloin A from chloroform/methanol solvent polarity. The characterization of the isolated compound was performed by spectroscopy techniques corresponding to UV, IR, 1H- and 13C-NMR spectroscopy. It was formulated as ointment using polyethylene glycol (PEG) and both the ointment and the isolated compound were probed for in vitro antimicrobial activity.
Results
Aloin A has been isolated from chloroform/methanol solvent mixture. The structure has been explicated as (10S)-10-β-d-glucopyranosyl-1,8-dihydroxy-3-(hydroxymethyl)-9(10H)-anthracenone(1S)-1,5-anhydro-1-[(9S)-4,5-dihydroxy-2-(hydroxymethyl)-10-oxo-9,10-dihydro-9-anthracenyl]-d-glucitol. The minimum inhibitory concentration (MIC) of the isolated aloin A on the pathogens ranged from 2.5 to 5.0 mg/ml and 0.32 to 5.0 mg/ml for both aloin A and the formulated ointment respectively. It was further revealed that the activity of aloin A showed dose dependence against all the test microorganisms. There was no significant difference in the activity of the drug against K. pneumoniae, S. aureus, E. coli, C. albicans and T. flavus (P > 0.05) when the concentration was raised from 2.5 to 5 mg/ml, however, there was significant difference (P ˂ 0.05) in activity against P. aeruginosa. The formulated ointment exhibited dose dependent activity against all test microorganisms. At low concentrations, the ointment showed no significant difference in diameter zone of inhibition against all test microorganisms (P > 0.05) except P. aeruginosa which exhibited a highly significant difference (P < 0.05).
Conclusion
Both the isolated aloin A and its formulated ointment demonstrated substantial inhibition of growth of the pathogenic strains. These findings sturdily suggest that aloin A is a nascent drug that could be explored as skin and wound transmittable agent.
Similar content being viewed by others
Introduction
Currently, one significant problem in human health is the less efficiency of commercial antibiotics against several pathogenic bacterial and fungal isolates. Specifically emphasized in literature among few others is Staphylococcus aureus, a gram-positive bacterium from Staphylococcaceae family, and considered one of the world’s most important infectious agents triggering disease spates linked to food consumption, poorly treated wounds, and hospital-associated infections [1, 2]. S. aureus is frequently described as being responsible for a variety of human and animal diseases and its epidemiological significance is mainly due to their ability of becoming highly resistant to common antimicrobials [3,4,5] and to a lesser degree to some of the major types of antibiotics [6, 7]. In the last decades, evolution of resistance, for example, to methicillin, has become an enormous problem for treatment of S. aureus infections. Candida albicans is a member of the normal human microbiome. However, it is an opportunistic fungal pathogen associated with infections such as thrush, vaginal yeast infections and diaper rash [8]. One of the commonest causes of vaginitis among middle age women is attributed to candida infection. The predisposing factors include drug addiction, obesity, intake of birth control pills, pregnancy, antibiotic therapy, hormone therapy and diabetes mellitus. The mortality rate of candida infections is reported to be 40% [9, 10].
Thus, scientists have increased research programs to develop new and more effective antimicrobial molecules and several plants have been used in diverse ways to extract potential antimicrobial compounds. Different authors have shown that plants have naturally bioactive compounds that could act alone or in synergy with antibiotics against bacterial isolates [11, 12]. Throughout the world today, extensive research work are on-going regarding therapeutic applications of herbal plants which are of unlimited abundance and are believe to improve quality of life. One of the medicinal plants which is unique in terms of geographic distribution, species abundance and chemical composition is the aloe plant [13].
The aloe plant is known to have several species of which Aloe barbadensis Miller (Aloe vera L.) and Aloe arborescens are grown commercially [14]. Traditionally, Aloe vera gel is used for both external and internal applications. Topically, it has been used for treatment of wounds, minor burns and skin irritations and internally for constipation, coughs, ulcers, diabetes, headaches, arthritis and immune-system deficiencies [15]. Thus, we set this study in which we isolated and characterized aloin A and investigated its antimicrobial potential against different isolates of important pathogens highly associated with outbreak of diseases and antibiotic resistance phenomena.
Methods
Collection of plant material
Plant material, Aloe barbadensis Miller leaves, were purchased from Parks and Gardens in Kumasi in the Ashanti Region, Ghana. Officials followed the prescribed legislation and guidelines in collection and sale of the plant material. It was subsequently identified and authenticated by a plant taxonomist at the herbarium of Ghana Herbaria, Northern Savanna Biodiversity:Savanna Herbarium. The voucher specimen (number: SH 722) was deposited in the herbarium.
Preparation of plant crude extracts
Aloe barbadensis Miller leaves were washed with distilled water and dried under sunshade for 4 weeks to ensure the leaves were devoid of moisture. The dried leaves were then pounded with the aid of mortar and pestle to obtain uniform powder, which was stored in an air-tight clean container. Powdered leaf sample (1.5 kg) was macerated in 5 l of absolute ethanol for 72 h at room temperature. The mixture was periodically shaken to enhance the extraction of the bioactive phytochemicals. The mixture was then filtered and the solvent was removed using rotary evaporator at temperature of 40 °C, to give a dark gummy residue. The yield was calculated to be 155.6 g (10.3% w/w).
Isolation of aloin A by column chromatography
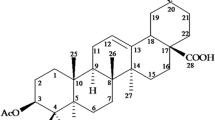
Applying the method described by Coopoosamy and Magwa [16], extract (52 g) was partitioned between n-hexane and water. The n-hexane portion (5.5 g) was further fractionated by column chromatography over silica gel H (60–120 μm mesh size) using solvents of increasing polarity (0–100% EtOAc in n-hexane) and a total of 44 fractions were collected. The fraction obtained with 45–50% EtOAc in n-hexane was subsequently subjected to gel filtration (Sephadex LH-20), eluted with CHCl3 followed by MeOH:CHCl3 (5:95), producing a total of 30 fractions. Fractions 9–18, totaling 0.25 g, were combined and subjected to column chromatography over silica gel H, using solvent system CHCl3:MeOH (0–30%). A total of 22 fractions (20 ml each) were collected. Fractions 22–27 (pinkish color) were eluted with CHCl3:MeOH (90:10, v/v) to give a total of 30 fractions. Fractions 9–18 were combined (0.25 g) and further fractionated by column chromatography over silica gel H using CHCl3:MeOH (90:10 v/v). Major compound with minor impurity co-eluted at 90% chloroform as a yellow-powder and was separated using preparative TLC over silica gel F254 60 coated on glass plates 20 cm × 20 cm (solvent system: MeOH:CHCl3, 0.5:9.5) to afford the compound aloin A (Fig. 1), a total amount of 39.6 mg.
Aloin A was reconstituted in dimethylsulphoxide (DMSO) to obtain the desired concentrations of 20 mg/ml, 10 mg/ml, 2.5 mg/ml, 1.25 mg/ml, 0.63 mg/ml, 0.32 mg/ml, 0.16 mg/ml, 0.08 mg/ml and 0.04 mg/ml. Aliquots were stored at − 80 °C until needed.
The identity of the isolated aloin A was confirmed based on the following: The UV and IR spectra were recorded on Beckman DU-7400 and Perkin Elmer FT-IR spectrometers respectively. 1H and 13C (500 MHz) NMR spectra were recorded on a Bruker AMX 500 instrument with chemical shift data reported in parts per million (ppm) relative to the solvent used with field gradient BBI (inverse) probe.
Formulation of aloin A-polyethylene glycol (PEG) ointment
Using a method described by Donkor et al. [17], polyethylene glycol 4000 (PEG 4000) and Polyethylene glycol 400 (PEG 400), 30 g each, was weighed into a beaker and melted on a thermostatic water bath at 45 °C until liquefied. It was then stirred with a glass rod under tap water at room temperature until congealed. Aloin A isolated from Aloe barbadensis Miller leaf extract was formulated with PEG ointment. The following quantities 20, 10, 5, 2.5, and 1.25 mg of aloin A were weighed into cleaned labeled separate beakers and 1.0 g of the PEG ointment was then added to each beaker and warmed at 70 °C while stirring continuously with a sterile glass rod for about 30 min. The mixtures were allowed to cool at room temperature to produce an aloin A‒PEG ointment at varying concentrations of 20 mg g−1, 10 mg g−1, 5 mg g−1, 2.5 mg g−1, 1.25 mg g−1.
Test microorganisms
Clinical isolates of P. aeruginosa, E. coli, K. pneumoniae, S. aureus, C. albicans and T. flavus were used for the current study. They were obtained from the Microbiology Department of the Tamale Teaching Hospital, Northern Region, Ghana. Bacteria were maintained at temperatures between 2 and 8 °C on nutrient broth, and fungi at 4 °C on potato dextrose agar.
Agar well diffusion assay
The method described by [18, 19] was used for the agar well diffusion assay.
Test for antifungal activity
The method described by Suurbaar et al. [19] was applied to explore the antifungal activity of aloin A. The fungal spores were washed from the surface of agar plates with sterile 0.85% saline containing 0.1% Tween 80 (v/v). The spore suspension was adjusted with sterile saline to a concentration of approximately 1.0 × 107 cfu/ml. The inocula were stored at 4 °C for further use. Dilutions of the inocula were cultured on solid potato dextrose agar to verify the absence of contamination and to check the validity of the inoculum.
Inoculum preparation for minimum inhibitory concentration (MIC) and minimum bactericidal concentrations (MBC)
Using the method described by Suurbaar et al. [19], inoculum for the MIC and MBC test was prepared. At least three to five well isolated colonies of the same morphology from agar plate culture were taken. Using a sterile loop, the top of each colony was touched and the loop was transferred into a tube containing 5 ml of normal saline and then vortexed. The broth culture was incubated at 37 °C and monitored for approximately 4 h until it achieved the turbidity of 0.5 McFarland standard (1.5 × 108 cfu).
Determination of minimum bactericidal concentration (MBC) and minimum inhibitory concentration (MIC)
The method described by [18, 19] was employed for the determination of MBCs and MICs.
Determination of minimum fungicidal concentration (MFC)
The method described by Donkor et al. [18] was used to determine the minimum fungicidal concentrations (MFCs). A commercial standard, Fluconazole (Sigma), was used as positive control (1–3000 μg/ml) and DMSO (99.9%) as negative control. All experiments were performed in triplicate and repeated two times for reproducibility.
Statistical analysis
Means and standard deviation were calculated for the zones of inhibition measured for the two sets of experiments in each case. These means were statistically compared using One-way ANOVA followed by Tukey Multiple Comparison Test to determine if they were significantly different at P < 0.05.
Results and discussion
Isolation and identification of Aloin A
Aloe barbadensis Miller is native to Ghana and has been used by the indigenous people as a basis of therapy for several illnesses. There has been successful accomplishment of isolation and purification of many compounds from aloe species [20, 21]. The compound, aloin A (1,8-dihydroxy-3-hydroxymethyl-9-antracenone) (Fig. 1), was isolated from n-hexane/water branch extract of Aloe barbadensis leaf through chromatograph processes using different solvent systems with yield of 27%. Coopoosamy and Magwa [16] reported 30% yield of aloin A isolated from Aloe excelsa, using CHCl3:MeOH (90:10 v/v). Renuka et al. [22] used HPLC method and reported 37.2% yield of Aloin content with DimamonsilC-18 (250 mm × 4.6 mm 5 µl) column, methanol–water–acetic acid (42:58:0.5) mobile phase. Minale et al. [23] also reported 28.4% yield of Aloin A isolated from the leaf latex of Aloe sinana Reynolds using a mixture of chloroform and methanol (4:1). The varying yields could be attributed to the different species of the plant, the different portions of the leaf used, different solvent polarity and the method employed.
The spectral data (IR, 1H and 13C NMR chemical shift assignments) (Table 1) were consistent with the data reported by Coopoosamy and Magwa [16] who had previously isolated the compound from Aloe excelsa. Although it is not a novel compound, its formulation with polyethylene glycol (PEG) as an ointment against wound pathogenic microorganisms has been accomplished in this work for the first time.
Antimicrobial studies of aloin A
In this research, the leaves were used because traditionally they have been used to treat wound and skin related diseases in Ghana. Antimicrobial activity of aloin A against the six (6) selected clinical isolates exhibited low MIC values (Table 2) revealing that the compound was effective against the entire microorganisms used in this research. All the test microorganisms showed high sensitivity towards the compound with maximum activity recorded against P. aeruginosa with zone of inhibition of 15.9 ± 0.2 mm at a concentration of 20 mg/ml, and low activity recorded for E. coli, K. pneumoniae, S. aureus, C. albicans and T. flavus with zones of inhibition 14.0 ± 1.4, 13.5 ± 0.7, 13.5 ± 0.7, 12.5 ± 0.3 mm and 12.3 ± 0.0 respectively (Fig. 2; Additional file 1). The observed results further revealed that the activity of aloin A showed dose dependence against all the test microorganisms. There was no significant difference in the activity of the drug against K. pneumoniae, S. aureus, E. coli, C. albicans and T. flavus (P > 0.05) when the concentration was raised from 2.5 to 5 mg/ml, however, there was significant difference (P ˂ 0.05) in activity against P. aeruginosa. Moreover, at 20 mg/ml, the drug showed significant higher activity against the two fungi compared with fluconazole, the positive control (Fig. 2; Additional file 1). However, the positive control (chloramphenicol) for the bacteria used in this research showed significant higher activity compared with aloin A but the negative control (DMSO—99.99%) showed no inhibitory activity. Minale et al. [23] recounted aloin A isolated from A. sinana exhibiting strong activity against the different strains of E. coli, S. typhi Ty2, Shigella, S. aureus and V. cholera and was found to be comparable to ciprofloxacin. They further stated the isolated compound showed broad spectrum antibacterial activity against both Gram-positive and Gram-negative bacteria. As stated by Asamenew et al. [24], aloin A which was isolated from Aloe harlana Reynolds also showed activity against E. coli, S. typhi Ty2, Shigella and V. cholera bacterial strains. B. pumilus and B. subtilis were found to be the most resistant bacterial strains to aloinoside—a precursor of aloin A—whereas aloin showed weak activity against these bacterial strains with MIC value of 200 µg/ml. Additionally, E. coli that causes a spectrum of disease ranging from diarrhea to the life threatening disease, hemolytic uremic syndrome, was also the most inhibited bacterial pathogen by aloinoside with MIC value of 10 µg/ml.
There are contrasting reports in scientific literature concerning the toxicity of aloin. It is reported that aloin exhibited antitumor activity against experimental murine tumors with no harmful side effects on the host metabolism [25]. In another research, Celestino et al. [26] indicated that A. ferox resin, found to contain 33.5% anthranoid glycosides derivates expressed as aloin, was nontoxic when its laxative activity was investigated in vivo. On the contrary, animal studies have indicated aloin to have induced dose-related increased incidences and severities of mucosal and goblet cell hyperplasia in male rats [27]. Aloin is also suggested to exhibit antibacterial property for certain intestinal commensal bacteria and decreases butyrate-production in a dose-dependent manner [28]. These contradictory results could be attributed to the route of administration; the method of production which may result in the inclusion of other products, thus affecting the percentage purity of aloin; as well as in vitro or in vivo studies [29].
Antimicrobial studies of formulated Aloin A-PEG ointment
We sought to test activity of aloin A–PEG ointment against the selected microorganisms using agar well diffusion method. Polyethylene glycol (PEG) is a biodegradable synthetic polymer of ethylene oxide units. PEG is reported to be highly biocompatible and non-immunogenic [30]. Its applications focus in majority on drug delivery and targeted diagnostics. PEG shows outstanding toxicological safety vis-à-vis acute and chronic oral toxicity, embryotoxicity or skin compatibility [31]. PEGs have been used for many years in cosmetics, foodstuffs [32] and the pharmaceutical industries. Extra significant assets of PEG is the solvent power for numerous substances that are sparingly soluble in water [33].
The antimicrobial effect of formulated aloin A-PEG ointment showed substantial activity against all the microorganisms with average zone of inhibition of 16 mm at 20 mg/g (Fig. 3; Additional file 2). The formulated ointment exhibited dose dependent activity against all test microorganisms. When the concentration of ointment was raised from 2.5 to 5 mg/g, there was no significant difference in diameter zone of inhibition against all test microorganisms (P > 0.05) except P. aeruginosa which exhibited significant difference (P < 0.05). However, at higher concentration there was highly significant difference (P < 0.05) in diameter zones of inhibition of all test microorganisms. Similar trends were recorded as the concentration of the ointment was increased up to 20 mg/g (P < 0.05). Similarly, the activity of aloin A alone, and its formulated ointment recorded significant activity against both fungi (C. albicans and T. flavus) than the positive control (fluconazole) used in this current research. However, chloramphenicol, positive control for the bacteria employed for this research expressed very high activity against P. aeruginosa, E. coli, K. pneumoniae and S. aureus than the formulated ointment (Fig. 3; Additional file 2).
The extensively spread of resistance to antimicrobial drugs is promoting revival in pursuit of new drug candidates for the treatment of diseases triggered by bacteria and fungi. Consequently, the in vitro activity of both aloin A and the aloin A–PEG ointment on the above disease causing bacterial and fungal isolates is highly substantial. Nonetheless, in vivo antimicrobial and toxicity studies need to be conducted before the products could be used in combatting bacterial and fungal infection.
Conclusion
We have successfully isolated aloin A from the leaves of Aloe barbadensis Miller, characterized using 1H- and 13C-NMR as well as IR spectroscopy. We further tested the isolated compound alone and in combination with polyethylene glycol against some selected microorganisms such as P. aeruginosa, E. coli, K. pneumoniae, S. aureus, C. albicans and T. flavus. This research gives a scientific validation to the fact that aloin A, one of the bioactive components in Aloe barbadensis was extracted substantially in hexane/water/ethyl acetate mixture and exhibited highly promising bacterial and fungal inhibitory activity.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Additional files.
Abbreviations
- A. vera :
-
Aloe vera
- K. pneumoniae :
-
Klebsiella pneumoniae
- E. coli :
-
Escherichia coli
- P. aeruginosa :
-
Pseudomonas aeruginosa
- S. aureus :
-
Staphylococcus aureus
- C. albicans :
-
Candida albicans
- T. flavus :
-
Talaromyces flavus
- MIC:
-
minimum inhibitory contration
- MBC:
-
minimum bactericidal concentration
- MFC:
-
minimum fungicidal concentration
- SD:
-
standard deviation
References
Fetsch A, Contzen M, Hartelt K, Kleiser A, Maassen S, Rau J et al (2014) Staphylococcus aureus food-poisoning outbreak associated with the consumption of ice-cream. Int J Food Microbiol 187:1–6
Tong SY, Chen LF, Fowler VG (2012) Colonization, pathogenicity, host susceptibility and therapeutics for Staphylococcus aureus: what is the clinical relevance? Semin Immunopathol 34(2):185–200
Jamali H, Paydar M, Radmehr B, Ismail S, Dadrasnia A (2015) Prevalence and antimicrobial resistance of Staphylococcus aureus isolated from raw milk and dairy products. Food Control 54:383–388
Gao J, Ferreri M, Yu F, Liu X, Chen L, Su J et al (2012) Molecular types and antibiotic resistance of Staphylococcus aureus isolates from bovine mastitis in a single herd in China. Vet J 192(3):550–552
Aires T, Serrão EA, Engelen AH (2016) Host and environmental specificity in bacterial communities associated to two highly invasive marine species (genus Asparagopsis). Front Microbiol 7:559
Mandal SM, Ghosh AK, Pati BR (2015) Dissemination of antibiotic resistance in methicillin-resistant Staphylococcus aureus and vancomycin-resistant S. aureus strains isolated from hospital effluents. Am J Infect Control 43(12):e87–e88
Dhand A, Sakoulas G (2014) Daptomycin in combination with other antibiotics for the treatment of complicated methicillin-resistant Staphylococcus aureus bacteremia. Clin Ther 36(10):1303–1316
Nobile CJ, Jonhson AD (2015) Candida albicans biofilms and human disease. Annu Rev Microbiol 69:71–92
David OM, Jide AA (2017) Anti-candidal activities of leaf extracts of Sansevieria aethiopica (Thunb): a medicinal plant used in the treatment of oral Candidiasis in Eastern Cape of South Africa. J Pharm Microbiol. 3(1):3
Mayer FL, Wilson D, Hube B (2013) Candida albicans pathogenicity mechanisms. Virulence 4(2):119–128
Bellio P, Segatore B, Mancini A, Di Pietro L, Bottoni C, Sabatini A et al (2015) Interaction between lichen secondary metabolites and antibiotics against clinical isolates methicillin-resistant Staphylococcus aureus strains. Phytomedicine 22(2):223–230
Chusri S, Siriyong T, Na-Phatthalung P, Voravuthikunchai SP (2014) Synergistic effects of ethnomedicinal plants of Apocynaceae family and antibiotics against clinical isolates of Acinetobacter baumannii. Asian Pac J Trop Med 7(6):456–461
Atherton P (1998) Aloe vera: magic or medicine? Nurs Stand 12(41):49–54
Kokate C (2005) Pharmacognosy, 13th edn. Nirali Prakashan, Pune
Eshun K, He Q (2004) Aloe vera: a valuable ingredient for the food, pharmaceutical and cosmetic industries—a review. Crit Rev Food Sci Nutr 44(2):91–96
Coopoosamy RM, Magwa ML (2006) Antibacterial activity of aloe emodin and aloin A isolated from Aloe excelsa. Afr J Biotechnol 5(11):1092–1094
Donkor A-M, Glover RLK, Boateng JK, Gakpo VY (2012) Antibacterial activity of the fruit extract of Physalis angulata and its formulation. J Med Biomed Sci 1(4):21–26
Donkor A-M, Mosobil R, Suurbaar J (2016) In vitro bacteriostatic and bactericidal activities of Senna alata, Ricinus communis and Lannea barteri extracts against wound and skin disease causing bacteria. J Anal Pharm Res 3(1):00046. https://doi.org/10.15406/japlr.2016.03.00046
Suurbaar J, Mosobil R, Donkor A-M (2017) Antibacterial and antifungal activities and phytochemical profile of leaf extract from different extractants of Ricinus communis against selected pathogens. BMC Res Notes 10(1):660
Choche T, Shende S, Kadu P (2014) Extraction and identification of bioactive components from Aloe barbadensis Miller. Res Rev J Pharmacogn Phytochem 2:14–23
Lawrence R, Tripathi P, Jeyakumar E (2009) Isolation, purification and evaluation of antibacterial agents from Aloe vera. Braz J Microbiol 40(4):906–915
Renuka R, Nancy D, Ranandkumar S, Reyaz A, Sathya K, Indra AP (2012) Assessment of antibiotic, antioxidant activity and aloin content in Aloe Barbadensis Miller gel extract. J Pharm Res 5(6):2481–2484
Minale G, Bisrat D, Asres K, Mazumder A (2014) In vitro antimicrobial activities of anthrones from the leaf latex of Aloe sinana Reynolds. Int J Green Pharm 8(1):7–12
Asamenew G, Bisrat D, Mazumder A, Asres K (2011) In vitro antimicrobial and antioxidant activities of anthrone and chromone from the latex of Aloe harlana Reynolds. Phytother Res 25(12):1756–1760
Esmat A, Said M, Khalil S (2015) Aloin: a natural antitumor anthraquinone glycoside with iron chelating and non-atherogenic activities. Pharm Biol 53(1):138–146
Celestino V, Maranhão H, Vasconcelos C, Lima C, Medeiros G, Araújo A et al (2013) Acute toxicity and laxative activity of Aloe ferox resin. Rev Bras Farmacogn 23(2):279–283
Boudreau M, Olson G, Tryndyak V, Bryant M, Felton R, Beland F (2017) Aloin, a component of the Aloe vera plant leaf, induces pathological changes and modulates the composition of microbiota in the large intestines of F344/N male rats. Toxicol Sci 158(2):302–318
Gokulan K, Kolluru P, Cerniglia C, Khare S (2019) Dose-dependent effects of Aloin on the intestinal bacterial community structure, short chain fatty acids metabolism and intestinal epithelial cell permeability. Front Microbiol. https://doi.org/10.3389/fmicb.2019.00474
Williams L, Burdock G, Shin E, Kim S, Jo T, Jones K et al (2010) Safety studies conducted on a proprietary high-purity aloe vera inner leaf fillet preparation, Qmatrix®. Regul Toxicol Pharmacol 57(1):90–98
Zhu J (2010) Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 31(17):4639–4656
Hermansky S, Neptun D, Loughran K, Leung H (1995) Effects of polyethylene glycol 400 (PEG 400) following 13 weeks of gavage treatment in Fischer-344 rats. Food Chem Toxicol 33(2):139–149
World Health Organisation (1980) Polyethylene glycol. In: Evaluation of certain food additives: twenty-third report of the Joint FAO/WHO Expert Committee on Food Additives, Geneva, Technical Report Series, vol 648. pp 17–18
Özdemir C, Güner A (2007) Solubility profiles of poly (ethylene glycol)/solvent systems, I: qualitative comparison of solubility parameter approaches. Eur Polym J 43(7):3068–3093
Acknowledgements
The authors would like to acknowledge the support of the technical staff of the Microbiology Department, Navrongo Health Research Centre (NHRC) and the Department of Applied Biology, University for Development Studies. We would also like to thank the staff of NHRC for administrative assistance.
Funding
This study was supported in part by the Faculty of Applied Sciences, University for Development Studies.
Author information
Authors and Affiliations
Contributions
AMD, NK conceived and designed the study; NK collected the plant samples and performed the experiments; MND performed statistical/data analysis; AMD, MND, NK wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Antimicrobial activity of aloin A (mm). The data represent diameter of inhibition of the compound against P. aeruginosa, E. coli, K. pneumoniae, S. aureus, C. albicans and T. flavus. The average of three wells treated on the same day was recorded. The experiment was repeated twice and day-to-day variation was found to be within one fold of the presented data.
Additional file 2.
Antimicrobial activity of the formulated aloin A ointment (mm). The data represent diameter of inhibition of the formulated ointment against P. aeruginosa, E. coli, K. pneumonia, S. aureus, C. albicans and T. flavus. The average of three wells treated on the same day was recorded. The experiment was repeated twice and day-to-day variation was found to be within one fold of the presented data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Donkor, AM., Donkor, M.N. & Kuubabongnaa, N. Evaluation of anti-infective potencies of formulated aloin A ointment and aloin A isolated from Aloe barbadensis Miller. BMC Chemistry 14, 8 (2020). https://doi.org/10.1186/s13065-020-0659-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-020-0659-7