Abstract
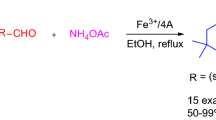
A facile and convenient method for the synthesis of acridines and its derivatives was developed through one-pot, three-component condensation reaction of aromatic aldehydes, 5,5-dimethyl-1,3-cyclohexanedione, aryl amines or ammonium acetates in the presence of a catalytic amount of cobalt–alanine metal complex using aqueous ethanol as a reaction medium is reported. The present described novel methodology offers several advantages over the traditional methods reported in the literature, such as mild reaction conditions, inexpensive catalyst, short reaction times, excellent yields of products, simplicity and easy workup are the advantages of this procedure.
Similar content being viewed by others
Introduction
Multi-component reactions (MCRs) have been paid much attention by synthetic organic chemists worldwide due to the effectiveness of multiple component reactions at building functionalized, novel drug discovery procedures and allow the fast, automated and high-throughput generation of organic compounds [1]. Thus we believe that discovering and developing new and novel bond formation of C–N, C–O and C–S bonds by MCRs is usual in numerous heterocyclic compounds is an important pursuit in pharmaceutical, biological and material science [2]. Consequently, the development of new multicomponent reactions towards biomedical and industrial scaffolds is inevitable at the present time. Therefore, in the last decade research in academia and industry has increasingly emphasized on the use of MCRs [3,4,5,6,7,8,9,10,11].
Acridine-1,8-dione and acridine derivatives are well known polyfunctionalized 1,4-dihydropyridines (DHPs) [12]. These derivatives of DHPs have an important ring skeleton and reported wide range of pharmaceutical and biological properties, including antitumor [13], antitubercular [14], antimalaria [15], antibacterial [16], antihypertensive [17], fungicidal [18], anticancer [19], anti-inflammatory [20] and diabetes [21]. These derivatives are also commercially used as calcium channel blockers [22,23,24]. Further, these compounds are also useful for the treatment of angina pectoris [25], hypertension [26,27,28] and Alzheimer’ disease [29]. Few of these compounds are used as effective drug for the treatment of congestive heart failure [30]. 1,4-Dihydropyridine derivatives are used in numerous bioactive compounds [31,32,33]. 1,8-Dioxo-decahydroacridines derivatives are using as dyes [34,35,36] and also as photoinitiators [37]. Furthermore, these derivatives have the applications in material science like semiconductors [38] and in spectroscopy as luminescent agent [39].
Due to the broad utility of acridines, there has been intense demand for the development of new and efficient synthetic protocols for the preparation of these important class of molecules have attracted a large number of organic chemists. Recently, many methods have reported in the literature for the synthesis of acridine derivatives containing 1,4-dihydropyridines, involves the three-component cyclocondensation reaction of 5,5-dimethyl-1,3-cyclohexanedione (dimedone), aromatic aldehydes and various aniline or ammonium acetate in the presence of a several catalysts such as Amberlyst-15 [40], sulfonic acid functionalized silica (SBSSA) [41], ammonium chloride, Proline [12], Zn(OAc)2·H2O or l-proline [42], triethylbenzylammoniumchloride(TEBAC) [43], ZnO nanoparticles [44], nano-Fe3O4 [45], CeCl3·7H2O [46], silica-bonded N-propyl sulfamic acid (SBNPSA) [47]. ionic liquids [48, 49] such as 1-methyl imidazolium trifluoroacetate ([Hmim]TFA) [50] and Bronsted acidic imidazolium salts containing perfluoroalkyl tails [51], microwave irradiation [52, 53], p-dodecylbenzenesulfonicacid (DBSA) [54] and PMA-SiO2 [55].
However, some of these reported methods for the synthesis of 1,8-dioxodecahydroacridine have limitations such as low yields, unpleasant experimental procedure, reagents are expensive or the use of an excess of catalyst, generation of polluting effluents and prolonged reaction times. Therefore, there is scope for further innovation of methods with milder reaction conditions, short reaction times, increase in variation of the substituents in the components and better yields for the synthesis of 1,8-dioxodecahydroacridine, the discovery of new methodologies using new and efficient catalyst is highly desirable.
In continuation to our effort in developing novel transition metal complexes with amino acids for various organic transformations [56,57,58], herein we would like to report an efficient method for the synthesis of 1,8-dioxodecahydroacridine derivatives through one-pot three component cyclisation reaction catalyzed by cobalt–alanine complex under an aqueous ethanol solvent system.
Results and discussions
Initially, we studied the one-pot three component condensation reaction of benzaldehyde (1 mmol), dimedone (2 mmol) and aniline (1 mmol) as a model reaction in the presence of cobalt–alanine complex (5 mol%) as a catalyst in aqueous ethanol solvent system and the product was obtained in excellent yield (Scheme 1).
After successful of the model reaction, we studied for the optimization of the reaction conditions with respect to temperature, time, solvent and molar ratio of the catalyst. It was observed that 5 mol% of catalyst was enough to complete the reaction and the desired 1,8-dioxodecahydroacridines products were obtained in high yield. The results are summarized in Table 1.
Further, we performed a screening of various solvents such as acetonitrile, DCM, DMF, EtOH and without solvent system for this reaction (Table 2, entries 1–5). we found ethanol (Table 2, entry 4) was the best solvent to afford the desired products in higher yield in shorter reaction time.
To evaluate the scope and the generality of this new protocol for various aldehydes and amines under optimized conditions. A series of aromatic aldehydes bearing either electron-donating or electron-withdrawing substituents reacted successfully with dimedone and aromatic amines or ammonium acetate to afford a wide range of substituted 1,8-dioxodecahydroacridines products in high yields in a shorter reaction time and the results are summarized in Table 3. We explored further the electronic effect of various substituents present on the aldehyde component. We noticed that a wide range of aldehydes having both electron-donating and electron-withdrawing substituents are equally facile for the reaction, and gave the corresponding 1,8-dioxodecahydroacridine derivatives in very good yields. We have observed most of the electron-donating aldehydes reacted in a shorter reaction time and gave the corresponding 1,8-dioxodecahydroacridine derivatives in good yields than electron-withdrawing aldehydes.
The physical and spectral data of synthesized compounds were (4a–4v) found to be in agreement with the reported data.
A plausible mechanism for the formation of the 1,8-dioxodecahydroacridine products using cobalt–alanine metal complex as a catalyst has presented in Scheme 2.
We propose that the cobalt–alanine metal complex induces the polarization of the carbonyl groups, of aldehyde (1) and 1,3-diketone (2). Further, we assume that cobalt–alanine metal complex triggers the 1,3-diketone (2) and facilitates the formation of corresponding imine through a condensation reaction with amine 3. The obtained imine, further undergoes tautomerizes to form enamine. Therefore, cobalt–alanine metal complex facilitates the formation of two reactive intermediate species I and II which subsequently react each other via Michael addition and forms the adduct, which further tautomerize. The tautomerized Michael adduct undergoes simple intramolecular ring closure and give the final desired products 4a–4v by loss of water molecule.
Spectral data for selected compounds
3,3,6,6-Tetramethyl-9,10-diphenyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4a)
IR (KBr disc) cm−1: 3120, 2984, 2848, 1664, 1583, 1467, 1437, 1385, 1312, 1244, 1184, 1065, 860, 787, 714, 684. 1H NMR (500 MHz CDCl3): δ 0.86 (s, 6H, 2CH3), 0.94 (s, 6H, 2CH3), 2.14 (d, 2H, J = 16.4 Hz,), 2.23 (d, 2H, J = 16.4 Hz,), 2.24–2.41 (m, 4H, 2CH2), 5.52 (s, 1H), 7.01 (s, 1H, ArH), 7.04 (d, J = 6.2 Hz, 2H, ArH), 7.12–7.28 (m, 5H, ArH), 7.34 (d, J = 6.2 Hz, 2H, ArH). 13C NMR (125 MHz, CDCl3): δ 22.8, 23.4, 36.2, 42.2, 52.4, 123.8, 124.8, 126.6, 127.2, 128.2, 130.6, 132.2, 136.2, 145.8, 151.6, 212.8.
3,3,6,6-Tetramethyl-9-(4-methoxyphenyl)-10-phenyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4b)
IR (KBr disc) cm−1: 3118, 2964, 2902, 2882, 1654, 1614, 1604, 1572, 1502, 1484, 1342, 1274, 1228, 1168, 1058, 856, 734, 658. 1H NMR (500 MHz, CDCl3): 0.83 (s, 6H, 2CH3), 1.02 (s, 6H, 2CH3), 1.24 (d, 2H, J = 14.6 Hz,), 2.14 (d, 2H, J = 14.6 Hz,), 2.25–244 (m, 4H, 2CH2), 3.94 (s, 3H, OCH3), 5.29 (s, 1H), 6.64 (s, 1H, ArH), 6.98 (d, J = 7.6 Hz, 2H, ArH), 7.14–7.28 (m, 3H, ArH), 7.39 (d, J = 7.4 Hz, 2H, ArH). 13C NMR (125 MHz, CDCl3): δ 22.3, 23.8, 35.2, 42.4, 52.5, 125.2, 126.8, 127.5, 129.2, 131.5, 134.2, 136.9, 146.8, 148.3, 150.3, 166.2, 212.6.
3,3,6,6-Tetramethyl-9-(4-cyanophenyl)-10-phenyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4d)
IR (KBr disc) cm−1: 2984, 2912, 2284, 1672, 1548, 1484, 1322, 1277, 1231, 1164, 1123, 1112, 1052, 842, 722, 580. 1H–NMR (500 MHz, CDCl3): δ 0.72 (s, 6H, 2CH3), 0.92 (s, 6H, 2CH3), 1.62 (d, J = 14.5 Hz, 2H, CH2), 2.06 (d, J = 14.5 Hz, 2H, CH2), 2.11 (d, J = 12.2 Hz, 2H, CH2), 2.24 (d, J = 12.2 Hz, 2H, CH2), 5.28 (s, 1H), 7.22 (d, J = 6.0 Hz, 2H, ArH), 7.48 (d, J = 6.4 Hz, 2H, ArH), 7.52 (d, J = 6.0 Hz, 2H, ArH), 7.64 (m, 3H, ArH). 13C-NMR (125 MHz, CDCl3): δ 24.2, 29.8, 30.8, 32.2, 42.4, 52.4, 111.2, 113.8, 121.4, 127.6, 130.7, 132.7, 137.6, 150.2, 154.1, 195.2.
3,3,6,6-Tetramethyl-9-(4-nitrophenyl)-10-phenyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4e)
IR (KBr disc) cm−1: 2982, 1654, 1568, 1544, 1368, 1258, 1146, 1122, 1102, 1068, 882, 856, 724, 588, 526. 1H-NMR (500 MHz, CDCl3): δ 0.74 (s, 6H, 2CH3), 0.92 (s, 6H, 2CH3), 1.35 (d, J = 14.8 Hz, 2H, CH2), 2.08 (d, J = 14.8 Hz, 2H, CH2), 2.12 (d, J = 12.4 Hz, 2H, CH2), 2.18 (d, J = 12.4 Hz, 2H, CH2), 5.16 (s, 1H, CH), 7.14 (d, J = 7.2 Hz, 2H, ArH), 7.58 (d, J = 7.2 Hz, 2H, ArH), 7.62 (s, 1H, ArH), 7.68 (d, J = 8.2 Hz, 2H, ArH), 8.16 (d, J = 8.2 Hz, 2H, ArH). 13C-NMR (125 MHz, CDCl3): δ 24.2, 28.2, 31.6, 36.2, 42.4, 52.3, 113.2, 123.8, 129.2, 130.2, 136.4, 147.8, 151.4, 155.8, 195.8.
3,3,6,6-Tetramethyl-9-(4-hydroxyphenyl)-10-phenyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4f)
IR (KBr disc) cm−1: 3418, 2976, 2912, 2882, 1658, 1584, 1512, 1488, 1433, 1367, 1242, 1184, 1054, 822, 728, 712, 686. 1H NMR (500 MHz, CDCl3): δ 0.82 (s, 6H, 2CH3), 0.92 (s, 6H, 2CH3), 1.48 (d, J = 15.4 Hz, 2H), 2.02 (d, J = 15.4, 2H), 2.28–248 (m, 4H, 2CH2), 5.48 (s, 1H), 7.12 (d, J = 6.8 Hz, 2H, ArH), 7.18–7.23 (m, 4H, ArH), 7.36 (d, J = 6.4 Hz 2H, ArH), 9.11 (s, 1H, OH). 13C NMR (125 MHz, CDCl3): δ 22.8, 23.6, 33.2, 41.2, 55.2, 123.2, 127.8, 129.2, 131.9, 136.2, 138.9, 148.4, 152.7, 153.5, 165.2, 212.6.
3,3,6,6-Tetramethyl-9-(4-chlorophenyl)-10-phenyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4g)
1H-NMR (500 MHz, CDCl3): δ 0.78 (s, 6H, 2CH3), 0.82 (s, 6H, 2CH3), 1.69 (d, J = 16.6 Hz, 2H, CH2), 2. 21 (d, J = 16.6 Hz, 2H, CH2), 2.08 (d, J = 14.8 Hz, 2H, CH2), 2.14 (d, J = 14.8 Hz, 2H, CH2), 5.21 (s, 1H, CH), 7.14 (d, J = 7.6 Hz, 2H, ArH), 7.21 (d, J = 7.6 Hz, 2H, ArH), 7.38 (d, J = 8.6 Hz, 2H, ArH), 7.44 (m, 3H, ArH).
3,3,6,6-Tetramethyl-9-(4-bromophenyl)-10-phenyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4h)
IR (KBr disc) cm−1: 3024, 2986, 2848, 1665, 1642, 1564, 1474, 1432, 1422, 1402, 1384, 1342, 1248, 1218, 1184, 1136, 1054, 1038, 1024, 936, 875, 741, 712, 684. 1H NMR (500 MHz, CDCl3): δ 0.74 (s, 6H, 2CH3), 0.86 (s, 6H, 2CH3), 1.72 (d, J = 16.8 Hz, 2H, 2CH), 2.18 (d, J = 16.8 Hz, 2H, 2CH), 2.21–2.28 (m, 4H, 2CH2), 5.18 (s, 1 H, CH), 7.26 (d, J = 6.8 Hz, 2H, ArH), 7.42–7.48 (m, 4H, ArH), 7.62–7.66 (m, 3H, ArH). 13C NMR (125 MHz, CDCl3): δ 24.6, 28.2, 31.8, 33.6, 43.5, 51.2, 113.2, 117.7, 128.2, 129.8, 130.6, 132.6, 139.1, 145.2, 151.2, 196.8.
3,3,6,6-Tetramethyl-9-(3-nitrophenyl)-10-phenyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4k)
1H-NMR (500 MHz, CDCl3): δ 0.82 (s, 6H, 2CH3), 0.94 (s, 6H, 2CH3), 1.78 (d, J = 16.6 Hz, 2H, CH2), 2.08 (d, J = 16.4 Hz, 2H, CH2), 2.16 (d, J = 13.6 Hz, 2H, CH2), 2.28 (d, J = 13.6 Hz, 2H, CH2), 5.42 (s, 1H, CH), 7.34 (m, 1H, ArH), 7.46 (m, 2H, ArH), 7.64 (m, 3H, ArH), 7.94 (m, 2H, ArH), 8.38 (s, 1H, ArH).
3,3,6,6-Tetramethyl-9-(4-methoxyphenyl)-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4l)
IR (KBr disc) cm−1: 3184, 1648, 1617, 1462, 1366, 1223, 1138, 1084, 968, 748, 646. 1H NMR (500 MHz, CDCl3): δ 0.94 (s, 6H, 2 CH3), 1.05 (s, 6H, 2CH3), 2.09–2.16 (dd, 4H), 2.19–2.28 (m, 4H, 2 CH2), 3.66 (s, 3H), 5.04 (s, 1H), 6.69–6.73 (d, J = 8.4 Hz, 2H, ArH), 7.23–7.26 (d, J = 7.6 Hz, 2H, ArH), 8.22 (s, 1H, NH).
3,3,6,6-Tetramethyl-9-(4-nitrophenyl)-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4o)
1H-NMR (500 MHz, CDCl3) δ 0.98 (s, 6H, 2CH3), 1.08 (s, 6H, 2CH3), 2.14 (d, J = 16.5 Hz, 2H), 2.34 (d, J = 16.2 Hz, 2H), 2.38 (d, J = 14.8 Hz, 2H), 2.52 (d, J = 14.8 Hz, 2H), 5.24 (s, 1H, CH), 6.18 (s br., 1H, NH), 7.68 (d, J = 7.8 Hz, 2H, arom-H), 8.16 (d, J = 7.8 Hz, 2H, arom-H).
3,3,6,6-Tetramethyl-9-(2,3-dichlorophenyl)-10-phenyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4p)
IR (KBr disc) cm−1: 2954, 2936, 1658, 1642, 1585, 1464, 1342, 1268, 1176, 1049, 1024, 842, 738, 582. 1H-NMR (500 MHz, CDCl3): δ 0.84 (s, 6H, 2CH3), 0.98 (s, 6H, 2CH3), 1.76 (d, J = 17.6 Hz, 2H, CH2), 2.16 (d, J = 17.6 Hz, 2H, CH2), 2.34 (m, 4H, CH2), 5.28 (s, 1H, CH), 7.24 (m, 2H, ArH), 7.38 (m, 2H, ArH), 7.48 (s 1H, ArH), 7.52 (m, 3H, ArH). 13C-NMR (125 MHz, CDCl3): δ 26.2, 30.4, 31.6, 43.2, 51.8, 113.2, 126.8, 129.2, 130.2, 131.3, 133.2, 136.5, 145.2, 151.7, 196.2.
3,3,6,6-Tetramethyl-9-(2,3-dimethoxyphenyl)-10-phenyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4q)
IR (KBr disc) cm−1: 3412, 1654, 1454, 1382, 1231, 1130, 1065, 978, 768, 645. 1H NMR (500 MHz, CDCl3): δ 0.88 (s, 6H, 2CH3), 0.94 (s, 6H, 2CH3), 1.86–2.24 (dd, 4H), 2.32–2.46 (dd, 4H), 3.84 (s, 3H, OCH3), 3.94 (s, 3H, OCH3), 5.12 (s, 1H), 6.72–6.84 (m, 3H, ArH), 9.12 (s, 1H, NH).
Conclusion
In conclusion, we have developed a facile and an efficient protocol for the synthesis of 1,8-dioxodecahydroacridines using cobalt–alanine metal complex as a catalyst in aqueous ethanol as solvent via one-pot three-component condensation of aromatic aldehydes, dimedone, aniline or ammonium acetate. Significant advantages of this study are reasonably simple experimental workup procedure and catalyst preparation, ease of product isolation, high to excellent yields, short reaction time and using catalytic amount of cobalt–alanine metal complex, are notable advantages of the present methodology.
Experimental procedure
Materials and methods
All chemicals were purchased from Sigma Aldrich and used as received without further purification. All chemicals and reagents used in the present study were of analytical grade. The reactions were monitored by TLC using silicagel plates. The FTIR spectra were recorded on a Shimadzu JASCO FTIR-460 plus spectrometer using KBr pellets or neat. The UV–visible spectra of the compounds were recorded on Shimadzu UV-2100 spectrophotometer. The morphology of synthesized complex was characterized by Scanning electron microscopy (SEM) on a JEOL-JSM-6390 LV. 1H NMR and 13C NMR spectra were recorded on a Brucker DRX 500 AVANCE (500 MHz) spectrometer using CDCl3 as solvent and TMS as internal standard. The elemental analysis of the complexes were recorded by using Perkin-Elmer CHN-2400 analyzer their results were found to be good agreement with the calculated values. Photoluminance spectra of the complexes and ligands were recorded on LUMINA fluorescence spectrometer of Thermo Scientific Co. USA. The XRD measurements were performed on Schimadzu DX-6000 using Cu for Kα-particle source.
General procedure for the preparation of cobalt–alanine complex
It is commonly well-known that a metal ion can bind two amino acids to form an amino acid metal complex (Fig. 1). Therefore, amino acid-metal complexes were prepared in hot ethanol solvent by reacting the corresponding metal ion and amino acid in a 1:2 molar ratio.
The synthesis of cobalt–alanine metal complex was achieved in a two steps. In a first step, the CoCl2·6H2O (2.4 g) metal salt was dissolved in a hot ethanol solvent and l-alanine (1.8 g) amino acid ligand also dissolved in ethanol separately. In the second step, the resultant solution of ligand was slowly added drop by drop into the metal salt solution under vigorous stirring. Once the addition of ligand solution was completed, then 0.01 M Na2CO3 solution also added slowly to adjust the PH around 6.5 to 8.5 for the formation of Co–alanine metal complex. The reaction mixture was refluxed for 3 h at 80 °C under vigorous stirring (Scheme 3) [56,57,58]. The reaction progress was observed by TLC. The mobile phase condition was n-butanol:acetone:acetic acid:water (7:7:2:4) used as a solvent system. The TLC plate was run in this solvent system and the obtained amino acid metal complex was moved well on the TLC plate and also visualized by using solution of ninhydrin. After completion of the reaction, as indicated by TLC, the mixture was allowed to cooled at room temperature; thus the obtained solid crude product was separated by filtration and the crude product was recrystallized in acetone and diethyl ether solvent system with constant stirring under reflux for a period of time, later it was allowed to cool. The resultant solid product was filtered and dried under vacuum. The obtained pure solid product was in a purple color.
IR (KBr, ν cm−1): 3084 (νOH), 1620 (νC=N), 1014 (νCo–O), 485 (νCo–N).
Elemental analysis: C: 30.14%, H: 5.52%, N: 11.82%, Co: 25.24%.
The synthesized cobalt–alanine metal complex was characterized by using photoluminescence spectroscopy, which provided an evidence of complexation. The recorded emission spectra showed an interesting evidence for the complex formation. The emissions at λmax 556–566 and 660–730 nm provide evidence that the metal atoms are transferring energy to the ligand (alanine) hence; promoting the photoluminescence to the organic ligand (Fig. 2).
Further evidence to suggest this justification was revealed by the powder XRD analysis of the complex. The obtained new peaks were observed at 30–40 θ which clearly provides the evidence for the formation of complex (Fig. 3).
The final evidence of complex composition was predicted from by SEM analysis. The SEM-micrograph for the cobalt–alanine has shown in Fig. 4. The morphology, texture and shape of the synthesized complex with varying thickness in the range of 5 to 10 µm are seen. The morphology of this complex was seen as rods at different magnifications.
General procedure for the synthesis of 1,8-dioxodecahydroacridines catalyzed by cobalt–alanine metal complex
A mixture of an aromatic aldehyde 1 (1 mmol), 5,5-dimethyl-1,3-cyclohexanedione 2 (2 mmol), aromatic amine or ammonium acetate 3 (1.2 mmol) and cobalt–alanine complex (5 mol%) in ethanol (10 mL) was stirred at reflux. The progress of the reaction was monitored by TLC. After completion of the reaction as indicated by TLC, the mixture was cooled to room temperature and filtered. The filtrate was concentrated to obtained the crude product. The crude products were purified and recrystallized from EtOH and water mixture to obtain pure products in high yields (Scheme 4).
Abbreviations
- MCR:
-
multi-component reactions
- DHPs:
-
1,4-dihydropyridines
- SBSSA:
-
sulfonic acid functionalized silica
- TEBAC:
-
triethylbenzylammoniumchloride
- SBNPSA:
-
silica-bonded N-propyl sulfamic acid
- [Hmim]TFA:
-
1-methyl imidazolium trifluoroacetate
- DBSA:
-
p-dodecylbenzenesulfonicacid
- DCM:
-
dichloromethane
- DMF:
-
dimethylformamide
- EtOH:
-
ethanol
- SEM:
-
scanning electron microscope
- FT-IR:
-
Fourier transform infrared spectroscopy
- TLC:
-
thin layer chromatography
References
Negwar M (1994) In organic-chemical drugs and their synonyms. Akademie, Berlin
Dekamin MG, Mokhtari Z, Karimi Z (2011) Nano-ordered B-MCM-41: an efficient and recoverable solid acid catalyst for three-component Strecker reaction of carbonyl catalyst for three-component Strecker reaction of carbonyl. Sci Iran Trans C 18:1356–1364
Gerencser J, Dormon G, Darvas F (2006) Meldrum’s acid in multicomponent reaction: applications to combinatorial and diversity-oriented synthesis. QSAR Comb Sci 25:439–448
Ramon DJ, Yus M (2005) Asymmetric multicomponent reactions (AMCRs): the new frontier. Angew Chem Int Ed 44:1602–1634
Domling A, Ugi I (2000) Multicomponent reactions with isocyanides. Angew Chem Int Ed 39:3168–3210
Strubing D, Neumann H, Klaus S, Hubner S, Beller M (2005) A facile and efficient synthesis of enyne-reaction precursors by multicomponent reactions. Tetrahedron 61:11333–11344
Heydari A, Arefi A, Khaksar S, Shiroodi RK (2007) Guanidine hydrochloride: an active and simple catalyst for Strecker type reaction. J Mol Catal A 271:142–144
Guillena G, Ramon DJ, Yus M (2007) Organocatalytic enantioselective multicomponent reactions (OEMCRs). Tetrahedron Asymmetry 18:693–700
Yu L, Chen B, Huang X (2007) Multicomponent reactions of allenes, diaryl diselenides, and nucleophiles in the presence of iodosobenzene diacetate: direct synthesis of 3-functionalized-2-arylselenyl substituted allyl derivatives. Tetrahedron Lett 48:925–927
Lee D, Sello JK, Schreiber SL (2000) Pairwise use of complexity-generating reactions in diversity-oriented organic synthesis. Org Lett 2:709–712
Zhu J (2003) Recent developments in the isonitrile-based multicomponent synthesis of heterocycles. Eur J Org Chem 7:1133–1144
Venkatesan K, Pujari SS, Srinivasan KV (2009) Proline-catalyzed simple and efficient synthesis of 1,8-dioxodecahydroacridines in aqueous ethanol medium. Synth Commun 39:228–244
Mikata Y, Yokoyama M, Mogami K, Kato M, Okura I, Chikira M, Yano S (1998) Intercalator-linked cisplatin: synthesis and antitumor activity of cis-dichloroplatinum (II) complexes connected to acridine and phenylquinolines by one ethylene chain. Inorg Chim Acta 279:51–57
Sirisha K, Bikshapathi D, Achaiah G, Reddy VM (2011) Synthesis, antibacterial and antimycobacterial activities of some new 4-aryl/heteroaryl-2,6-dimethyl-3,5-bis-N-(aryl)-carbamoyl-1,4-dihydropyridines. Eur J Med Chem 46:1564–1571
Spalding DP, Chapin EC, Mosher HS (1954) Heterocyclic basic compounds. XV. Benzacridine derivatives. J Org Chem 19:357–364
Solanki MJ, Vachharajani PR, Dubal GG, Shah VH (2011) Synthesis and antimicrobial evaluation of some newer symmetrical 1,4-dihydropyridines. Int J ChemTech Res 3:1139–1144
Ogawa T, Nakato A, Tsuchida K, Hatayama K (1993) Synthesis and antihypertensive activities of new 1,4-dihydropyridine derivatives containing a nitrooxy moiety at the 3-ester position. Chem Pharm Bull 41:108–116
Wainwright M (2001) Acridine—a neglected antibacterial chromophore. J Antimicrob Chemother 47:1–13
Sirisha K, Achaiah G, Reddy VM (2010) Facile synthesis and antibacterial, antitubercular, and anticancer activities of novel 1,4-dihydropyridines. Arch Pharm 343:342–352
Jain SM, Kant R, Devi S, Dahr KL, Singh S, Bani S, Singh GB (1990) Indian J Chem Sect B 29:95
Godfraid T, Miller R, Wibo M (1986) Calcium antagonism and calcium entry blockade. Pharmacol Rev 38:321–416
Bossert F, Vater W (1989) 1,4-Dihydropyridines—a basis for developing new drugs. Med Res Rev 9:291–324
Berkan O, Sarac B, Simsek R, Yildirim S, Sariogli Y, Safak C (2002) Vasorelaxing properties of some phenylacridine type potassium channel openers in isolated rabbit thoracic arteries. Eur J Med Chem 37:519–523
Stou DM, Meyers AI (1982) Recent advances in the chemistry of dihydropyridines. Chem Rev 82:223–243
Antman E, Muller J, Goldberg S, Macalpin R, Rubenfire M, Tabatznik B, Liang C, Heupler F, Achuff S, Reichek N, Geltman E, Kerin NZ, Neff RK, Raunwald E (1980) Nifedipine therapy for coronary–artery spasm—experience in 127 patients. N Engl J Med 302:1269–1273
Guazzi M, Olivari M, Polese A, Fiorentini C, Margrini F, Moruzzi P (1977) Nifedipine, a new antihypertensive with rapid action. Clin Pharmacol Ther 22:528–532
Hornung RS, Gould BA, Jones RI, Sonecha TN, Raferty EB (1983) Nifedipine tablets for systemic hypertension: a study using continuous ambulatory intraarterial recording. Am J Cardiol 51:1323–1325
Bossert F, Meyer H, Wehinger E (1981) 4-Aryldihydropyridines, a new class of highly active calcium antagonists. Angew Chem Int Ed Engl 20:762–769
Bretzel RG, Bollen CC, Maeser E, Federlin KF (1992) Drugs Future 17:465
Vo D, Matowe WC, Ramesh M, Iqbal N, Wolowyk MW, Howlett SE, Knaus E (1995) Syntheses, calcium channel agonist-antagonist modulation activities, and voltage-clamp studies of isopropyl 1,4-dihydro-2,6-dimethyl-3-nitro-4-pyridinylpyridine-5-carboxylate racemates and enantiomers. J Med Chem 38:2851–2859
Delfourne E, Roubin C, Bastide J (2000) The first synthesis of the pentacyclic pyridoacridine marine alkaloids: arnoamines A and B. J Org Chem 65:5476–5479
Ferlin MG, Marzano C, Chiarelotto G, Bordin F, Baccichetti F (2000) Synthesis and antiproliferative activity of some variously substituted acridineand azacridine derivatives. Eur J Med Chem 35:827–837
Gordeev MF, Patel DV, Gordon EM (1996) Approaches to combinatorial synthesis of heterocycles: a solid-phase synthesis of 1,4-dihydropyridines. J Org Chem 61:924–928
Shanmugasundaram P, Murugan P, Ramakrishnan VT (1996) Synthesis of acridinedione derivatives as laser dyes. Heteroat Chem 7:17–22
Murugan P, Shanmugasundaram P, Ramakrishnan VT, Venkatachalapathy B, Srividya N, Ramamurthy P, Gunasekaran K, Velmurugan D (1998) Synthesis and laser properties of 9-alkyl-3,3,6,6-tetramethyl-1,2,3,4,5,6,7,8,9,10-decahydroacridine-1,8-dione derivatives. J Chem Soc Perkin Trans 2:999–1004
Islam A, Murugan P, Hwang KC, Cheng CH (2003) Blue lightemitting devices based on 1,8-acridinedione derivatives. Synth Met 139:347–353
Tu SJ, Miao C, Gao Y, Fang F, Zhuang Q, Feng Y, Shi D (2004) Novel cascade reaction of aryl aldoxime with dimedone under microwave irradiation: the synthesis of N-hydroxylacridine. Synlett 2:255–258
Papagni A, Campiglio P, Campione M (2008) Synthesis and physical properties of polyfluoro-acridines bearing perfluoroalkyl chains. J Fluor Chem 129:294–300
Reddy BVS, Antony A, Yadav JS (2010) Novel intramolecular aza-Diels–Alder reaction: a facile synthesis of trans-fused 5H-chromeno[2,3-c] acridine derivatives. Tetrahedron Lett 51:3071–3074
Das B, Thirupathi P, Mahender I, Reddy VS, Rao YK (2006) Amberlyst-15: an efficient reusable heterogeneous catalyst for the synthesis of 1,8-dioxo-octahydroxanthenes and 1,8-dioxo-decahydroacridines. J Mol Catal A 247:233–239
Niknam K, Panahi F, Saberi D, Mohagheghnejad M (2010) Silica-bonded S-sulfonic acid as recyclable catalyst for the synthesis of 1,8-dioxo-decahydroacridines and 1,8-dioxo-octahydroxanthenes. J Heterocycl Chem 47:292–300
Balalaie S, Chadegani F, Darviche F, Bijanzadeh HR (2009) One-pot synthesis of 1,8-dioxo-decahydroacridine derivatives in aqueous media. Chin J Chem 27:1953–1956
Wang XS, Shi DQ, Zhang YF, Wang SH, Tu SJ (2004) Chin J Org Chem 24:430–432
Safaei-Ghomi J, Ghasemzadeh MA, Zahedi S (2013) ZnO nanoparticles: a highly effective and readily recyclable catalyst for the one-pot synthesis of 1,8-dioxo-decahydroacridine and 1,8-dioxooctahydro-xanthene derivatives. J Mexi Chem Soc 57:1–7
Ghasemzadeh MA, Safaei-Ghomi J, Molaei H (2012) Fe3O4 nanoparticles: as an efficient, green and magnetically reusable catalyst for the one-pot synthesis of 1, 8-dioxo-decahydroacridine derivatives under solvent-free conditions. C R Chim 15:969–974
Fan X, Li Y, Zhang X, Qu G, Wang J (2007) An efficient and green preparation of 9-arylacridine-1,8-dione derivatives. J Heteroat Chem 18:786–790
Rashedian F, Saberib D, Niknam K (2010) Silica-bonded N-propyl sulfamic acid: a recyclable catalyst for the synthesis of 1,8-dioxo-decahydroacridines, 1,8-dioxo-octahydroxanthenes and quinoxalines. J Chin Chem Soc 57:998–1006
Li YL, Zhang MM, Wang XS, Shi DQ, Tu SJ, Wei XY, Zong ZM (2005) J Chem Res 44:600–605
Zhang MM, Wang XS, Jiang H, Shi DQ, Tu SJ, Wei XY, Zong ZM (2006) An improved and benign synthesis of 9,10-diarylacridine-1,8-dione and indenoquinoline derivatives from 3-anilino-5,5-dimethylcyclohex-2-enones, benzaldehydes, and 1,3-dicarbonyl compounds in an ionic liquid medium. Synthesis 24:4187–4199
Dabiri M, Baghbanzadeh M, Arzroomchilar E (2008) 1-Methylimidazolium triflouroacetate ([Hmim]TFA): an efficient reusable acidic ionic liquid for the synthesis of 1,8-dioxo-octahydroxanthenes and 1,8-dioxo-decahydroacridines. Catal Commun 9:939–942
Shen W, Wang LM, Tian H, Tang J, Yu JJ (2009) Brønsted acidic imidazolium salts containing perfluoroalkyl tails catalyzed one-pot synthesis of 1,8-dioxo-decahydroacridines in water. J Fluor Chem 130:522–527
Miao CB, Tu SJ, Gao Y, Feng YJ, Feng JC (2002) Microwave-prompted reaction of cinnamonitrile derivatives with 5,5-dimethyl-1,3-cyclohexanedione. Chin J Chem 20:703–706
Wang XS, Shi DQ, Wang SH, Tu SJ (2003) Chin J Org Chem 23:1291–1293
Jin TS, Zhang JS, Guo TT, Wang AQ, Li TS (2004) One-pot clean synthesis of 1,8-dioxo-decahydroacridines catalyzed by p-dodecylbenezenesulfonic acid in aqueous media. Synthesis 12:2001–2005
Subramanyam M, Varala R, Sreenivasulu R, Rao MVB, Rao KP (2018) A facile, efficient and convenient synthesis of 1,8-dioxodecahydroacridines with PMA-SiO2 reusable catalyst. Lett Org Chem 15:915–921
Merajuddin SA, Mubarak AT, Alam MM, Halima AAA (2013) Cyclohexane oxidation: synthesis and characterization of amino acid metal complexes and their catalytic activity evaluation. Int J Basic Appl Chem Sci 3:29–40
Merajuddin SA, Mubarak AT, Fouda AA, Alam MM, Sabr SA (2013) Cyclohexane oxidation: synthesis of 1,10 phenanthroline Cu (II) complexes by classical, microwave and hydrothermal methods and their catalytic activity evaluation. Int J Basic Appl Chem Sci 3:69–78
Merajuddin SA, Mubarak AT, Alam MM, Halima AAA (2014) Cyclohexane oxidation: synthesis of iron (III)-amino acid and amino acid Schiff base complexes and their catalytic activity evaluation. Am Chem Sci J 4:600–615
Authors’ contributions
MMA, ATM, MAA, SMA and AMF designed and performed the research. MMA, SMA and AMF did the sample collection. MMA, ATM, MAA and SMA analyzed the data and interpreted the results. MMA, MAA and AMF wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors are thankful to Dean of Scientific Research, King Khalid University and the College of Science. Department of Chemistry for providing facilities to carry out our research work.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets generated and analysed during the current study available from the corresponding author on reasonable request.
Funding
This work was supported by the Dean of Scientific Research, King Khalid University for the financial support is greatly appreciated for the General Research Project under grant number [G. P. R. 193/38].
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Alam, M.M., Mubarak, A.T., Assiri, M.A. et al. A facile and efficient synthesis of 1,8-dioxodecahydroacridines derivatives catalyzed by cobalt–alanine metal complex under aqueous ethanol media. BMC Chemistry 13, 40 (2019). https://doi.org/10.1186/s13065-019-0545-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-019-0545-3