Abstract
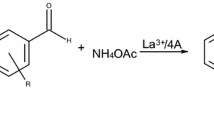
A series of 9-aryl-hexahydroacridine-1,8-diones are synthetized with good to excellent yields (50–99%) via a one-pot four-component reaction of dimedone, aromatic aldehydes and ammonium acetate in the presence of 4 Å molecular sieves modified with iron(III) as an efficient heterogeneous catalyst, in ethanol. The process offers the advantages of high yields, mild reaction conditions and easy work-up procedure. The catalyst can be reused without significant loss of activity.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
During the past years, multicomponent reactions (MCRs) have emerged as valuable and efficient tools in the hands of synthetic organic chemists, since they have several advantages over the classical synthetic strategies. MCRs provide complex organic compounds in a single step using simple and readily available substrates without the isolation of any intermediates under shorter reaction times, yielding less side products with lower energy consumption and waste production, thus leading to environmentally more friendly processes [1].
Acridinediones are a highly important class of organic compounds, since they possess a wide range of pharmaceutical and biological activities such as a positive ionotropic effect promoting the entry of calcium to the intracellular space [2], anticancer activity [3], enzyme and tumour cell inhibition [4], antimicrobial activity and cytotoxicity [5]. They have structural similarity to 1,4-dihydropyridines (1,4-DHPs), which are well known intermediates in the synthesis of several pharmaceuticals including those for the treatment of hypertension and cardiovascular diseases [6]. Acridine-1,8-diones can also be used as laser dyes with very high lasing-efficiencies [7, 8].
Due to their importance, several strategies have been developed for the synthesis of 9-aryl-acridine-1,8-diones, including the thermal reaction of dimedone, different aromatic aldehydes and aqueous ammonia in ethanol [9], the microwave-irradiation-aided solvent-free reaction of dimedone and an aldehyde using alumina supported ammonium acetate and catalytic N,N-dimethylformamide [10], or ammonium bicarbonate [11], in water under microwave irradiation [12], and in pure water without any additives [13]. The use of ionic liquids alone [14, 15] and in the presence of CeCl3·7H2O [16] was also reported for this conversion. Numerous protocols have been developed for the synthesis using different catalysts. As homogenous catalysts, CuSO4·5H2O [17] and ceric ammonium nitrate and PEG [18] are described for the synthesis, and we can also find examples for heterogeneous catalysts such as HY-Zeolite [19], carbon-based solid acid (CBSA) [20], Fe3O4@SiO2@Ni–Zn–Fe LDH [21], mesoporous silica nanoparticles (MSNs) [22], and Fe3O4@SiO2 nanoparticles [23].
Although the reported methods have their own advantages and limitations, a simple, easily accessible and reusable heterogeneous catalyst may have a synthetic importance.
The main research profile of our group is to elaborate new heterogeneous catalytic methods for the preparation of organic compounds in the presence of supported metal catalysts. Several metals have been used successfully in different organic syntheses, such as palladium [24,25,26,27], nickel [28], copper [29,30,31,32], titanium [33, 34], lanthanum [35, 36] and zinc [37] on different supports (Mg:La 3:1 mixed oxide, 4 Å molecular sieves). In this paper we present a simple method for the one-pot four-component synthesis of 9-aryl-acridine-1,8-dione derivatives from dimedone, aldehydes and ammonium acetate in the presence of a slightly basic, 4 Å molecular sieves-supported iron catalyst (Fe3+/4A).
2 Experimental
Morphology of the catalyst samples was investigated by a JEOL 6380LVa (JEOL, Tokyo, Japan) type scanning electron microscope and elemental mapping was also accomplished using the energy-dispersive X-ray detector of the equipment. Each specimen was fixed by conductive double-sided carbon adhesive tape and sputtered by gold (using a JEOL 1200 instrument). Applied accelerating voltage and working distance were between 15 and 30 kV and 10 and 12 mm, respectively. In the case of the comparison of the fresh and the used catalysts, the samples were examined without sputtering with gold and were not fixed on a carbon tape.
The nitrogen adsorption/desorption isotherms were measured at − 196 °C with a computer-controlled Nova 200e (Quantachrome) instrument. The apparent surface area (SBET) was calculated using the Brunauer–Emmett–Teller (BET) model. The total pore volume (Vt) was derived from the amount of vapour adsorbed at p/p0 → 1, assuming that the pores were already filled with liquid adsorbate. The micropore volume (W0) was derived from the Dubinin–Radushkevich (DR) plot. Prior to the adsorption measurement, the samples were evacuated at 110 °C for 24 h.
1H and 13C NMR spectra were made on BRUKER Avance-500 instrument using TMS as an internal standard in CDCl3. All compounds and solvents were purchased from Merck Hungary Ltd.
2.1 Preparation of the Catalyst
Four Angstrom molecular sieves (4A) were impregnated with FeCl3 × 6H2O as follows: 1 mmol of the metal salt was dissolved in 100 ml of deionised water and stirred with 1 g 4A at room temperature for 24 h. The solid was filtered, washed with deionised water and with acetone, then dried in an oven at 150 °C for 1 h. Samples were heated at 120 °C for 1 h before the reaction.
2.2 Determination of the pH of the Catalyst
The catalyst (1 g) was stirred in 30 mL deionised water under continuous measuring of the pH. The values were accepted after reaching a constant value at least during 10 min.
2.3 Typical Reaction Conditions
2.3.1 General Procedure for the One-Pot Synthesis of Acridinedione Derivatives
A typical reaction was carried out in a 10 mL flask. Dimedone (2 mmol), aldehyde (1 mmol), ammonium acetate (3 mmol), Fe3+/4A (0.1 g) and ethanol (3 mL) were stirred at reflux temperature for 14 h. The progression of the reaction was monitored by TLC. After completion, the solid was filtered, and washed with ethanol, then the filtrate was evaporated. The residue was extracted with dichloromethane and 0.25 M NaOH solution. The organic phase was dried over anhydrous sodium sulphate, filtered and the solvent was evaporated. The product was subjected to 1H and 13C NMR spectroscopy.
All products have satisfactory spectral data (1H and 13C NMR). The spectral data of the known compounds were identical with those reported in the literature.
2.3.2 Characterisation of the Products
9-Phenyl-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8-dione,4a yellow solid. 1H NMR (500 MHz, CDCl3) δ (ppm): 0.95 (s, 6H); 1.06 (s, 6H); 2.13-2.30 (m, 8H); 5.09 (s, 1H); 7.06 (t, J = 7.5 Hz, 1H); 7.19 (t, J = 7.5 Hz, 2H); 7.34 (d, J = 7.0 Hz, 2H); 7.96 (s, 1H). 13C NMR (125 MHz, CDCl3) δ (ppm): 27.17, 29.63, 32.67, 33.71, 40.77, 50.97, 113.31, 126.04, 128.01, 146.70, 149.22, 195.94. Anal. Calcd. for C23H27NO2: C 79.08, H 7.74, N 4.01%, found: C 79.12, H 7.79, N 3.98%.
9-(3-Bromophenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8-dione,4b yellow solid. 1H NMR (500 MHz, CDCl3) δ (ppm): 0.97 (s, 6H); 1.08 (s, 6H); 2.13-2.38 (m, 8H); 5.02 (s, 1H); 7.05 (t, J = 8.0 Hz, 1H); 7.17 (d, J = 8.0 Hz, 1H); 7.32 (d, J = 6.0 Hz, 1H); 7.40 (s, 1H); 8.16 (s, 1H). 13C NMR (125 MHz, CDCl3) δ (ppm): 26.96, 29.29, 32.35, 39.91, 40.55, 50.54, 112.37, 121.74, 126.95, 128.59, 129.16, 130.66, 148.59, 195.00. Anal. Calcd. for C23H26BrNO2: C 64.49, H 6.07, N 3.27%, found: C 64.52, H 6.12, N 3.25%.
9-(4-Bromophenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8-dione,4c yellow solid. 1H NMR (500 MHz, CDCl3) δ (ppm): 0.95 (s, 6H); 1.07 (s, 6H); 2.12-2.32 (m, 8H); 5.03 (s, 1H); 7.22 (d, J = 8.5 Hz, 2H); 7.29 (d, J = 8.5 Hz, 2H); 8.45 (s, 1H). 13C NMR (125 MHz, CDCl3) δ (ppm): 27.17, 29.65, 32.63, 40.67, 50.90, 112.70, 119.65, 129.99, 130.97, 145.99, 149.44, 195.79. Anal. Calcd. for C23H26BrNO2: C 64.49, H 6.07, N 3.27%, found: C 64.54, H 6.09, N 3.29%.
9-(2-Chlorophenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8-dione, 4d yellow solid. 1H NMR (500 MHz, CDCl3) δ (ppm): 0.95 (s, 6H); 1.07 (s, 6H); 2.02-2.39 (m, 8H); 5.25 (s, 1H); 6.98 (t, J = 8.0 Hz, 1H); 7.09 (t, J = 7.5 Hz, 1H); 7.18 (d, J = 7.5 Hz, 1H); 7.39 (d, J = 8.0 Hz, 1H); 8.84 (s, 1H). 13C NMR (125 MHz, CDCl3) δ (ppm): 26.86, 29.34, 32.08, 39.91, 40.31, 50.60, 111.60, 125.89, 126.64, 129.19, 131.91, 132.86, 143.82, 149.38, 194.87. Anal. Calcd. for C23H26ClNO2: C 72.06, H 6.79, N 3.66%, found: C 72.11, H 6.82, N 3.61%.
9-(4-Chlorophenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8-dione,4e yellow solid. 1H NMR (500 MHz, CDCl3) δ (ppm): 0.95 (s, 6H); 1.07 (s, 6H); 2.17-2.29 (m, 8H); 5.07 (s, 1H); 7.16 (d, J = 8.5 Hz, 2H); 7.29 (d, J = 8.5 Hz, 2H); 8.49 (s, 1H). 13C NMR (125 MHz, CDCl3) δ (ppm): 27.17, 29.69, 32.72, 40.70, 50.98, 112.85, 128.19, 129.57, 131.65, 145.39, 149.79, 196.16. Anal. Calcd. for C23H26ClNO2: C 72.06, H 6.79, N 3.66%, found: C 72.09, H 6.80, N 3.63%.
9-(2-Fluorophenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8-dione,4f yellow solid. 1H NMR (500 MHz, CDCl3) δ (ppm): 0.94 (s, 6H); 1.05 (s, 6H); 2.10-2.30 (m, 8H); 5.24 (s, 1H); 6.89 (t, J = 9.5 Hz, 1H); 6.99-7.08 (m, 2H); 7.45 (t, J = 7.5 Hz, 1H); 8.01 (s, 1H). 13C NMR (125 MHz, CDCl3) δ (ppm): 26.91, 29.64, 32.59, 40.69, 50.92, 111.77, 115.20, 123.56, 127.64, 131.69, 133.04, 149.71, 195.81. Anal. Calcd. for C23H26FNO2: C 75.20, H 7.08, N 3.81%, found: C 75.25, H 7.11, N 3.75%.
9-(2-Methylphenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8-dione,4g yellow solid. 1H NMR (500 MHz, CDCl3) δ (ppm): 0.92 (s, 6H); 1.06 (s, 6H); 2.05-2.37 (m, 8H); 2.84 (s, 3H); 5.06 (s, 1H); 6.91 (t, J = 8.0 Hz, 1H); 6.97 (t, J = 7.5 Hz, 2H); 7.08 (d, J = 7.5 Hz, 1H); 8.39 (s, 1H). 13C NMR (125 MHz, CDCl3) δ (ppm): 19.34, 26.39, 28.92, 31.89, 40.05, 50.30, 113.92, 125.01, 127.58, 128.96, 129.02, 129.06, 135.56, 146.09, 148.14, 195.07. Anal. Calcd. for C24H29NO2: C 79.34, H 7.99, N 3.86%, found: C 79.37, H 8.04, N 3.81%.
9-(3-Methylphenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8-dione,4h yellow solid. 1H NMR (500 MHz, CDCl3) δ (ppm): 0.95 (s, 6H); 1.06 (s, 6H); 2.10-2.32 (m, 11H); 5.01 (s, 1H); 6.85 (d, J = 7.0 Hz, 1H); 7.03-7.09 (m, 2H); 7.14 (s, 1H); 8.40 (s, 1H). 13C NMR (125 MHz, CDCl3) δ (ppm): 21.56, 27.05, 29.57, 32.50, 40.57, 50.88, 112.98, 125.01, 126.55, 127.68, 128.92, 136.94, 146.78, 149.11, 149.17, 195.56. Anal. Calcd. for C24H29NO2: C 79.34, H 7.99, N 3.86%, found: C 79.35, H 8.02, N 3.83%.
9-(4-Methylphenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8-dione,4i yellow solid. 1H NMR (500 MHz, CDCl3) δ (ppm): 0.95 (s, 6H); 1.05 (s, 6H); 2.12-2.28 (m, 11H); 5.06 (s, 1H); 6.99 (d, J = 8.0 Hz, 2H); 7.22 (d, J = 8.0 Hz, 2H); 8.50 (s, 1H). 13C NMR (125 MHz, CDCl3) δ (ppm): 21.09, 27.08, 29.55, 32.55, 40.51, 50.99, 113.09, 127.88, 128.67, 135.14, 143.86, 149.79, 196.13. Anal. Calcd. for C24H29NO2: C 79.34, H 7.99, N 3.86%, found: C 79.38, H 8.05, N 3.86%.
9-(2-Methoxyphenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8-dione,4k yellow solid. 1H NMR (500 MHz, CDCl3) δ (ppm): 0.90 (s, 6H); 1.04 (s, 6H); 2.02-2.33 (m, 8H); 3.78 (s, 3H); 5.16 (s, 1H); 6.75-6.80 (m, 2H); 7.03 (t, J = 7.5 Hz, 1H); 7.37 (d, J = 7.0 Hz, 1H); 8.62 (s, 1H). 13C NMR (125 MHz, CDCl3) δ (ppm): 26.44, 29.67, 32.28, 50.89, 55.19, 110.66, 111.37, 119.76, 126.86, 131.57, 133.94, 149.62, 157.63, 195.21. Anal. Calcd. for C24H29NO3: C 75.99, H 7.65, N 3.69%, found: C 76.03, H 7.68, N 3.64%.
9-(4-Methoxyphenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8-dione,4l yellow solid. 1H NMR (500 MHz, CDCl3) δ (ppm): 0.95 (s, 6H); 1.06 (s, 6H); 2.12-2.28 (m, 8H); 3.67 (s, 3H); 5.04 (s, 1H); 6.71 (d, J = 8.5 Hz, 2H); 7.24 (d, J = 8.5 Hz, 2H); 7.92 (s, 1H). 13C NMR (125 MHz, CDCl3) δ (ppm): 27.11, 29.57, 32.59, 40.66, 50.89, 55.01, 113.29, 113.39, 128.93, 139.18, 148.96, 157.64, 195.99. Anal. Calcd. for C24H29NO3: C 75.99, H 7.65, N 3.69%, found: C 76.05, H 7.71, N 3.65%.
9-(2-Nitrophenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8-dione,4m yellow solid. 1H NMR (500 MHz, CDCl3) δ (ppm): 0.92 (s, 6H); 1.03 (s, 6H); 2.07–2.32 (m, 8H); 5.30 (s, 1H); 7.19 (t, J = 8.5 Hz, 1H); 7.45–7.50 (m, 2H); 7.71 (d, J = 8.0 Hz, 1H); 8.20 (s, 1H). 13C NMR (125 MHz, CDCl3) δ (ppm): 27.27, 29.17, 32.35, 40.55, 50.66, 112.15, 123.89, 126.31, 130.82, 130.84, 131.98, 141.27, 149.71, 195.17. Anal. Calcd. for C23H26N2O4: C 70.05, H 6.60, N 7.11%, found: C 70.11, H 6.65, N 7.05%.
9-(3-Nitrophenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8-dione,4m yellow solid. 1H NMR (500 MHz, CDCl3) δ (ppm): 0.95 (s, 6H); 1.09 (s, 6H); 2.11-2.43 (m, 8H); 5.15 (s, 1H); 7.37 (t, J = 7.5 Hz, 1H); 7.80 (d, J = 7.5 Hz, 1H); 7.92 (d, J = 7.5 Hz, 1H); 8.11 (s, 1H); 8.66 (s, 1H). 13C NMR (125 MHz, CDCl3) δ (ppm): 27.03, 29.47, 32.50, 40.54, 50.65, 111.95, 120.82, 122.57, 128.50, 134.96, 149.65, 195.31. Anal. Calcd. for C23H26N2O4: C 70.05, H 6.60, N 7.11%, found: C 70.09, H 6.67, N 7.06%.
9-(4-Nitrophenyl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8-dione,4n yellow solid. 1H NMR (500 MHz, CDCl3) δ (ppm): 0.94 (s, 6H); 1.08 (s, 6H); 2.11–2.42 (m, 8H); 5.14 (s, 1H); 7.51 (d, J = 8.0 Hz, 2H); 8.06 (d, J = 8.0 Hz, 2H); 11.91 (s, 1H). 13C NMR (125 MHz, CDCl3) δ (ppm): 27.08, 29.51, 32.47, 34.49, 50.68, 111.57, 123.02, 129.04,145.82, 149.82, 154.66, 195.09. Anal. Calcd. for C23H26N2O4: C 70.05, H 6.60, N 7.11%, found: C 70.12, H 6.65, N 7.04%.
9-Propyl-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8-dione,4o yellow solid. 1H NMR (500 MHz, CDCl3) δ (ppm): 0.79 (s, 3H); 1.08 (s, 12H); 1.21–1.29 (m, 2H); 2.18 (s, 4H); 2.31 (s, 4H); 3.27 (s, 2H); 3.93 (s, 1H); 8.72 (s, 1H). 13C NMR (125 MHz, CDCl3) δ (ppm): 14.75, 18.92, 27.31, 30.03, 32.56, 37.97, 40.87, 51.32, 66.10, 112.29, 150.59, 196.06. Anal. Calcd. for C20H29NO2: C 76.19, H 9.21, N 4.44%, found: C 76.25, H 9.27, N 4.43%.
3 Results and Discussion
We investigated the structure of the Fe3+/4A catalyst by scanning electron microscopy (SEM). During the impregnation procedure the molecular sieves support preserved its characteristic cuboctahedron shape as can be seen on Fig. 1. The average particle size ranged from 6 to 8 μm, and the particles are well defined both in shape and size. The iron is evenly distributed on the surface of the support, EDS measurement showed 5.14 w/w% iron on the surface. The iron content of the catalyst determined by ICP-OES is concordant with the theoretical value (5.3 w/w%), thus it can be concluded that the metal is mainly situated on the surface of the support. The nitrogen adsorption/desorption measurements showed a considerable decrease in the surface of the support as well, the specific surface of 4A dropped from 800 to 106.065 m2/g. The total pore volume of the molecular sieves altered from 0.3 to 0.11 cm3/g, the micropore volume was 0.022 cm3/g. The catalysts pH value is 8.42, so it is in the slightly basic region.
Recently, we reported the efficient synthesis of polyhydroquinolines via Hantzsch reaction in the presence of a molecular sieves supported lanthanum catalyst [36]. As acridinediones are structurally similar to 1,4-DHPs, first we tested our La3+/4A catalyst in the synthesis of 9-aryl-acridine-1,8-dione derivatives. As a model reaction, we investigated the reaction of dimedone, 4-chlorobenzaldehyde and ammonium acetate applying the previously elaborated reaction conditions. With the use of 1.5 mmol ammonium acetate, the yield of the desired product was only 67%, hence we attempted to increase the yield of the product applying excess of ammonium acetate. The results are summarised in Table 1.
Since the yields were not satisfactory, we investigated the model reaction applying other molecular sieves supported metal catalysts. The results are shown in Table 2.
The best result was obtained with Fe3+/4A, the desired product was formed with complete conversion (99% isolated yield, Table 2, entry 7). In the case of the Cu2+/4A catalyst, the metal dissolved into the reaction mixture from the surface of the support probably because of a complex formation between copper and ammonium acetate, thus contaminated the product. Titanium, zinc and zirconium were less active; while in their 1H NMR spectra small impurities could be detected.
Based on the reaction conditions elaborated, a wide range of aromatic aldehydes were reacted with dimedone and ammonium acetate in the Fe3+/4A catalysed one-pot synthesis of 9-aryl-acridine-1,8-diones. The results are summarized in Table 3.
Benzaldehyde and other substituted aromatic aldehydes containing electron-withdrawing or electron-donating groups were tested in the reaction and gave the desired product in good to excellent yields. There was no significant substituent effect observed; only the nitro-derivatives (Table 3, entries 12–14) showed slightly weaker reactivity. In the case of the 2-nitro-derivate, another product could be isolated from the reaction mixture, which, based on the NMR spectra, proved to be the enol form of the desired product. The structure of this enol form can be seen on Fig. 2. This form might be stabilized by the H-bond formation between the hydroxyl and the nitro groups.
As an example for aliphatic aldehydes, we examined the reaction of butyraldehyde (Table 3, entry 15), that gave the desired product with moderate yield, no secondary product was observed. We also investigated the applicability of heteroaryl aldehydes (furfural, thiophene-2-carbaldehyde) in the condensation reaction, but in these cases complex reaction mixtures were formed.
We propose a plausible mechanism for the synthesis of 9-aryl-acridine-1,8-dione derivatives involving a Knoevenagel condensation, a Michael addition, and an intramolecular ring closure in the presence of a 4Å molecular sieves-supported iron catalyst (Scheme 1). The mechanism is similar to the one described by Dam et al. [23]. We have identified two intermediates, thus the Knoevenagel intermediate (D) could be detected in the GC–MS and NMR spectra when the reaction was interrupted before completion. When the reaction was carried out in the absence of ammonium acetate, intermediate E could be isolated. The role of the iron might be to facilitate the reaction steps through the coordination of the heteroatoms in the transition states.
The workup of the reaction mixtures was rather simple; at the end of the reaction the catalyst was filtered out and washed with ethanol. The filtrate was evaporated, and the crude product obtained was extracted with DCM and 0.25 M NaOH solution. The organic phase was dried over anhydrous sodium sulphate, filtered and the solvent was evaporated.
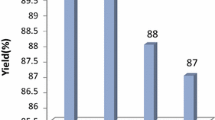
The reusability of the catalysts was examined in the reaction of dimedone, 4-chlorobenzaldehyde and ammonium acetate. After 14 h reflux, the reaction mixture was worked up as described above, then the catalyst was heated at ca. 150 °C for 1 h. The catalyst was reused in two more runs without the significant loss of its activity. The yields in the 1st, 2nd and 3rd runs were 99, 98 and 98%, respectively. These results clearly demonstrate the reusability of the Fe3+/4A catalyst.
The use of a heterogeneous catalyst often induces a dispute over whether the reaction takes place on the solid surface of the catalyst or in the liquid phase through the leaching of the metal. To determine the heterogeneous or homogeneous nature of the reaction, we used the hot filtration test. The reaction is interrupted; the catalyst is filtered out, than the filtrate is reacted further. In case of the leaching of the metal, the conversion necessarily increases further, otherwise the reaction stops without the catalyst. We carried out the hot filtration test in the reaction of dimedone, 4-chlorobenzaldehyde and ammonium acetate. After 5 h the catalyst was filtered, the filtrate was divided into two fractions, one was worked up, and the other was reacted further for the rest of the reaction time. After 14 h, the latter one was worked up as well. The two fractions were subjected to 1H NMR spectroscopy. There was no difference between the two spectra, thus the leaching of the metal was not observable.
In order to evaluate the efficiency of our catalyst with respect to the previously reported catalysts for the preparation of acridinedione derivatives, we compared our results with the methods reported in the literature for this synthesis. The comparison is shown in Table 4. In contrast to the homogeneous catalysts, e.g. CuSO4·5H2O and CAN, a heterogeneous catalyst has several advantages; it can be easily separated from the reaction mixture thus the product is not contaminated by the catalyst. Although solvent-free methods have been described, during the isolation and purification of the products, solvents had to be applied and the preparation of the catalysts have often been a complex procedure consisting of several steps (e.g. CBSA, Fe3O4@SiO2@Ni–Zn–Fe LDH, MSNs, Fe3O4@SiO2 nanoparticles). As it is clear from Table 4, our Fe3+/4A catalyst is competitive with the published catalysts taking into account its efficacy and the simplicity of its preparation and the yield obtained. Further advantage of our catalyst, that it is reusable several times without any regeneration and without loss of activity.
4 Conclusions
In conclusion, iron on 4 Å molecular sieves support proved to be efficient catalyst for the one-pot, four-component synthesis of 9-aryl-acridine-1,8-dione derivatives under mild, slightly basic conditions. The desired products were formed with good to excellent yields (50–99%). The preparation of the catalyst is very simple and it can be reused several times without the significant loss of its activity.
References
Zhu J, Bienayme H (2005) Multicomponent reactions. Wiley, New York. ISBN: 3-527-30806-7.
Schramm M, Thomas G, Towart R, Franchowiak G (1983) Nature 303:535–537
Ramesh KB, Pasha MA (2014) Bioorg Med Chem Lett 24:3907–3913
Alvala M, Bhatnagar S, Ravi A, Jeankumar VU, Manjashetty TH, Yogeeswari P, Sriram D (2012) Bioorg Med Chem Lett 22:3256–3260
Patel MM, Mali MD, Patel SK (2010) Bioorg Med Chem Lett 20:6324–6326
Edraki N, Mehdipour AR, Khoshneviszadeh M, Miri R (2009) Drug Discov Today 14:1058–1066
Shanmugashundaran P, Prabahar KJ, Ramakrishnan VT (1993) J Heterocycl Chem 30:1003–1007
Srividya N, Ramamurthy P, Shanmugashundaran P, Ramakrishnan VT (1996) J Org Chem 61:5083–5089
Martín N, Quinteiro M, Seoane C, Soto JL, Mora A, Suárez M, Ochoa E, Morales A, del Bosque JR (1995) J Heterocycl Chem 32:235–238
Suárez M, Loupy A, Salfrán E, Morán L, Rolando E (1999) Heterocycles 51:21–27
Tu S-J, Lu Z, Shi D, Yao C, Gao Y, Guo C (2002) Synth Commun 32:2181–2185
Singh SK, Singh KN (2011) J Heterocycl Chem 48:69–73
Wang G-W, Xia J-J, Miao C-B, Wu X-L (2006) Bull Chem Soc Jpn 79:454–459
Li Y-L, Zhang M-M, Wang X-S, Shi D-Q, Tu S-J, Wei X-Y, Zong Z-M (2005) J Chem Res 2005:600–602
Shi D-Q, Ni S-N, Yang F, Shi J-W, Dou G-L, Li X-Y, Wang X-S (2008) J Heterocycl Chem 45:653–660
Fan X, Li Y, Zhang X, Qu G, Wang (2007) J Heteroatom Chem 18:786–790
Pasha MA, Khan R-ur-R, Ramesh KB (2016) Can Chem Trans 4:90–98
Kidwai M, Bhatnagar D (2010) Chem Pap 64:825–828
Nikpassand M, Mamaghani M, Tabatabaeian K (2009) Molecules 14:1468–1474
Davoodnia A, Khojastehnezhad A, Tavakoli-Hoseini N (2011) Bull Korean Chem Soc 32:2243–2248
Gilanizadeh M, Zeynizadeh B (2019) Polycycl Aromat Comp. https://doi.org/10.1080/10406638.2019.1567560
Nasresfahani Z, Kassaee MZ (2015) Catal Commun 60:100–104
Dam B, Nandi S, Pal AK (2014) Tetrahedron Lett 55:5236–5240
Cwik A, Hell Z, Figueras F (2006) Adv Synth Catal 348:523–530
Cwik A, Hell Z, Figueras F (2006) Tetrahedron Lett 47:3023–3026
Cwik A, Hell Z, Figueras F (2005) Org Biomol Chem 3:4307–4309
Németh J, Hell Z (2013) React Kinet Mech Catal 111:115–121
Kiss A, Hell Z, Bálint M (2010) Org Biomol Chem 8:331–335
Fodor A, Kiss A, Debreczeni N, Hell Z, Gresits I (2010) Org Biomol Chem 8:4575–4581
Kiss A, Hell Z (2013) Synth Comm 43:1778–1786
Kiss A, Hell Z (2011) Tetrahedron Lett 52:6021–6023
Magyar Á, Hell Z (2016) Monatsh Chem 147:1583–1589
Magyar Á, Nagy B, Hell Z (2015) Catal Lett 145:1876–1879
Magyar Á, Hell Z (2019) Synlett 30:89–93
Magyar Á, Hell Z (2016) Catal Lett 146:1153–1162
Magyar Á, Hell Z (2017) Period Polytech Chem Eng 61:278–282
Magyar Á, Hell Z (2018) Green Proc Synth 7:316–322
Acknowledgments
Open access funding provided by Budapest University of Technology and Economics (BME). Á. M. is grateful to the József Varga Foundation for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Magyar, Á., Hell, Z. An Efficient One-Pot Four-Component Synthesis of 9-Aryl-Hexahydroacridine-1,8-Dione Derivatives in the Presence of a Molecular Sieves Supported Iron Catalyst. Catal Lett 149, 2528–2534 (2019). https://doi.org/10.1007/s10562-019-02845-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02845-0