Abstract
Background
We investigated the associations of alcohol with percentage of epithelium, stroma, fibroglandular tissue (epithelium + stroma), and fat in benign breast biopsy samples.
Methods
We included 857 cancer-free women with biopsy-confirmed benign breast disease within the Nurses’ Health Study (NHS) and NHSII cohorts. Percentage of each tissue was measured on whole slide images using a deep-learning algorithm and then log-transformed. Alcohol consumption (recent and cumulative average) was assessed with semi-quantitative food frequency questionnaires. Regression estimates were adjusted for known breast cancer risk factors. All tests were 2-sided.
Results
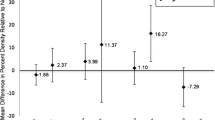
Alcohol was inversely associated with % of stroma and fibroglandular tissue (recent ≥ 22 g/day vs. none: stroma: β = − 0.08, 95% Confidence Interval [CI] − 0.13; − 0.03; fibroglandular: β = − 0.08, 95% CI − 0.13; − 0.04; cumulative ≥ 22 g/day vs. none: stroma: β = − 0.08, 95% CI − 0.13; − 0.02; fibroglandular: β = − 0.09, 95% CI − 0.14; − 0.04) and positively associated with fat % (recent ≥ 22 g/day vs. none: β = 0.30, 95% CI 0.03; 0.57; cumulative ≥ 22 g/day vs. none: β = 0.32, 95% CI 0.04; 0.61). In stratified analysis, alcohol consumption was not associated with tissue measures in premenopausal women. In postmenopausal women, cumulative alcohol use was inversely associated with % of stroma and fibroglandular tissue and positively associated with fat % (≥ 22 g/day vs. none: stroma: β = − 0.16, 95% CI − 0.28; − 0.07; fibroglandular: β = − 0.18, 95% CI − 0.28; − 0.07; fat: β = 0.61, 95% CI 0.01; 1.22), with similar results for recent alcohol use.
Conclusion
Our findings suggest that alcohol consumption is associated with smaller % of stroma and fibroglandular tissue and a greater % of fat in postmenopausal women. Future studies are warranted to confirm our findings and to elucidate the underlying biological mechanisms.
Similar content being viewed by others
Introduction
Previous studies have consistently linked alcohol consumption with an increased breast cancer risk, with approximately 10–40% risk increase with 15–30 g (1–2 drinks) per day consumption and about 7% risk increase for each additional drink of alcohol [1–4]. Multiple pathways have been suggested as possible explanations for these associations, including alteration of estrogen levels, gene expression changes and carcinogenic properties of ethanol metabolites that result from their ability to form protein and DNA adducts, disrupt normal anti-oxidative defense system and DNA repair, and cause genomic instability via indirect effect on DNA methylation [3, 5].
As summarized in a recent review, in some previous studies alcohol has been associated with increased mammographic breast density, a well-established and strong breast cancer risk factor reflective of relative proportions of epithelium, stroma, and fat on the woman’s mammogram [6]. The positive associations with percent mammographic density (the proportion of epithelium and stroma [i.e., fibroglandular tissue] out of the entire breast area) were apparent in both pre- and postmenopausal women [6]. Other studies found no associations with percent density or absolute dense and non-dense areas (reflective of absolute area occupied by fibroglandular tissue and by fat, respectively) [7, 8]. The exact mechanism for the effects of alcohol on the breast tissue composition remains unclear [9]. Some of the hypothesized biological pathways that may explain the association between alcohol consumption and breast density include an increase in endogenous estrogen [10], increased aromatase activity [11], and alterations in the growth hormone insulin-like growth factor (IGF) axis [12], all of which may increase epithelial proliferation in the breast and subsequently increase breast density [13]. To the best of our knowledge, no study has examined histologic measures of breast tissue composition in normal breast tissue of cancer-free women. While radiological findings from mammograms provide information on overall relative abundance of fibrogladular structures and fat in the breast, they do not allow segmentation of epithelium from stroma. As these two tissue types have specific contributions to breast carcinogenesis, it is important to be able to consider them separately in etiological studies. In this study, we aimed to assess the associations of alcohol consumption with the extent of epithelial, stromal, fibroglandular (i.e., combined epithelium and stroma), and fat tissue in non-malignant breast tissue from benign breast biopsy samples using prospective data in cancer-free women from the Nurses’ Health Study (NHS) and Nurses’ Health Study II (NHSII) and a deep-learning computational pathology method for tissue composition assessment. The non-malignant tissue from breast biopsy samples served as a proxy for normal breast tissue. Based on the findings on positive associations of alcohol consumption and breast cancer, we hypothesized that alcohol use will be positively associated with the extent of epithelium and/or stroma.
Materials and methods
Study population
Our analysis included cancer-free women (controls) from the nested case–control study of breast cancer conducted among the subcohort of women with biopsy-confirmed benign breast disease (BBD) in the NHS and NHSII cohorts [14, 15]. These prospective cohorts followed registered nurses in the United States who were 30–55 years (NHS) or 25–42 years old (NHSII) at enrollment. After administration of the baseline questionnaire, the information on breast cancer risk factors (Body Mass Index [BMI], reproductive history, and postmenopausal hormones) and any diagnoses of cancer or other diseases was updated through biennial questionnaires. Cancer diagnoses were then confirmed via medical record review [16, 17]. Details of this nested case–control study and BBD assessment have been previously described [14, 15].
Early NHS questionnaires (1976, 1978, and 1980) asked whether the participant had ever been diagnosed with ‘fibrocystic disease’ or ‘other BBD’ and whether she had been hospitalized in relation to this diagnosis. Beginning in 1982, the NHS questionnaires specifically asked about a history of biopsy-confirmed BBD. The initial 1989 NHS II questionnaire and all subsequent biennial questionnaires also asked participants to report any BBD diagnosis and to indicate whether it was confirmed by biopsy or aspiration.
Cases were women with biopsy-confirmed BBD who reported a breast cancer diagnosis during 1976–1998 for the NHS and 1989–1999 for the NHSII following their BBD diagnosis. Using incidence density sampling, four women with biopsy-confirmed BBD who were free of breast cancer at the time of the matching case’s diagnosis (controls) were matched to the respective breast cancer case on year of birth and year of benign breast biopsy [18]. Only controls from this nested case–control study were used to examine the associations of alcohol with the tissue composition. BBD pathology records and archived biopsy specimens were obtained from the women’s hospital pathology departments. Women were excluded if they had evidence of in situ or invasive carcinoma or unknown lesion type at the time of benign breast biopsy (n = 12). Out of 1920 controls, 857 had tissue readings and information on alcohol consumption and were included in this analysis (Fig. 1). Women with and without available tissue readings had similar distributions of breast cancer risk factors.
All study procedures were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required. Consent was obtained or implied by return of questionnaires.
Benign breast biopsy confirmation and BBD subtypes
Hematoxylin and eosin (H&E) breast tissue slides were retrieved for biopsy-confirmed BBD patients who gave permission to review their biopsy records. The slides were independently reviewed by one of three pathologists in a blinded fashion, i.e. the evaluating pathologists were blinded to type of BBD noted on the original diagnosis [19, 20]. Any slide identified as having either questionable atypia or atypia was jointly reviewed by two pathologists. For each set of slides, a detailed work sheet was completed and the benign breast biopsy was classified according to the categories of Page et al. [21] as non-proliferative, proliferative without atypia, or atypical hyperplasia [14].
Whole slide image acquisition
H&E slides were digitized into whole slide images at 20× or 40× using the Panoramic SCAN 150 (3DHISTECH Ltd, Budapest, Hungary). For women with good-quality slides, up to six slides from different tissue blocks were digitized. H&E slides that were not digitized were due to poor quality, slides too thick to fit into scanner, and plastic mounting coverslips. Attempts to create new H&E slides were not always possible due to missing (or returned to hospital) blocks, old-style blocks not created using tissue cassettes, or poor-quality blocks [22]. Out of all controls in the original nested case–control study (n = 1920), 1083 (or 56%) had their slides successfully digitized into whole slide images (WSI) (Fig. 1). Women with and without available tissue readings had similar distributions of breast cancer risk factors [23].
Quantification of epithelium, stroma, and fat
Whole slide images were processed using a deep-learning computational pathology method to segment BBD tissues into epithelial, stroma, and fat regions. Tissue image analysis included normal terminal duct lobular units (TDLUs) and BBD lesions. Details of the image analysis method and its performance are described elsewhere [23, 24]. Briefly, to evaluate the tissue segmentation network, precision, recall, and Dice similarity coefficient were calculated using the held-out test set (n = 48). Dice similarity coefficient is the harmonic mean of precision (i.e., sensitivity) and recall (i.e., positive predictive value) and assesses how accurate the automated segmentation compares with ground truth on a pixel-wise basis. The range for Dice similarity coefficient is from 0 to 1, with 1 indicating perfect overlap. The majority of the precision, recall, and Dice similarity coefficient values of the tissue segmentation network and nuclei detection were > 0.75 [24]. For more details about the nuclear segmentation network, please refer to the previously published methods paper by Vellal et al. [24].
For each whole slide image, our method computed total, epithelial, stromal, and adipose tissue areas in pixels. We next calculated the average percent of each tissue type out of the total area across all available slides for each woman (median = 3, range 1–4), weighted by the total tissue area of the slides. In our sample, we observed low heterogeneity between tissue measures across available slides for a woman, with coefficients of intra-class variation ranging between 0.51 and 0.71. The approach of using weighted average to summarize whole tissue slide or core-level data into woman-level expression has been widely used in previous studies, including our own, to account for tissue composition and heterogeneity within the tissue sections/cores, to reduce the measurement error, and to reliably link tissue markers to breast cancer and its risk factors; it demonstrated high reproducibility for associations with breast cancer risk and in prognostic algorithms in clinical trials [25–33]. The distribution of breast cancer risk factors and tissue measures were similar in women with 1 versus 4 slides.
We examined associations of alcohol with percentage of each of these individual tissue regions as well as combined epithelium and stroma (fibroglandular area).
Assessment of alcohol consumption
Information on alcohol consumption was obtained from semi-quantitative food frequency questionnaires (FFQ) [34]. In NHS, questions regarding alcohol consumption were asked in 1980, 1984, 1986, and 1990. Women reported their average consumption of beer, wine, and liquor separately in the prior year. One drink was considered equal to one can or bottle of beer, a 4-ounce glass of wine, or one drink or shot of liquor. Participants were asked to select from the following categories: almost never, 1–3 per month, 1 per week, 2–4 per week, 5–6 per week, 1 per day, 2–3 per day, 4–6 per day, ≥ 6 per day. Similarly, women in NHS II answered questions on alcohol consumption in the 1989 and 1991 questionnaires. In 1991, the questions were expanded to include red wine, white wine, light beer, regular beer, and liquor. Total alcohol consumption per questionnaire cycle was calculated as the sum of the daily number of drinks multiplied by the average alcohol content per type of alcoholic beverage (12.8 g for regular beer, 11.3 for light beer, 11.0 g for wine, and 14.0 g for liquor) [20, 35]. Alcohol consumption in these cohorts has been shown to be valid and highly reproducible in repeated assessments [36].
Women were assigned the alcohol exposure from the cycle closest to the date of the benign biopsy. If alcohol consumption was missing from the questionnaire before the biopsy date, the exposure from the preceding cycle was used (9% of women in our sample) [20]. The Spearman correlation between reported alcohol consumption in questionnaire cycles was 0.80 (P < 0.0001) or greater for all consecutive cycles [37]. In the current analysis, we used both a continuous (per 11 g/day [1 drink/day]) as well as categorical measure of alcohol consumption (0 [reference], < 11 g/day [< 1 drink/day], 11–< 22 g/day [1–< 2 drinks/day], and ≥ 22 g/day [≥ 2 drinks/day]). Median levels within respective categories were used for the test of trend. We also examined cumulative average alcohol consumption using all available data from before the biopsy date, with the same variable modeling approaches.
Covariate information
Information on breast cancer risk factors was obtained from the biennial questionnaires closest to the date of the biopsy. Women were considered to be postmenopausal if they reported: (1) no menstrual periods within the 12 months before biopsy with natural menopause, (2) bilateral oophorectomy, or (3) hysterectomy with one or both ovaries retained, and were 54 years or older for ever smokers or 56 years or older for never smokers [38, 39].
Statistical analysis
We used multivariable linear regression to examine the associations of alcohol consumption with proportion of epithelial, stromal, fibroglandular, and fat tissues. Because tissue type measures were non-normally distributed, Both visual inspection of Q–Q plots for all tissue measures and formal statistical tests demonstrated that residuals were not normally distributed (p values for all tests < 0.01); therefore, we used log-transformed values for all tissue measures in all the regression analyses to improve normality of the error distribution. The risk estimates were adjusted for age (continuous), BMI (continuous), a family history of breast cancer (yes vs. no), combined parity/age at first birth (parous with first birth before age 25, parous with first birth at or after age 25, nulliparous, unknown), age at menarche (< 12, 12, 13, > 13), menopausal status/postmenopausal hormone use (pre-, post-/no hormones, post-/past hormone use, post-/current hormone use, post-/unknown hormone use status), and study cohort (NHS, NHSII). Finally, to account for potential influence of BBD lesions on the study findings, we additionally adjusted all models for type of the BBD.
Differences in the associations of alcohol with tissue measures in pre- versus postmenopausal women were evaluated with two-way interactions and using Wald Chi-square test. We used respective medians within each of the alcohol consumption categories to model the interaction as well as the continuous alcohol consumption. All tests were 2-sided and statistical significance in all the analyses was assessed at 0.05 level. The analyses were performed using SAS software (version 9.4, SAS Institute, Cary, NC, USA).
Results
In this study of 857 women, 260 (30.3%) had non-proliferative disease, 484 (56.5%) had proliferative disease without atypia, and 113 (13.2%) had atypical hyperplasia, consistent with previously reported distributions of these BBD subtypes [20]. The average age at the biopsy was 47 years (range 19–73 years). A majority of the women were premenopausal at the biopsy (62.5%). Age-adjusted characteristics of pre- and postmenopausal women in the study by their cumulative average alcohol consumption status are presented in Table 1. In our study, 18.1% consumed ≥ 11 g (≥ 1 drinks) of alcohol per day at the time of biopsy and 17.7% had the cumulative average consumption of ≥ 11 g (≥ 1 drinks). Recent and cumulative average alcohol use were highly correlated (correlation coefficient r = 0.90, p < 0.001). The average percentage of epithelium, stroma, and fat was 9.0% (range 0.7–52.2%), 72.4% (23.6–99.0%), and 18.6% (0–71.3%), respectively.
In multivariable analysis (Table 2), alcohol consumption was inversely associated with proportion of stroma and fibroglandular tissue; these associations were most pronounced for recent consumption of ≥ 22 g (≥ 2 drinks) per day (stroma: β = − 0.08, 95% Confidence Interval [CI] − 0.13; − 0.03; fibroglandular: β = − 0.08, 95% CI − 0.13; − 0.04) and cumulative average consumption of ≥ 22 g (≥ 2 drinks) per day (stroma β = − 0.08, 95% CI − 0.13; − 0.02; fibroglandular: β = − 0.09, 95% CI − 0.14; − 0.04). Alcohol consumption of ≥ 22 g/day was also positively associated with proportion of fat (β = 0.30, 95% CI 0.03; 0.57 for recent and β = 0.32, 95% CI 0.04; 0.61 for cumulative average). Alcohol was not associated with proportion of epithelium.
In stratified analysis by menopausal status (Tables 3 and 4), alcohol consumption was inversely associated with proportion of stroma and fibroglandular tissue and positively associated with proportion of fat in postmenopausal women. The strongest associations were observed for recent alcohol consumption of ≥ 22 g/day (stroma: β = − 0.14, 95% CI − 0.24; − 0.05; fibroglandular: β = − 0.16, 95% CI − 0.25; − 0.08; fat: β = 0.79, 95% CI 0.28; 1.31) and cumulative average consumption of ≥ 22 g/day (stroma β = − 0.16, 95% CI − 0.28; − 0.05; fibroglandular: β = − 0.18, 95% CI − 0.28; − 0.07; fat: β = 0.61, 95% CI 0.01; 1.22). Alcohol consumption was not associated with tissue measures in premenopausal women. We found significant interactions of alcohol consumption with menopausal status for recent continuous alcohol consumption in relation to fibroglandular tissue (p-interaction = 0.02) and interactions of continuous cumulative alcohol use with menopausal status in relation to stroma (p-interaction = 0.02), fat (p-interaction = 0.01), and fibroglandular tissue (p-interaction = 0.01) that were driven by positive associations in postmenopausal women and no associations observed among premenopausal women. A few other interactions between menopausal status and alcohol consumption also showed marginal significance (Additional file 1: Table S1).
Discussion
In this study of 857 cancer-free women, alcohol consumption was inversely associated with proportion of stroma and fibroglandular tissue and positively associated with fat, with more pronounced effects for recent consumption of ≥ 22 g/day (≥ 2 drinks/day) and cumulative average consumption of ≥ 22 g/day (≥ 2 drinks/day). In stratified analysis by menopausal status, these associations persisted in postmenopausal women only.
We found an inverse association of alcohol with stroma and fibroglandular tissue. Previous studies of alcohol and mammographic breast density generally have shown positive associations [6]. The findings in postmenopausal women have been conflicting [40, 41]. Mammographic breast density is more reflective of alterations in stromal composition rather than epithelium [42]. In contrast to our a priori hypothesis, our results do not suggest that alcohol could influence breast density by its influence on stroma. However, our results are based on a direct measurement of biopsy sample retrieved from a specific area of the breast while studies of mammographic density take into account the overall breast density pattern of the entire breast or the average density measurements of both breasts. Second, some of the previous studies are based on radiologist-assisted density estimation while our study employs computerized assessment of breast histopathological images.
In terms of the potential mechanisms behind alcohol-associated increase in breast cancer risk, by reducing the extent of stroma, alcohol may be potentially suppressing the protective role of stroma in sustaining normal breast tissue structure and function via a variety of signaling mechanisms that control and regulate normal processes and suppress malignant transformation [43–47]. So these findings could potentially offer new and previously unrecognized mechanisms of alcohol’s contributions to breast carcinogenesis such as epithelial-stromal interactions. However, future studies are warranted to confirm our findings and to elucidate biological mechanisms behind the observed associations.
We also observed positive associations of alcohol with the proportion of fat. In our earlier study of alcohol and breast density in postmenopausal women from another nested case–control study within NHS/NHSII, we reported an inverse association of alcohol with absolute non-dense area on the mammogram, which is represented by adipose tissue [48]. Similar association was also observed in another study in postmenopausal women [41]. This inverse association could potentially be explained by alcohol’s fat-reducing effect on various tissues, including breast, due to high energy demanding nature of the microsomal ethanol oxidation which dominates in women [49] as well as alcohol-associated increase in thermogenesis [41, 50]. However, our findings from the current study with direct measurement of tissue composition suggests a greater proportion of fat in women with greater alcohol consumption limited to postmenopausal women. During menopausal transition, breast tissue undergoes significant remodeling with involution of breast lobules with a reduction in the number and size of the acini per lobule and replacement of the intralobular stroma with dense collagen and, eventually, fatty tissue [51]. Some studies in animal models also suggest that estrogen may promote involution by exacerbating inflammation, cell death and adipocytes repopulation [52]. Thus, the associations observed in postmenopausal women could potentially be reflective of the effects of alcohol on involution, via either direct or estrogen-mediated mechanisms. Additionally, much higher levels of estrogens in premenopausal as compared to postmenopausal women may have dominating effects on breast tissue composition as compared to any effects of alcohol. These conflicting results could potentially be explained by the reasons noted above.
To our knowledge, this is the first study to explore the associations of alcohol with the proportion of epithelium, stroma, fibroglandular, and fat tissues using breast histopathological images. The analysis used data from the NHS and NHSII, established cohorts with more than 30 years of follow-up, confirmed benign breast disease status, and comprehensive information on breast cancer risk factors. Our study has a few limitations. Despite the prospective nature of the cohort, potential errors in reporting of alcohol consumption are possible. However, previous validation studies suggest reasonable reproducibility and validity of the data from food frequency questionnaires for the use in studies of associations between diet and health outcomes in epidemiologic studies [53, 54]. High accuracy in self-reported alcohol consumption has been reported in both men and women, including the NHS [36, 55, 56]. There was a high correlation between alcohol intake reported on FFQ and that assessed by multiple week diet records over the same period (r = 0.90). Moreover, four years after completing the diet record, another assessment was done to collect self-reported alcohol intake over the previous 4 years. These measures were highly correlated as well (r = 0.84). This evidence suggests that a FFQ provides reliable and sufficiently accurate information on alcohol intake over an extended period of time for use in epidemiologic investigations [36]. Next, breast tissue composition was measured on a whole tissue section from a biopsy which represents a smaller piece of tissue and it is unclear how generalizable it is to the rest of the breast, which may also explain the differences in our findings as compared to those for associations between alcohol and mammographic breast density. However, our previous work demonstrates that this sampling approach still provides strong evidence for a priori hypotheses and meaningful findings for breast tissue involution [57], identification of markers associated with breast cancer risk [14, 26, 58], and associations with known breast cancer risk factors, suggesting that this limitation has minimal impact on research findings [59]. Further, a study from Mayo BBD cohort has shown a large concordance in lobular involution across all four quadrants of the breast [51], suggesting low heterogeneity and thus minimum potential measurement error. Finally, our study includes only cancer-free women with clinically-indicated biopsy resulting in BBD diagnosis and since the analysis of the whole slide images included both the background normal tissue and benign lesions, the findings are expected to be generalizable to cancer-free women with BBD.
Conclusions
Our findings suggest that alcohol consumption is associated with smaller proportion of stroma and fibroglandular tissue and a greater proportion of fat in postmenopausal women. Future studies are warranted to confirm our findings and to elucidate the underlying biological mechanisms.
Availability of data and materials
Data are available upon reasonable written request. According to standard controlled access procedure, applications to use NHS/NHSII/HPFS resources will be reviewed by our External Collaborators Committee for scientific aims, evaluation of the fit of the data for the proposed methodology, and verification that the proposed use meets the guidelines of the Ethics and Governance Framework and the consent that was provided by the participants. Investigators wishing to use NHS/NHSII/HPFS data are asked to submit a brief description of the proposed project (go to https://www.nurseshealthstudy.org/researchers (contact email: nhsaccess@channing.harvard.edu) and https://sites.sph.harvard.edu/hpfs/for-collaborators/for details.
Abbreviations
- BBD:
-
Benign breast disease
- BMI:
-
Body Mass Index
- CI:
-
Confidence interval
- FFQ:
-
Food frequency questionnaire
- H&E:
-
Hematoxylin and eosin
- NHS:
-
Nurses’ Health Study
References
Scoccianti C, Lauby-Secretan B, Bello PY, Chajes V, Romieu I. Female breast cancer and alcohol consumption: a review of the literature. Am J Prev Med. 2014;46(3 Suppl 1):S16-25.
Boyle P, Boffetta P. Alcohol consumption and breast cancer risk. Breast Cancer Res. 2009;11(Suppl 3):S3.
McDonald JA, Goyal A, Terry MB. Alcohol intake and breast cancer risk: weighing the overall evidence. Curr Breast Cancer Rep. 2013. https://doi.org/10.1007/s12609-12013-10114-z.
Hirko KA, Chen WY, Willett WC, Rosner BA, Hankinson SE, Beck AH, Tamimi RM, Eliassen AH. Alcohol consumption and risk of breast cancer by molecular subtype: prospective analysis of the Nurses’ Health Study after 26 years of follow-up. Int J Cancer. 2016;138(5):1094–101.
Wang J, Heng YJ, Eliassen AH, Tamimi RM, Hazra A, Carey VJ, Ambrosone CB, de Andrade VP, Brufsky A, Couch FJ, et al. Alcohol consumption and breast tumor gene expression. Breast Cancer Res. 2017;19(1):108.
Ziembicki S, Zhu J, Tse E, Martin LJ, Minkin S, Boyd NF. The association between alcohol consumption and breast density: a systematic review and meta-analysis. Cancer Epidemiol Biomark Prev. 2017;26(2):170–8.
Liu Y, Tamimi RM, Colditz GA, Bertrand KA. Alcohol consumption across the life course and mammographic density in premenopausal women. Breast Cancer Res Treat. 2018;167(2):529–35.
Gapstur SM, López P, Colangelo LA, Wolfman J, Van Horn L, Hendrick RE. Associations of breast cancer risk factors with breast density in Hispanic women. Cancer Epidemiol Biomark Prev. 2003;12(10):1074–80.
Singletary KW, Gapstur SM. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA. 2001;286(17):2143–51.
Frydenberg H, Flote VG, Larsson IM, Barrett ES, Furberg AS, Ursin G, Wilsgaard T, Ellison PT, McTiernan A, Hjartåker A, et al. Alcohol consumption, endogenous estrogen and mammographic density among premenopausal women. Breast Cancer Res. 2015;17(1):103.
Qureshi SA, Couto E, Hofvind S, Wu AH, Ursin G. Alcohol intake and mammographic density in postmenopausal Norwegian women. Breast Cancer Res Treat. 2012;131(3):993–1002.
Pollak M. Insulin-like growth factor physiology and cancer risk. Eur J Cancer. 2000;36(10):1224–8.
Pettersson A, Graff RE, Ursin G, Silva IDS, McCormack V, Baglietto L, et al. Mammographic density phenotypes and risk of breast cancer: a meta-analysis. JNCI J Cancer Inst. 2014. https://doi.org/10.1093/jnci/dju078.
Tamimi RM, Colditz GA, Wang Y, Collins LC, Hu R, Rosner B, Irie HY, Connolly JL, Schnitt SJ. Expression of IGF1R in normal breast tissue and subsequent risk of breast cancer. Breast Cancer Res Treat. 2011;128(1):243–50.
Collins LC, Baer HJ, Tamimi RM, Connolly JL, Colditz GA, Schnitt SJ. The influence of family history on breast cancer risk in women with biopsy-confirmed benign breast disease: results from the Nurses’ Health Study. Cancer. 2006;107(6):1240–7.
Tamimi RM, Byrne C, Colditz GA, Hankinson SE. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2007;99(15):1178–87.
Vachon CM, Kuni CC, Anderson K, Anderson VE, Sellers TA. Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States). Cancer Causes Control. 2000;11(7):653–62.
Tamimi RM, Rosner B, Colditz GA. Evaluation of a breast cancer risk prediction model expanded to include category of prior benign breast disease lesion. Cancer. 2010;116(21):4944–53.
Jacobs TW, Byrne C, Colditz G, Connolly JL, Schnitt SJ. Pathologic features of breast cancers in women with previous benign breast disease. Am J Clin Pathol. 2001;115(3):362–9.
Tamimi RM, Byrne C, Baer HJ, Rosner B, Schnitt SJ, Connolly JL, Colditz GA. Benign breast disease, recent alcohol consumption, and risk of breast cancer: a nested case–control study. Breast Cancer Res. 2005;7(4):R555.
Page DL, Dupont WD, Rogers LW, Rados MS. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer. 1985;55(11):2698–708.
Kensler KH, Liu EZF, Wetstein SC, Onken AM, Luffman CI, Baker GM, Collins LC, Schnitt SJ, Bret-Mounet VC, Veta M, et al. Automated quantitative measures of terminal duct lobular unit involution and breast cancer risk. Cancer Epidemiol Biomark Prev. 2020;29(11):2358–68.
Yaghjyan L, Austin-Datta RJ, Oh H, Heng YJ, Vellal AD, Sirinukunwattana K, Baker GM, Collins LC, Murthy D, Rosner B, et al. Associations of reproductive breast cancer risk factors with breast tissue composition. Breast Cancer Res. 2021;23(1):70.
Vellal AD, Sirinukunwattan K, Kensler KH, Baker GM, Stancu AL, Pyle ME, et al. Deep learning image analysis of benign breast disease to identify subsequent risk of breast cancer. JNCI Cancer Spectr. 2021. https://doi.org/10.1093/jncics/pkaa119.
Yaghjyan L, Pettersson A, Colditz GA, Collins LC, Schnitt SJ, Beck AH, Rosner B, Vachon C, Tamimi RM. Postmenopausal mammographic breast density and subsequent breast cancer risk according to selected tissue markers. Br J Cancer. 2015;113(7):1104–13.
Oh H, Eliassen AH, Wang M, Smith-Warner SA, Beck AH, Schnitt SJ, Collins LC, Connolly JL, Montaser-Kouhsari L, Polyak K, et al. Expression of estrogen receptor, progesterone receptor, and Ki67 in normal breast tissue in relation to subsequent risk of breast cancer. NPJ Breast Cancer. 2016;2:16032.
Beca F, Kensler K, Glass B, Schnitt SJ, Tamimi RM, Beck AH. EZH2 protein expression in normal breast epithelium and risk of breast cancer: results from the Nurses’ Health Studies. Breast Cancer Res. 2017;19(1):21.
Sisti JS, Collins LC, Beck AH, Tamimi RM, Rosner BA, Eliassen AH. Reproductive risk factors in relation to molecular subtypes of breast cancer: results from the nurses’ health studies. Int J Cancer. 2016;138(10):2346–56.
Tamimi RM, Baer HJ, Marotti J, Galan M, Galaburda L, Fu Y, Deitz AC, Connolly JL, Schnitt SJ, Colditz GA, et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res BCR. 2008;10(4):R67–R67.
Yaghjyan L, Colditz GA, Collins LC, Schnitt SJ, Rosner B, Vachon C, Tamimi RM. Mammographic breast density and subsequent risk of breast cancer in postmenopausal women according to tumor characteristics. J Natl Cancer Inst. 2011;103(15):1179–89.
Howat WJ, Blows FM, Provenzano E, Brook MN, Morris L, Gazinska P, Johnson N, McDuffus L-A, Miller J, Sawyer EJ, et al. Performance of automated scoring of ER, PR, HER2, CK5/6 and EGFR in breast cancer tissue microarrays in the breast cancer association consortium. J Pathol Clin Res. 2015;1(1):18–32.
Knutsvik G, Stefansson IM, Aziz S, Arnes J, Eide J, Collett K, Akslen LA. Evaluation of Ki67 expression across distinct categories of breast cancer specimens: a population-based study of matched surgical specimens, core needle biopsies and tissue microarrays. PLoS ONE. 2014;9(11):e112121.
Bartlett JMS, Christiansen J, Gustavson M, et al. Validation of the IHC4 breast cancer prognostic algorithm using multiple approaches on the multinational TEAM clinical trial. Arch Pathol Lab Med. 2016;140(1):66–74.
Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC. Dietary protein sources in early adulthood and breast cancer incidence: prospective cohort study. BMJ. 2014;348:g3437.
Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306(17):1884–90.
Glovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, Willett WC. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133(8):810–7.
Tamimi RM, Byrne C, Baer HJ, Rosner B, Schnitt SJ, Connolly JL, Colditz GA. Benign breast disease, recent alcohol consumption, and risk of breast cancer: a nested case-control study. Breast Cancer Res BCR. 2005;7(4):R555-562.
Willett W, Stampfer MJ, Bain C, Lipnick R, Speizer FE, Rosner B, Cramer D, Hennekens CH. Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117(6):651–8.
Stampfer MJ, Willett WC, Colditz GA, Rosner B, Speizer FE, Hennekens CH. A prospective study of postmenopausal estrogen therapy and coronary heart disease. N Engl J Med. 1985;313(17):1044–9.
Rice MS, Bertrand KA, Lajous M, Tamimi RM, Torres G, López-Ridaura R, Romieu I. Reproductive and lifestyle risk factors and mammographic density in Mexican women. Ann Epidemiol. 2015;25(11):868–73.
Brand JS, Czene K, Eriksson L, Trinh T, Bhoo-Pathy N, Hall P, Celebioglu F. Influence of lifestyle factors on mammographic density in postmenopausal women. PLoS ONE. 2013;8(12):e81876.
Ironside AJ, Jones JL. Stromal characteristics may hold the key to mammographic density: the evidence to date. Oncotarget. 2016;7(21):31550–62.
Conklin MW, Keely PJ. Why the stroma matters in breast cancer: insights into breast cancer patient outcomes through the examination of stromal biomarkers. Cell Adh Migr. 2012;6(3):249–60.
Barcellos-Hoff MH, Medina D. New highlights on stroma-epithelial interactions in breast cancer. Breast Cancer Res BCR. 2005;7(1):33–6.
Nazari SS, Mukherjee P. An overview of mammographic density and its association with breast cancer. Breast Cancer. 2018;25(3):259–67.
Kim JB, Stein R, O’Hare MJ. Tumour-stromal interactions in breast cancer: the role of stroma in tumourigenesis. Tumor Biol. 2005;26(4):173–85.
Buchsbaum RJ, Oh SY. Breast cancer-associated fibroblasts: where we are and where we need to go. Cancers. 2016. https://doi.org/10.3390/cancers8020019.
Yaghjyan L, Colditz G, Eliassen H, Rosner B, Gasparova A, Tamimi RM. Interactions of alcohol and postmenopausal hormone use in regards to mammographic breast density. Cancer Causes Control. 2018;29(8):751–8.
Lands WE, Zakhari S. The case of the missing calories. Am J Clin Nutr. 1991;54(1):47–8.
Westerterp KR. Diet induced thermogenesis. Nutr Metab. 2004;1:5–5.
Ghosh K, Hartmann LC, Reynolds C, Visscher DW, Brandt KR, Vierkant RA, Scott CG, Radisky DC, Sellers TA, Pankratz VS, et al. Association between mammographic density and age-related lobular involution of the breast. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(13):2207–12.
Lim CL, Or YZ, Ong Z, Chung HH, Hayashi H, Shrestha S, Chiba S, Lin F, Lin VCL. Estrogen exacerbates mammary involution through neutrophil-dependent and -independent mechanism. Elife. 2020;9:e57274.
Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69(2):243–9.
Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility, and validity, of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65.
Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003;98:1–12.
Feunekes GI, Veer PV, van Staveren WA, Kok FJ. Alcohol intake assessment: the sober facts. Am J Epidemiol. 1999;150(1):105–12.
Rice MS, Tamimi RM, Connolly JL, Collins LC, Shen D, Pollak MN, Rosner B, Hankinson SE, Tworoger SS. Insulin-like growth factor-1, insulin-like growth factor binding protein-3 and lobule type in the Nurses’ health study II. Breast Cancer Res. 2012;14(2):1–7.
Huh SJ, Oh H, Peterson MA, Almendro V, Hu R, Bowden M, Lis RL, Cotter MB, Loda M, Barry WT, et al. The proliferative activity of mammary epithelial cells in normal tissue predicts breast cancer risk in premenopausal women. Cancer Res. 2016;76(7):1926–34.
Oh H, Eliassen AH, Beck AH, Rosner B, Schnitt SJ, Collins LC, Connolly JL, Montaser-Kouhsari L, Willett WC, Tamimi RM. Breast cancer risk factors in relation to estrogen receptor, progesterone receptor, insulin-like growth factor-1 receptor, and Ki67 expression in normal breast tissue. NPJ Breast Cancer. 2017;3(1):39.
Acknowledgements
The authors would like to acknowledge the contribution to this study from central cancer registries supported through the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and/or the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. Central registries may also be supported by state agencies, universities, and cancer centers. Participating central cancer registries include the following: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Indiana, Iowa, Kentucky, Louisiana, Massachusetts, Maine, Maryland, Michigan, Mississippi, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Puerto Rico, Rhode Island, Seattle SEER Registry, South Carolina, Tennessee, Texas, Utah, Virginia, West Virginia, Wyoming.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (CA240341 to L.Y., CA131332, CA175080, P01 CA087969 to R.M.T., UM1 CA186107 to Meir Stampfer, U01 CA176726 to Walter Willett), Avon Foundation for Women, Susan G. Komen for the Cure®, and Breast Cancer Research Foundation.
Author information
Authors and Affiliations
Contributions
Conceptualization: LY, RMT. Data curation: RT, YJH. Formal analysis: LY, BAR. Methodology: LY, RMT, YJH, GMB. Supervision: LY. Writing—original draft: LY, RMT. Writing—review & editing: LY, RMT, YJH, GMB, and BAR. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required. Consent was obtained or implied by return of questionnaires.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Table S1.
Interactions of alcohol with menopausal status in relation to proportion of breast tissue composition (p-values).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yaghjyan, L., Heng, Y.J., Baker, G.M. et al. Associations of alcohol consumption with breast tissue composition. Breast Cancer Res 25, 33 (2023). https://doi.org/10.1186/s13058-023-01638-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13058-023-01638-z