Abstract
Background
There is currently a lack of evidence for the comparative effectiveness of Andexanet alpha and four-factor prothrombin complex concentrate (4F-PCC) in anticoagulation reversal of direct oral anticoagulants (DOACs). The primary aim of our systematic review was to verify which drug is more effective in reducing short-term all-cause mortality. The secondary aim was to determine which of the two reverting strategies is less affected by thromboembolic events.
Methods
A systematic review and meta-analysis was performed.
Results
Twenty-two studies were analysed in the systematic review and quantitative synthesis. In all-cause short-term mortality, Andexanet alpha showed a risk ratio (RR) of 0.71(95% CI 0.37–1.34) in RCTs and PSMs, compared to 4F-PCC (I2 = 81%). Considering the retrospective studies, the pooled RR resulted in 0.84 (95% CI 0.69–1.01) for the common effects model and 0.82 (95% CI 0.63–1.07) for the random effects model (I2 = 34.2%). Regarding the incidence of thromboembolic events, for RCTs and PSMs, the common and the random effects model exhibited a RR of 1.74 (95% CI 1.09–2.77), and 1.71 (95% CI 1.01–2.89), respectively, for Andexanet alpha compared to 4F-PCC (I2 = 0%). Considering the retrospective studies, the pooled RR resulted in 1.21 (95% CI 0.87–1.69) for the common effects model and 1.18 (95% CI 0.86–1.62) for the random effects model (I2 = 0%).
Conclusion
Considering a large group of both retrospective and controlled studies, Andexanet alpha did not show a statistically significant advantage over 4F-PCC in terms of mortality. In the analysis of the controlled studies alone, Andexanet alpha is associated with an increased risk of thromboembolic events.
Clinical trial registration
PROSPERO: International prospective register of systematic reviews, 2024, CRD42024548768.
Similar content being viewed by others
Background
Direct oral anticoagulants (DOACs) are a class of drugs that act directly by inhibiting thrombin action or factor Xa, inhibiting both the intrinsic and the extrinsic pathways of the coagulation cascade. They possess advantages over the older vitamin K antagonists. In fact, their effects are more predictable than the latter and no laboratory monitoring is required. DOACs have been proven effective in treating thrombosis, such as deep venous thrombosis or pulmonary thromboembolism, as well as in preventing thromboembolic events in some pro-thrombotic conditions, such as atrial fibrillation [1, 2]. In case of haemorrhagic events, however, it becomes essential to counteract their anticoagulant effect effectively and quickly, without causing an increase in thromboembolic events. While the administration of vitamin K can reverse the effects of older vitamin K antagonists, there are several strategies to reverse the anticoagulant effect of DOACs. A monoclonal antibody (idarucizumab) has been developed to directly counteract the anticoagulant effects of dabigatran, which is a direct thrombin inhibitor [3]. For factor X inhibitors (such as rivaroxaban, apixaban, and edoxaban), the main reversal drug is the prothrombin complex (currently Four-factor prothrombin complex concentrate, i.e., 4F-PCC), which restores the level of molecules involved in the coagulation cascade [4]. Andexanet alpha is a modified recombinant inactive form of factor Xa that has been developed in recent years [5]. By binding and sequestering the molecules of the factor Xa inhibitor, it restores the thrombin generation mechanism. There is currently a lack of evidence for the comparative effectiveness of Andexanet alpha and 4F-PCC. A recently released randomized controlled trial, the ANNEXA-I trial raised further attention to the evidence gap, since results had shown that Andexanet alpha is more effective in limiting the expansion of hematoma in cases of intracerebral haemorrhage, but its use is associated with a greater incidence of thromboembolic events [6]. The scientific discussion on the topic was also fostered due to some intrinsic limitations of the study. In fact, it has been argued the outcome chosen as a measure of the effectiveness of the intervention is not patient-centred and therefore may be considered only indirectly relevant from the clinical point of view.
To fill the gap in the field with updated evidence, the primary aim of our systematic review was to verify which drug (Andexanet alpha or 4F-PCC) is more effective in reducing short-term all-cause mortality in anticoagulation reversal. We would verify this outcome both in cases of intracerebral haemorrhage (ICH) and non-intracerebral haemorrhage (such as gastrointestinal haemorrhage, traumatic haemorrhage, etc.). The secondary aim was to determine which of the two reverting strategies is less affected by thromboembolic events.
Methods
A systematic review and meta-analysis of the literature was performed. The protocol of this review was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO, CRD42024548768), and we reported this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement reporting guidelines [7].
Eligibility criteria, search strategy and data collection
We considered any study that investigated the administration of Andexanet alpha or 4F-PCC to reverse an anticoagulation effect caused by a DOAC in cases of haemorrhage. Our search included randomized controlled trials (RCTs), observational prospective/retrospective studies, retrospective studies with propensity score matching and interventional studies. Qualitative studies, editorials, comments, letters to the editor, conference papers, case reports, clinical guidelines, or literature reviews with or without meta-analysis were excluded. Studies involving non-adult participants (i.e., < 18 years old), pregnant patients, animal subjects, and those that did not report outcome data were also excluded.
Searches were conducted using the electronic biomedical databases PubMed, Scopus, and Cumulative Index to Nursing and Allied Health Literature (CINHAL). To ensure a comprehensive synthesis of the available literature, existing meta-analyses on the same topic retrieved during the screening phase were retrieved and analysed to select relevant studies for inclusion. Search strings for each database were developed by one researcher (DO). Search strings were peer-reviewed prior to the execution [8] by an experienced researcher (FF) following the Peer Review of Electronic Search Strategies (PRESS) guidelines checklist [9]. Search results were imported into the Covidence platform by Veritas Health Innovation Ltd. The selection process consisted of two phases: title/abstract screening and full-text screening. After duplicate results were removed, the screening was performed independently and blindly by two researchers (TB and DO). When there were disagreements regarding article eligibility, a consensus was reached by rediscussing conflicting cases, and the final decision was made after discussion until a consensus was reached involving a third researcher (AB). The following data were extracted: name of the author(s), year, study design, sample size(s), the indication of reversal of anticoagulation (i.e., ICH or non-ICH), number of deaths in the intervention group and in the control group, thromboembolic events in the intervention group and in the control group. If available, the number of patients with a Rankin score > 3 in the intervention and control groups was reported. Any other variables reported in the studies were analysed and included when relevant to the systematic review's question. An electronic data extraction form was implemented using the Covidence platform and piloted with at least three of the articles selected to ensure its usefulness, appropriateness, and feasibility [8, 10]. The data was extracted cooperatively by two data extractors (DO and TB) who were previously trained and had the appropriate topic knowledge. Rediscussing conflicting cases led to a consensus for data extraction, and the final decision was made after the consensus was reached.
Risk of bias assessment
The risk of bias (RoB) was assessed independently by two authors (DO and FF). For the controlled trials, the Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB2) was used to assess the risk of bias [11]. In all remaining included studies, Robins-I (Risk Of Bias In Non-randomized Studies—of Interventions) was utilized [12]. In case of conflicting judgments, the authors discussed until they reached a consensus to resolve any disagreements.
Statistical analysis
In the execution of the meta-analysis, a binary outcome (number of events for each of the two groups) was identified. Fixed-effect and random-effect analyses were conducted. The risk ratio was calculated using the Mantel–Haenszel method in the initial case. In the second case, the inverse-variance method was used. The I2 statistic was used to assess between-study inconsistency. The meta-analysis findings were presented using forest plots.
Our research focused on the 'outliers' to identify the potential causes of heterogeneity in studies. We conducted an Influence Analysis to determine the most influential cases that determine the heterogeneity between studies. The indication for anticoagulation reversal (ICH or non-ICH) was used to plan an analysis of the sample subgroups. The main features of the included studies were used to conduct a meta-regression to identify the possible causes of heterogeneity between studies. To evaluate publication bias, a Funnel Plot was utilized.
Analyses were performed using R version 4.3.2, using the packages meta, dmetar, tidyverse, metafor, ggplot2, gridExtra, and robvis.
Results
Study selection and characteristics
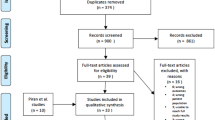
Six hundred twenty-three records were found during the initial identification process. We analysed 112 studies through full text after weeding out non-relevant and duplicate records (Fig. 1). Twenty-two studies were analysed in the systematic review and quantitative synthesis (Table 1).
Six studies were RCTs or retrospective studies in which the authors prepared some form of attenuation of the imbalance between the characteristics of the two groups (i.e., Propensity Score Matching, PSMs) [6, 13,14,15,16,17]. Three of these studies included patients with ICH [6, 13, 14]. Two studies considered patients with haemorrhages other than ICH [15, 16] and one considered both types of haemorrhagic events [17]. Only 4 studies reported the incidence of thrombotic events in the two groups of patients [6, 13,14,15]. The rate of patients with Rankin score was not reported in any of these studies [6, 13,14,15,16,17].
Sixteen studies were retrospective [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. Eleven studies included patients with ICH [18,19,20, 22,23,24, 27, 28, 30,31,32], while four studies included patients with non-ICH [21, 25, 26, 33], and one study included a mixed population [29]. Twelve studies reported the thrombotic events rate alongside the mortality rate. Only three studies reported the rate of patients with Rankin scores [18, 19, 30].
Risk of bias
Patient selection was the primary cause of bias in the studies that were included (Fig. 2). Retrospective studies were more susceptible to bias. Several studies have grouped and compared two distinct populations [20, 25, 29, 30]. The populations do not appear to be fully comparable, either because they were from different and independent previous trials or because the two groups in the study were enrolled at different times. The use of this methodological selection exposes these studies to a critical risk of bias. The inclusion of two study groups in a non-consecutive or sequential manner made the implicated studies more likely to influence researchers' awareness of the outcome for one of the two drugs studied. The controlled studies also raised concerns about the actual balance of the two study groups.
Quantitative synthesis for all-cause short-term mortality
The random effects model showed a risk ratio (RR) of 0.71 (95% CI 0.37–1.34) for Andexanet alpha group compared to 4F-PCC (as reference) in RCTs and PSMSs, as shown in Fig. 3. Since the confidence interval crosses the unit, the difference was not statistically significant. By subdividing the population based on the indication to anticoagulation reversal, the studies about ICH showed a RR of 0.94 (95% CI 0.04–20.40; I2 = 41.2%), the non-ICH studies showed a RR of 0.73 (95% CI 0.00–640.25; I2 = 83.8%), and the mixed population study showed a RR of 0.43 (95% CI 0.29–0.63) (Fig. 3S in Supplemental Material).
Forest plots for all-cause short-term mortality. A Forest plot for the controlled (RCT and PSM) studies. The random effects model exhibited a risk ratio (RR) of 0.71 (95% CI 0.37–1.34). Since the confidence interval crosses the unit, the difference was not statistically significant. B Forest plot for the retrospective studies. The pooled RR resulted in 0.84 (95% CI 0.69–1.01) for the common effects model and 0.82 (95% CI 0.63–1.07) for the random effects model
In the pooled model, the I2 was 81%. The study by Cohen et al. appeared to be particularly distant from the results of the other studies. When this study was removed, the model showed that the Andexanet alpha group had an RR of 0.82 (95% CI 0.38–1.79; I2 = 65.3%). According to meta-regression analysis, the study design type led to around 8% of heterogeneity (R2 = 7.83%). The funnel plot did not show a significant publication bias (Egger’s test p = 0.47) (Fig. 4).
Funnel plot for all-cause short-term mortality. A Funnel plot for the controlled (RCT and PSM) studies. Egger’s test was not statistically significant (p = 0.47). B Funnel plot for the retrospective studies. Egger’s test was statistically significant (p = 0.03). Some studies show an excess reduction of mortality in favour of the Andexanet alpha group
Considering the retrospective studies analysing Andexanet alpha versus 4F-PCC groups, the pooled RR resulted in 0.84 (95% CI 0.69–1.01) for the common effects model and 0.82 (95% CI 0.63–1.07) for the random effects model (Fig. 3). The pooled I2 was 34.2%. By subdividing the population based on the indication for anticoagulation reversal, the studies on Andexanet alpha versus 4F-PCC in ICH patients showed a RR of 0.79 (95% CI 0.63–1.00, I2 = 41.0%) (Fig. 4S in Supplemental Material). The non-ICH studies showed a RR of 0.93 (95% CI 0.65–1.32, I2 = 41.9%), and the mixed population study showed a RR of 0.89 (95% CI 0.37–2.12).
The study that differed most from the results of the other studies was that by Siepen et al. However, excluding it, the RR did not change substantially (RR 0.90; 95% CI 0.74–1.10 for the common effects model; RR 0.91; 95% CI 0.71–1.15 for the random effects model), although a reduction of the between-studies inconsistency was achieved (I2 = 12.4%). At meta-regression analysis, the different indications for anticoagulation reversal were not significantly responsible for a residual heterogeneity (R2 = 0%). The funnel plot showed a significant publication bias (Egger’s test p = 0.03) (Fig. 4).
Quantitative synthesis for the thromboembolic events
For RCTs and PSMSs, the common and random effects models respectively showed an RR of 1.74 (95% CI 1.09–2.77) and 1.71 (95% CI 1.01–2.89) for Andexanet alpha compared to 4F-PCC (Fig. 5). The pooled I2 was 0.
Forest plots for thromboembolic events. A For RCTs and PSMs, the common and the random effects model exhibited a RR of 1.74 (95% CI 1.09–2.77], and 1.71 (95% CI 1.01–2.89), respectively. B Forest plot for the retrospective studies. The pooled RR resulted in 0.84 (95% CI 0.69–1.01) for the common effects model and 0.82 (95% CI 0.63–1.07) for the random effects model. B Considering the retrospective studies, the pooled RR resulted in 1.21 (95% CI 0.87–1.69) for the common effects model and 1.18 (95% CI 0.86–1.62) for the random effects model
By subdividing the population based on the indication for anticoagulation reversal, the studies on the ICH population showed a RR for Andexanet alpha of 2.02 (95% CI 1.17–3.47) for the common effects model and 1.97 (95% CI 1.16–3.35); I2 = 41.2%) for the random effects model. In the non-ICH population studies, Andexanet alpha was found to have an RR of 1.13 (0.44–2.85) for the common effects model and 1.13 (0.44–2.85) for the random effects model compared to 4F-PCC (Fig. 7S in Supplemental Material). The funnel plot did not show a significant publication bias (Egger’s test p = 0.59) (Fig. 6).
Considering the retrospective studies, the pooled RR resulted in 1.21 (95% CI 0.87–1.69) for the common effects model and 1.18 (95% CI 0.86–1.62) for the random effects model for Andexanet alpha group vs 4F-PCC group (Fig. 5). The pooled I2 was 0.
By subdividing the population based on the indication for anticoagulation reversal, the studies about the ICH population showed a RR of 1.16 (95% CI 0.76–1.77, I2 = 0%) for the common effects model and 1.14 (95% CI 0.77–1.68) for Andexanet group (Fig. 8S in Supplemental Material). The non-ICH population studies showed a RR of 1.62 (95% CI 0.81- 3.25; I2 = 26%) for the common effects model, and 1.62 (95% CI 0.24–11.07) for the random effects model; for the mixed population study the RR was 0.88 (95% CI 0.34–2.23) for both the models. The funnel plot did not show a significant publication bias (Egger’s test p = 0.22) (Fig. 6).
Discussion
This review provides a summary of the evidence on the effectiveness of Andexanet alpha compared to 4F-PCC for short-term all-cause mortality. We found no statistically significant difference in the two comparison groups either in the controlled studies (RCTs and PSMs) or in the retrospective studies, either in the case of ICH or in the case of non-ICH. In this respect, while the high between-studies inconsistency makes the conclusions of the controlled studies less reliable, the low inconsistency of the pooled retrospective studies supports this conclusion. Regarding the incidence of thromboembolic events, the analysis of controlled studies shows an increase in relative risk in the Andexanet alpha group compared to the 4F-PCC group. This effect seems to be particularly significant for ICH-population studies. This conclusion is not confirmed by the analysis of retrospective studies, for which there is no different incidence of thromboembolic events between the two groups.
The rate of ICH due to factor Xa inhibitors is believed to be about one in 500–1000 per patient-year [34] and the rate of non-intracranial haemorrhages is near 19 per 100 patient-year [35]. Although DOACs seem at least as safe as the old Vitamin K antagonists in terms of the incidence of ICH (7% vs 11%) [36], evidence for the best reversal strategy for the anticoagulant effect is still lacking. Recently, the first (and so far, unique) RCT that directly compares Andexanet alpha to the usual therapy (i.e., 4F-PCC) was published, called ANNEXA-I [6]. The expectation of the results of this study was proportional to the controversies arising from its publication. The main criticisms were the primary outcome and the high patient rate in the control group without any treatment. The primary outcome of the trial was the expansion of intracerebral hematoma less than 35% of the volume at 12 h after administration of the drug. There was a statistically significant difference for the group of patients who were given Andexanet alpha (67.0% vs 53.1%) in this regard. However, the 30-day mortality rate was not statistically significant between the two groups. This result was achieved even though approximately 15% of patients in the control group did not receive any treatment (the so-called “passive reversal” strategy). In addition, the intervention group showed a higher rate of thromboembolic events (i.e., myocardial infarction, ischemic stroke, etc.) than the control group (10.3% vs 5.6%). Our meta-analysis confirms that there are no significant differences in short-term all-cause mortality between the two groups, which is consistent with the results of most of the studies (both controlled and retrospective). However, the ANNEXA-I study, like most of the studies included in this meta-analysis, was not designed to address mortality as a primary outcome. In fact, mortality is influenced by the location of bleeding, the patient's clinical condition, as well as the extent of bleeding [37]. In addition, ANNEXA-I is the only trial that has set inclusion criteria that exclude GCS score < 7 or NHISS score > 35, as well as scheduled surgery less than 12 h. Due to their retrospective nature, other studies on ICH patients do not have any specific exclusion criteria for severity of bleeding. However, ANNEXA-I did not demonstrate any significant advantage in using Andexanet-alpha over 4F-PCC in terms of short-term mortality. Although in this study patients were theoretically less severe than other studies, the overall mortality rate in the ANNEXA-I study was not significantly different from other studies on patients with ICH (23% for ANNEXA-I vs 27%, median value in the other studies). In this respect, the mortality rate was homogeneous (i.e., around 20%) for studies on non-ICH patients, while in studies on ICH population, the mortality rate varied from 8%, as reported by Oh et al. to 47% of Milioglou et al. (Table 2S in Supplemental Material).
Regarding disability, we found that most studies did not report this outcome or reported it in a non-standardized manner. This limitation prevents the possibility of evaluating the effectiveness of anticoagulation reversal based on this patient-centred outcome.
Our meta-analysis differs significantly from previous ones in terms of the risk of thromboembolic events in the Andexanet alpha group compared to the 4F-PCC group. In fact, we found a statistically significant higher incidence of thromboembolic events in the group receiving Andexanet alpha than in the 4F-PCC group (for controlled studies but not for retrospective studies). The data analysis shows that the ANNEXA-I trial has the most significant impact. In fact, this study has a weight of almost 60%. Most studies included in our meta-analysis were not published at the time of the previous meta-analyses [38,39,40]. For example, most studies in our meta-analysis were not included by Shrestha et al. and by da Luz et al. because they were not published during their meta-analyses [38, 39]. Compared to the meta-analysis of Chaudhary et al., our meta-analysis also includes studies that have enrolled patients with extra-cranial bleeding [41]. However, the main difference is related to the inclusion of the ANNEXA-I trial which, as already highlighted, drags the result of the meta-analysis regarding the safety outcome.
The effectiveness of Andexanet alpha in thrombin generation was found to be higher than 4F-PCC in a recent ex vivo study, but no significant difference was observed in the remaining haemostatic reversal tests [42]. The restoration of coagulative cascade factors is confirmed by previous in vitro studies, with Andexanet alpha showing greater thrombin restoring ability at low DOACs concentrations [43, 44]. However, regarding the main outcome of this meta-analysis, namely mortality, Andexanet alpha does not appear to be more effective than the current standard reference (i.e., 4F-PCC). The relevance of this result is based on the higher cost of Andexanet alpha compared to 4F-PCC. Due to the increased cost in comparison to the current 4F-PCC strategy, certain clinicians may be seeking an advantage to achieve strong patient-centred outcomes [45]. In addition, concerns about safety in terms of increased incidence of thromboembolic events—although demonstrated only in patients with ICH (but by the only RCT published so far)—are to be considered carefully and deserve to be investigated with additional RCTs.
Limitations
The main limitation of our meta-analysis lies in the impossibility of establishing disability (or functional recovery) as an outcome, which may be considered one of the most important (if not the most important at all) in terms of patient-centred outcomes research. This limitation is due to the low frequency with which studies explicitly or standardize this data. This is crucial since mortality, despite being another patient-centred outcome, is only indirectly linked to the use of a single drug and, instead, is due to various causal factors.
The nearest mortality was only considered because it has a more significant correlation with the haemorrhagic event than the 30-day mortality, which is not in line with previous meta-analyses' choices [40]. It must be noted that in certain studies, such as the nearest mortality study, 30-day mortality was taken into consideration.
Further limitation is the large rate of inconsistency between studies that we found in the primary outcome analysis of controlled studies. This, as already mentioned, is linked to the different types and qualities of the aggregate studies. Our analysis reveals that the quality of any of the studies considered is generally not optimal, especially for the selection and imbalance of the two groups of patients. Therefore, RCTs that are both high-quality and methodologically correct are needed.
Conclusion
Considering a large group of both retrospective and controlled studies, Andexanet alpha did not show a statistically significant advantage over 4F-PCC in terms of mortality. In the analysis of the controlled studies alone, Andexanet alpha is associated with an increased risk of thromboembolic events.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- DOACs:
-
Direct oral anticoagulants
- 4F-PCC:
-
Four-factor prothrombin complex concentrate
- ICH:
-
Intracerebral haemorrhage
- PROSPERO:
-
International prospective register of systematic reviews
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- RCT:
-
Randomized controlled trial(s)
- PRESS:
-
Peer review of electronic search strategies
- RoB:
-
Risk of bias
- RoB2:
-
Revised Cochrane risk-of-bias tool for randomized trials
- Robins-I:
-
Risk of bias in non-randomized studies of interventions
- PSM:
-
Propensity score matching
- RR:
-
Risk ratio
- 95% CI:
-
95% Confidence interval
References
Vinogradova Y, Coupland C, Hill T, Hippisley-Cox J. Risks and benefits of direct oral anticoagulants versus warfarin in a real world setting: cohort study in primary care. BMJ. 2018;362:k2505. https://doi.org/10.1136/bmj.k2505. Erratum in: BMJ. 2018; 363:k4413.
Gómez-Outes A, Terleira-Fernández AI, Lecumberri R, Suárez-Gea ML, Vargas-Castrillón E. Direct oral anticoagulants in the treatment of acute venous thromboembolism: a systematic review and meta-analysis. Thromb Res. 2014;134(4):774–82. https://doi.org/10.1016/j.thromres.2014.06.020.
Pollack CV Jr. Evidence supporting idarucizumab for the reversal of dabigatran. Am J Emerg Med. 2016;34(11S):33–8. https://doi.org/10.1016/j.ajem.2016.09.051.
Milioglou I, Farmakis I, Neudeker M, Hussain Z, Guha A, Giannakoulas G, Kotoula V, Papaioannou M. Prothrombin complex concentrate in major bleeding associated with DOACs; an updated systematic review and meta-analysis. J Thromb Thrombol. 2021;52(4):1137–50. https://doi.org/10.1007/s11239-021-02480-w.
Sartori M, Cosmi B. Andexanet alfa to reverse the anticoagulant activity of factor Xa inhibitors: a review of design, development and potential place in therapy. J Thromb Thrombol. 2018;45(3):345–52. https://doi.org/10.1007/s11239-018-1617-2.
Connolly SJ, Sharma M, Cohen AT, Demchuk AM, Członkowska A, Lindgren AG, Molina CA, Bereczki D, Toni D, Seiffge DJ, Tanne D, Sandset EC, Tsivgoulis G, Christensen H, Beyer-Westendorf J, Coutinho JM, Crowther M, Verhamme P, Amarenco P, Roine RO, Mikulik R, Lemmens R, Veltkamp R, Middeldorp S, Robinson TG, Milling TJ Jr, Tedim-Cruz V, Lang W, Himmelmann A, Ladenvall P, Knutsson M, Ekholm E, Law A, Taylor A, Karyakina T, Xu L, Tsiplova K, Poli S, Kallmünzer B, Gumbinger C, Shoamanesh A, ANNEXA-I Investigators. Andexanet for Factor Xa Inhibitor-Associated Acute Intracerebral Hemorrhage. N Engl J Med. 2024;390(19):1745–55. https://doi.org/10.1056/NEJMoa2313040.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. https://doi.org/10.1136/bmj.n160.
Franco JVA, Garrote V, Vietto V, Escobar Liquitay CM, Solà I. Search strategies (filters) to identify systematic reviews in MEDLINE and embase. Cochrane Database Syst Rev. 2020;2020(7):MR000054. https://doi.org/10.1002/14651858.MR000054.
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. https://doi.org/10.1016/j.jclinepi.2016.01.021.
Büchter RB, Weise A, Pieper D. Development, testing and use of data extraction forms in systematic reviews: a review of methodological guidance. BMC Med Res Methodol. 2020;20(1):259. https://doi.org/10.1186/s12874-020-01143-3.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. https://doi.org/10.1136/bmj.i4919.
Costa OS, Connolly SJ, Sharma M, Beyer-Westendorf J, Christoph MJ, Lovelace B, Coleman CI. Andexanet alfa versus four-factor prothrombin complex concentrate for the reversal of apixaban- or rivaroxaban-associated intracranial hemorrhage: a propensity score-overlap weighted analysis. Crit Care. 2022;26(1):180. https://doi.org/10.1186/s13054-022-04043-8.
Parsels KA, Seabury RW, Zyck S, Miller CD, Krishnamurthy S, Darko W, Probst LA, Latorre JG, Cwikla GM, Feldman EA. Andexanet alfa effectiveness and safety versus four-factor prothrombin complex concentrate (4F-PCC) in intracranial hemorrhage while on apixaban or rivaroxaban: A single-center, retrospective, matched cohort analysis. Am J Emerg Med. 2022. https://doi.org/10.1016/j.ajem.2022.02.036.
Keinath JJ, Lekura J, Hauser CD, Bajwa MK, Bloome ME, Kalus JS, Jones MC. Deterioration free discharge comparison of andexanet-alfa and prothrombin complex concentrates (PCC) for reversal of factor Xa inhibitor associated bleeds. J Thromb Thrombol. 2023;56(2):315–22. https://doi.org/10.1007/s11239-023-02840-8.
Sutton SS, Magagnoli J, Cummings TH, Dettling T, Lovelace B, Christoph MJ, Hardin JW. Real-world clinical outcomes among US Veterans with oral factor xa inhibitor-related major bleeding treated with andexanet alfa or 4-factor prothrombin complex concentrate. J Thromb Thrombolysis. 2023;56(1):137–146. https://doi.org/10.1007/s11239-023-02820-y. Erratum in: J Thromb Thrombolysis. 2023.
Cohen AT, Lewis M, Connor A, Connolly SJ, Yue P, Curnutte J, Alikhan R, MacCallum P, Tan J, Green L. Thirty-day mortality with andexanet alfa compared with prothrombin complex concentrate therapy for life-threatening direct oral anticoagulant-related bleeding. J Am Coll Emerg Phys Open. 2022;3(2):e12655. https://doi.org/10.1002/emp2.12655.
Barra ME, Das AS, Hayes BD, Rosenthal ES, Rosovsky RP, Fuh L, Patel AB, Goldstein JN, Roberts RJ. Evaluation of andexanet alfa and four-factor prothrombin complex concentrate (4F-PCC) for reversal of rivaroxaban- and apixaban-associated intracranial hemorrhages. J Thromb Haemost. 2020;18(7):1637–47. https://doi.org/10.1111/jth.14838.
Pham H, Medford WG, Horst S, Levesque M, Ragoonanan D, Price C, Colbassani H, Piper K, Chastain K. Andexanet alfa versus four-factor prothrombin complex concentrate for the reversal of apixaban- or rivaroxaban-associated intracranial hemorrhages. Am J Emerg Med. 2022;55:38–44. https://doi.org/10.1016/j.ajem.2022.02.029.
Siepen BM, Polymeris A, Shoamanesh A, Connolly S, Steiner T, Poli S, Lemmens R, Goeldlin MB, Müller M, Branca M, Rauch J, Meinel T, Kaesmacher J, Z’Graggen W, Arnold M, Fischer U, Peters N, Engelter ST, Lyrer P, Seiffge D. Andexanet alfa versus non-specific treatments for intracerebral hemorrhage in patients taking factor Xa inhibitors: individual patient data analysis of ANNEXA-4 and TICH-NOAC. Int J Stroke. 2024. https://doi.org/10.1177/17474930241230209.
Schmidt LE, Hinton MS, Martin ND. Real-world reversal of factor Xa inhibition in the setting of major life-threatening bleeding or urgent surgery. J Pharm Pract. 2024;37(1):74–9. https://doi.org/10.1177/08971900221125516.
Oh ES, Schulze P, Diaz F, Shah K, Rios J, Silverman ME. The use of andexanet alfa and 4-factor prothrombin complex concentrate in intracranial hemorrhage. Am J Emerg Med. 2023;64:74–7. https://doi.org/10.1016/j.ajem.2022.11.023.
Troyer C, Nguyen W, Xie A, Wimer D. Retrospective review of Andexanet Alfa versus 4-factor prothrombin complex concentrate for reversal of DOAC-associated intracranial hemorrhage. J Thromb Thrombol. 2023;55(1):149–55. https://doi.org/10.1007/s11239-022-02715-4.
Vestal ML, Hodulik K, Mando-Vandrick J, James ML, Ortel TL, Fuller M, Notini M, Friedland M, Welsby IJ. Andexanet alfa and four-factor prothrombin complex concentrate for reversal of apixaban and rivaroxaban in patients diagnosed with intracranial hemorrhage. J Thromb Thrombolysis. 2022;53(1):167–75. https://doi.org/10.1007/s11239-021-02495-3.
Stevens VM, Trujillo TC, Kiser TH, MacLaren R, Reynolds PM, Mueller SW. Retrospective comparison of andexanet alfa and 4-factor prothrombin complex for reversal of factor Xa-inhibitor related bleeding. Clin Appl Thromb Hemost. 2021. https://doi.org/10.1177/10760296211039020.
Sadek E, Curtiss W, Andrews J, Hecht J. Four-factor prothrombin complex concentrate versus andexanet alfa for the reversal of traumatic brain injuries. Emerg Med J. 2024;41(3):162–7. https://doi.org/10.1136/emermed-2023-213229.
Irizarry-Gatell VM, Bacchus MW, De Leo EK, Zhang Y, Lagasse CA, Khanna AY, Harris NS, Zumberg MS. The use of andexanet alfa vs. 4-factor prothrombin complex concentrates in the setting of life-threatening intracranial hemorrhage. Blood Coagul Fibrinol. 2024;35(3):94–100. https://doi.org/10.1097/MBC.0000000000001279.
Lipski M, Pasciolla S, Wojcik K, Jankowitz B, Igneri LA. Comparison of 4-factor prothrombin complex concentrate and andexanet alfa for reversal of apixaban and rivaroxaban in the setting of intracranial hemorrhage. J Thromb Thrombolysis. 2023;55(3):519–26. https://doi.org/10.1007/s11239-022-02752-z.
Singer AJ, Concha M, Williams J, Brown CS, Fernandes R, Thode HC Jr, Kirchman M, Rabinstein AA. Treatment of factor-Xa inhibitor-associated bleeding with andexanet alfa or 4 factor PCC: a multicenter feasibility retrospective study. West J Emerg Med. 2023;24(5):939–49. https://doi.org/10.5811/westjem.60587.
Huttner HB, Gerner ST, Kuramatsu JB, Connolly SJ, Beyer-Westendorf J, Demchuk AM, Middeldorp S, Zotova E, Altevers J, Andersohn F, Christoph MJ, Yue P, Stross L, Schwab S. Hematoma expansion and clinical outcomes in patients with factor-Xa inhibitor-related atraumatic intracerebral hemorrhage treated within the ANNEXA-4 trial versus real-world usual care. Stroke. 2022;53(2):532–43. https://doi.org/10.1161/STROKEAHA.121.034572.
Ammar AA, Ammar MA, Owusu KA, Brown SC, Kaddouh F, Elsamadicy AA, Acosta JN, Falcone GJ. Andexanet alfa versus 4-factor prothrombin complex concentrate for reversal of factor xa inhibitors in intracranial hemorrhage. Neurocrit Care. 2021;35(1):255–61. https://doi.org/10.1007/s12028-020-01161-5.
Milioglou L, Liao K, Traeger J, McKenzie C, Burrelli C, Khunayfir AKB, Makii J, Hoffer A. Reversal of factor Xa inhibitors associated intracranial haemorrhage at a tertiary medical centre. Blood Coagul Fibrinol. 2022;33(5):261–5. https://doi.org/10.1097/MBC.0000000000001128.
Koo SJ, Hussain Y, Booth DY, Desai P, Oh ES, Rios J, Audley K. Four-factor prothrombin complex concentrate versus andexanet alfa for direct oral anticoagulant reversal. J Am Pharm Assoc. 2003;64(2):395–401. https://doi.org/10.1016/j.japh.2023.11.015.
Heath M, Hall B, De Leon J, Gillespie R, Hasara S, Henricks B, Lakshmi M, Watson D, Wilson K. Comparative hemostatic efficacy of 4F-PCC in patients with intracranial hemorrhage on factor Xa inhibitors versus warfarin. Am J Emerg Med. 2022;57:149–52. https://doi.org/10.1016/j.ajem.2022.04.044.
Gue Y, Bloomfield D, Freedholm D, Lip GYH. Comparing the real-world and clinical trial bleeding rates associated with oral anticoagulation treatment for atrial fibrillation. J Clin Med. 2024;13(8):2277. https://doi.org/10.3390/jcm13082277.
Karamian A, Seifi A, Karamian A, Lucke-Wold B. Incidence of intracranial bleeding in mild traumatic brain injury patients taking oral anticoagulants: a systematic review and meta-analysis. J Neurol. 2024. https://doi.org/10.1007/s00415-024-12424-y.
Houben R, Schreuder FHBM, Bekelaar KJ, Claessens D, van Oostenbrugge RJ, Staals J. Predicting prognosis of intracerebral hemorrhage (ICH): performance of ICH score is not improved by adding oral anticoagulant use. Front Neurol. 2018;9:100. https://doi.org/10.3389/fneur.2018.00100.
Shrestha DB, Budhathoki P, Adhikari A, Shrestha S, Khati N, Mir WAY, Joshi T, Shrestha A. Efficacy and safety of andexanet alfa for bleeding caused by factor Xa inhibitors: a systematic review and meta-analysis. Cureus. 2021;13(12):e20632. https://doi.org/10.7759/cureus.20632.
da Luz LT, Marchand M, Nascimento B, Tien H, Nathens A, Shah P. Efficacy and safety of the drugs used to reverse direct oral anticoagulants: a systematic review and meta-analysis. Transfusion. 2017;57(7):1834–46. https://doi.org/10.1111/trf.14096.Erratum.In:Transfusion.2017;57(12):3069.
Luo C, Chen F, Chen YH, Zhao CF, Feng CZ, Liu HX, Luo DZQ. Prothrombin complex concentrates and andexanet for management of direct factor Xa inhibitor related bleeding: a meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25(6):2637–53. https://doi.org/10.26355/eurrev_202103_25428.
Chaudhary R, Singh A, Chaudhary R, Bashline M, Houghton DE, Rabinstein A, Adamski J, Arndt R, Ou NN, Rudis MI, Brown CS, Wieruszewski ED, Wanek M, Brinkman NJ, Linderbaum JA, Sorenson MA, Atkinson JL, Thompson KM, Aiyer AN, McBane RD 2nd. Evaluation of direct oral anticoagulant reversal agents in intracranial hemorrhage: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(11):e2240145. https://doi.org/10.1001/jamanetworkopen.2022.40145.
Rayatdoost F, Deventer K, Rossaint R, Schöchl H, Grottke O. Comparative analysis of andexanet alfa and prothrombin complex concentrate in reversing anticoagulation by rivaroxaban ex vivo. Br J Anaesth. 2024;132(2):251–9. https://doi.org/10.1016/j.bja.2023.10.018.
Lu G, Lin J, Bui K, Curnutte JT, Conley PB. Andexanet versus prothrombin complex concentrates: Differences in reversal of factor Xa inhibitors in in vitro thrombin generation. Res Pract Thromb Haemost. 2020;4(8):1282–94. https://doi.org/10.1002/rth2.12418.
Brinkman HJM, Zuurveld M, Meijers JCM. In vitro reversal of direct factor Xa inhibitors: direct comparison of andexanet alfa and prothrombin complex concentrates Cofact and Beriplex/Kcentra. Res Pract Thromb Haemost. 2022;6(5):e12775. https://doi.org/10.1002/rth2.12775.
Frontera JA, Bhatt P, Lalchan R, Yaghi S, Ahuja T, Papadopoulos J, Joset D. Cost comparison of andexanet versus prothrombin complex concentrates for direct factor Xa inhibitor reversal after hemorrhage. J Thromb Thrombolysis. 2020;49(1):121–31. https://doi.org/10.1007/s11239-019-01973-z.
Acknowledgements
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
DO was responsible for research and data extraction, statistical analysis, and the first draft of the manuscript; FF was responsible for the selection of titles and supervised the data extraction; IC was responsible for the selection of titles and supervised the data extraction; AB was responsible for the research and data extraction; and TB, EA and MS supervised the research.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Orso, D., Fonda, F., Brussa, A. et al. Andexanet alpha versus four-factor prothrombin complex concentrate in DOACs anticoagulation reversal: an updated systematic review and meta-analysis. Crit Care 28, 221 (2024). https://doi.org/10.1186/s13054-024-05014-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-024-05014-x