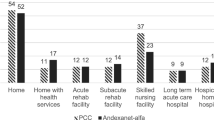
Abstract
Andexanet-alpha is a specific reversal agent for direct factor Xa inhibitors (dFXaI). We aimed to project utilization rates and cost of andexanet for reversal of dFXaI-related major hemorrhage compared to 4-factor prothrombin complex concentrates (4F-PCC). A retrospective, multicenter review was conducted between 1/1/2014 and 7/15/2018 of patients who received 4F-PCC for reversal of dFXaI-related life-threatening hemorrhages. Total hospital reimbursements/patient were calculated based on national average MS-DRG payments adjusting for Medicare discounts. The projected cost for andexanet (based on dose and insurance) and % reimbursement/patient was compared to the actual cost of 4F-PCC. Hemostasis at 24 h (excellent/good vs. poor) and 30-day thrombotic complications were assessed. Of 126 patients who received 4F-PCC to reverse dFXaI, 46 (~ 10 per-year) met inclusion criteria. The median projected cost of andexanet was $22,120/patient, compared to $5670/patient for 4F-PCC (P < 0.001). The median hospital reimbursement was $11,492/hospitalization. The projected cost of andexanet alone would exceed the entire hospital reimbursement in 74% of patients by a median of $7604, while 4F-PCC cost exceeded the total hospital payments in 7% of patients in the same cohort (P < 0.001). Hemostasis was excellent/good in 72% of patients post-4F-PCC, compared to 82% in andexanet trials. Thromboembolic events occurred in 4% of patients following 4F-PCC versus 10% in andexanet trials. The projected cost of andexanet would exceed the national average hospital reimbursement/patient in nearly 75% of patients by over $7500/hospitalization. 4F-PCC was significantly less expensive, had lower rates of thrombosis, but also lower rates of good/excellent hemostasis compared to published data for andexanet.


Similar content being viewed by others
References
Connolly SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, Lawrence JH, Yue P, Bronson MD, Lu G, Conley PB, Verhamme P, Schmidt J, Middeldorp S, Cohen AT, Beyer-Westendorf J, Albaladejo P, Lopez-Sendon J, Demchuk AM, Pallin DJ, Concha M, Goodman S, Leeds J, Souza S, Siegal DM, Zotova E, Meeks B, Ahmad S, Nakamya J, Milling TJ Jr, Investigators A- (2019) Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med 380(14):1326–1335. https://doi.org/10.1056/NEJMoa1814051
Schulman S, Gross PL, Ritchie B, Nahirniak S, Lin Y, Lieberman L, Carrier M, Crowther MA, Ghosh I, Lazo-Langner A, Zondag M, Study Investigators (2018) Prothrombin complex concentrate for major bleeding on factor Xa inhibitors: a prospective cohort study. Thromb Haemost 118(5):842–851. https://doi.org/10.1055/s-0038-1636541
Majeed A, Agren A, Holmstrom M, Bruzelius M, Chaireti R, Odeberg J, Hempel EL, Magnusson M, Frisk T, Schulman S (2017) Management of rivaroxaban- or apixaban-associated major bleeding with prothrombin complex concentrates: a cohort study. Blood 130(15):1706–1712. https://doi.org/10.1182/blood-2017-05-782060
Santibanez M, Lesch CA, Lin L, Berger K (2018) Tolerability and effectiveness of 4-factor prothrombin complex concentrate (4F-PCC) for warfarin and non-warfarin reversals. J Crit Care 48:183–190. https://doi.org/10.1016/j.jcrc.2018.08.031
Allison TA, Lin PJ, Gass JA, Chong K, Prater SJ, Escobar MA, Hartman HD (2018) Evaluation of the use of low-dose 4-factor prothrombin complex concentrate in the reversal of direct oral anticoagulants in bleeding patients. J Intensive Care Med. https://doi.org/10.1177/0885066618800657
Udayachalerm S, Rattanasiri S, Angkananard T, Attia J, Sansanayudh N, Thakkinstian A (2018) The reversal of bleeding caused by New Oral Anticoagulants (NOACs): a systematic review and meta-analysis. Clin Appl Thromb Hemost. https://doi.org/10.1177/1076029618796339
ATLS Subcommittee, American College of Surgeons’ Committee on Trauma, International ATLS Working Group (2012) Advanced trauma life support (ATLS®): the ninth edition. J Trauma Acute Care Surg 74(5):1363–1366
Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis (2005) Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 3(4):692–694. https://doi.org/10.1111/j.1538-7836.2005.01204.x
Frontera JA, Lewin JJ III, Rabinstein AA, Aisiku IP, Alexandrov AW, Cook AM, Del Zoppo GJ, Kumar M, Peerschke EI, Stiefel MF, Teitelbaum JS, Wartenberg KE, Zerfoss CL (2016) Guideline for reversal of antithrombotics in intracranial hemorrhage: executive Summary”. A statement for healthcare professionals from the Neurocritical Care Society and the Society of Critical Care Medicine. Crit Care Med 44(12):2251–2257. https://doi.org/10.1097/CCM.0000000000002057
Frontera JA, Lewin JJ 3rd, Rabinstein AA, Aisiku IP, Alexandrov AW, Cook AM, del Zoppo GJ, Kumar MA, Peerschke EI, Stiefel MF, Teitelbaum JS, Wartenberg KE, Zerfoss CL (2016) Guideline for reversal of antithrombotics in intracranial hemorrhage: a statement for healthcare professionals from the Neurocritical Care Society and Society of Critical Care Medicine. Neurocrit Care 24(1):6–46. https://doi.org/10.1007/s12028-015-0222-x
FDA (2019) Highlights of prescribing information: Andexxa. https://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM606687.pdf. Accessed 7 Mar 2019
CMS.gov (2019) New Medical Services and New Technologies. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-payment/AcuteInpatientPPS/newtech.html. Accessed 7 Mar 2019
Services DoHaH (2018) Federal Register 83 (160):41355-41362
CMS.gov (2016) Medicare-Provider-Charge-Data. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Inpatient2016.html. Accessed 7 Mar 2019
Connolly SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, Lawrence JH, Yue P, Bronson MD, Lu G, Conley PB, Verhamme P, Schmidt J, Middeldorp S, Cohen AT, Beyer-Westendorf J, Albaladejo P, Lopez-Sendon J, Demchuk AM, Pallin DJ, Concha M, Goodman S, Leeds J, Souza S, Siegal DM, Zotova E, Meeks B, Ahmad S, Nakamya J, Milling TJ Jr, ANNEXA-4 Investigators (2019) Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. https://doi.org/10.1056/NEJMoa1814051
Sarode R, Milling TJ Jr, Refaai MA, Mangione A, Schneider A, Durn BL, Goldstein JN (2013) Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation 128(11):1234–1243. https://doi.org/10.1161/CIRCULATIONAHA.113.002283
Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, Khoury J (1996) The ABCs of measuring intracerebral hemorrhage volumes. Stroke 27(8):1304–1305
Gebel JM, Sila CA, Sloan MA, Granger CB, Weisenberger JP, Green CL, Topol EJ, Mahaffey KW (1998) Comparison of the ABC/2 estimation technique to computer-assisted volumetric analysis of intraparenchymal and subdural hematomas complicating the GUSTO-1 trial. Stroke 29(9):1799–1801
Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES, Jr., MacDonald RL, Mayer SA (2006) Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery 59(1):21–27; discussion 21–27. https://doi.org/10.1227/01.neu.0000218821.34014.1b
Tomaselli GF, Mahaffey KW, Cuker A, Dobesh PP, Doherty JU, Eikelboom JW, Florido R, Hucker W, Mehran R, Messe SR, Pollack CV Jr, Rodriguez F, Sarode R, Siegal D, Wiggins BS (2017) 2017 ACC Expert Consensus Decision Pathway on management of bleeding in patients on oral anticoagulants: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 70(24):3042–3067. https://doi.org/10.1016/j.jacc.2017.09.1085
Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Collins R, Camm AJ, Heidbuchel H, ESC Scientific Document Group (2018) The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 39(16):1330–1393. https://doi.org/10.1093/eurheartj/ehy136
Niessner A, Tamargo J, Morais J, Koller L, Wassmann S, Husted SE, Torp-Pedersen C, Kjeldsen K, Lewis BS, Drexel H, Kaski JC, Atar D, Storey RF, Lip GYH, Verheugt FWA, Agewall S (2017) Reversal strategies for non-vitamin K antagonist oral anticoagulants: a critical appraisal of available evidence and recommendations for clinical management—a joint position paper of the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy and European Society of Cardiology Working Group on Thrombosis. Eur Heart J 38(22):1710–1716. https://doi.org/10.1093/eurheartj/ehv676
Lip GYH, Banerjee A, Boriani G, Chiang CE, Fargo R, Freedman B, Lane DA, Ruff CT, Turakhia M, Werring D, Patel S, Moores L (2018) Antithrombotic therapy for atrial fibrillation: CHEST Guideline and Expert Panel Report. Chest 154(5):1121–1201. https://doi.org/10.1016/j.chest.2018.07.040
Levy JH, Ageno W, Chan NC, Crowther M, Verhamme P, Weitz JI, Subcommittee on Control of Anticoagulation (2016) When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost 14(3):623–627. https://doi.org/10.1111/jth.13227
Pollack CV Jr, Reilly PA, van Ryn J, Eikelboom JW, Glund S, Bernstein RA, Dubiel R, Huisman MV, Hylek EM, Kam CW, Kamphuisen PW, Kreuzer J, Levy JH, Royle G, Sellke FW, Stangier J, Steiner T, Verhamme P, Wang B, Young L, Weitz JI (2017) Idarucizumab for dabigatran reversal—full cohort analysis. N Engl J Med 377(5):431–441. https://doi.org/10.1056/NEJMoa1707278
Piran S, Khatib R, Schulman S, Majeed A, Holbrook A, Witt DM, Wiercioch W, Schunemann HJ, Nieuwlaat R (2019) Management of direct factor Xa inhibitor-related major bleeding with prothrombin complex concentrate: a meta-analysis. Blood Adv 3(2):158–167. https://doi.org/10.1182/bloodadvances.2018024133
NCT03661528 (2019) Trial of andexanet in ICH patients receiving an oral FXa inhibitor. clinicaltrials.gov. Accessed 15 Apr 2019
Acknowledgements
This study was approved by the NYU Langone Hospitals IRB.
Author information
Authors and Affiliations
Contributions
J.A.F, T.A., J.P., S.Y. and D.J. contributed to study concept and design. J.A.F., P.B., R.L. and D.J. contributed to data acquisition and analysis. J.A.F reviewed and finalized the statistical analysis. J.A.F. and D.J. prepared the first draft of the manuscript. J.A.F, P.B., R.L., T.A., J.P., S.Y. and D.J. contributed to drafting and finalizing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Frontera, J.A., Bhatt, P., Lalchan, R. et al. Cost comparison of andexanet versus prothrombin complex concentrates for direct factor Xa inhibitor reversal after hemorrhage. J Thromb Thrombolysis 49, 121–131 (2020). https://doi.org/10.1007/s11239-019-01973-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-019-01973-z