Abstract
Acute brain injury (ABI) covers various clinical entities that may require invasive mechanical ventilation (MV) in the intensive care unit (ICU). The goal of MV, which is to protect the lung and the brain from further injury, may be difficult to achieve in the most severe forms of lung or brain injury. This narrative review aims to address the respiratory issues and ventilator management, specific to ABI patients in the ICU.
Similar content being viewed by others
Introduction
In patients with acute brain injury (ABI) the delivery of mechanical ventilation (MV) in the intensive care unit (ICU) involves adequate timing for intubation, lung protective ventilation (LPV), brain protection, and weaning. Unlike non-neurocritical patients, ABI patients usually have no primary respiratory indication for ventilator support, but often require prolonged MV, although they are often able to breathe spontaneously [1,2,3]. Better understanding the complex relationship between brain and respiration/ventilation and providing judicious respiratory support are critical issues.
Respiratory challenges in patients with ABI
Severe ABI refers to a sudden event that results in brain damage and reduced perfusion leading to reduced alertness. ABI is heterogeneous and covers different subtypes, notably traumatic brain injury (TBI), subarachnoid hemorrhage (SAH), intracranial bleeding and hypoxic ischemic brain injury. Since the brain is surrounded by the inextensible skull, any change affecting brain volume would result in an increase in intracranial pressure (ICP) and impairment of cerebral blood flow upon depletion of the compensatory reserve. Therefore, perfusion is tightly regulated by cerebral autoregulation (CA) to preserve cerebral blood flow facing variations in systemic pressure and metabolism. CA is a potent modulator of cerebral vasoreactivity [4]. Changes in PaCO2 alters CA: both hypo and hypercapnia can induce cerebral ischemia from the reduction of perfusion through vasoconstriction or vasodilatation, respectively, the latter also promoting higher ICP.
Respiratory dysfunction is common in ABI and can result from either dysregulation of the breathing patterns or acute lung injury.
Dysregulated respiratory centers function
The brainstem contains the respiratory centers responsible for regulating breathing. In ABI, the respiratory centers can be dysregulated either through direct damage to the brainstem or indirectly through an increase in ICP and mass effects due to cerebral hemorrhage or edema. Damage to the respiratory center leads to impaired respiratory drive [5].
They regulate the respiratory response to stabilize CO2, e.g., after an increase in PaCO2 and low pH in the cerebrospinal fluid or brain tissue [6]. The peripheral chemoreceptors located in the carotid body and the lungs affect drive by modifying the sensitivity and threshold of central chemoreceptors, providing faster and more intense response to changes in hypoxemia, PaCO2 and pH [7]. In addition, the lung mechanoreceptors are stretch receptors activated by lung inflation and inhibit central chemoreceptors that terminate inspiration during the Hering–Breuer inhibitory reflex [8]. The respiratory drive pathway can be compromised not only because of respiratory biochemical input (respiratory acidosis or hypoxemia) and/or a mechanical input such as atelectasis, but also from the primary brain injury [9].
Acute lung injury
Apart from a damaged or dysregulated respiratory center, ABI is commonly associated with acute lung injury, such as neurogenic pulmonary edema (NPE), lung inflammation, acute respiratory distress syndrome (ARDS), aspiration pneumonia, ventilator-associated pneumonia (VAP) and lung contusion [10,11,12].
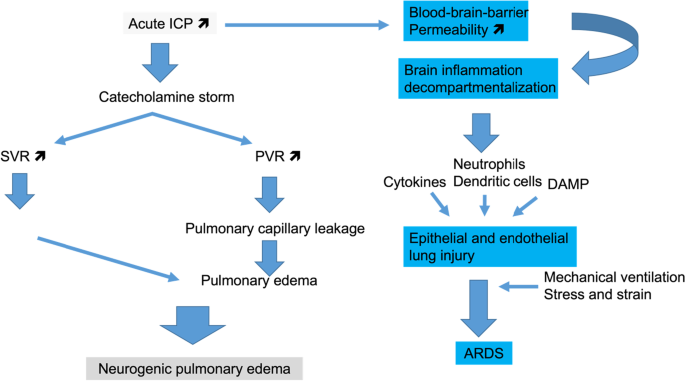
The most common ABIs associated with NPE are SAH, aneurysm rupture and TBI. NPE is typically characterized by the presence of respiratory distress, hypoxemia and bilateral alveolar opacities with diffuse infiltrates in both lungs in the absence of any other cause of respiratory failure [13, 14]. Thus, NPE is like the most severe form of acute hypoxemic respiratory failure, e.g. the ARDS, but with a different pathophysiology (Fig. 1).
Pathways of acute lung injury directly related to acute brain injury. High intracranial pressure (ICP) can promote two different sequences of events that end up into neurogenic pulmonary edema or acute respiratory distress syndrome (ARDS). Both of them may coexist in a given patient. SVR: systemic vascular resistance, PVR: pulmonary vascular resistance, LV: left ventricle, DAMP: damage-associated molecules pattern
Typically, in the presence of increased ICP, a massive neural sympathetic discharge from anatomical regions such as the hypothalamus, the basal portion of the preoptic nucleus and periventricular system can occur [15]. This central sympathetic discharge likely induces pulmonary and systemic vasoconstriction or impairment in vascular permeability, promoting pulmonary edema [16].
In addition to the catecholamines storm that leads to NPE, a massive release of cytokines from the injured brain can contribute to cytokine-induced inflammation leading to ARDS [17,18,19]. The mechanisms of neuro-immunomodulation and ARDS have been recently reviewed [20]. Lung contusion due to multiple trauma that TBI patients frequently experience represents an additional risk factor for ARDS [21].
The reduced consciousness also makes the ABI patients more susceptible for aspiration pneumonia, impaired mucus clearance and VAP than ICU patients without ABI [19, 22, 23]. In a recent large European cohort of about thousand patients with TBI, one out of five developed VAP after a median interval of 5 days (interquartile range 3–7 days, indicating that 75% of episodes occurred in the first week of MV) [24]. Systematic performance of bronchoalveolar lavage within 24 h after intubation of trauma patients showed that 80% of samples grew some microorganisms and 30% are suspect of early pneumonia [22].
In summary, ABI can affect the respiratory system through different mechanisms and may interfere with evaluation of cerebral recovery and further delay the initiation of the weaning process. The respiratory support should simultaneously accomodate the interplay between ICP, PaCO2 and cerebral perfusion, and deliver the lung protective ventilation, e.g. protecting the brain and the lung simultaneoulsy, which are potential contradictory goals.
Ventilatory management of patients with ABI
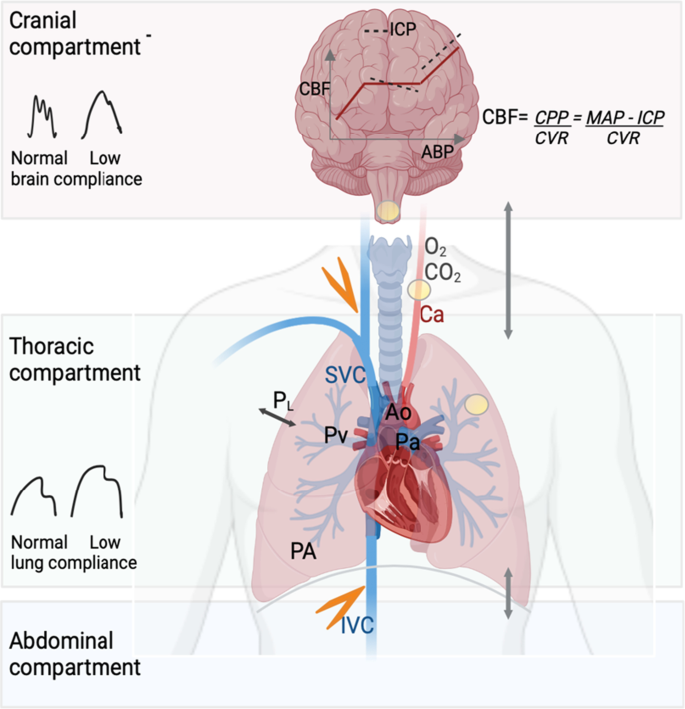
Figure 2 summarizes the brain–lung interactions during mechanical ventilation, the role of PaCO2 and the CA.
Schematic representation of the lung–brain interactions. During positive pressure mechanical ventilation, cerebral blood flow (CBF) can be reduced from different sources. The transmission of airway pressure to the cardiovascular structures depends on the pleural pressure and thus on the transpulmonary pressure (PL) and lung compliance. With normal lung compliance, the higher the airway pressure, the higher the right atrial pressure, which can lead to a reduction in venous return (orange flash). Increase in abdominal pressure counteracts this effect in normal conditions. Increased tidal volume increases pulmonary venous pressure (Pv). These changes result in lower right ventricular ejection volume, and thus, cardiac output (CO) will decrease. CO reduction is limited by the fact that the increased intrathoracic pressure will decrease the left ventricle afterload. Despite changes in CO and arterial pressure, cerebral autoregulation maintains CBF and intracranial pressure (ICP) within a certain range of arterial pressure. However, ICP is highly dependent on venous outflow from the cranial cavity. Positive pressure ventilation with increased right atrial pressure can reduce venous outflow from the cranial cavity and thereby increase ICP. In patients with impaired pulmonary compliance (i.e., severe acute respiratory distress syndrome), the effects of positive pressure mechanical ventilation on alveolar pressure (PA) and PL are often attenuated. Hypoxemia (low PaO2) and hypercapnia (high PaCO2) both increase pulmonary artery pressure (Pa) and pulmonary vascular resistance, thereby increasing right ventricular afterload. Alterations in PaCO2, PaO2 and hydrogen ion also trigger chemoreceptors (yellow circles) to send signals to the respiratory center to regulate respiratory drive. At the level of cerebral circulation, hypercapnia increases CBF and hypocapnia has the opposite effect. The interaction between low brain compliance, cerebral autoregulation and different levels of CO2 has not been studied. Ao aorta, PaCO2 partial pressure of carbon dioxide, PaO2 partial pressure of oxygen, Ca carotid artery, cardiac output (CO), CBF cerebral blood flow, CPP cerebral perfusion pressure, CVR cerebral vascular resistance, ICP intracranial pressure, IVC inferior vena cava, MAP mean arterial pressure, Pa pulmonary artery pressure, PA alveolar pressure, Pv pulmonary venous pressure, PaO2 partial pressure of oxygen, PL transpulmonary pressure, SVC superior vena cava
Oxygen and carbon dioxide targets
The safety range of oxygenation targets in ABI patients is uncertain. Traditionally, the goal of oxygen supplementation in patients with ABI has been to avoid hypoxia [25]. Recent research supports the need to consider an upper limit of oxygen supplementation [26, 27]. The CENTER-TBI, which is a large European, multicenter observational study, showed that the median highest arterial oxygen partial pressure (PaO2) level during the first week after ICU admission was 134 mmHg [26]. The maximal and mean PaO2 were independently associated with an unfavorable functional neurologic outcome or death at 6 months. However, a cut-off for upper limit of PaO2 related to worse outcome was not defined [2]. By contrast, no difference in outcome based on PaO2 levels ranging from 60 to > 300 mmHg was found by others [28, 29]. A post hoc analysis of a randomized trial, identified PaO2 thresholds of 150 and 200 mmHg associated with better functional neurological outcome [30]. This is supported by a meta-analysis of observational studies on adult ABI patients where hyperoxemia (PaO2 cut-off point > 200 mmHg) was associated with poor neurological outcome [31]. Among randomized clinical trials (RCTs) performed in the ICU setting, the adverse effects of hyperoxemia has not been confirmed [32, 33]. Recent meta-analysis of RCTs did not support to set an upper safety PaO2 target in all-comer critically ill patients [34]. Cerebral oxygen supply by the use of microdialysis or brain tissue oxygen monitoring may serve as a marker for impending cerebral hypoxia [35]. Oxygen targeted by continuous monitoring of brain tissue oxygen will be addressed in an ongoing study [36]. To conclude, the role of excessive hyperoxemia is uncertain and avoiding it seems to be a reasonable strategy.
Hypocapnia is used to control acute bursts of high ICP. Recently, a retrospective analysis found that mild hypocapnia (30–34 mmHg) might be associated to better cerebrovascular reactivity and did not worsen cerebral energy metabolism [37]. Guidelines suggest that PaCO2 should be maintained in the normal or low normal reference range when ICP is high [3, 38]. The increased cerebral blood flow that follows hypercapnia might have a role in delayed cerebral ischemia (DCI), if ICP is controlled with external ventricular drainage [39]. Since patients with ABI are a heterogeneous group, future studies should identify those patients who may benefit from higher PaCO2 targets.
While invasive MV represents the standard management strategy to achieve above mentioned physiological goals for ABI patients, non-invasive methods could be an option in some circumstances.
High-flow oxygen and non-invasive ventilation
There is limited evidence for use of high-flow oxygen and non-invasive ventilation (NIV) in ABI patients, at variance of acute hypoxemic respiratory failure in other patient populations [40, 41]. Coma is a contraindication for NIV unless it is due to acute hypercapnia in patients with chronic obstructive pulmonary disease [42]. Skull base fracture is a relative contradiction for use of high flow and NIV. Chest trauma might also complicate the use of NIV although some studies showed it feasible in case of isolated chest trauma [43]. To our knowledge, there are no published RCTs on the use of NIV in patients with TBI. Clinicians need to consider the individual patient´s clinical status and coexisting respiratory abnormalities when making decisions about the use of NIV. However, most often, ABI compromises the airway, warranting early intubation, sometimes after a brief attempt of NIV.
Invasive mechanical ventilation
Indications for endotracheal intubation are to provide airway protection, treat hypoxemia and inadequate ventilation, management of brain edema with tight PaCO2 targets and reduction of cerebral metabolism.
Table 1 provides an overview of the ventilation management, fluid strategies, and indication for steroid use in patients with ABI with and without high ICP and/or ARDS based on the studies included in this review.
Specific types of ABI patients and ventilatory management
The management of ABI in the acute phase is mainly driven by the goal to ensure adequate cerebral perfusion, by cerebral perfusion pressure or ICP-oriented targets [38, 58].
The different forms of severe ABI have disease-specific characteristics that can influence ventilation management. Patients with severe TBI are most treated with ICP monitoring, which serves as a guide to ICU management [38, 59]. Strict low-range PaCO2 and PEEP targets are used for ICP control. Recently, CA as part of ICP management and the interaction of PEEP and LPV with CA has been evaluated [44, 47].
ICU management of patients with SAH targets prevention of rebleeding, intraventricular hemorrhage, and later-stage DCI [60]. In contrast to TBI, generalized contusion and cytotoxic edema are not the primary pathophysiological problem. Since these patients commonly have external ventricular drainage, strict PaCO2 targets for ICP monitoring are not required as often as in patients with TBI. In the DCI phase, microdialysis or brain tissue oxygen is sometimes used to detect local cerebral hypoxia and set PaO2 targets [61, 62]. An assessment of neurological function and reduced sedation is required to diagnose DCI. Spontaneous ventilation with the following broader PaCO2 target is therefore used more liberally than in TBI patients.
Most patients with severe intracerebral hemorrhage have systemic hypertension. Lowering blood pressure, rather than measures to lower ICP, has been the focus of general management in the ICU [63]. Treatment in the stroke unit is associated with a better outcome, but this does not necessarily improve when the patient is admitted to the ICU [64]. The indication for the use of ICP monitoring and targets is unclear and is often derived from the TBI literature.
Ventilatory settings for ABI patients with no lung injury
In non-ARDS patients, no difference in patient outcome was found in two large multicenter RCTs between low (6 ml/kg predicted body weight) vs. intermediate (10 ml/kg) tidal volume (VT) [45] and low (5 cmH2O) vs. high (8 cmH2O) PEEP [48]. As these trials were not dedicated to ABI patients, a small proportion of the patients had ABI. Limiting VT with concomitant permissive hypercapnia is difficult to carry out with a simultaneous ICP control. Dead space reduction by replacing heat-moisture exchangers with heated-humidifiers is feasible and can set low VT without increasing PaCO2 [65]. High PEEP has shown contradictory results about the response related to brain and lung compliance [66,67,68]. Measurement of transpulmonary pressure could not clarify which ABI patients had adverse effect on high PEEP [44]. PEEP adjustment is therefore recommended only during rigorous ICP monitoring and curve analysis if brain compliance is suspected to be low [44]. The role of respiratory mechanics variables other than PEEP and VT has been explored in recent observational studies. A sub-analysis of the Target Temperature Management-2 trial found that respiratory rate, driving pressure, and mechanical power were independently associated with 6-month mortality in post-cardiac arrest survivors [69]. Mechanical power (MP) might also be associated with mortality in patients with ABI from other causes [70, 71]. A recent observational study found that the MP during the first week of MV was associated with poor outcome independently on oxygenation [71]. MP might also be related to PEEP-induced high ICP [44].
Ventilatory management of ABI patients with concurrent ARDS
As detailed earlier in the ventilator management of patients with ABI some conflicting physiological goals may arise when aiming at protecting both the lung and the brain [72]. Typical, permissive hypercapnia as part of LPV may have cerebral side effects leading to a complex if not impossible evaluation of the benefit-risk ratio. While in patients without lung injury the value of strict LPV remains debated and cerebral physiology may predominantly drive patient management, the situation is more complex in case of established ARDS [11, 73]. Permissive hypercapnia might be feasible if ICP is controlled with external ventricular drainage. Another option is to increase respiratory rate up to the limit of Auto-PEEP or plateau pressure. Since respiratory rate contributes to MP, compensating for low VT by increasing respiratory rate might not be the solution for regulating PaCO2 in ABI. RCTs are required to assess the interplay between inflammatory and mechanical stress of the lungs in this population, as well as potential interventions and their impact on long-term outcomes on both cerebral and pulmonary recovery (weaning of MV).
Prone position in patients with moderate to severe ARDS is a cornerstone treatment that might be considered in patients with co-existing ABI. Since prone position might affect PaCO2 and venous return from the brain, ICP monitoring is strongly advised in acute, severe ABI [3, 74]. High ICP was a non-inclusion criterion in Proseva trial [52]. The role of alveolar recruitment maneuvers to improve oxygenation in ARDS is uncertain [53]. In patients with acute, severe ABI, the role of the recruitment maneuver with cardiopulmonary interaction, should lead to caution when using this maneuver.
Asynchronies between patient to ventilator might be the clinical consequences of the alterations in the respiratory drive or prolonged MV.
During assisted mechanical ventilation, the critical determinant of respiratory drive and work of breathing is the set peak flow. An insufficient peak flow is associated with higher drive and work of breathing. Low peak flow leads to air hunger and excessive peak flow leads to excessively short inspiratory time associated with asynchrony and breath stacking. Highest peak flows increase respiratory rates because of shortened inspiratory time. In fact, a shorter inspiratory time decreases the negative feedback derived from lung inflation, resulting in a higher ventilatory frequency. Physiologically, high volume or lung inflation reduces the respiratory drive and the peak flow becomes less relevant. Flow dyssynchrony is typical in situations of high drive or of activation of respiratory muscles after time-initiated ventilator cycles during controlled MV called “respiratory entrainment” secondary to a sustained activation of the Hering–Breuer reflex and C3, C5 spinal reflex leading to reverse triggering [77]. Luo et al. investigated the patient–ventilator asynchrony in mechanically ventilated brain-injured patients and found that the prevalence of asynchrony was 38% higher than in patients without brain injury [78], while the most prevalent type of asynchrony was ineffective triggering, characterized by a lower drive in terms of P0.1 values. The asynchrony index was similar after stroke, craniotomy for brain tumor or TBI, and significantly lower during pressure control/assisted ventilation than during other ventilation modes and higher during combined use of opioids and sedatives. Similarly, to non-neurological patients, asynchrony is a sign of uncoupling between the neuronal input and muscular efficiency and is associated with prolonged MV [78]. Recently, in patients with ABI, patient-ventilator asynchrony was monitored with esophageal pressure monitoring [78]. It was demonstrated that asynchrony, in particular ineffective trigging is common and associated with combination of analgesia and sedation strategy. The prevalence of ventilator-induced diaphragm dysfunction in brain-injured patients is likely to play an important role but remains to be investigated [79].
Additional management
Two additional strategies relevant for the ventilatory management will be briefly discussed in this part: fluid balance and steroids.
Fluid management in ABI. The FACCT trial in ARDS patients (with no ABI) found that a restrictive strategy, as compared to the liberal fluid strategy, was similar in terms of patient mortality but was associated with less days spent under invasive MV [54]. In ABI patients, in addition to careful adjustment of the ventilator pressures, ensuring that patients are euvolemic might protect against the adverse effects of higher ventilator pressures and fluctuations in PaCO2. Fluid management must consider administrating fluids vs the risk of cerebral edema due to disruption of the blood brain barrier and cell damage.
In ABI patients with or without ARDS, the restrictive fluid strategy is therefore recommended to prevent any further brain edema. However, it is important to take care to avoid hypovolemia in order to achieve the cerebral perfusion pressure goal [55, 75].
Steroids. In patients with ARDS, steroids may play a beneficial role in the acute phase [56]. In TBI, the MRC-CRASH trial found that mortality was higher with methylprednisolone than with placebo; therefore, steroid use is not recommended [57]. In general, steroid use is not recommended in any form of ABI patients with acute cerebral swelling due to a lack of evidence [76].
Weaning
Prolonged MV may further delay rehabilitation and ICU discharge, increasing the risk of long-term sequelae and complications with physical, psychological and psychiatric as well as cognitive symptoms as part of the post-intensive care syndrome which does also affect patients’ relatives [80]. Also, in patients with ABI, there has been increased focus on weaning practices and early rehabilitation [81, 82].
Uncertainty persists about the best approach to successful weaning of ABI patients and involves sedation practices, weaning criteria, and timing for tracheotomy. Traditionally, critically ill patients have been treated with deep sedation and immobilization, which prolong time to extubation [83]. This practice is in particularly common in patients with ABI, where deep sedation has been used to reduce cerebral metabolism, prevent intracranial hypertension and for fever control. Recently, ABCDEF bundle (Assess, prevent, and manage pain; Both spontaneous awakening and breathing trials: Choice of Analgesia and Sedation; Delirium assess, prevent, and manage; Early Mobility and Exercise; Family engagement/ empowerment) received acknowledgement within intensive care medicine [84]. Among ABI patients, data are lacking to precisely decipher the relative contributions of sequelae of the initial cerebral insult and intensive care acquired long-term neurological, muscular and cognitive symptoms. Patients with ABI have higher extubation failure than patients without [85, 86].
Several factors may contribute to extend the duration of MV, some are non-modifiable such as the brain injury itself, recovery of consciousness being one key factor for weaning readiness; others, like management-related factors may be modified such as deep and prolonged sedation and screening practice for weaning readiness [87, 88]. Once extubated, patients with ABI are at high risk of re-intubation, mainly because of respiratory and airway failure due to dysphagia, lack of muscle strength and poor cough. In a large international observational study, that evaluated 1512 patients with ABI who were ventilated more than 24 h with an initial Glasgow coma scale (GCS) ≤ 12, 19% of patients were reintubated within 5 days of extubation. Authors identified predictors of extubation failure at day 5 which were combined in a score which may be easy to use at the bedside and comprising the following variables associated with extubation success: TBI, vigorous cough, gag reflex, swallowing attempts, endotracheal suctioning less than twice per hour, GCS motor component at 6, body temperature normal or low. The score area under the receiver operating characteristics curve was 0.65 (95% confidence interval, 0.53–0.76) in the validation cohort, clinical evaluation of cut-offs with high or low positive predictive value may be warranted to decipher potential clinical use of this score. Of note, 21% of the patients of this large international cohort did not undergo usual weaning toward extubation but underwent direct tracheostomy. The optimal timing of eventual tracheostomy of ICU patients has been the matter of long-lasting debates, with a current lack of firm benefit of early tracheostomy. A recent large scale retrospective study including 1538 patients, specifically evaluated timing of tracheostomy among the subgroup of 498 patients with a GCS below 8 at ICU admission and showed a lack of significant link between tracheostomy timing and patient outcome [89]. An observational study on 1358 patients with TBI showed an association between late tracheostomy and poor neurological outcome [90]. However, this finding was not confirmed by the SETPOINT2 RCT on patients with stroke [91]. Current guidelines recommend considering tracheostomy in patients who failed an extubation or have persistent reduced level of consciousness with no recommendation on optimal timing of tracheostomy [3]. Focusing on measures to reduce sedation/analgesia with early weaning of patients with severe ABI are the target of future studies (NCT04291235, NCT04080440).
Conclusions
Neurologically ill patients have specific challenging respiratory problems and lung–brain interactions. Optimal delivery of MV has not been studied extensively in this setting and RCTs are scarce. Recent interest in the combination of neuro- and respiratory monitoring and large multicenter trials in ABI patients advances our knowledge in terms of optimized treatment and outcomes. The future will offer the opportunity to combine physiological studies and the use of big data analysis to identify predictors and optimize ventilation strategies for patients with severe ABI.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- ABI:
-
Acute brain injury
- ARDS:
-
Acute respiratory distress syndrome
- CA:
-
Cerebral autoregulation
- DCI:
-
Delayed cerebral ischemia
- GCS:
-
Glasgow coma scale
- ICP:
-
Intracranial pressure
- ICU:
-
Intensive care unit
- LPV:
-
Lung protective ventilation
- MP:
-
Mechanical power
- MV:
-
Mechanical ventilation
- NPE:
-
Neurogenic pulmonary edema
- NIV:
-
Non-invasive ventilation
- PaCO2 :
-
Arterial carbon dioxide partial pressure
- PaO2 :
-
Arterial oxygen partial pressure
- PEEP:
-
Positive end-expiratory pressure
- RCT:
-
Randomized controlled trial
- SAH:
-
Subarachnoid hemorrhage
- TBI:
-
Traumatic brain injury
- VT:
-
Tidal volume
- VAP:
-
Ventilator associated pneumonia
References
Pelosi P, Ferguson ND, Frutos-Vivar F, Anzueto A, Putensen C, Raymondos K, et al. Management and outcome of mechanically ventilated neurologic patients. Crit Care Med. 2011;39(6):1482–92.
Ziaka M, Exadaktylos A. Brain–lung interactions and mechanical ventilation in patients with isolated brain injury. Crit Care. 2021;25(1):1–10.
Robba C, Poole D, McNett M, Asehnoune K, Bösel J, Bruder N, et al. Mechanical ventilation in patients with acute brain injury: recommendations of the European Society of Intensive Care Medicine consensus. Intensive Care Med. 2020;46(12):2397–410.
Meng L, Gelb AW. Regulation of cerebral autoregulation by carbon dioxide. Anesthesiology. 2015;122:196–205.
North JB, Jennett S. Abnormal breathing patterns associated with acute brain damage. Arch Neurol. 1974;31(5):338–44.
Smith CA, Rodman JR, Chenuel BJA, Henderson KS, Dempsey JA. Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol. 2006;100(1):13–9.
Vaporidi K, Akoumianaki E, Telias I, Goligher EC, Brochard L, Georgopoulos D. Respiratory drive in critically Ill patients pathophysiology and clinical implications. Am J Respir Crit Care Med. 2020;201(1):20–32.
Hamilton RD, Winning AJ, Horner RL, Guz A. The effect of lung inflation on breathing in man during wakefulness and sleep. Respir Physiol. 1988;73(2):145–54.
Spinelli E, Mauri T, Beitler JR, Pesenti A, Brodie D. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med. 2020;46(4):606–18.
Johnson NJ, Carlbom DJ, Gaieski DF. Ventilator management and respiratory care after cardiac arrest: oxygenation, ventilation, infection, and injury. Chest. 2018;153(6):1466–77.
Aisiku IP, Yamal JM, Doshi P, Rubin ML, Benoit JS, Hannay J, et al. The incidence of ARDS and associated mortality in severe TBI using the Berlin definition. J Trauma Acute Care Surg. 2016;80(2):308–12.
Shih JA, Robertson HK, Issa MS, Grossestreuer AV, Donnino MW, Berg KM, et al. Acute respiratory distress syndrome after in-hospital cardiac arrest. Resuscitation. 2022;177:78–84.
Busl KM, Bleck TP. Neurogenic pulmonary edema. Crit Care Med. 2015;43(8):1710–5.
Davison DL, Terek M, Chawla LS. Neurogenic pulmonary edema. Crit Care. 2012;16(2):1–7.
Mrozek S, Constantin JM, Geeraerts T. Brain-lung crosstalk: Implications for neurocritical care patients. World J Crit Care Med. 2015;4(3):163–78.
Theodore J, Robin ED. Pathogenesis of neurogenic pulmonary oedema. The Lancet. 1975;306(7938):749–51.
Kalsotra A, Zhao J, Anakk S, Dash PK, Strobel HW. Brain trauma leads to enhanced lung inflammation and injury: evidence for role of P4504Fs in resolution. J Cereb Blood Flow Metab. 2007;27(5):963–74.
Fisher AJ, Donnelly SC, Hirani N, Burdick MD, Strieter RM, Dark JH, et al. Enhanced pulmonary inflammation in organ donors following fatal non-traumatic brain injury. Lancet. 1999;353(9162):1412–3.
Hu PJ, Pittet JF, Kerby JD, Bosarge PL, Wagener BM. Acute brain trauma, lung injury, and pneumonia: more than just altered mental status and decreased airway protection. Am J Physiol Lung Cell Mol Physiol. 2017;313(1):L1-15.
Ziaka M, Exadaktylos A. ARDS associated acute brain injury: from the lung to the brain. Eur J Med Res. 2022;27(1):1–11.
Schieren M, Wappler F, Wafaisade A, Lefering R, Sakka SG, Kaufmann J, et al. Impact of blunt chest trauma on outcome after traumatic brain injury- a matched-pair analysis of the TraumaRegister DGU®. Scand J Trauma Resusc Emerg Med. 2020;28(1):1–7.
Harrell KN, Lee WB, Rooks HJ, Briscoe WE, Capote W, Dart BW, et al. Early pneumonia diagnosis decreases ventilator-associated pneumonia rates in trauma population. J Trauma Acute Care Surg. 2023;94:30–5.
François B, Cariou A, Clere-Jehl R, Dequin PF, Renon-Carron F, Daix T, et al. Prevention of early ventilator-associated pneumonia after cardiac arrest. N Engl J Med. 2019;381(19):1831–42.
Robba C, Rebora P, Banzato E, Wiegers EJA, Stocchetti N, Menon DK, et al. Incidence, risk factors, and effects on outcome of ventilator-associated pneumonia in patients with traumatic brain injury: analysis of a large, multicenter, prospective. Observational Longitudinal Study Chest. 2020;158(6):2292–303.
McHugh GS, Engel DC, Butcher I, Steyerberg EW, Lu J, Mushkudiani N, et al. Prognostic value of secondary insults in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):287–93.
Rezoagli E, Petrosino M, Rebora P, Menon DK, Mondello S, Cooper DJ, et al. High arterial oxygen levels and supplemental oxygen administration in traumatic brain injury: insights from CENTER-TBI and OzENTER-TBI. Intensive Care Med. 2022;48:1709–25.
Singer M, Young PJ, Laffey JG, Asfar P, Taccone FS, Skrifvars MB, et al. Dangers of hyperoxia. Crit Care. 2021;25:1–15.
Weeden M, Bailey M, Gabbe B, Pilcher D, Bellomo R, Udy A. Functional outcomes in patients admitted to the intensive care unit with traumatic brain injury and exposed to hyperoxia: a retrospective multicentre cohort study. Neurocrit Care. 2021;34(2):441–8.
Schmidt H, Kjaergaard J, Hassager C, Mølstrøm S, Grand J, Borregaard B, et al. Oxygen targets in comatose survivors of cardiac arrest. N Engl J Med. 2022;387(16):1467–76.
Alali AS, Temkin N, Vavilala MS, Lele AV, Barber J, Dikmen S, et al. Matching early arterial oxygenation to long-term outcome in severe traumatic brain injury: target values. J Neurosurg. 2019;132(2):537–44.
Hirunpattarasilp C, Shiina H, Na-Ek N, Attwell D. The effect of hyperoxemia on neurological outcomes of adult patients: a systematic review and meta-analysis. Neurocrit Care. 2022;36(3):1027–43.
Lång M, Skrifvars MB, Siironen J, Tanskanen P, Ala-Peijari M, Koivisto T, et al. A pilot study of hyperoxemia on neurological injury, inflammation and oxidative stress. Acta Anaesthesiol Scand. 2018;62(6):801–10.
Semler MW, Casey JD, Lloyd BD, Hastings PG, Hays MA, Stollings JL, et al. Oxygen-saturation targets for critically ill adults receiving mechanical ventilation. N Engl J Med. 2022;387(19):1759–69.
Barbateskovic M, Schjørring OL, Krauss SR, Meyhoff CS, Jakobsen JC, Rasmussen BS, et al. Higher vs lower oxygenation strategies in acutely ill adults: a systematic review with meta-analysis and trial sequential analysis. Chest. 2021;159(1):154–73.
Veldeman M, Albanna W, Weiss M, Park S, Hoellig A, Clusmann H, et al. Invasive multimodal neuromonitoring in aneurysmal subarachnoid hemorrhage: a systematic review. Stroke. 2021;52:3624–32.
Bernard F, Barsan W, Diaz-Arrastia R, Merck LH, Yeatts S, Shutter LA. Brain oxygen optimization in severe traumatic brain injury (BOOST-3): a multicentre, randomised, blinded-endpoint, comparative effectiveness study of brain tissue oxygen and intracranial pressure monitoring versus intracranial pressure alone. BMJ Open. 2022;12(3):60188.
Svedung Wettervik T, Howells T, Hillered L, Nilsson P, Engquist H, Lewén A, et al. Mild hyperventilation in traumatic brain injury—relation to cerebral energy metabolism, pressure autoregulation, and clinical outcome. World Neurosurg. 2020;133:e567–75.
Chesnut R, Aguilera S, Buki A, Bulger E, Citerio G, Cooper DJ, et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2020;46:919–29.
Stetter C, Weidner F, Lilla N, Weiland J, Kunze E, Ernestus RI, et al. Therapeutic hypercapnia for prevention of secondary ischemia after severe subarachnoid hemorrhage: physiological responses to continuous hypercapnia. Sci Rep. 2021;11(1):1–11.
Kea B, NKJ BurnsKEA A. Cochrane library Cochrane database of systematic reviews noninvasive positive-pressure ventilation as a weaning strategy for intubated adults with respiratory failure (review) noninvasive positive-pressure ventilation as a weaning strategy for intubated a. Cochrane Database Syst Rev. 2013;4127.
Frat JP, Brugiere B, Ragot S, Chatellier D, Veinstein A, Goudet V, et al. Sequential application of oxygen therapy via high-flow nasal cannula and noninvasive ventilation in acute respiratory failure: an observational pilot study. Respir Care. 2015;60(2):170–8.
Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29(5):1033–56.
Chiumello D, Coppola S, Froio S, Gregoretti C, Consonni D. Noninvasive ventilation in chest trauma: systematic review and meta-analysis. Intensive Care Med. 2013;39(7):1171–80.
Beqiri E, Smielewski P, Guérin C, Czosnyka M, Robba C, Bjertnæs L, et al. Neurological and respiratory effects of lung protective ventilation in acute brain injury patients without lung injury: brain vent, a single centre randomized interventional study. Crit Care. 2023;27(1):115.
Simonis FD, Serpa Neto A, Binnekade JM, Braber A, Bruin KCM, Determann RM, et al. Effect of a low vs intermediate tidal volume strategy on ventilator-free days in intensive care unit patients without ARDS. JAMA. 2018;320(18):1872.
Network TARDS. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–8.
Giardina A, Cardim D, Ciliberti P, Battaglini D, Ball L, Kasprowicz M, et al. Effects of positive end-expiratory pressure on cerebral hemodynamics in acute brain injury patients. Front Physiol. 2023;14:690.
Algera AG, Pisani L, Serpa Neto A, den Boer SS, Bosch FFH, Bruin K, et al. Effect of a lower vs higher positive end-expiratory pressure strategy on ventilator-free days in ICU patients without ARDS: a randomized clinical trial. JAMA. 2020;324(24):2509–20.
Boone MD, Jinadasa SP, Mueller A, Shaefi S, Kasper EM, Hanafy KA, et al. The effect of positive end-expiratory pressure on intracranial pressure and cerebral hemodynamics. Neurocrit Care. 2016;26:174–81.
Robba C, Ball L, Nogas S, Battaglini D, Messina A, Brunetti I, et al. Effects of positive end-expiratory pressure on lung recruitment, respiratory mechanics, and intracranial pressure in mechanically ventilated brain-injured patients. Front Physiol. 2021;12:1684.
Hirunpattarasilp C, Shiina H, Na-Ek N, Attwell D. The effect of hyperoxemia on neurological outcomes of adult patients: a systematic review and meta-analysis. Neurocrit Care. 2022;36:1027–43.
Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–68.
Cavalcanti AB, Suzumura ÉA, Laranjeira LN, Paisani DDM, Damiani LP, Guimarães HP, et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome a randomized clinical trial. JAMA. 2017;318(14):1335–45.
Wiedemann HP, Clinic C, Wheeler AP, Bernard GR, University V, Taylor Thompson B, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–75.
Wiegers EJAB, JoanneCooper JD, et al. Fluid balance and outcome in critically ill patients with traumatic brain injury (CENTER-TBI and OzENTER-TBI): a prospective, multicentre, comparative effectiveness study. Lancet Neurol. 2021;20(8):627–38.
Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267–76.
Crash TC. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury—outcomes at 6 months. The Lancet. 2005;365(9475):1957–9.
Le-Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy GM, et al. Consensus summary statement of the international multidisciplinary consensus conference on multimodality monitoring in neurocritical care: a statement for healthcare professionals from the neurocritical care society and the European Society of Intensive C. Intensive Care Med. 2014;40(9):1189–209.
Meyfroidt G, Bouzat P, Casaer MP, Chesnut R, Hamada SR, Helbok R, et al. Management of moderate to severe traumatic brain injury: an update for the intensivist. Intensive Care Med. 2022;48(6):649–66.
Hoh BL, Ko NU, Amin-Hanjani S, Hsiang-Yi Chou S, Cruz-Flores S, Dangayach NS, et al. 2023 Guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2023.
Torné R, Culebras D, Sanchez-Etayo G, García-García S, Muñoz G, Llull L, et al. Double hemispheric microdialysis study in poor-grade SAH patients. Sci Rep. 2020;10(1):1–9.
Helbok R, Kofler M, Schiefecker AJ, Gaasch M, Rass V, Pfausler B, et al. Clinical use of cerebral microdialysis in patients with aneurysmal subarachnoid hemorrhage-State of the art. Front Neurol. 2017;8:1.
Greenberg SM, Ziai WC, Cordonnier C, Dowlatshahi D, Francis B, Goldstein JN, et al. 2022 Guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2022;53:E282-361.
Ungerer MN, Ringleb P, Reuter B, Stock C, Ippen F, Hyrenbach S, et al. Stroke unit admission is associated with better outcome and lower mortality in patients with intracerebral hemorrhage. Eur J Neurol. 2020;27(5):825–32.
Pitoni S, D’Arrigo S, Grieco DL, Idone FA, Santantonio MT, Di Giannatale P, et al. Tidal volume lowering by instrumental dead space reduction in brain-injured ARDS patients: effects on respiratory mechanics, gas exchange, and cerebral hemodynamics. Neurocrit Care. 2021;34(1):21–30.
Chen H, Chen K, Xu JQ, Zhang YR, Yu RG, Zhou JX. Intracranial pressure responsiveness to positive end-expiratory pressure is influenced by chest wall elastance: a physiological study in patients with aneurysmal subarachnoid hemorrhage. BMC Neurol. 2018;18(1):124.
Boone MD, Jinadasa SP, Mueller A, Shaefi S, Kasper EM, Hanafy KA, et al. The effect of positive end-expiratory pressure on intracranial pressure and cerebral hemodynamics. Neurocrit Care. 2016;26(2):174–81.
Robba C, Ball L, Nogas S, Battaglini D, Messina A, Brunetti I, et al. Effects of positive end-expiratory pressure on lung recruitment, respiratory mechanics, and intracranial pressure in mechanically ventilated brain-injured patients. Front Physiol. 2021;12:1684.
Robba C, Badenes R, Battaglini D, Ball L, Brunetti I, Jakobsen JC, et al. Ventilatory settings in the initial 72 h and their association with outcome in out-of-hospital cardiac arrest patients: a preplanned secondary analysis of the targeted hypothermia versus targeted normothermia after out-of-hospital cardiac arrest (TTM2) tr. Intensive Care Med. 2022;48(8):1024–38.
Jiang X, Zhu Y, Zhen S, Wang L. Mechanical power of ventilation is associated with mortality in neurocritical patients: a cohort study. J Clin Monit Comput. 2022;36(6):1621–8.
Wahlster S, Sharma M, Taran S, Town JA, Stevens RD, Cinotti R, et al. Utilization of mechanical power and associations with clinical outcomes in brain injured patients: a secondary analysis of the extubation strategies in neuro-intensive care unit patients and associations with outcome (ENIO) trial. Crit Care. 2023;27(1):156.
Robba C, Camporota L, Citerio G. Acute respiratory distress syndrome complicating traumatic brain injury. Can opposite strategies converge? Intensive Care Med. 2023;49(5):583–6.
Elmer J, Hou P, Wilcox SR, Chang Y, Schreiber H, Okechukwu I, et al. Acute respiratory distress syndrome after spontaneous intracerebral hemorrhage. Crit Care Med. 2013;41(8):1992.
Roth C, Ferbert A, Deinsberger W, Kleffmann J, Kästner S, Godau J, et al. Does prone positioning increase intracranial pressure? A retrospective analysis of patients with acute brain injury and acute respiratory failure. Neurocrit Care. 2014;21(2):186–91.
Oddo M, Poole D, Helbok R, Meyfroidt G, Stocchetti N, Bouzat P, et al. Fluid therapy in neurointensive care patients: ESICM consensus and clinical practice recommendations. Intensive Care Med. 2018;44:23.
Cook AM, Morgan Jones G, Hawryluk GWJ, Mailloux P, McLaughlin D, Papangelou A, et al. Guidelines for the acute treatment of cerebral edema in neurocritical care patients. Neurocrit Care. 2020;32(3):647–66.
Albaiceta GM, Brochard L, Dos Santos CC, Fernández R, Georgopoulos D, Girard T, et al. The central nervous system during lung injury and mechanical ventilation: a narrative review. Br J Anaesth. 2021;127(4):648–59.
Luo XY, He X, Zhou YM, Wang YM, Chen JR, Chen GQ, et al. Patient–ventilator asynchrony in acute brain-injured patients: a prospective observational study. Ann Intensive Care. 2020;10:1–10.
Goligher EC, Dres M, Patel BK, Sahetya SK, Beitler JR, Telias I, et al. Lung- and diaphragm-protective ventilation. Am J Respir Crit Care Med. 2020;202(7):950–61.
Herridge MS, Azoulay É. Outcomes after critical illness. N Engl J Med. 2023;388(10):913–24.
Cinotti R, Mijangos JC, Pelosi P, Haenggi M, Gurjar M, Schultz MJ, et al. Extubation in neurocritical care patients: the ENIO international prospective study. Intensive Care Med. 2022;48(11):1539–50.
Asehnoune K, Mrozek S, Perrigault PF, Seguin P, Dahyot-Fizelier C, Lasocki S, et al. A multi-faceted strategy to reduce ventilation-associated mortality in brain-injured patients. The BI-VILI project: a nationwide quality improvement project. Intensive Care Med. 2017;43(7):957–70.
Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186(8):724–31.
Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:825–73.
Tejerina EE, Robba C, del Campo-Albendea L, Pelosi P, Muriel A, Peñuelas O, et al. Weaning outcomes in patients with brain injury. Neurocrit Care. 2022;37(3):649–59.
Taran S, Angeloni N, Pinto R, McCredie V, Schultz M, Robba C, et al. Prognostic factors associated with extubation failure in acutely brain-injured patients: a systematic review and meta-analysis. Can J Anesth. 2022;69(1 SUPPL):S109–11.
Pham T, Serpa Neto A, Pelosi P, Laffey JG, De Haro C, Lorente JA, et al. Outcomes of patients presenting with mild acute respiratory distress syndrome: insights from the LUNG SAFE study. Anesthesiology. 2019;130(2):263–83.
Burns KEA, Rizvi L, Cook DJ, Lebovic G, Dodek P, Villar J, et al. Ventilator weaning and discontinuation practices for critically ill patients. JAMA J Am Med Assoc. 2021;325:1173–84.
Tanaka A, Uchiyama A, Kitamura T, Sakaguchi R, Komukai S, Matsuyama T, et al. Association between early tracheostomy and patient outcomes in critically ill patients on mechanical ventilation: a multicenter cohort study. J Intensive Care. 2022;10(1):1–10.
Robba C, Galimberti S, Graziano F, Wiegers EJA, Lingsma HF, Iaquaniello C, et al. Tracheostomy practice and timing in traumatic brain-injured patients: a CENTER-TBI study. Intensive Care Med. 2020;46(5):983–94.
Bösel J, Niesen WD, Salih F, Morris NA, Ragland JT, Gough B, et al. Effect of early vs standard approach to tracheostomy on functional outcome at 6 months among patients with severe stroke receiving mechanical ventilation: the SETPOINT2 randomized clinical trial. JAMA J Am Med Assoc. 2022;327(19):1899–909.
Acknowledgements
Not applicable.
Funding
Shirin K. Frisvold was funded by Northern Norway Regional Health Authority (Grant no. 181021).
Author information
Authors and Affiliations
Contributions
Each author has made substantial contributions to the conception of the work and substantively revised it; approved the submitted version; agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Frisvold, S., Coppola, S., Ehrmann, S. et al. Respiratory challenges and ventilatory management in different types of acute brain-injured patients. Crit Care 27, 247 (2023). https://doi.org/10.1186/s13054-023-04532-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04532-4