Abstract
Background
Limiting tidal volume (VT), plateau pressure, and driving pressure is essential during the acute respiratory distress syndrome (ARDS), but may be challenging when brain injury coexists due to the risk of hypercapnia. Because lowering dead space enhances CO2 clearance, we conducted a study to determine whether and to what extent replacing heat and moisture exchangers (HME) with heated humidifiers (HH) facilitate safe VT lowering in brain-injured patients with ARDS.
Methods
Brain-injured patients (head trauma or spontaneous cerebral hemorrhage with Glasgow Coma Scale at admission < 9) with mild and moderate ARDS received three ventilatory strategies in a sequential order during continuous paralysis: (1) HME with VT to obtain a PaCO2 within 30–35 mmHg (HME1); (2) HH with VT titrated to obtain the same PaCO2 (HH); and (3) HME1 settings resumed (HME2). Arterial blood gases, static and quasi-static respiratory mechanics, alveolar recruitment by multiple pressure–volume curves, intracranial pressure, cerebral perfusion pressure, mean arterial pressure, and mean flow velocity in the middle cerebral artery by transcranial Doppler were recorded. Dead space was measured and partitioned by volumetric capnography.
Results
Eighteen brain-injured patients were studied: 7 (39%) had mild and 11 (61%) had moderate ARDS. At inclusion, median [interquartile range] PaO2/FiO2 was 173 [146–213] and median PEEP was 8 cmH2O [5–9]. HH allowed to reduce VT by 120 ml [95% CI: 98–144], VT/kg predicted body weight by 1.8 ml/kg [95% CI: 1.5–2.1], plateau pressure and driving pressure by 3.7 cmH2O [2.9–4.3], without affecting PaCO2, alveolar recruitment, and oxygenation. This was permitted by lower airway (− 84 ml [95% CI: − 79 to − 89]) and total dead space (− 86 ml [95% CI: − 73 to − 98]). Sixteen patients (89%) showed driving pressure equal or lower than 14 cmH2O while on HH, as compared to 7 (39%) and 8 (44%) during HME1 and HME2 (p < 0.001). No changes in mean arterial pressure, cerebral perfusion pressure, intracranial pressure, and middle cerebral artery mean flow velocity were documented during HH.
Conclusion
The dead space reduction provided by HH allows to safely reduce VT without modifying PaCO2 nor cerebral perfusion. This permits to provide a wider proportion of brain-injured ARDS patients with less injurious ventilation.
Similar content being viewed by others
Background
Acute respiratory distress syndrome (ARDS) affects up to 30% of critically ill patients with acute brain injury [1,2,3,4,5], representing an independent predictor of worse clinical outcome [6].
The use of low tidal volume (VT) to limit plateau pressure and driving pressure (i.e., plateau pressure–positive end-expiratory pressure, ∆P) reduces ventilator-induced lung injury (VILI) and improves survival in ARDS patients [7,8,9]. Nonetheless, lower VT yield increased risk of hypercapnia, which is deleterious [10], especially in patients with brain injury: In this particular subset of patients, tight control of arterial partial pressure of carbon dioxide (PaCO2) is needed to prevent any secondary brain injury due to increases in cerebral blood flow and intracranial pressure [11].
Consequently, in brain-injured patients with ARDS, two competing priorities arise: use of low VT for lung protection and tight PaCO2 control to maintain proper cerebral blood flow and prevent undue intracranial pressure increases. The optimal balance between brain and lung protection during mechanical ventilation is not well established, and no recommendation exists on ventilatory management of these patients. In clinical practice, patients with acute brain injury and ARDS often receive VT exceeding 6 ml/kg of predicted body weight (PBW) [12,13,14,15,16].
Heat and moisture exchangers (HME) and heated humidifiers (HH) are used for gas conditioning during invasive mechanical ventilation. Although they are simpler to use, HMEs carry relevant instrumental dead space and decrease the proportion of VT contributing to alveolar ventilation. Previous authors highlighted that replacing HME with HH decreases dead space, promotes CO2 clearance and allows VT and plateau pressure reduction during ARDS [17,18,19,20]: however, no data clarify to what extent ∆P is reduced by this approach and whether this is safe in patients with concomitant brain injury, for whom tight control of PaCO2 is mandatory and any intervention has to be evaluated also from the perspective of cerebral hemodynamics.
We conducted a physiological study to elucidate to what extent VT reduction with HH allows to limit ∆P and whether this is safe in terms of cerebral hemodynamics.
Methods
The study was conducted in the general intensive care unit (ICU) of a university hospital in Rome, Italy, according to the principles of the Declaration of Helsinki. The study protocol was reviewed and approved by the local institutional ethics committee. Written informed consent was obtained according to committee recommendation.
Patients
Acute brain-injured patients with ARDS were screened for enrollment. Acute brain injury was defined as a traumatic brain injury or a non-traumatic cerebral hemorrhage with a Glasgow Coma Scale at admission < 9. Diagnosis of ARDS was based on the criteria established by Berlin definition [21]. Patients were eligible for inclusion if they had acute brain injury, developed ARDS, and were monitored with invasive intracranial pressure for clinical purposes, with stable pressure values < 20 mmHg.
For safety reasons, because lowering VT may cause alveolar derecruitment and hypoxemia, patients with severe ARDS (PaO2/FiO2 < 100 mmHg) were not considered for inclusion in the study. Other non-inclusion criteria were: age < 18, pregnancy, severe hemodynamic instability, contraindication to muscular paralysis, leaking chest tube, and decompressive craniotomy.
All patients were lying in the semi-seated position, intubated, sedated, paralyzed (cisatracurium 0.1 mg/kg), and mechanically ventilated in volume-controlled mode with an I-to-E ratio set at 1:2. A standard bitube circuit with Y-piece and HME filter (Hygrobac; DAR: dead space 84 ml, resistance 1.0 cmH2O/L/s) was used in the stabilization phase. Ventilatory parameters were set by the attending physician, who was not involved in the study, but was specifically asked to optimize the ventilator settings to obtain a PaCO2 between 30 and 35 mmHg and PaO2 > 70 mmHg or a SpO2 ≥ 98%, as per standard of care in brain-injured patients.
Study Protocol
Two humidification devices were used: HME (Hygrobac; DAR: dead space 84 ml, resistance 1.0 cmH2)/L/s) and HH (MR850, Fisher & Paykel, Auckland, New Zealand).
This crossover study was organized into three phases. In phase I (HME1), a HME placed distally to the Y-piece of the circuit, as in the stabilization phase. Mechanical ventilation, as prescribed by the attending physician, was maintained for 30 min without any changes in the settings: Afterward, all relevant data were collected. In phase II (HH), the HME was removed and a HH was placed in the inspiratory limb of the circuit and VT was titrated (20–30 ml decrease every 10 min) to obtain PaCO2 equal to the one detected at the end of HME1; study data were collected 30 min after reaching the target PaCO2 level. In phase III (HME2), an HME was placed again distally to the Y-piece of the circuit and all baseline settings were resumed.
All patients received cisatracurium continuous infusion, at a standard dose of 35 mg/h [22]. Patients’ sedation, vasopressor dose, set PEEP, respiratory rate, FiO2, and I-to-E ratio were kept unchanged over the entire course of the experiment.
Endotracheal suctioning was performed at study entry and was not repeated over the course of the study period, unless specifically required.
Measurements
The following parameters were continuously monitored (SC7000 Monitor, Siemens, Erlangen, Germany) during the study: heart rate, arterial blood pressure, intracranial pressure, cerebral perfusion pressure, and SpO2.
Blood flow velocity in the middle cerebral artery was measured at the end of each study step with a 2 MHz pulsed Doppler ultrasound device (transcranial Doppler [TCD] H21—Hitachi Medical System Europe, Zug Switzerland).
The ventilator (ServoVentilator 900C, Siemens-Elema, Sweden) and a mainstream capnograph (CO2 analyzer 930, Siemens-Elema, Sweden) were connected to a personal computer. The ventilator system transducers produced signals representing pressure in the expiratory line, ventilator flow rate, and CO2 at airway opening. These signals were filtered to avoid aliasing and were converted from analog to digital at 50 Hz. The flow signal was calibrated under BTPS (body temperature and pressure, saturated) conditions with a 1-L syringe. Pressure was calibrated using a water manometer and CO2 using a gas mixture with a known composition.
Tidal volume was measured as digital integration of expiratory flow signal. Tidal volume/kg of predicted body weight (PBW) was computed, with PBW calculated as described elsewhere [7].
Total PEEP (PEEPTOT) was measured during end-expiratory occlusions, while airway plateau pressure (PPLAT) was measured during a 2-second end-inspiratory occlusion. Driving pressure (∆P) was computed as the difference between PPLAT and PEEPTOT. Static respiratory system compliance (CRS) was calculated as VT/∆P. Total, airway, and alveolar dead space was computed using volumetric capnography, according to a method validated elsewhere [23, 24]. Respiratory system mechanics, gas exchange, physiological dead space, and hemodynamics were measured in each phase of the protocol.
Elastic pressure–volume curves at set and zero PEEP were recorded in each phase of the study during low sinusoidal flow inflation, according to a method previously described in detail [25,26,27,28,29]. The linear CRS was calculated as the steeper segment between the lower inflection point and upper inflection point of the curve at zero PEEP. The derecruited volume from set PEEP to zero PEEP was measured (Rec) and consisted in the volume difference between the pressure–volume curves recorded at set PEEP and zero PEEP that were graphically superimposed and compared at an elastic pressure of 20 cmH2O [30, 31]. Rec was also normalized to the applied level of set PEEP: Rec/PEEPTOT was computed as the ratio between Rec and PEEPTOT, and patients were classified as having a highly recruitable profile when Rec/PEEPTOT > 14.5 ml/cmH2O [32].
Endpoints
Primary endpoint of this physiological study was to assess during isocapnic conditions the gain provided by HH in terms of VT, PPLAT, and, ∆P reduction, as compared to HME.
Safety endpoints were the effects of a low VT strategy on cerebral perfusion, as defined by cerebral perfusion pressure and blood flow velocity in the middle cerebral artery, and on respiratory mechanics and lung recruitment, as defined by lower and upper inflection points, linear and static CRS, Rec, and Rec/PEEPTOT.
Sample Size Calculation
Given the physiological design of the study, we did not perform a formal sample size calculation. Based on other investigations on the topic [17, 18, 20], we planned to enroll 15–18 patients that appear an adequate sample to draw conclusions on the specific endpoints addressed in the present investigation.
Statistical Analysis
Categorical data are showed as number of events (%). Continuous data are presented as median [interquartile range] and were analyzed using Friedman test for repeated measures. Post hoc paired comparisons were performed with Wilcoxon sum-rank test. Mean differences (95% CI) are displayed for most significant results. Distribution of categorical variables in the three study steps was compared with the Cochrane Q test: Paired comparisons were performed with the McNamar test.
Two-sided p value ≤ 0.05 was considered statistically significant. Analysis was performed using SPSS (version 20.0).
Results
Eighteen patients met inclusion criteria and were enrolled in the study. Demographics and clinical characteristics are shown in Table 1.
Consistently with the design of the protocol, no changes in PaCO2, respiratory rate, set, and total PEEP were found among the three study steps (all p > 0.05; Table 2, Fig. 1).
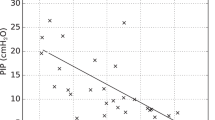
Tidal volume, plateau pressure, driving pressure, total dead space, airway dead space, and alveolar tidal volume were significantly lower during HH as compared to HME1 (all p < 0.05; Table 2, Figs. 2, 3).
Tidal volume, plateau pressure, and driving pressure, in the three study steps. Individual data are displayed. Horizontal line indicating driving pressure = 14 cmH2O is showed: Note that 16/18 (89%) of patients have a driving pressure ≤ 14 cmH2O in the HH step, as compared to 7/18 (39%) and 8/18 (44%) during HME1 and HME2 (p < 0.001). * indicates p < 0.05 for comparison HME1 versus HH; ° indicates p < 0.05 for comparison HME2 versus HH
Partitioning of dead space in the three study steps. Median and interquartile ranges are displayed. Total dead space was lower during HH, as compared to HME1 and HME2 (p < 0.001). The reduction in dead space was due to a lower airway dead space in the HH step. * indicates p < 0.05 for comparison HME1 versus HH; ° indicates p < 0.05 for comparison HME2 versus HH
Consistent with these findings, static Crs was higher during HH and lower tidal volume ventilation than during HME1 (p = 0.008; Table 1). No significant effects on PaO2/FiO2 ratio, linear CRS, alveolar dead space, lower and upper inflection point, Rec, Rec/PEEPTOT, and the proportion of patients with Rec/PEEPTOT > 14.5 ml/cmH2O (all p > 0.05; Table 2) have been detected.
Heart rate, arterial pressure, intracranial and cerebral perfusion pressure, and flow velocity in the middle cerebral artery were similar in the three study steps (Table 2; Fig. 1; all p > 0.10).
The use of HHs, as compared to HMEs, decreased total dead space (− 86 [95% CI: − 73 to − 98] ml, p < 0.001) due to significantly lower airway dead space (− 84 [95% CI: − 79 to − 89] ml, p < 0.001), without affecting alveolar dead space (Table 2, Fig. 3).
The application of HH allowed an average VT reduction of 120 [95% CI: 98–144] ml (p < 0.001) along with a decrease in VT/kg PBW of 1.8 [95% CI: 1.5–2.1] ml/kg (p < 0.001) (Fig. 2). The use of lower tidal volume was associated with an increase in 2.3 ml/cmH2O in static CRS [95% CI: 0.6–4.1] (p = 0.08) and with lower PPLAT and ∆P (both − 3.7 [95% CI: − 2.9 to − 4.3] cmH2O, p < 0.001); 16/18 (89%) of patients showed a ∆P ≤ 14 cmH2O in the HH step, as compared to 7/18 (39%) and 8/18 (44%) during HME1 and HME2 (p < 0.001).
Discussion
Our results show that, in brain-injured patients with ARDS, the use of HHs permits to reduce tidal volume and ∆P without affecting cerebral hemodynamics and arterial CO2 tension.
Consistently with previous investigations [17, 18, 20, 33] HHs, as compared to HMEs, significantly reduced total and airway dead space. The measured dead space reduction provided by HHs was 86 [95% CI: 73–98] ml and is consistent with the 90-ml instrumental dead space declared by HME manufacturer. In our study, this allowed to reduce VT/kg PBW by 1.8 [95% CI: 1.5–2.1] ml/kg and ∆P by 3.7 [95% CI: − 2.9 to − 4.3] cmH2O.
Several strategies have been proposed to mitigate VILI and improve clinical outcome during ARDS: Among these, the most convincing are lower VT, prone positioning and, possibly, mid-to-high PEEP with/without muscle paralysis in most severe patients [7, 22, 34,35,36,37]. Prone positioning may yield increases in intracranial pressure [38]; the use of high PEEP may not be safe in all brain-injured patients due to its possible detrimental effects on central venous pressure, venous return, cardiac output, and intracranial pressure [39]; thus, lowering VT appears as the only available intervention to enhance lung protection in this context [40]. This appears of crucial importance when brain injury coexists, as these patients are burdened by high risk of respiratory complications, high tracheostomy rates, prolonged mechanical ventilation, and worse clinical outcome [2, 13, 41, 42].
The ∆P, which is VT normalized to CRS and is a surrogate of the dynamic strain [43], represents the final mediator of ventilator settings effects on clinical outcome [44, 45]: Although a safe threshold for this parameter has not been identified yet, patients with ∆P ≤ 14 cmH2O show improved survival [46]. Our protocol led to an increase in the proportion of patients showing ∆P ≤ 14 cmH2O from 39 to 89%, thus suggesting a possible clinical benefit by this approach. Although VT and ∆P reductions are among the most important modifiable factors capable of improving survival during ARDS [47], the use of HH was not associated with improved clinical outcome in wide unselected cohorts of mechanically ventilated patients [48]. In previous studies, however, the use of HH was not systematically accompanied by VT reduction as it is in our protocol, so that any possible benefit could have been underestimated.
In our study, the use of low VT leads to a significant increase in static CRS without affecting the linear compliance measured between lower and upper inflection point. Lung volume, as defined by Rec, did not change nor patients’ position varied among the study steps, and chest wall elastance was likely constant over the entire course of the study, thus suggesting that any observed change in respiratory mechanics reflects variations in lung mechanics: In particular, the results inhering static and quasi-static compliance indicate some degree of lung overdistention when higher VT were used, as already suggested by other authors [20, 49, 50].
Although previous data indicate that lower VT can favor alveolar derecruitment [7, 51, 52], we do not report significant derecruitment or oxygenation worsening during VT reduction. Lung volume change as a response to PEEP may significantly vary among patients according to different degrees of lung recruitability [53, 54]. Accordingly, only 17–22% of our patients showed a high recruitability profile (i.e., > 14.5 ml/cmH2O of PEEP), as compared to 50% of patients in previous ARDS cohorts [32], so that the scarce derecruitment effect of lower tidal volume observed in our study may be explained by this particular characteristic of the studied population. In this sense, because of the risk of further impairment in oxygenation that can be fatal in brain-injured subjects, we did not enroll patients with severe ARDS who, indeed, show the highest lung recruitability profile [54, 55]. Moreover, higher PEEP (up to 20 cmH2O or further) may be required to achieve optimal lung recruitment [56] and such values may be difficult to apply in brain-injured patients.
Finally, and most importantly, our approach is simple, easily bedside available and showed a broad safety spectrum: No hemodynamic instability, abrupt increases in end tidal CO2 (EtCO2) and intracranial pressure, decreases in SpO2 and cerebral perfusion pressure, or any other adverse events were detected over the course of the entire study. Similarly, the use of low VT was not associated with changes in cerebral perfusion pressure or blood flow velocity in the middle cerebral artery.
The main limitation of the present study is its sequential crossover design, since the predetermined order of interventions may have affected the outcome. However, we tried to mitigate this aspect introducing a HME2 step, when all the baseline conditions were resumed. The substantial equivalence between most of the parameters in step HME1 and HME2 suggests that the patients were not subject to changes in respiratory, hemodynamic, and cerebral conditions during any of the study period, thus contributing to the strength and reproducibility of our findings. The small differences between HME1 and HME2 can be ascribed to the limited sample and the statistical rank-based test used for the analysis. Finally, initial tidal volumes and respiratory rates reflect individual clinician’s attitude in the treatment of patients with brain injury, and a strictly low-tidal ventilation strategy was not applied at baseline. This is consistent with previous reports, indicating that patients with brain injury are often exposed non-protective ventilation settings [12,13,14,15,16]. Indeed, the aim of this study was limited to the assessment of the physiological effects of changing from an HME device to HH.
Conclusions
The use of HH in patients with brain injury and ARDS reduces instrumental dead space and allows to reduce tidal volume and driving pressure in isocapnic conditions, with no alveolar derecruitment, hypoxemia, changes in cerebral perfusion pressure nor blood flow. This increases the proportion of patients receiving mechanical ventilation within safety limits. Given its safeness and strong pathophysiological plausibility, we deem this intervention can be recommended among the first-line ventilatory management in brain-injured ARDS patients.
References
Kahn JM, Caldwell EC, Deem S, Newell DW, Heckbert SR, Rubenfeld GD. Acute lung injury in patients with subarachnoid hemorrhage: incidence, risk factors, and outcome. Crit Care Med. 2006;34:196–202.
Mascia L, Sakr Y, Pasero D, Payen D, Reinhart K, Vincent J-L, et al. Extracranial complications in patients with acute brain injury: a post hoc analysis of the SOAP study. Intensive Care Med. 2008;34:720–7.
Zygun DA, Kortbeek JB, Fick GH, Laupland KB, Doig CJ. Non-neurologic organ dysfunction in severe traumatic brain injury. Crit Care Med. 2005;33:654–60.
Wartenberg KE, Schmidt JM, Claassen J, Temes RE, Frontera JA, Ostapkovich N, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34:617–23 (quiz 624).
Rincon F, Ghosh S, Dey S, Maltenfort M, Vibbert M, Urtecho J, et al. Impact of acute lung injury and acute respiratory distress syndrome after traumatic brain injury in the United States. Neurosurgery. 2012;71:795–803.
Holland MC, Mackersie RC, Morabito D, Campbell AR, Kivett VA, Patel R, et al. The development of acute lung injury is associated with worse neurologic outcome in patients with severe traumatic brain injury. J Trauma. 2003;55:106–11.
Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8.
Neto AS, Hemmes SNT, Barbas CSV, Beiderlinden M, Fernandez-Bustamante A, Futier E, et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med. 2016;4:272–80.
Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, et al. An official american thoracic society/european society of intensive care medicine/society of critical care medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195:1253–63.
Nin N, Muriel A, Peñuelas O, Brochard L, Lorente JA, Ferguson ND, et al. Severe hypercapnia and outcome of mechanically ventilated patients with moderate or severe acute respiratory distress syndrome. Intensive Care Med. 2017;43:200–8.
Radermacher P, Maggiore SM, Mercat A. Fifty years of research in ARDS. Gas exchange in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;196:964–84.
Mascia L. Acute lung injury in patients with severe brain injury: a double hit model. Neurocrit Care. 2009;11:417–26.
Pelosi P, Ferguson ND, Frutos-Vivar F, Anzueto A, Putensen C, Raymondos K, et al. Management and outcome of mechanically ventilated neurologic patients. Crit Care Med. 2011;39:1482–92.
Mascia L, Zavala E, Bosma K, Pasero D, Decaroli D, Andrews P, et al. High tidal volume is associated with the development of acute lung injury after severe brain injury: an international observational study. Crit Care Med. 2007;35:1815–20.
Asehnoune K, Mrozek S, Perrigault PF, Seguin P, Dahyot-Fizelier C, Lasocki S, et al. A multi-faceted strategy to reduce ventilation-associated mortality in brain-injured patients. The BI-VILI project: a nationwide quality improvement project. Intensive Care Med. 2017;43:957–70.
Marhong JD, Ferguson ND, Singh JM. Ventilation practices in subarachnoid hemorrhage: a cohort study exploring the use of lung protective ventilation. Neurocrit Care. 2014;21:178–85.
Prin S, Chergui K, Augarde R, Page B, Jardin F, Vieillard-Baron A. Ability and safety of a heated humidifier to control hypercapnic acidosis in severe ARDS. Intensive Care Med. 2002;28:1756–60.
Prat G, Renault A, Tonnelier J-M, Goetghebeur D, Oger E, Boles J-M, et al. Influence of the humidification device during acute respiratory distress syndrome. Intensive Care Med. 2003;29:2211–5.
Richecoeur J, Lu Q, Vieira SR, Puybasset L, Kalfon P, Coriat P, et al. Expiratory washout versus optimization of mechanical ventilation during permissive hypercapnia in patients with severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:77–85.
Morán I, Bellapart J, Vari A, Mancebo J. Heat and moisture exchangers and heated humidifiers in acute lung injury/acute respiratory distress syndrome patients. Effects on respiratory mechanics and gas exchange. Intensive Care Med. 2006;32:524–31.
ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–33.
Papazian L, Forel J-M, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–16.
Tusman G, Sipmann FS, Borges JB, Hedenstierna G, Bohm SH. Validation of Bohr dead space measured by volumetric capnography. Intensive Care Med. 2011;37:870–4.
Kreit WJ. Volume capnography in the intensive care unit: physiological principles, measurements, and calculations. Ann Am Thorac Soc. 2019;16:291–300.
Servillo G, Svantesson C, Beydon L, Roupie E, Brochard L, Lemaire F, et al. Pressure-volume curves in acute respiratory failure: automated low flow inflation versus occlusion. Am J Respir Crit Care Med. 1997;155:1629–36.
Jonson B, Richard JC, Straus C, Mancebo J, Lemaire F, Brochard L. Pressure-volume curves and compliance in acute lung injury: evidence of recruitment above the lower inflection point. Am J Respir Crit Care Med. 1999;159:1172–8.
Svantesson C, Drefeldt B, Sigurdsson S, Larsson A, Brochard L, Jonson B. A single computer-controlled mechanical insufflation allows determination of the pressure-volume relationship of the respiratory system. J Clin Monit Comput. 1999;15:9–16.
Ranieri VM, Giuliani R, Fiore T, Dambrosio M, Milic-Emili J. Volume-pressure curve of the respiratory system predicts effects of PEEP in ARDS: “occlusion” versus “constant flow” technique. Am J Respir Crit Care Med. 1994;149:19–27.
Scaramuzzo G, Spadaro S, Waldmann AD, Böhm SH, Ragazzi R, Marangoni E, et al. Heterogeneity of regional inflection points from pressure-volume curves assessed by electrical impedance tomography. Crit Care. 2019;23:119.
Maggiore SM, Richard JC, Brochard L. What has been learnt from P/V curves in patients with acute lung injury/acute respiratory distress syndrome. Eur Respir J Suppl. 2003;42:22s–6s.
Maggiore SM, Jonson B, Richard JC, Jaber S, Lemaire F, Brochard L. Alveolar derecruitment at decremental positive end-expiratory pressure levels in acute lung injury: comparison with the lower inflection point, oxygenation, and compliance. Am J Respir Crit Care Med. 2001;164:795–801.
Dellamonica J, Lerolle N, Sargentini C, Beduneau G, Di Marco F, Mercat A, et al. PEEP-induced changes in lung volume in acute respiratory distress syndrome. Two methods to estimate alveolar recruitment. Intensive Care Med. 2011;37:1595–604.
Campbell RS, Davis K, Johannigman JA, Branson RD. The effects of passive humidifier dead space on respiratory variables in paralyzed and spontaneously breathing patients. Respir Care. 2000;45:306–12.
Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators, Cavalcanti AB, Suzumura ÉA, Laranjeira LN, Paisani DDM, Damiani LP, et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low peep on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318:1335–45.
Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303:865–73.
Guérin C, Reignier J, Richard J-C, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–68.
National Heart, Lung and and Blood Institute PETAL Clinical Trials Network, Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380:1997–2008.
Roth C, Ferbert A, Deinsberger W, Kleffmann J, Kästner S, Godau J, et al. Does prone positioning increase intracranial pressure? A retrospective analysis of patients with acute brain injury and acute respiratory failure. Neurocrit Care. 2014;21:186–91.
Caricato A, Conti G, Della Corte F, Mancino A, Santilli F, Sandroni C, et al. Effects of PEEP on the intracranial system of patients with head injury and subarachnoid hemorrhage: the role of respiratory system compliance. J Trauma. 2005;58:571–6.
Tejerina E, Pelosi P, Muriel A, Peñuelas O, Sutherasan Y, Frutos-Vivar F, et al. Association between ventilatory settings and development of acute respiratory distress syndrome in mechanically ventilated patients due to brain injury. J Crit Care. 2017;38:341–5.
Vargas M, Sutherasan Y, Antonelli M, Brunetti I, Corcione A, Laffey JG, et al. Tracheostomy procedures in the intensive care unit: an international survey. Crit Care. 2015;19:291.
Abe T, Madotto F, Pham T, Nagata I, Uchida M, Tamiya N, et al. Epidemiology and patterns of tracheostomy practice in patients with acute respiratory distress syndrome in ICUs across 50 countries. Crit Care. 2018;22:195.
Grieco DL, Russo A, Romanò B, Anzellotti GM, Ciocchetti P, Torrini F, et al. Lung volumes, respiratory mechanics and dynamic strain during general anaesthesia. Br J Anaesth. 2018;121:1156–65.
Grieco DL, Chen L, Dres M, Brochard L. Should we use driving pressure to set tidal volume? Curr Opin Crit Care. 2017;23:38–44.
Amato MBP, Meade MO, Slutsky AS, Brochard L, EL Costa V, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–55.
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800.
Laffey JG, Bellani G, Pham T, Fan E, Madotto F, Bajwa EK, et al. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med. 2016;42:1865–76.
Vargas M, Chiumello D, Sutherasan Y, Ball L, Esquinas AM, Pelosi P, et al. Heat and moisture exchangers (HMEs) and heated humidifiers (HHs) in adult critically ill patients: a systematic review, meta-analysis and meta-regression of randomized controlled trials. Crit Care. 2017;21:123.
Henderson WR, Chen L, Amato MBP, Brochard LJ. Fifty years of research in ARDS. Respiratory mechanics in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;196:822–33.
Xie J, Jin F, Pan C, Liu S, Liu L, Xu J, et al. The effects of low tidal ventilation on lung strain correlate with respiratory system compliance. Crit Care. 2017;21:23.
Richard J-C, Brochard L, Vandelet P, Breton L, Maggiore SM, Jonson B, et al. Respective effects of end-expiratory and end-inspiratory pressures on alveolar recruitment in acute lung injury. Crit Care Med. 2003;31:89–92.
Richard JC, Maggiore SM, Jonson B, Mancebo J, Lemaire F, Brochard L. Influence of tidal volume on alveolar recruitment. Respective role of PEEP and a recruitment maneuver. Am J Respir Crit Care Med. 2001;163:1609–13.
Chen L, Del Sorbo L, Grieco DL, Junhasavasdikul D, Rittayamai N, Soliman I, et al. Potential for lung recruitment estimated by the recruitment-to-inflation ratio in acute respiratory distress syndrome. A Clinical Trial. Am J Respir Crit Care Med. 2020;201:178–87.
Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–86.
Chiumello D, Marino A, Brioni M, Cigada I, Menga F, Colombo A, et al. Lung recruitment assessed by respiratory mechanics and computed tomography in patients with acute respiratory distress syndrome. What is the relationship? Am J Respir Crit Care Med. 2016;193:1254–63.
Cressoni M, Chiumello D, Algieri I, Brioni M, Chiurazzi C, Colombo A, et al. Opening pressures and atelectrauma in acute respiratory distress syndrome. Intensive Care Med. 2017;43:603–11.
Funding
Support was provided solely from institutional and/or departmental sources. Outside of the present work, Dr. Grieco is supported by research grants by SIAARTI and ESICM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DLG has received payments for travel expenses by Maquet, Air Liquide, and reports non-financial support by Dimar. MA has received payments for personal fees by Orion and Pfizer, and reports a research grant by Estor. DLG and MA disclose a research grant by General Electric Healthcare.
Ethical approval/Informed consent
The study was conducted in the general intensive care unit (ICU) of a university hospital in Rome, Italy. Written informed consent was obtained from all study participants. The study protocol was approved by the Institutional Review Board of the Catholic University of the Sacred Heart of Rome, Italy (IRB approval number 8060/13) and we have adhered to ethical considerations in the protection of all patients involved according to the principles of the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pitoni, S., D’Arrigo, S., Grieco, D.L. et al. Tidal Volume Lowering by Instrumental Dead Space Reduction in Brain-Injured ARDS Patients: Effects on Respiratory Mechanics, Gas Exchange, and Cerebral Hemodynamics. Neurocrit Care 34, 21–30 (2021). https://doi.org/10.1007/s12028-020-00969-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-020-00969-5