Abstract
Background
Magnetic resonance imaging (MRI) carries prognostic importance after traumatic brain injury (TBI), especially when computed tomography (CT) fails to fully explain the level of unconsciousness. However, in critically ill patients, the risk of deterioration during transfer needs to be balanced against the benefit of detecting prognostically relevant information on MRI. We therefore aimed to assess if day of injury serum protein biomarkers could identify critically ill TBI patients in whom the risks of transfer are compensated by the likelihood of detecting management-altering neuroimaging findings.
Methods
Data were obtained from the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study. Eligibility criteria included: TBI patients aged ≥ 16 years, Glasgow Coma Score (GCS) < 13 or patient intubated with unrecorded pre-intubation GCS, CT with Marshall score < 3, serum biomarkers (GFAP, NFL, NSE, S100B, Tau, UCH-L1) sampled ≤ 24 h of injury, MRI < 30 days of injury. The degree of axonal injury on MRI was graded using the Adams-Gentry classification. The association between serum concentrations of biomarkers and Adams-Gentry stage was assessed and the optimum threshold concentration identified, assuming different minimum sensitivities for the detection of brainstem injury (Adams-Gentry stage 3). A cost–benefit analysis for the USA and UK health care settings was also performed.
Results
Among 65 included patients (30 moderate-severe, 35 unrecorded) axonal injury was detected in 54 (83%) and brainstem involvement in 33 (51%). In patients with moderate-severe TBI, brainstem injury was associated with higher concentrations of NSE, Tau, UCH-L1 and GFAP. If the clinician did not want to miss any brainstem injury, NSE could have avoided MRI transfers in up to 20% of patients. If a 94% sensitivity was accepted considering potential transfer-related complications, GFAP could have avoided 30% of transfers. There was no added net cost, with savings up to £99 (UK) or $612 (US). No associations between proteins and axonal injury were found in intubated patients without a recorded pre-intubation GCS.
Conclusions
Serum protein biomarkers show potential to safely reduce the number of transfers to MRI in critically ill patients with moderate-severe TBI at no added cost.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Traumatic brain injury (TBI) accounts for 300,000 hospitalizations in the US and 1.5 million hospitalization in Europe every year [1]. Some patients present with a low Glasgow Coma Scale score (GCS) [2] without evidence of mass lesion or raised intracranial pressure on computed tomography (CT). The low GCS may be caused either by CT-occult traumatic (diffuse) axonal injury, or by more reversible factors such as alcohol, drugs and/or seizures. Other patients are emergently intubated at the injury scene to manage extra-cranial injuries and arrive at the hospital without a recorded pre-intubation GCS. In these patients, a normal CT does not preclude the presence of CT-occult injury.
Magnetic resonance imaging (MRI) is increasingly used to detect such CT-occult axonal injury. High-grade axonal injury (particularly brainstem injury) is clinically important, since it tends to drive outcome [3, 4]. According to the Adams-Gentry grading, foci confined to the hemispheres indicate stage 1, foci involving the corpus callosum stage 2 and foci in the brainstem stage 3 [5, 6]. Such information is critical when considering interventions such as decompressive craniectomy, which can increase survival in refractory intracranial hypertension, but at the risk of unacceptable disability in patients in whom outcome is driven by the primary injury rather than secondary insults of intracranial hypertension [7].
The prognostic benefit of MRI, however, needs to be weighed against the clinical risk of patient transfer to the scanner, even if MRI is available within the same hospital. Previous studies found that one in four intra-hospital transfers of ventilated patients is associated with complications (1.5% with life-threatening complications), which occur at twice the rate seen in non-transferred patients [8, 9]. A serum biomarker of axonal injury as a triage tool for MRI would thus be useful. Serum protein biomarkers, especially glial fibrillary acidic protein, have been shown to detect CT-occult (axonal) injury in mild TBI, but their utility in moderate-severe TBI is still unclear [10]. To be useful, serum biomarkers would need to prove clinically safe (i.e., reach an acceptable minimum sensitivity for the detection of brainstem injury) and affordable (i.e., not generating large additional costs).
We therefore aimed to investigate if serum protein biomarkers could identify critically ill TBI patients for MRI.
Methods
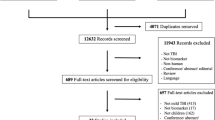
Patients were selected from the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study [11]. Clinical data was accessed via the Neurobot platform (RRID/SCR_017004, core data, version 3.0; International Neuroinformatics Coordinating Facility; released November 24, 2020).
The present analysis included all patients in whom the CT did not fully explain the GCS, defined as:
-
(1)
a CT within 24 h of injury without evidence of raised intracranial pressure or mass lesion i.e., Marshall score < 3 [12].
-
(2)
PLUS
-
a.
a GCS < 13 (“moderate-severe sub-cohort”) OR
-
b.
a GCS that was unrecorded prior to intubation (“unrecorded sub-cohort”).
-
a.
In addition, all patients must have been aged ≥ 16 years, undergone MRI within 30 days of injury, and had serum protein biomarkers sampled within 24 h. Glial fibrillary acidic protein (GFAP), neurofilament light (NFL), neuron-specific enolase (NSE), S100 calcium-binding protein B (S100B), total tau (Tau) and ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) were assayed as described previously [13].
Image acquisition, and reporting
CT images were acquired according to local site protocols, were reported centrally by trained investigators blinded to outcome and assigned a Marshall score [12, 14].
MR images were obtained following study-specific protocols (https://www.center-tbi.eu/project/mri-study-protocols) and included T1-weighted, T2-weighted, fluid-attenuated inversion recovery, susceptibility-weighted and diffusion-weighted images. The location of axonal injury on MRI was reported in Cambridge by one neurotrauma research clinician blinded to patient characteristics (SR) and reviewed by a second neurotrauma research clinician (VFJN). The degree of axonal injury was scored using the Adams-Gentry classification [5, 6]. For axonal brainstem injury we recorded if known adverse features were present, i.e., injury that was bilateral, dorsal, pontine or associated with Duret hemorrhage or contusion [4, 15, 16].
Statistical analysis
The associations between proteins and the Adams-Gentry stage or the presence of brainstem injury was assessed with a two-sided Jonckheere-Terpstra test and a Mann–Whitney U test, respectively. Statistical analysis was conducted in R 4.2.0 (R Project for Statistical Computing). The significance threshold for p values was set at 0.05 and all p values adjusted for multiple comparisons using the Benjamini–Hochberg method [17].
For each protein we identified the serum concentration where specificity would be maximal given a minimum sensitivity of (a) 90% or (b) 100%, using the R package OptimalCutpoints. The cost–benefit analysis was conducted with the perspectives of the UK and the USA health care systems (Additional file 1: Methods).
Results
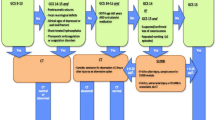
Inclusion criteria were met by 65 patients (30 in the moderate-severe sub-cohort, 35 in the unrecorded sub-cohort), of which 49 (75%) were male, 60 (92%) were intubated and 39 (60%) had sustained major extra-cranial injuries; the median age was 40 years (range 16–82) (Additional file 1: Table S1). The study population was younger and more severely injured than the whole CENTER-TBI population (Additional file 1: Table S2). The MRI was performed at a median of 6 days (range 0–29), showed axonal injury in 54 (83%) and brainstem involvement 33 (51%) patients (Additional file 1: Table S3).
Axonal injury burden irrespective of location was associated with serum GFAP concentrations (Additional file 1: Fig. S1). The Adams-Gentry stage was associated with GFAP, NSE and UCH-L1 in the moderate-severe sub-cohort but was not associated with any proteins in the unrecorded sub-cohort or the overall study cohort (Additional file 1: Table S4).
Adams-Gentry stage 3 (brainstem involvement) was associated with GFAP (borderline statistical significance), NSE, Tau and UCH-L1 in the moderate-severe sub-cohort but was not associated with any proteins in the unrecorded sub-cohort or the overall study cohort (Table 1).
In the moderate-severe sub-cohort protein biomarkers showed potential for MRI triage. Assuming a minimum sensitivity of 90% for the detection of brainstem injury, the best specificity was achieved by GFAP, avoiding 30% of MRI transfers whilst missing 1 in 20 brainstem injuries (Table 2). If a sensitivity of 100% was desired, then NSE performed best, avoiding 20% of MRI transfers whilst not missing any brainstem injury (Table 2 and Additional file 1: Table S5). Both approaches were cost-saving.
The prevalence of adverse features of brainstem injury is documented in Additional file 1: Table S3. In the moderate-severe sub-cohort, GFAP, NSE and UCH-L1 were associated with adverse features (Additional file 1: Table S6).
Discussion
This study evaluated whether protein biomarkers could help avoid high-risk clinical transfers for MRI in critically ill TBI patients in whom the CT may not fully explain the GCS.
The risk of deterioration during transfer varies widely depending on, for example, the patient’s clinical status, the availability of trained personnel and the distance to the MRI scanner [8, 9]. Depending on this estimated risk a clinician may tolerate different levels of sensitivity for protein biomarkers. We identified NSE as the most promising biomarker in patients at lower risk of transfer-related complications and GFAP as the most promising biomarker when transfer-related adverse events are likely. In both scenarios, protein biomarkers were not only affordable but cost-saving.
We found GFAP, NSE, UCH-L1 outperformed axonal markers (NFL and Tau) for the detection of axonal injury. Sampling within 24 h may have discriminated against the slower to peak NFL. Protein biomarker concentrations have previously been found using CT to reflect the total burden of injury irrespective of lesion type or location [18]. The complex pathophysiology after TBI including traumatic vascular injury, blood brain barrier disruption and a host inflammatory response means biomarker elevations may not only result from axonal injury.
We considered whether the poor relationship between biomarkers and MRI in the unrecorded sub-cohort might be due to a lower severity of brain stem injury in these emergently intubated patients, which however was not the case. Despite additional exploration of the data, we were unable to find a satisfactory explanation. Possible confounds that we are unable to test for in our data include high volume transfusions which may have diluted biomarker levels; second insults not reflected in admission biomarker levels; or a Type II error due to relatively small sample sizes in subgroups, which may be addressed in a larger study.
Limitations
The sample size of 65, while large for a prospective study of early MRI after moderate-to-severe TBI, requires external validation in larger cohorts. This would also enable more refined analysis of the influence of lesion location and type. Furthermore, Quanterix assay kits are currently only available for research purposes. However, an alternative platform is already licensed for GFAP and UCH-L1 and more are likely to be approved in the future [19]. Our time window for MRI ranged from 0 to 30 days. As imaging features change during this timeframe, future studies should aim for a more uniform imaging timepoint [4, 20]. Our findings should therefore not be interpreted as a call to change clinical practice, but as an encouragement to repeat this study in a larger cohort.
Conclusion
Serum protein biomarkers show promise as a triage tool for MRI in TBI patients where the CT does not fully explain a low GCS, but not in patients with an unrecorded GCS. Findings require validation in a larger cohort.
Availability of data and materials
CENTER-TBI investigators are strong proponents of data sharing to advance TBI research. De-identified patient data is available upon request, subject to approval by the CENTER-TBI Management Committee. Proposals can be submitted online at https://www.center-tbi.eu/data and will be assessed for methodological soundness. Shared data can be used without a time limit but in the context of a data sharing agreement and in accordance with the regulatory restrictions of the original CENTER-TBI study. The statistical analysis code is freely available at https://github.com/DrSophieRichter/MRI_triage.
Abbreviations
- TBI:
-
Traumatic brain injury
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- GFAP:
-
Glial fibrillary acidic protein
- NFL:
-
Neurofilament light
- NSE:
-
Neuron-specific enolase
- S100B:
-
Calcium-binding protein B
- Tau:
-
Total tau
- UCH-L1:
-
Ubiquitin carboxy-terminal hydrolase L1
References
Maas AIR, Menon DK, Adelson PD, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987–1048. https://doi.org/10.1016/s1474-4422(17)30371-x.
Teasdale G, Jennett B. Assessment of coma and impaired consciousness A practical scale. Lancet. 1974;13(2):81–4. https://doi.org/10.1016/s0140-6736(74)91639-0.
Haghbayan H, Boutin A, Laflamme M, et al. The prognostic value of MRI in moderate and severe traumatic brain injury: a systematic review and meta-analysis. Crit Care Med. 2017;45(12):e1280–8. https://doi.org/10.1097/CCM.0000000000002731.
Tjerkaski J, Nystrom H, Raj R, et al. Extended analysis of axonal injuries detected using magnetic resonance imaging in critically Ill traumatic brain injury patients. J Neurotrauma. 2022;39(1–2):58–66. https://doi.org/10.1089/neu.2021.0159.
Hume Adams J, Doyle D, Ford I, Gennarelli A, Graham DI, Mclellan DR. Diffuse axonal injury in head injury: Definition, diagnosis and grading. Histopathology. 1989;15(1):49–59. https://doi.org/10.1111/j.1365-2559.1989.tb03040.x.
Gentry LR. Imaging of closed head injury. Radiology. 1994;191(1):1–17. https://doi.org/10.1148/radiology.191.1.8134551.
Hutchinson PJ, Kolias AG, Timofeev IS, et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016;375(12):1119–30. https://doi.org/10.1056/nejmoa1605215.
Schwebel C, Clec’h C, Magne S, et al. Safety of intrahospital transport in ventilated critically ill patients: a multicenter cohort study. Crit Care Med. 2013;41(8):1919–28. https://doi.org/10.1097/CCM.0b013e31828a3bbd.
Murata M, Nakagawa N, Kawasaki T, et al. Adverse events during intrahospital transport of critically ill patients: a systematic review and meta-analysis. Am J Emerg Med. 2022;52:13–9. https://doi.org/10.1016/j.ajem.2021.11.021.
Yue JK, Upadhyayula PS, Avalos LN, Deng H, Wang KKW. The role of blood biomarkers for magnetic resonance imaging diagnosis of traumatic brain injury. Medicina. 2020;56(2):87. https://doi.org/10.3390/medicina56020087.
Maas AI, Menon DK, Steyerberg EW, et al. Collaborative European NeuroTrauma effectiveness research in traumatic brain injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery. 2015;76(1):67–80. https://doi.org/10.1227/NEU.0000000000000575.
Marshall LF, Marshall SB, Klauber MR, et al. A new classification of head injury based on computerized tomography. J Neurosurg. 1991;75:S14–20. https://doi.org/10.3171/sup.1991.75.1s.0s14.
Czeiter E, Amrein K, Gravesteijn BY, et al. Blood biomarkers on admission in acute traumatic brain injury: relations to severity, CT findings and care path in the CENTER-TBI study. EBioMedicine. 2020;56:102785. https://doi.org/10.1016/j.ebiom.2020.102785.
Vande Vyvere T, Wilms G, Claes L, et al. Central versus local radiological reading of acute computed tomography characteristics in multi-center traumatic brain injury research. J Neurotrauma. 2019;36(7):1080–92. https://doi.org/10.1089/neu.2018.6061.
Izzy S, Mazwi NL, Martinez S, et al. Revisiting Grade 3 diffuse axonal injury: not all brainstem microbleeds are prognostically equal. Neurocrit Care. 2017;27(2):199–207. https://doi.org/10.1007/s12028-017-0399-2.
Williams JR, Nieblas-Bedolla E, Feroze A, et al. Prognostic value of hemorrhagic brainstem injury on early computed tomography: a TRACK-TBI study. Neurocrit Care. 2021;35(2):335–46. https://doi.org/10.1007/s12028-021-01263-8.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x.
Whitehouse DP, Monteiro M, Czeiter E, et al. Relationship of admission blood proteomic biomarkers levels to lesion type and lesion burden in traumatic brain injury: A CENTER-TBI study. EBioMedicine. 2022;75:103777. https://doi.org/10.1016/j.ebiom.2021.103777.
US Food and Drug Administration. FDA authorizes marketing of first blood test to aid in the evaluation of concussion in adults. Feb 14, 2018
Richter S, Winzeck S, Kornaropoulos EN, et al. Neuroanatomical substrates and symptoms associated with magnetic resonance imaging of patients with mild traumatic brain injury. JAMA Netw Open. 2021;4(3):e210994. https://doi.org/10.1001/jamanetworkopen.2021.0994.
Acknowledgements
The CENTER-TBI MRI Sub-Study Participants and Investigators: Krisztina Amrein, MD, János Szentágothai Research Centre, University of Pécs, Pécs, Hungary; Nada Andelic, MD Ph.D., Division of Surgery and Clinical Neuroscience, Department of Physical Medicine and Rehabilitation, Oslo University Hospital and University of Oslo, Oslo, Norway; Lasse Andreassen, MD, Department of Neurosurgery, University Hospital Northern Norway, Tromso, Norway; Audny Anke, MD, Department of Physical Medicine and Rehabilitation, University Hospital Northern Norway, Tromso, Norway; Philippe Azouvi, MD Ph.D.; Raymond Poincare hospital, Assistance Publique – Hopitaux de Paris, Paris, France; Bo‑Michael Bellander, MD Ph.D., Department of Neurosurgery and Anesthesia and intensive care medicine, Karolinska University Hospital, Stockholm, Sweden; Habib Benali MD, Anesthesie-Réanimation, Assistance Publique – Hopitaux de Paris, Paris, France; Andras Buki, DSc, Örebro University, School of Medical Sciences, Örebro, Sweden, János Szentágothai Research Centre, University of Pécs, Pécs, Hungary; Alessio Caccioppola, MD, Neuro ICU, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy; Emiliana Calappi, MD, Neuro ICU, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy; Marco Carbonara, MD, Neuro ICU, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy; Giuseppe Citerio, MD Ph.D., School of Medicine and Surgery, Università Milano Bicocca; NeuroIntensive Care, ASST di Monza, Milan, Italy; Monza, Italy; Hans Clusmann, MD, Department of Neurosurgery, Medical Faculty RWTH Aachen University, Aachen, Germany; Mark Coburn, MD, Department of Anaesthesiology, University Hospital of Aachen and Department of Anesthesiology and Intensive Care Medicine, University Hospital Bonn, Aachen, Germany; Bonn Germany; Jonathan Coles, MD Ph.D., Department of Anesthesia and Neurointensive Care, Cambridge University Hospital NHS Foundation Trust, Cambridge, UK; Marta Correia, Ph.D., Radiology/MRI department, MRC Cognition and Brain Sciences Unit, Cambridge, UK; Endre Czeiter Ph.D., Department of Neurosurgery, Medical School, University of Pécs, Hungary and Neurotrauma Research Group, János Szentágothai Research Centre, University of Pécs, Pécs, Hungary; Véronique De Keyser, MSc, Department of Neurosurgery, Antwerp University Hospital and University of Antwerp, Edegem, Belgium; Vincent Degos, MD, Anesthesie-Réanimation, Assistance Publique – Hopitaux de Paris, Paris, France; Bart Depreitere, MD Ph.D., Department of Neurosurgery, University Hospitals Leuven, Leuven, Belgium; Live Eikenes, Ph.D., Department of Circulation and Medical Imaging, Norwegian University of Science and Technology, NTNU, Trondheim, Norway; Erzsébet Ezer, MD, Department of Anaesthesiology and Intensive Therapy, University of Pécs, Pécs, Hungary; Kelly Foks, MD Ph.D., Department of Neurology, Erasmus MC, Rotterdam, the Netherlands; Shirin Frisvold, Department of Anesthesiology and Intensive care, University Hospital Northern Norway, Tromso, Norway; Damien Galanaud, MD, Anesthesie-Réanimation, Assistance Publique – Hopitaux de Paris, Paris, France; Alexandre Ghuysen, MD, Emergency Department, CHU, Liège, Belgium; Ben Glocker, Ph.D., Department of Computing, Imperial College London, London, UK; Asta Haberg, Ph.D., Department of Neuromedicine and Movement Science, Norwegian University of Science and Technology, NTNU; Department of Physical Medicine and Rehabilitation, St.Olavs Hospital, Trondheim University Hospital, Trondheim, Norway; Iain Haitsma, MD, Department of Neurosurgery, Erasmus MC, Rotterdam, the Netherlands; Eirik Helseth, Department of Neurosurgery, Oslo University Hospital, Oslo, Norway; Peter J. Hutchinson, MD Ph.D., Division of Neurosurgery, Department of Clinical Neurosciences, Addenbrooke’s Hospital and University of Cambridge, Cambridge, UK; Evgenios Kornaropoulos, Ph.D., Division of Anaesthesia, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK; Noémi Kovács, Ph.D., Hungarian Brain Research Program—Grant No. KTIA_13_NAP-A-II/8, University of Pécs, Pécs, Hungary; Ana Kowark, MD, Department of Anaesthesiology, University Hospital of Aachen, Aachen, Germany; Steven Laureys, MD Ph.D., Cyclotron Research Center , University of Liège, Liège, Belgium; Didier Ledoux, MD Ph.D., Cyclotron Research Center , University of Liège, Liège, Belgium; Hester Lingsma, Ph.D., Department of Public Health, Erasmus Medical Center-University Medical Center, Rotterdam, The Netherlands; Andrew I.R. Maas, MD Ph.D., Department of Neurosurgery, Antwerp University Hospital and University of Antwerp, Edegem, Belgium; Geoffrey Manley, MD Ph.D., Department of Neurological Surgery, University of California, San Francisco, California, USA; David K. Menon MD Ph.D., Division of Anaesthesia, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK; Tomas Menovsky, MD Ph.D., Department of Neurosurgery, Antwerp University Hospital and University of Antwerp, Edegem, Belgium; Benoit Misset, MD, Cyclotron Research Center, University of Liège, Liège, Belgium; Visakh Muraleedharan, MSc, Karolinska Institutet, INCF International Neuroinformatics Coordinating Facility, Stockholm, Sweden; Ingeborg Nakken, MSc, Department of Radiology and Nuclear Medicine, St.Olavs Hospital, Trondheim University Hospital,Trondheim, Norway; Virginia Newcombe, MD Ph.D., Division of Anaesthesia, University of Cambridge, Addenbrooke’s Hospital Cambridge, UK; Wibeke Nordhøy, Ph.D., Department of Diagnostic Physics, Clinic of Radiology and Nuclear Medicine, Oslo University Hospital, Oslo, Norway; József Nyirádi, Ph.D., János Szentágothai Research Centre, University of Pécs, Pécs, Hungary; Fabrizio Ortolano, MD, Neuro ICU, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy; Paul M. Parizel, MD Ph.D. FRANZCR, David Hartley Chair of Radiology, Royal Perth Hospital (RPH) and University of Western Australia (UWA), Perth, WA 6000, Australia; Vincent Perlbarg, Ph.D., Anesthesie-Réanimation, Assistance Publique – Hopitaux de Paris, Paris, France, Paolo Persona; MD, Department of Anesthesia and Intensive Care, Azienda Ospedaliera Università di Padova, Padova, Italy; Wilco Peul, MD Ph.D., Dept. of Neurosurgery, Leiden University Medical Center, Leiden, The Netherlands and Dept. of Neurosurgery, Medical Center Haaglanden, The Hague, The Netherlands; Jussi P. Posti, MD Ph.D., Division of Clinical Neurosciences, Department of Neurosurgery and Turku Brain Injury Centre, Turku University Hospital and University of Turku, Turku, Finland; Louis Puybasset, MD Ph.D., Department of Anesthesiology and Critical Care, Pitié -Salpêtrière Teaching Hospital, Assistance Publique, Hôpitaux de Paris and University Pierre et Marie Curie, Paris, France; Sophie Richter, MD, Division of Anaesthesia, University of Cambridge, Addenbrooke’s Hospital Cambridge, UK; Cecilie Roe, MD, Department of Physical Medicine and Rehabilitation, Oslo University Hospital/University of Oslo, Oslo, Norway; Olav Roise, MD, Division of Orthopedics, Oslo University Hospital; Institute of Clinical Medicine, Faculty of medicine, University of Oslo, Oslo, Norway; Rolf Rossaint, MD, Department of Anaesthesiology, University Hospital of Aachen, Aachen, Germany; Sandra Rossi MD, Department of Anesthesia and Intensive Care, Azienda Ospedaliera Università di Padova Padova, Italy; Daniel Rueckert Ph.D., Department of Computing, Imperial College London, London, UK, Toril Skandsen, MD Ph.D., Department of Neuromedicine and Movement Science, Norwegian University of Science and Technology, NTNU; Department of Physical Medicine and Rehabilitation, St.Olavs Hospital, Trondheim University Hospital, Trondheim, Norway; Abayomi Sorinola, MD, Department of Neurosurgery, University of Pécs, Pécs, Hungary; Emmanuel Stamatakis, Ph.D., Division of Anaesthesia, University of Cambridge, Addenbrooke’s Hospital Cambridge, UK; Ewout W. Steyerberg, Ph.D., Department of Public Health, Erasmus Medical Center-University Medical Center; Dept. of Department of Biomedical Data Sciences, Leiden University Medical Center, Rotterdam, The Netherlands; Leiden, The Netherlands; Nino Stocchetti, MD, Department of Pathophysiology and Transplantation, Milan University, and Neuroscience ICU, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milano, Italy; Riikka Takala, MD Ph.D., Perioperative Services, Intensive Care Medicine and Pain Management, Turku University Hospital and University of Turku, Turku, Finland; Viktória Tamás, MD, Department of Neurosurgery, University of Pécs, Pécs, Hungary; Olli Tenovuo, MD Ph.D., Department of Clinical Neurosciences and Turku Brain Injury Centre, Turku University Hospital and University of Turku, Turku, Finland; Zoltán Vámos MD, Department of Anaesthesiology and Intensive Therapy, University of Pécs, Pécs, Hungary; Gregory Van der Steen, MSc, Department of Neurosurgery, Antwerp University Hospital and University of Antwerp, Edegem, Belgium; Wim Van Hecke, Ph.D., icoMetrix NV, Leuven, Belgium; Thijs Vande Vyvere, Ph.D., icoMetrix NV, Leuven, Belgium; Jan Verheyden, Ph.D., icoMetrix NV, Leuven, Belgium; Anne Vik, MD Ph.D., Department of Neuromedicine and Movement Science, Norwegian University of Science and Technology, NTNU; Department of Neurosurgery, St.Olavs Hospital, Trondheim University Hospital, Trondheim, Norway; Victor Volovici, MD Ph.D., Department of Neurosurgery, Erasmus MC Rotterdam, The Netherlands; Lars T. Westlye, Ph.D., Norwegian Centre for Mental Disorders Research (NORMENT), Division of Mental Health and Addiction, Oslo University Hospital and Institute of Clinical Medicine, University of Oslo and Department of Psychology, University of Oslo, Oslo, Norway; Guy Williams, Ph.D., Division of Anaesthesia, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK; Stefan Winzeck, Ph.D., Department of Computing, Imperial College London, London, UK; Peter Ylén, Ph.D., VTT Technical Research Centre, Tampere, Finland; Tommaso Zoerle, MD, Neuro ICU, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy.
Funding
Data collection was supported by the European Union 7th Framework Program (EC grant 602150), with additional funding from Hannelore Kohl Stiftung (Germany), OneMind (USA) and Integra LifeSciences Corporation (USA), NeuroTrauma Sciences (USA). Infrastructure was provided by the NIHR Cambridge Biomedical Research Centre and the NIHR Cambridge Clinical Research Facility, which is a partnership between Cambridge University Hospitals NHS (National Health Service) Foundation Trust and the University of Cambridge, funded by the NIHR. Individuals were supported by a Wellcome Trust Ph.D. Fellowship (222213/Z/20/Z) (SR) and by the Academy of Medical Sciences/The Health Foundation (UK) (VFJN). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. The funders had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed, through their participation in the CENTER-TBI study or directly, to data acquisition. DKM conceptualized the study. DKM, SR and VFJN designed the analysis. SR and VFJN reported magnetic resonance images. GS provided United States cost data. SR performed the statistical analysis and drafted the initial manuscript. All authors contributed to the interpretation of the data particular to their area of expertise and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for CENTER-TBI was obtained in accordance with all relevant laws and regulations for each recruiting site. Details may be found at: https://www.center-tbi.eu/project/ethical-approval. Informed consent from the patient or legal representative/next of kin was obtained for all participants. Whilst the patient was comatose assent was given by a proxy (next of kin) and consent sought when the patient regained capacity.
Consent for publication
Not applicable.
Competing interests
DKM received personal fees from Lantmannen AB, GlaxoSmithKline plc, Calico Life Sciences LLC, Integra Neurosciences, and NeuroTrauma Sciences, LLC; and grants from GlaxoSmithKline plc and Lantmannen AB, outside the presented work. VFJN holds a grant from Roche Pharmaceuticals paid to the institution for an analysis outside the presented work. KW reports stock options with Gryphon Bio. AIRM declares personal fees from NeuroTrauma Sciences and Novartis and participated in the DSMB of PresSura Neuro during the conduct of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplemental methods, figures and tables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Richter, S., Winzeck, S., Czeiter, E. et al. Serum biomarkers identify critically ill traumatic brain injury patients for MRI. Crit Care 26, 369 (2022). https://doi.org/10.1186/s13054-022-04250-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-04250-3