Abstract
Background
The Scandinavian Neurotrauma Committee (SNC) has recommended the use of serum S100B as a biomarker for mild low-risk Traumatic brain injuries (TBI). This study aimed to assess the adherence to the SNC guidelines in clinical practice and the diagnostic performance of S100B in patients with TBI. The aims of this study were to examine adherence to the SNC guideline and the diagnostic accuracy of serum protein S100B.
Methods
Data of consecutive patients of 18 years and above who presented to the emergency department (ED) at Helsingborg Hospital with isolated head injuries, were retrieved from hospital records. Patients with multitrauma, follow-up visits, and visits managed by a nurse without physician involvement were excluded.
Results
A total of 1671 patients were included of which 93 (5.6%) had intracranial hemorrhage. CT scans were performed in 62% of patients. S100B was measured in 26% of patients and 30% of all measurements targeted the low-risk mild head injuries indicated by the guideline. S100B's recommended cut-off value (≥ 0.10 µg/L) had a 100% sensitivity, 47% specificity, 10.1% positive predictive value, and 100% negative predictive value—if applied to the target SNC category (SNC 4). If applied to all patients tested, the sensitivity was 93% for traumatic intracranial hemorrhage (TICH). Current ED practices were adherent to the SNC guideline in 55% of patients. Non-adherent practices occurred in 64% of patients with low-risk mild head injuries (SNC4) including overtesting or undertesting of S100B and CT scans.
Conclusion
Adherence to guidelines was low and associated with a higher admission rate than non-adherence practice but no significant increase in missed TICH or death associated with non-adherence to guideline was found. In routine care, we found that the sensitivity and NPV of serum protein S100B was excellent and safely ruled out TICH when measured in the patient category recommended by the guideline. However, measuring serum protein S100B in patients not recommended by the guideline rendered unacceptably low sensitivity with possible missed TICHs as a consequence. To further delineate the magnitude and impact of non-adherence, more studies are needed.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
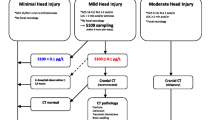
In Europe, the incidence of traumatic brain injuiry (TBI) is noted at around 300 cases/100 000 people/year [1]. It causes great morbidity, mortality and many emergency department (ED) visits [2]. Moreover, TBI is challenging for ED physicians to manage because signs and symptoms are not always indicative of the extent of brain injury [3, 4]. Because TBI is so common and sometimes difficult to assess medically, adjuncts are needed to aid the clinicians. The gold standard for diagnosing traumatic intracranial hemorrhage (TICH) is computerized tomography (CT) of the head. It is very accurate but disadvantages include exposing the patient to potentially harmful ionizing radiation and a relatively high cost [5,6,7,8]. Clinical practice guidelines (CPGs) can help risk stratify and select patients that should undergo a CT-head scan (e.g. Canadian CT Head Rule, National Institute for health and care excellence (NICE) and Scandinavian Neurotrauma Committee (SNC)). Theses algorithms have high negative predictive values (> 99%) but lower specificity (45–60% [9, 10] for detecting TICH requiring neurosurgical intervention. The specificity for TICH might be as low as 34% [11]. However, these numbers are derived from validation studies and theoretical retrospective applications where the guidelines are tested under ideal conditions. Furthermore, the adherence to CPGs in TBI varies widely but adherence to the SNC CPG has been reported at 40–60% [12, 13]. Guideline adherence has been reported to reduce TBI-mortality [12]. The SNC guideline recommends using serum protein S100B level to rule out TICH. Please see Fig. 1 for graphical illustration of the SNC guideline [14]. S100B is the most studied biomarker for ruling out TICH but others exist as well. The clinical cutoff of 0.1 µg/l of serum protein S100B level is set to ensure that no dangerous TICHs are missed and has a negative predictive value of > 99% but a specificity as low as 30–50% [15, 16]. It is only recommended in SNC category 4 and only as a “rule-out” test. The low specificity is of little importance in this category because if no S100B-test was available, all patients would be prescribed a head-CT.
Scandinavian Neurotrauma Committee Guidelines: a name and a number have been added to each category with red color to simplify category referencing throughout the present article (SNC1–5). SNC stands for Scandinavian Neurotrauma Committee. Adapted and published with permission from original author Johan Undén
The primary aim of this study was to investigate the adherence to the Scandinavian Neurotrauma Committee head trauma guidelines. The secondary aim was to assess the diagnostic accuracy of brain biomarker serum protein S100B.
Material and methods
Data on consecutive patients presenting to the ED at Helsingborg General Hospital with isolated head injury was collected retrospectively through medical records. Data collection was performed in patients registered between January 1, 2017, and December 31, 2017.
The hospital provides secondary care for 300,000 people which generates 70,000 ED visits per annum. Tertiary neurosurgical care is provided at Skane’s University Hospital, 40 km away. Multitrauma patients were managed according to ATLS™. The in-hospital guideline for traumatic brain injury during the study period was the SNC guideline [14].
The inclusion criteria were adult patients (≥ 18 years) attending the ED with “head trauma” as the chief complaint. Exclusion criteria were multitrauma, follow-up visits, visits managed by a nurse without physician’s involvement and confidential medical records. Some included patients had additional minor injuries, however, all patients triaged as multitrauma (n = 647) were excluded. The multitrauma definition used was in accordance with the 2014 Berlin definition [17]. This was done to ensure that the cohort was representative of ED patients with minor traumatic brain injury that are managed according to a head trauma CPG.
To make results of the present study clear and easy to understand, a modification of the original SNC flow chart has been made where a name and a number have been added to each risk category (SNC1–5). This is shown in Fig. 1.
The primary aim (adherence) was assessed in all SNC categories (Categories 1–5) and outcome measures were number of CTs, serum protein S100B assays, admissions, neurosurgical interventions and deaths. The secondary aim (diagnostic accuracy of serum protein S100B level) was reported for SNC category 4 separately and for all S100B-measurements together. The purpose of this was to outline how diagnostic accuracy was affected by S100B-measurement not indicated by the guideline. Outcome measures for secondary aim are further described in “statistical analysis” below.
To make assessment of level of consciousness internationally valid, it was converted from Reaction Level Scale (RLS) to Glasgow Coma Scale (GCS) and reported as GCS throughout the study. Earlier articles have reported good correlation between RLS1–2 and GCS 14–15 but differences between RLS3 and GCS13–8. Because of this, level of consciousness was only reported as GCS15-14 and GCS < 14 [18, 19]. Loss of consciousness was defined as any length of loss of partial or complete loss of perception of oneself and/or the surroundings.
Guidelines for retrospective reviews developed by Vassar and Holzmann [20] were followed.
Statistical analysis
Data was analyzed with SPSS version 25 for Mac. Histograms and Shapiro-Wilks formula were used to test for normal distribution. Statistical significance was set to p < 0.05. Central tendencies were presented as medians with interquartile range when non-parametric. Descriptive statistics were used to describe the material. Serum protein S100B level diagnostic accuracy was evaluated with sensitivity, specificity, negative predictive value, positive predictive value and Receiver operator Characteristics (ROC) curve with area under the curve (AUC) assessment. Contingency tables were tested using the χ2 test or Fisher’s test when applicable.
Results
This study included 1671 patients with head injuries with a median age of 64 years (interquartile range 39–80), and 47% were females. See Fig. 2 for inclusion process and distribution of different SNC categories. Other demographic, clinical and laboratory characteristics of the studied patients are summarized in Table 1. Head injury was minimal (SNC5) in 44.3%, mild (SNC2–4) in 54.5%, and moderate (SNC1) in 0.7% of all patients. Ten patients had severe head injuries and were therefore not classified according to SNC. The proportion of patients admitted to the hospital, received neurosurgical interventions, or died increased with the severity of head injuries (see Table 2). CT-scans were performed in 1039 patients (62.2%), serum protein S100B level was assessed in 434 patients (26.0%) of which 131 where in the recommended SNC category 4. CT scan detected intracranial hemorrhage (ICH) in 93 patients (5.6%; 95% confidence interval (CI) 4.5–6.7%). Of these 93 ICHs, 27 were subdural, 2 were epidural, 12 were categorized as subarachnoid, 8 were contusions and 44 were not clearly described in the radiology report.
In the SNC Category 4 of 323 patients, 229 (70.9%) underwent a head-CT and 24 (7.4%) had ICHs. See Table 2 for information on number of investigations and outcomes.
Adherence
Current ED practices were adherent to the SNC guideline in 912 (54.6%) patients with regards to head-CTs, serum protein S100B level assays and admissions/discharges. CTs and S100B-assays were prescribed in accordance with the guideline in 77.8% and 77.8% of cases, respectively. Non-adherence existed in 51.1% the patients with minimal head injuries (SNC5), 63.8% of patients with low-risk mild head injuries (SNC4), and 28.8% of patients with higher risk for ICH (SNC0–3). Non-adherent practices included overtesting and undertesting of S100B and CT scan as described in Table 3.
Adherent and non-adherent practices resulted in 214 (55.2%) and 174 admissions (44.8%), respectively. A total of 147 admissions (37.9% of all hospital admissions, most of them in SNC4 and SNC5 categories) were not indicated by the guideline. Adherence to SNC guideline resulted in more admissions compared to non-adherence (p = 0.011). No statistically significant differences were seen in missed admissions, neurosurgical interventions and deaths (Table 4).
Serum protein S100B level assay
In the SNC4 category, 41% of the patients had an assay of serum protein S100B level. The S100B measurements in the SNC4 category represented 30% of all S100 measurements (all categories). S100B measurements were higher or equal to 0.10 µg/L (cut-off value) in 261 patients (60.1%).
Evaluation of the performance of S100B's cut-off value (≥ 0.10 µg/L) in SNC category 4 yielded a sensitivity of 100% (95% CI 76.8—100.0), a specificity of 47% (95% CI 37.7—56.5), a PPV of 10.1% (95% CI 8.6—11.7), and a NPV of 100%. ROC curve analysis of S100B measurements in SNC category 4 had an AUC of 0.79 (95% CI 0.71—0.86; p < 0.001) (Fig. 3A).
However, assay of S100B applied to any patient with TBI regardless of SNC classification, reduced the accuracy; the sensitivity was 93% (95% CI 75.7—99.1); specificity was 42% (95% CI 37.2—47.0); PPV was 8.7% (95% CI 7.6–9.8), and NPV was 99.0% (95% CI 96.2–99.7). If applied to all patients regardless of the SNC classification, the ROC curve showed an AUC of 0.72 (95% CI 0.63 − 0.81; p < 0.001) and similar optimal cut-off value of 0.11 µg/L (Fig. 3B).
Discussion
This was a retrospective review of the medical records of 1671 TBI patients to investigate the extent of the adherence to the SNC guidelines in real-life settings and the diagnostic accuracy of serum protein S100B level in TBI-patients. The results showed an adherence to SNC guidelines of 54.6%; the non-adherence was concentrated in patients with minimal and low-risk head injuries (SNC 5 and 4) [21].
Such findings run in line with previous reports that noted a substantial discrepancy between guidelines' recommendations and initial management of TBIs in real-life practice. In a recent systematic review, Cnossen et al. [22], noted that the adherence to SNC recommendations in real-life practice ranged between 50 and 60% only. According to their results, non-adherence was strongly associated with prolonged hospitalization. In two reports by Heskestad et al. [23, 24], the rate of non-adherence to SNC recommendations ranged from 50 to 63%; the majority of non-compliance was reported amongst patients with minimal and low-risk head injuries (SNC 5 and 4), and this was mainly in the form of performing unnecessary CT scans. The non-adherence led to over-triage and unnecessary hospital admissions. Another report found that the rate of non-compliance to SNC was 54.5%, 45.1%, and 2.2% in patients with minimal, mild, and moderate TBIs, respectively [25]. Several reasons can contribute to limited compliance to SNC guidelines in a real-life setting. Firstly, insufficient knowledge and misinterpretation of the guidelines may lead physicians to rely on their clinical judgment and experience [14]. Besides, many physicians may consider S100B measurement as a part of routine investigations for patients with suspected TBIs rather than considering it as a valid screening tool for assessing a selected patient group (e.g. SNC4) need for further investigations. The crowded and busy nature of the emergency department may also lead the physicians to seek rapid patient turnover without waiting for laboratory results or ordering biomarkers before risk-stratifying into SNC categories.
In a recent qualitative study on barriers to SNC guideline adherence, interviewees stated that the guideline was useful but that the most important measure to increase adherence would be to increase digital and physical availability of the guideline. Other factors included more concise, easily-read and well-illustrated guidelines as well as a culture that better promoted guideline utilization [26].
Serum S100B can play an important role in predicting patients with TICH who present to the ED with mild TBI and have a low-risk profile; hence, it can reduce the number of unnecessary CT scans [27]. In the present study, we provided real-world evidence that S100B is a useful biomarker for prediction of TICH in mild, low-risk, TBI patients when measured in accordance with present guidelines. At the current cut-off value of > 0.10 μg/l, serum protein S100B had an exccellent sensitivity (100.0%) and negative predictive value (NPV; 100.0%). In line with our findings, Jones et al. [28], demonstrated that the S100B had a NPV of 97.3% for ruling out ICH in patients with mild TBIs. Another recent report showed that the S100B had a 97% sensitivity and 92% NPV in patients with mild TBI [29]. Such findings were consistent with other recent reports [30,31,32]. In a previous systematic review and meta-analysis on twelve studies, Undén and Romner [27] reported that the serum S100B had a NPV of 99% for detection of TICH in patients with mild TBIs. However, we found that the sensitivity of S100B was lowered to an unacceptable level (93%) in routine clinical practice if not used according to SNC recommendations. Thus, it is very important to risk stratify TBI before using serum protein S100B, otherwise TICHs could potentially be missed.
On the other hand, we found that the serum S100B had a low specificity (47.0%) for detection of TICH in patients with mild TBIs, highlighting that serum S100B has limited utility as a single biomarker for TICH and cannot be used as a rule-in biomarker. In agreement with our findings, Stein et al. [33], reported that the serum S100B had a specificity of 53% in patients with mild TBIs. Thus, to minimize the false-positive results and unnecessary CT scans—particularly in patients with dark skin [34], the decision to perform S100B assay should be combined with clinical evaluation as it is not suited as a TBI-screening tool. Besides, the usefulness of additional investigations, such as electroencephalogram (EEG) and other blood markers [35], could be evaluated in future studies to improve the specificity of serum S100B in initial triage of patients with TBI.
While the present study poses additional insights concerning the adherence to SNC recommendations in real-life setting, we acknowledge the existence of certain limitations.
The choice to exclude multitrauma patients was done because the SNC guideline is not applicable in this subset of patients. The retrospective method has some limitations. Information bias occurs when reviewing medical records and handling missing data. Irrespective of our attempts to prevent this, only careful conclusions can be drawn from this study. The pragmatic way of handling missing data was deliberated in our study group and regarded as the best solution. Nevertheless, it precludes reliability measurements (e.g., confidence intervals) and direction of any bias cannot be quantified. Interpreting “Head-CT not performed” as the “no traumatic intracranial hemorrhage” can entail missed hemorrhages. However, because of the follow-up search for other ED-visits 6 months after the TBI it can be assumed that intracranial hemorrhages with severe consequences would have been found.
Besides, we did not correlate adherence rate with uneventful hospitalized patients or complication rates among discharged patients to reflect the impact of non-adherence to the SNC guideline on the clinical course of TBIs patients.
In conclusion, adherence to guidelines was low and associated with a higher admission rate than non-adherence practice but no significant increase in missed ICH or death associated with non-adherence to guideline was found. In routine care, we found that the sensitivity and NPV of serum protein S100B was excellent and safely ruled out TICH when measured in the patient category recommended by the guideline. However, measuring serum protein S100B in patients not recommended by the guideline rendered unacceptably low sensitivity with possible missed TICHs as a consequence. To further delineate the magnitude and impact of non-adherence, more studies are needed.
Availability of data and materials
Data will be made available upon reasonable request.
Abbreviations
- AUC:
-
Area under curve
- CT:
-
Computed tomography
- ED:
-
Emergency department
- EEG:
-
Electroencephalogram
- ICH:
-
Intracranial hemorrhage
- NPV:
-
Negative predictive value
- RLS:
-
Reaction Level Scale
- ROC:
-
Receiver operator Characteristics
- SNC:
-
Scandinavian Neurotrauma Committee
- TICH:
-
Traumatic intracranial hemorrhage
- TBI:
-
Traumatic brain injuries
References
Brazinova A, Rehorcikova V, Taylor MS, Buckova V, Majdan M, Psota M, et al. Epidemiology of traumatic brain injury in europe: a living systematic review. J Neurotrauma. 2021;38:1411–40.
Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. Surgical management of traumatic parenchymal lesions. Neurosurgery. 2006;58:S225–46.
Leach P, Childs C, Evans J, Johnston N, Protheroe R, King A. Transfer times for patients with extradural and subdural haematomas to neurosurgery in Greater Manchester. Br J Neurosurg. 2007;21:11–5.
Nakahara S, Matsuoka T, Ueno M, Mizushima Y, Ichikawa M, Yokota J, et al. Predictive factors for undertriage among severe blunt trauma patients: What enables them to slip through an established trauma triage protocol? J Trauma Injury Infect Crit Care. 2010;68:1044–51.
Paul AB, Oklu R, Saini S, Prabhakar AM. How much is that head CT? Price transparency and variability in radiology. J Am Coll Radiol. 2015;12:453–7.
Smith-Bindman R. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169:2078.
Brenner DJ. Should we be concerned about the rapid increase in CT usage? Rev Environ Health. 2010;25:63–8.
Aygun N, Masaryk TJ. Diagnostic imaging for intracerebral hemorrhage. Neurosurg Clin N Am. 2002;13(313–34):vi.
Bouida W, Marghli S, Souissi S, Ksibi H, Methammem M, Haguiga H, et al. Prediction value of the Canadian CT head rule and the New Orleans criteria for positive head CT scan and acute neurosurgical procedures in minor head trauma: a multicenter external validation study. Ann Emerg Med. 2013;61:521–7.
Svensson S, Vedin T, Clausen L, Larsson P-A, Edelhamre M. Application of NICE or SNC guidelines may reduce the need for computerized tomographies in patients with mild traumatic brain injury: a retrospective chart review and theoretical application of five guidelines. Scand J Trauma Resusc Emerg Med. 2019;27:99.
Undén L, Calcagnile O, Undén J, Reinstrup P, Bazarian J. Validation of the Scandinavian guidelines for initial management of minimal, mild and moderate traumatic brain injury in adults. BMC Med. 2015;13:292.
Cnossen MC, Scholten AC, Lingsma HF, Synnot A, Tavender E, Gantner D, et al. Adherence to guidelines in adult patients with traumatic brain injury: a living systematic review. J Neurotrauma. 2021;38:1072–85.
Vedin T, Edelhamre M, Karlsson M, Bergenheim M, Larsson P-A. Management of traumatic brain injury in the Emergency Department: guideline adherence and patient safety. Qual Manag Health Care. 2017;26:190–5.
Undén J, Ingebrigtsen T, Romner B. Scandinavian guidelines for initial management of minimal, mild and moderate head injuries in adults: An evidence and consensus-based update. BMC Med BioMed Cent. 2013;11:1–14.
Thelin E, Al Nimer F, Frostell A, Zetterberg H, Blennow K, Nyström H, et al. A serum protein biomarker panel improves outcome prediction in human traumatic brain injury. J Neurotrauma. 2019;36:2850–62.
Thelin EP, Nelson DW, Bellander B-M. A review of the clinical utility of serum S100B protein levels in the assessment of traumatic brain injury. Acta Neurochir. 2017;159:209–25.
Pape H-C, Lefering R, Butcher N, Peitzman A, Leenen L, Marzi I, et al. The definition of polytrauma revisited: An international consensus process and proposal of the new “Berlin definition.” J Trauma Acute Care Surg. 2014;77:780–6.
Starmark JE, Stålhammar D, Holmgren E, Rosander B. A comparison of the Glasgow Coma Scale and the Reaction Level Scale (RLS85). J Neurosurg. 1988;69:699–706.
Johnstone AJ, Lohlun JC, Miller JD, McIntosh CA, Gregori A, Brown R, et al. A comparison of the Glasgow Coma Scale and the Swedish Reaction Level Scale. Brain Inj. 1993;7:501–6.
Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013;10:12.
Sistrom CL, McKay NL. Costs, charges, and revenues for hospital diagnostic imaging procedures: differences by modality and hospital characteristics. J Am Coll Radiol. 2005;2:511–9.
Cnossen MC, Scholten AC, Lingsma HF, Synnot A, Tavender E, Gantner D, et al. Adherence to guidelines in adult patients with traumatic brain injury: a living systematic review. https://home.liebertpub.com/neu. Mary Ann Liebert, Inc., New Rochelle; 2021;38:1072–85.
Heskestad B, Waterloo K, Ingebrigtsen T, Romner B, Harr ME, Helseth E. An observational study of compliance with the Scandinavian guidelines for management of minimal, mild and moderate head injury. Scand J Trauma Resusc Emerg Med. 2012;20:1–7.
Heskestad B, Baardsen R, Helseth E, Ingebrigtsen T. Guideline compliance in management of minimal, mild, and moderate head injury: High frequency of noncompliance among individual physicians despite strong guideline support from clinical leaders. J Trauma Injury Infect Crit Care. 2008;65:1309–13.
Harr ME, Heskestad B, Ingebrigtsen T, Romner B, Rønning P, Helseth E. Alcohol consumption, blood alcohol concentration level and guideline compliance in hospital referred patients with minimal, mild and moderate head injuries. Scand J Trauma Resusc Emerg Med. 2011;19:25.
Vestlund S, Vedin T, Edelhamre M, Lindén M, Larsson P-A. Ways to improve guideline adherence in the emergency department: an interview study on the management of traumatic brain injuries. Eur J Trauma Emerg Surg. 2022. https://doi.org/10.1007/s00068-021-01853-3.
Undén J, Romner B. Can low serum levels of S100B predict normal CT findings after minor head injury in adults? An evidence-based review and meta-analysis. J Head Trauma Rehabil. 2010;25:228–40.
Jones CMC, Harmon C, McCann M, Gunyan H, Bazarian JJ. S100B outperforms clinical decision rules for the identification of intracranial injury on head CT scan after mild traumatic brain injury. Brain Inj. 2020;34:407–14.
Kahouadji S, Salamin P, Praz L, Coiffier J, Frochaux V, Durif J, et al. S100B blood level determination for early management of ski-related mild traumatic brain injury: a pilot study. Front Neurol. 2020;11:856.
Czeiter E, Amrein K, Gravesteijn BY, Lecky F, Menon DK, Mondello S, et al. Blood biomarkers on admission in acute traumatic brain injury: Relations to severity, CT findings and care path in the CENTER-TBI study. EBioMedicine. 2020;66:56.
Posti JP, Takala RSK, Lagerstedt L, Dickens AM, Hossain I, Mohammadian M, et al. Correlation of blood biomarkers and biomarker panels with traumatic findings on computed tomography after traumatic brain injury. J Neurotrauma. 2019;36:2178–89.
Okonkwo DO, Puffer RC, Puccio AM, Yuh EL, Yue JK, Diaz-Arrastia R, et al. Point-of-care platform blood biomarker testing of glial fibrillary acidic protein versus S100 calcium-binding protein B for prediction of traumatic brain injuries: a transforming research and clinical knowledge in traumatic brain injury study. J Neurotrauma. 2020;37:2460–7.
Stein SC, Fabbri A, Servadei F, Glick HA. A critical comparison of clinical decision instruments for computed tomographic scanning in mild closed traumatic brain injury in adolescents and adults. Ann Emerg Med. 2009;53:180–8.
Ben Abdesselam O, Vally J, Adem C, Foglietti MJ, Beaudeux JL. Reference values for serum S-100B protein depend on the race of individuals. Clin Chem. 2003;66:836–7.
Piazza O, Cotena S, Esposito G, De Robertis E, Tufano R. S100B is a sensitive but not specific prognostic index in comatose patients after cardiac arrest. Minerva Chir. 2005;60:477–80.
Acknowledgements
Thanks to Dr. Linus Clausen who performed substantial data collection. Thanks to Dr. Ahmed Fouad for helping with statistical analysis
Funding
Open access funding provided by Lund University. No funding was received to perform this study.
Author information
Authors and Affiliations
Contributions
Dr. Mohammed Faisal: Manuscript contribution and statistical analysis; Dr. Tomas Vedin: Manuscript contribution and overseeing of data collection process; Dr. Marcus Edelhamre: Manuscript contribution and overseeing of data collection process; Dr. Jakob Lundager Forberg: Manuscript contribution. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical permission was granted by National Review Board of Medical Ethics (NRBME), Sweden.
Consent for publication
Consent was waivered by the NRBME.
Competing interests
Authors report no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Faisal, M., Vedin, T., Edelhamre, M. et al. Diagnostic performance of biomarker S100B and guideline adherence in routine care of mild head trauma. Scand J Trauma Resusc Emerg Med 31, 3 (2023). https://doi.org/10.1186/s13049-022-01062-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13049-022-01062-w