Abstract
Background
The combination therapy of hydrocortisone, vitamin C, and thiamine has been proposed as a potential treatment in patients with sepsis and septic shock. However, subsequent trials have reported conflicting results in relation to survival outcomes. Hence, we performed this randomized controlled trial (RCT) to evaluate the efficacy and safety of early combination therapy among adult patients with septic shock.
Methods
This single-center, double-blind RCT enrolled adult patients with diagnosis of septic shock within 12 h from Northern Jiangsu People's Hospital between February 2019 and June 2021. Recruited patients were randomized 1:1 to receive intervention (hydrocortisone 200 mg daily, vitamin C 2 g every 6 h, and thiamine 200 mg every 12 h) or placebo (0.9% saline) for 5 days or until ICU discharge. The primary endpoint was 90-day mortality. The secondary endpoints included mortality at day 28, ICU discharge, and hospital discharge; shock reversal; 72-h Delta SOFA score; ICU-free days, vasopressor-free days, and ventilator support -free days up to day 28; ICU length of stay (LOS) and hospital LOS.
Results
Among 426 patients randomized, a total of 408 patients with septic shock were included in the per-protocol (PP) analysis, of which 203 were assigned to the intervention group and 205 to the placebo group. In the PP population, the primary outcome of 90-day mortality was 39.9% (81/203) and 39.0% (80/205) in the intervention and the placebo groups, respectively, and was not significantly different (P = 0.86). There was no significant difference between two groups in 28-day mortality (36.5% vs. 36.1%, P = 0.94) or the ICU mortality (31.5% vs. 28.8%, P = 0.55) and hospital mortality (34.5% vs. 33.2%, P = 0.78). No other secondary outcomes showed significant differences between two groups, including shock reversal, vasopressor-free days, and ICU LOS. Intention-to-treat analysis included all the 426 patients and confirmed these results (all P > 0.05).
Conclusion
Among adult patients with septic shock, early use of hydrocortisone, vitamin C, and thiamine combination therapy compared with placebo did not confer survival benefits.
Trial registration ClinicalTrials.gov: NCT03872011, registration date: March 12, 2019.
Graphic Abstract

Similar content being viewed by others
Introduction
Sepsis is a life-threatening condition that occurs due to a dysregulated host response to infection [1]. There were approximately 49 million cases of sepsis and 11 million sepsis-related deaths worldwide annually [2]. Septic shock, a subset of sepsis, is characterized by circulatory and cellular/metabolic abnormalities that are associated with a higher risk of mortality [3].
At present, there are no treatments directly targeting the pathogenesis of sepsis; therefore, management relies on early identification and treatment of infection through appropriate antibiotic therapy and/or source control, as well as the reversal of hemodynamic instability through fluid resuscitation and vasopressors, if necessary [3]. Therefore, safe, effective, affordable adjuvant interventions that focus on mitigating dysregulated host responses in addition to standard therapy are urgently required.
A previous retrospective before–after study of 94 patients showed that early use of combination therapy with intravenous vitamin C, hydrocortisone, and thiamine might prove to be effective in preventing progressive organ dysfunction including acute kidney injury and reducing the mortality of patients with severe sepsis and septic shock [4]. The promising results of this study have aroused great interest of the therapeutic effects of the combination therapy with vitamin C, thiamine, and hydrocortisone among sepsis and septic shock patients. However, recently published prospective, randomized controlled trials (RCTs) conducted since then reported conflicting results in relation to survival outcomes [5,6,7,8], and limited specific data are available in septic shock patients. The VITAMINS and ACTS trials, which included patients diagnosed of septic shock within 24 h, both demonstrated no significant mortality difference between the combination therapy and control groups [5, 9]. It was assumed that beneficial effect could be achieved if the combination therapy initiated early [6]. Therefore, we performed this randomized controlled clinical trial to evaluate the efficacy of early administration of hydrocortisone, vitamin C, and thiamine combination therapy for patients with diagnosis of septic shock within 12 h.
Methods
Study design and participants
This study was a single-center, double-blind RCT conducted in a 45-bed intensive care unit (ICU) of Northern Jiangsu People's Hospital in Yangzhou, China. The study was approved by the Human Research Ethics Committee of Northern Jiangsu People's Hospital (2019KY-145) and was registered at clinicaltrial.gov (NCT03872011). Written informed consent was obtained from patients or patients’ legally authorized representatives. Patients were enrolled from February 2019 through June 2021, with last patient follow-up in September 2021.
The inclusion criteria were as follows: (1) age 18 years old or older; (2) diagnosis of septic shock within 12 h. The exclusion criterion was the presence of any of the following: (1) systemic corticosteroid therapy within the last 3 months before septic shock; (2) high-dose steroid therapy; (3) immunosuppression; (4) pregnant; (5) known glucose-6 phosphate dehydrogenase (G-6PD) deficiency; (6) known hemochromatosis; (7) known allergy to vitamin C, hydrocortisone, or thiamine; (8) anticipated death from a preexisting disease within 90 days after randomization (as determined by the enrolling physician); and (9) refusal of the attending staff or patient family.
Study randomization and intervention
Patients were randomized 1:1 to receive intervention or placebo. The randomization was stratified according to a table of computer-generated random numbers. Throughout the study, patients, investigators, clinical staff, and research staff remained blinded to the allocated therapy, with the exception of designated nurses who were responsible for the preparation of both study drug and placebo. The designated nurses were not involved with clinical care or outcome evaluation. Blinding regarding the trial regimen was ensured by the supply of study drug and placebo in identical, masked bags.
When the patients were diagnosed as septic shock, they were primarily treated with aggressive fluid challenge, adequate antibiotics, and vasoactive agents, according to Surviving Sepsis Campaign guidelines [3]. For the use of vasoactive drugs, norepinephrine was the first choice. At least 30 mL/kg of IV crystalloid fluid was given within the first 3 h. If target mean arterial pressure (MAP) of 65 mmHg could not be achieved, norepinephrine would be initiated within first hour of hypotension during or after 1-h fluid resuscitation. Patients in the intervention group received hydrocortisone (200 mg daily), vitamin C (2 g every 6 h), and thiamine (200 mg every 12 h) for 5 days or until ICU discharge, whichever occurred first. Vitamin C and thiamine were diluted in 100 ml 0.9% sodium chloride, respectively, and intravenously administered to patients over 60 min. Hydrocortisone was administered as a continuous infusion over 24 h. In the placebo group, an identical volume of 0.9% saline from the placebo drug bag was administered to patients using the same protocol. Attending ICU clinicians were allowed to order open-label corticosteroid therapy in place of study hydrocortisone or placebo for patients as deemed necessary (e.g., hydrocortisone for refractory shock, methylprednisolone for acute exacerbation of chronic obstructive pulmonary disease). However, the vitamin C and thiamine or matching placebo would remain randomized and blinded. These participants would remain in the study and be followed for outcomes. Other monitoring and interventions during and after the intervention period could be used in both groups at the discretion of the attending physicians.
Definitions
Septic shock was defined as sepsis with persisting hypotension requiring vasopressors to maintain MAP ≥ 65 mmHg and having a serum lactate level > 2 mmol/L despite adequate volume resuscitation [10]. High-dose steroid therapy was defined as ≥ 2 mg/kg prednisone equivalent per day for > 5 d. Immunosuppression encompassed the following conditions: solid malignancies with a history of chemotherapy within the last 3 months, progressive metastatic disease, hematologic malignancies, solid organ transplantation, and HIV infection [11]. Length of stay (LOS) prior to randomization was defined as the time from arrival at emergency department (ED) or general ward to randomization. Diagnosis of refractory shock was dependent on the physicians’ clinical experience. Reversal of shock was defined as the maintenance of a systolic blood pressure of at least 90 mmHg without vasopressor support for at least 24 h [12]. Time to shock reversal was defined as the time from randomization to shock reversal. 72-h Delta Sequential Organ Failure Assessment (SOFA) score was calculated by subtracting the SOFA score at 72 h from the corresponding value at enrollment (ΔSOFA score = initial SOFA score at enrollment–SOFA score after 72 h). If the patient discharged within 72 h after being enrolled in the study, the SOFA score at discharge was used for the analysis. Appropriate antibiotic therapy was considered if the initially prescribed antibiotics were active against the identified pathogens, based on in vitro susceptibility testing. Fluid overload was defined as more than a 10% increase in body weight relative to baseline [(total fluid in − total fluid out) in liters/admission body weight × 100] during the course of administration of the intervention or placebo [13]. Blood gas analysis was evaluated by skilled nurses via the blood gas analyzer (Cobas b221, Roche Diagnostics); all sample tests were performed with standard factory settings. Blood glucose disturbance was considered if the blood gas analyzer reported “interference” while the same blood sample tested showed normal blood glucose reading in the central laboratory device (Cobas 8000, Roche Diagnostics), which was not affected by vitamin C.
Data collection
The following information was collected and analyzed for every enrolled patient: age, sex, locale before ICU admission (ED/general ward), chronic medical histories (hypertension, chronic obstructive pulmonary disease, coronary artery disease, diabetes mellitus, chronic renal disease, and malignancy), primary site of infection, laboratory results (complete blood count, coagulation profile, arterial blood gas analysis, blood biochemistry), LOS prior to randomization, time from diagnosis of septic shock to randomization, time from randomization to first study drug administration, time from randomization to first antibiotic administration, proportion of antibiotic administration before randomization, appropriateness of antimicrobials, open-label corticosteroid administration, amount of fluid administered before vasopressor, ventilator support (invasive mechanical ventilation, noninvasive ventilation and high-flow nasal cannula), and renal replacement therapy (RRT) requirements. Blood culture and cultures of specimens from the site of infection were routinely performed. Disease severity was assessed by Acute Physiology and Chronic Health Evaluation II (APACHE II) [14, 15] and SOFA scores [16].
Outcome measures
The primary outcome was all-cause mortality at day 90 after randomization. The key secondary outcomes included all-cause mortality at day 28, ICU discharge, and hospital discharge. Additional secondary outcomes included shock reversal rate; time to shock reversal; 72-h delta SOFA score; ICU-free days, vasopressor-free days and ventilator support-free days up to day 28 (patients who died before day 28 were assigned zero free days); ICU length of stay (LOS) and hospital LOS. The 90-day mortality and 28-day mortality were assessed by review of the medical records of the participant, or by contacting the participant by phone.
Statistical analyses
We determined that a population of 406 patients (203 patients in each group) would provide the trial with 90% power to detect an absolute difference of 15 percentage points (a conservative estimate based on about 32% benefit observed in the study by Marik et al. [4]) in 90-day all-cause mortality from an estimated baseline mortality of 40%, at an alpha level of 0.05. Assuming a dropout rate of 5%, the sample size was calculated as 426 patients.
Due to the fact that most of our data were not normally distributed, which was proved by Shapiro–Wilk test and Kolmogorov–Smirnov test, we presented the data as median with interquartile range (IQR) for numerical data and numbers with percentages for categorical data. Continuous variables were compared using the Wilcoxon rank-sum test, while categorical variables were compared using the chi-square test. Patient survival time was analyzed using Cox proportional hazards regression, with results reported as hazard ratios with 95% confidence intervals (CI) and presented using Kaplan–Meier curves with a log-rank test for comparison. Proportional hazards assumptions were confirmed by Schoenfeld residuals.
Post hoc subgroup analysis for the primary outcome was performed on four subgroups determined from baseline variables, namely abdominal cavity infection, APACHE II score, age and LOS prior to randomization, with the latter three subgroups created by splitting each variable at the median level to create high and low subgroups. Every factor in subgroup analyses was analyzed with rates of the primary endpoint by testing the treatment by factor interaction with the use of Cox models.
For patients lost to follow-up, a “last status carried forward” approach would be used. And the sensitivity analyses of the primary outcome were chosen to model “Worst–Best” and “Best–Worst” scenarios: (1) Only patients lost to follow-up in the intervention group were considered to be dead; (2) only patients lost to follow-up in the placebo group were considered to be dead. Moreover, we performed the sensitivity analyses of 72-h SOFA scores to model “worst-possible” and “best-possible” scenarios: (1) The worst-possible SOFA score (score of 24) was imputed for those participants who discharged within 72 h; (2) only those patients who survive 72 h were included (Additional file 1: Tables S1 and S2). P values < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS version 11.0.
Results
Patient characteristics
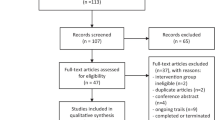
A total of 508 patients were screened for this study. Of these, 82 patients were excluded; the intention-to-treat (ITT) analysis included all the 426 patients. In addition, another 10 patients in the intervention group and 8 patients in the placebo group were excluded after randomization. The specific reasons for exclusion are shown in Fig. 1. Therefore, 408 patients were included in the per-protocol (PP) analysis, of which 203 were assigned to the intervention group and 205 to the placebo group. All patients were from Chinese Han population. The baseline characteristics of the ITT and PP population are presented in Table 1. Briefly, there were no differences between the groups with respect to age, sex, APACHE II score, SOFA score, locale before ICU admission, comorbidities, and primary site of infection (P > 0.05).
Baseline clinical and laboratory measurements of the ITT and PP population are presented in Table 2. The two groups were similar in terms of baseline biological variables. Both groups had similar characteristics, such as LOS prior to randomization, time from diagnosis of septic shock to randomization, time from randomization to the first study drug administration, time from randomization to the first antibiotic administration, proportion of antibiotic administration before randomization, appropriateness of antimicrobials, open-label corticosteroid administration, amount of fluid administered before vasopressor, need for ventilator support, and need for RRT (P > 0.05).
Primary outcome
In the ITT population, the primary outcome of 90-day mortality was 40.4% (86/213) and 39.0% (83/213) in the intervention and the placebo groups, respectively, and was not significantly different (P = 0.77) (Table 3). In addition, the Kaplan–Meier survival curve demonstrated that the 90-day survival was not significantly different between the two groups (HR 1.08; 95% CI 0.80–1.46; P = 0.62) (Fig. 2).
Kaplan–Meier estimates of survival rate distribution among patients in the intervention or placebo group. Panel A shows analysis of the intention-to-treat population. A log-rank (Mantel–Cox) test P = 0.62 for intergroup differences in survival rate distribution. Hazard ratio for mortality is 1.08; 95% CI 0.80–1.46. Panel B shows analysis of the per-protocol population. Log-rank (Mantel–Cox) test P = 0.67 for intergroup differences in survival rate distribution. Hazard ratio for mortality is 1.07; 95% CI 0.79–1.46
In the PP population, the primary outcome of 90-day mortality was 39.9% (81/203) and 39.0% (80/205) in the intervention and the placebo groups, respectively, and was not significantly different (P = 0.86) (Table 3). Simultaneously, the Kaplan–Meier survival curve demonstrated that the 90-day survival was not significantly different between the two groups (HR 1.07; 95% CI 0.79–1.46; P = 0.67) (Fig. 2).
Key secondary outcomes
In the ITT population, there was no significant difference between the intervention and placebo groups in 28-day mortality (37.1% vs. 36.2%, respectively, P = 0.84) or the ICU mortality (31.9% vs. 29.1%, respectively, P = 0.53) and hospital mortality (35.2% vs. 33.3%, respectively, P = 0.68) (Table 3).
In the PP population, there was no significant difference between the intervention and placebo groups in 28-day all-cause mortality (36.5% vs. 36.1%, respectively, P = 0.94) or in the mortality of ICU discharge (31.5% vs. 28.8%, respectively, P = 0.55) and hospital discharge (34.5% vs. 33.2%, respectively, P = 0.78) (Table 3).
Additional secondary outcomes
In the ITT population, the proportion of patients with reversal of shock was similar in the intervention and placebo groups (80.8% vs. 81.7%, respectively, P = 0.80). No significant differences were found in the time from randomization to shock reversal (3.0 vs. 2.0, respectively, P = 0.30); 72-h Delta SOFA score between the intervention and placebo groups (2.0 vs. 2.0, respectively, P = 0.59). Similarly, there was no statistically significant between-group difference in terms of 28-day cumulative ICU-free days (13.0 vs. 14.0, P = 0.67), vasopressor-free days (23.9 vs. 25.0, P = 0.26), or ventilator support–free days (17.6 vs. 19.3, P = 0.42) (Table 3). No other secondary outcomes showed significant differences between the intervention and placebo groups, including LOS in the ICU (7.0 vs. 6.0, P = 0.85) or LOS in the hospital (16.0 vs. 17.0, P = 0.35) (Table 3).
In the PP population, the proportion of patients with reversal of shock was similar in the intervention and placebo groups (80.8% vs. 82.0%, respectively, P = 0.76). There were no significant differences in the time from randomization to shock reversal (3.0 vs. 2.0, respectively, P = 0.40) and 72-h Delta SOFA score between the intervention and placebo groups (1.0 vs. 2.0, respectively, P = 0.65). Similarly, there was no statistically significant between-group difference in 28-day cumulative ICU-free days (13.0 vs. 14.0, P = 0.23), vasopressor-free days (23.9 vs. 25.0, P = 0.36), or ventilator support-free days (17.6 vs. 19.6, P = 0.51) (Table 3). No other secondary outcomes showed significant differences between the intervention and placebo groups, including LOS in the ICU (7.0 vs. 6.0, P = 0.93) or LOS in the hospital (16.0 vs. 17.0, P = 0.26) (Table 3).
Cox multivariate analysis demonstrated that after adjusting for potential confounding factors, age, APACHE II score, pneumonia, urinary tract infection, other sites of infection, bacteremia, time from randomization to the first antibiotic administration, lactate, need for ventilator support, and need for RRT remained independently associated with mortality (Additional file 1: Table S3).
Adverse events
In the safety population, the most common serious adverse events were severe hypernatremia (> 160 mmol/L) (occurring in 9 patients in the intervention group and 4 patients in the placebo group, P = 0.16) and fluid overload (occurring in 7 and 5 patients, respectively, P = 0.56). In addition, blood glucose disturbance was reported in 27 patients in the intervention group (12.7% vs. 0, P < 0.001) (Table 4).
Subgroup analysis
In the post hoc subgroup analysis, the 90-day mortality of the all subgroups was not significantly different between the intervention and placebo groups (P > 0.05 for all comparisons) (Additional file 1: Fig. S1).
Discussion
In this single-center, double-blind RCT of patients with septic shock, early initiation of hydrocortisone, vitamin C, and thiamine combination therapy compared with placebo did not significantly impact 90-day mortality and the secondary outcomes.
There is accumulative evidence indicating that vitamin C or thiamine deficiency is a common complication of septic shock patients, which is associated with immune dysfunction and poor prognosis [17]. The combination of hydrocortisone, vitamin C, and thiamine might show synergetic effect in ameliorating the systemic inflammatory response, preventing progressive organ dysfunction and reducing mortality of the septic shock patients [18].
In spite of theoretical plausibility of hydrocortisone, vitamin C, and thiamine combination therapy, our study’s results do not provide any significant survival benefit in septic shock patients, which is consistent with the results of previous RCTs [5, 6, 9, 19] and meta-analyses [20, 21]. It is worth pointing out that Marik et al. [4] and Chang et al. [22] highlighted that the early use of the combination therapy appeared to be associated with patients’ survival improvement. In our study, the diagnosis time of septic shock in included patients was within 12 h, which is earlier than previous studies [4, 22, 23]. However, early use of combination treatment in our study did not result in survival benefits.
The difference of our study from other studies should be considered when interpreting the discrepancies in efficacy of combination therapy. Firstly, the severity of organ failure is maybe a key determinant of the efficacy. The inclusion criteria of our research were restricted in patients with septic shock, a deteriorative subset of sepsis, while the research of Marik et al. [4] and Chang et al. [22] enrolled patients with sepsis and septic shock. Therefore, our included patients were more critically ill as evidenced by higher baseline SOFA scores (mean 10 points) compared with the included patients described in the research of Marik et al. (mean SOFA 8 points). Higher SOFA scores indicated an increased risk of death; it was supported by the VICTOR trial demonstrating that mortality benefit was observed only in a subset of patients with a lower SOFA score [6]. Moreover, our patients’ severity of organ failure is also corroborated by the significantly greater incidence of mechanical ventilation (72%) and the need for RRT (37%), compared with the patients included in the trial of Marik et al. (51%, 15%, respectively). In our study, the need for ventilator support and RRT was shown to be independent risk factors for mortality in patients with septic shock. And the LOVIT trial [24] also reported a high baseline severity of illness, which is maybe one of the reasons why there were no mortality benefits of vitamin C observed in the research.
Secondly, the selection of the primary endpoint should be taken into consideration. It should be pointed out that we adopted the 90-d mortality as the primary endpoint, rather than a change of the SOFA score, which was chosen as the primary endpoint in the ATESS [25], CITRIS-ALI [26], and ORANGES [27] studies. Given that the SOFA score focused on the early recovery of organ function and was assessed only if the patients remained alive during the assessment period, which could result in survivorship bias [25], the mortality endpoint was selected. However, the mortality endpoint, especially the long-term mortality, is theoretically diluted by many confounding factors [28]. However, our research did not observe any credible benefits either in non-mortality endpoints or in specific population.
Notably, our research differed from other studies in the distribution of infection sites. Abdominal infection accounted for approximately half of the cases of septic shock in this study. However, our subgroup analysis indicated that the treatment group did not show a better therapeutic effect than the control group, whether the subgroup patients had abdominal infections or not. The type of infection sites is maybe not a key determinant for the effectiveness of combination therapy. Future studies are needed to elucidate the issue.
In addition, there were no significant differences in shock reversal, or vasopressor-free days between the groups. This differs from the results of the ACTS study [9], which observed a longer shock-free days in the intervention group. In comparison with the ACTS study [9], in which hydrocortisone was given as intermittent boluses (50 mg every 6 h), hydrocortisone was given as a continuous infusion (200 mg/day) in this study. Repetitive bolus application of hydrocortisone compared with a continuous infusion likely results in higher peak serum and intracellular concentrations with greater binding to the glucocorticoid receptor and subsequently greater therapeutic effects [29]. However, hemodynamic improvement observed with the intervention in the ACTS study [9] may be related to corticosteroids alone, given that in the VITAMINS study [5], which hydrocortisone monotherapy was mandated in the control group, was not powered to detect difference in vasopressor-free days.
Intriguingly, the current study revealed a phenomenon of blood glucose measurement interference, in consistent with previous studies [30,31,32]. Our glucose-monitoring device adopted glucose oxidase method. Vitamin C was a strong reducing agent, thus interfering the results [32]. Our device reported “interference” reading results directly, rather than the falsely low testing results reported in some devices [30]. On the contrary, the falsely high testing results were also reported in devices adopting glucose dehydrogenase pyrroloquinoline quinone (GDH-PQQ) method [31, 33]. In this scenario, hypoglycemia may occur following insulin administration for factitious hyperglycemia [34]. Additionally, the hexokinase method was not affected by vitamin C [32]. Hence, it was adopted in our central laboratory device and recommended during the use of high-dose vitamin C.
Our study incorporates several strengths. Firstly, in terms of the timing of intervention, our study manifested an earlier application of combination therapy compared to previous studies [7, 9]. Therefore, it is possible to rule out the effect of time delay in the combination therapy application on the mortality of septic shock. Secondly, our trial investigated long-term combination therapy for septic shock. Most previous studies limited the use of combination therapy to 4 days [6, 9, 35] or even 2 days [25]. It was supposed that the lack of consistent benefits in trials mentioned above might also be due to insufficient dosage [36]. However, this trial might help to rule out the effect of duration of combination therapy on survival benefits of septic shock patients. Thirdly, to date, our trial shows a relatively larger sample size, especially compared with the trials in Chinese Han population. Fourthly, very few patients were lost to follow-up, thus minimizing attrition bias.
Some limitations of our study should be taken into consideration. First, the vitamin C and thiamine were infused over a set range of doses, and we did not measure vitamin C and thiamine levels as a guide to the dose or the duration of infusion, so it is unknown if this does/schedule was able to correct vitamin C or thiamine deficiency. Second, this was a single-center study, which may affect its external validity and generalizability.
Conclusions
In conclusion, among patients with septic shock, hydrocortisone, vitamin C, and thiamine did not appear to reduce the 90-day mortality compared with placebo. These data do not support routine use of this combination therapy for adult patients with septic shock.
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation II
- CI:
-
Confidence interval
- ED:
-
Emergency department
- GDH-PQQ:
-
Glucose dehydrogenase pyrroloquinoline quinone
- G-6PD:
-
Glucose-6 phosphate dehydrogenase
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- ITT:
-
Intention-to-treat
- LOS:
-
Length of stay
- MAP:
-
Mean arterial pressure
- PP:
-
Per-protocol
- RCTs:
-
Randomized controlled trials
- RRT:
-
Renal replacement therapy
- SOFA:
-
Sequential Organ Failure Assessment
References
Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–51.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet (London, England). 2020;395(10219):200–11.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving Sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–77.
Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin c, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 2017;151(6):1229–38.
Fujii T, Luethi N, Young PJ, Frei DR, Eastwood GM, French CJ, Deane AM, Shehabi Y, Hajjar LA, Oliveira G, et al. Effect of vitamin c, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the VITAMINS randomized clinical trial. JAMA. 2020;323(5):423–31.
Mohamed ZU, Prasannan P, Moni M, Edathadathil F, Prasanna P, Menon A, Nair S, Greeshma CR, Sathyapalan DT, Menon V, et al. Vitamin C therapy for routine care in septic shock (ViCTOR) trial: effect of intravenous vitamin c, thiamine, and hydrocortisone administration on inpatient mortality among patients with septic shock. Indian J Crit Care Med. 2020;24(8):653–61.
Sevransky JE, Rothman RE, Hager DN, Bernard GR, Brown SM, Buchman TG, Busse LW, Coopersmith CM, DeWilde C, Ely EW, et al. Effect of vitamin C, thiamine, and hydrocortisone on ventilator- and vasopressor-free days in patients with sepsis: the VICTAS randomized clinical trial. JAMA. 2021;325(8):742–50.
Wani SJ, Mufti SA, Jan RA, Shah SU, Qadri SM, Khan UH, Bagdadi F, Mehfooz N, Koul PA. Combination of vitamin C, thiamine and hydrocortisone added to standard treatment in the management of sepsis: results from an open label randomised controlled clinical trial and a review of the literature. Infect Dis (Lond). 2020;52(4):271–8.
Moskowitz A, Huang DT, Hou PC, Gong J, Doshi PB, Grossestreuer AV, Andersen LW, Ngo L, Sherwin RL, Berg KM, et al. Effect of ascorbic acid, corticosteroids, and thiamine on organ injury in septic shock: the ACTS randomized clinical trial. JAMA. 2020;324(7):642–50.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–10.
Jamme M, Daviaud F, Charpentier J, Marin N, Thy M, Hourmant Y, Mira JP, Pène F. Time course of septic shock in immunocompromised and nonimmunocompromised patients. Crit Care Med. 2017;45(12):2031–9.
Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111–24.
Gillespie RS, Seidel K, Symons JM. Effect of fluid overload and dose of replacement fluid on survival in hemofiltration. Pediatr Nephrol. 2004;19(12):1394–9.
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29.
Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. 1981;9(8):591–7.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–10.
Carr AC, Rosengrave PC, Bayer S, Chambers S, Mehrtens J, Shaw GM. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care. 2017;21(1):300.
Carr AC, Shaw GM, Fowler AA, Natarajan R. Ascorbate-dependent vasopressor synthesis: A rationale for vitamin C administration in severe sepsis and septic shock? Crit Care. 2015;19:418.
Hussein AA, Sabry NA, Abdalla MS, Farid SF. A prospective, randomised clinical study comparing triple therapy regimen to hydrocortisone monotherapy in reducing mortality in septic shock patients. Int J Clin Pract. 2021;75(9): e14376.
Na W, Shen H, Li Y, Qu D. Hydrocortisone, ascorbic acid, and thiamine (HAT) for sepsis and septic shock: a meta-analysis with sequential trial analysis. J Intensive Care. 2021;9(1):75.
Wu T, Hu C, Huang W, Xu Q, Hu B, Li J. Effect of combined hydrocortisone, ascorbic acid and thiamine for patients with sepsis and septic shock: a systematic review and meta-analysis. Shock. 2021;56(6):880–9.
Chang P, Liao Y, Guan J, Guo Y, Zhao M, Hu J, Zhou J, Wang H, Cen Z, Tang Y, et al. Combined treatment with hydrocortisone, vitamin C, and thiamine for sepsis and septic shock (HYVCTTSSS): a randomized controlled clinical trial. Chest. 2020;158:174–82.
Wald EL, Sanchez-Pinto LN, Smith CM, Moran T, Badke CM, Barhight MF, Malakooti MR. Hydrocortisone-ascorbic acid-thiamine use associated with lower mortality in pediatric septic shock. Am J Respir Crit Care Med. 2020;201(7):863–7.
Lamontagne F, Masse MH, Menard J, Sprague S, Pinto R, Heyland DK, Cook DJ, Battista MC, Day AG, Guyatt GH, et al. Intravenous vitamin c in adults with sepsis in the intensive care unit. N Engl J Med. 2022;386(25):2387–98.
Hwang SY, Ryoo SM, Park JE, Jo YH, Jang DH, Suh GJ, Kim T, Kim YJ, Kim S, Cho H, et al. Combination therapy of vitamin C and thiamine for septic shock: a multi-centre, double-blinded randomized, controlled study. Intensive Care Med. 2020;46(11):2015–25.
Fowler AA 3rd, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, Fisher B, Thacker LR 2nd, Natarajan R, Brophy DF, et al. Effect of vitamin c infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. 2019;322(13):1261–70.
Iglesias J, Vassallo AV, Patel VV, Sullivan JB, Cavanaugh J, Elbaga Y. Outcomes of metabolic resuscitation using ascorbic acid, thiamine, and glucocorticoids in the early treatment of sepsis: the ORANGES trial. Chest. 2020;158(1):164–73.
Jakobsen JC, Wetterslev J, Gluud C. Considerations on the strengths and limitations of using disease-related mortality as an outcome in clinical research. BMJ Evid-Based Med. 2021;26(3):127–30.
Marik PE. The role of glucocorticoids as adjunctive treatment for sepsis in the modern era. Lancet Respir Med. 2018;6(10):793–800.
Katzman BM, Kelley BR, Deobald GR, Myhre NK, Agger SA, Karon BS. Unintended consequence of high-dose vitamin c therapy for an oncology patient: evaluation of ascorbic acid interference with three hospital-use glucose meters. J Diabetes Sci Technol. 2021;15(4):897–900.
Tang Z, Du X, Louie RF, Kost GJ. Effects of drugs on glucose measurements with handheld glucose meters and a portable glucose analyzer. Am J Clin Pathol. 2000;113(1):75–86.
He J, Zheng G, Qian X, Sheng H, Chen B, Zhao B, Chen E, Mao E, Bian X. Effect of high-dose intravenous vitamin C on point-of-care blood glucose level in septic patients: a retrospective, single-center, observational case series. Curr Med Res Opin. 2021;37(4):555–65.
Kahn SA, Lentz CW. Fictitious hyperglycemia: point-of-care glucose measurement is inaccurate during high-dose vitamin C infusion for burn shock resuscitation. J Burn Care Res Off Publ Am Burn Assoc. 2015;36(2):e67-71.
Agarwal A, Basmaji J, Fernando SM, Ge FZ, Xiao Y, Faisal H, Honarmand K, Hylands M, Lau VI, Lewis K, et al. Administration of parenteral vitamin c in patients with severe infection: protocol for a systematic review and meta-analysis. JMIR Res Protoc. 2022;11(1): e33989.
Chang P, Liao Y, Guan J, Guo Y, Zhao M, Hu J, Zhou J, Wang H, Cen Z, Tang Y, et al. Combined treatment with hydrocortisone, vitamin c, and thiamine for sepsis and septic shock: a randomized controlled trial. Chest. 2020;158(1):174–82.
Jung SY, Lee MT, Baek MS, Kim WY. Vitamin C for >/= 5 days is associated with decreased hospital mortality in sepsis subgroups: a nationwide cohort study. Crit Care. 2022;26(1):3.
Acknowledgements
We thank all participants and their families for their involvement in this study. We also thank all ICU medical staff at Northern Jiangsu People’s Hospital, including medical students, nurses, and the attending physician of the trial patient.
Funding
This research was supported by Jiangsu Province “333 Projects” (BRA2020183), Scientific Research Project of Jiangsu Commission of Health (Z2020055), and Social Development Funds of Yangzhou City (YZ2021059). These funding sources had no role in the design of this study, its execution, analyses, interpretation of the data, or decision to submit results.
Author information
Authors and Affiliations
Contributions
QL conceived and designed the experiments, analyzed and interpreted the data, ensured that the accuracy and integrity of all work were appropriately maintained, wrote the first draft of the manuscript, and had full access to all the data in the study. RZ conceived and designed the experiments, supervised the implementation of the study, wrote and revised the manuscript, and had full access to all the data in the study. QC performed data acquisition, analyzed the data, performed the statistical analysis, and revised the manuscript. JY and JS performed the randomization of patients, analyzed the data, and revised the manuscript. XG conceived and designed the experiments, provided study supervision and critically revised the manuscript for important intellectual content, and had full access to all the data in the study. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study was approved by the Human Research Ethics Committee of Northern Jiangsu People's Hospital (2019KY-145) and was conducted in accordance with the Declaration of Helsinki and relevant clinical research regulations in China. Written informed consent was obtained from patients or patients’ legally authorized representatives.
Consent for publication
All authors have agreed to the publication of this manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Worst–Best/Best–Worst case analyses of the primary outcome. Table S2. Worst-possible/best-possible case analyses of the 72-h SOFA score. Table S3. Cox multivariate analysis of factors influencing 90-day mortality in patients. Fig. S1. Subgroup analysis of 90-day mortality. The forest map shows the grouped factors of the subgroup analysis, HR for 90-day mortality, 95% CI in each subgroup, and P value for interaction of treatment (intervention or placebo) and the factor. There were no significant interactions in any of the subgroups (P > 0.1 for all comparisons).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lyu, QQ., Zheng, RQ., Chen, QH. et al. Early administration of hydrocortisone, vitamin C, and thiamine in adult patients with septic shock: a randomized controlled clinical trial. Crit Care 26, 295 (2022). https://doi.org/10.1186/s13054-022-04175-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-04175-x