Abstract
Purpose
To evaluate the effects of early combination therapy with intravenous vitamin C and thiamine on recovery from organ failure in patients with septic shock.
Methods
The ascorbic acid and thiamine effect in septic shock (ATESS) trial was a multi-centre, double-blind, randomized, controlled trial conducted in four academic emergency departments, enrolling adult patients with septic shock from December 2018 through January 2020. Patients were randomly assigned in a 1:1 ratio to either the treatment group [intravenous vitamin C (50 mg/kg, maximum single dose 3 g) and thiamine (200 mg) administration every 12 h for a total of 48 h] or the placebo group (identical volume of 0.9% saline with the same protocol). The primary outcome was Δ Sequential Organ Failure Assessment (SOFA) score (SOFA score at enrolment–SOFA score after 72 h). Eighteen secondary outcomes were predefined, including shock reversal and 28-day mortality.
Results
A total of 111 patients were enrolled, of which 53 were assigned to the treatment group and 58 were assigned to the placebo group. There was no significant difference in ΔSOFA scores between the treatment group and the placebo group [3, interquartile range (IQR) − 1 to 5 vs. 3, IQR 0–4, respectively, p = 0.96]. Predefined secondary outcomes were also not significantly different between the groups.
Conclusion
In this study, vitamin C and thiamine administration in the early phase of septic shock did not improve organ function compared with placebo, despite improvements in vitamin C and thiamine levels.
Similar content being viewed by others
Introduction
Septic shock, a subset of sepsis, is characterized by severe circulatory and cellular metabolism abnormalities that are associated with a high risk of mortality [1]. Septic shock represents a major healthcare and socioeconomic burden worldwide. In a recent meta-analysis including studies from Europe and North America, mortality due to septic shock by the Sepsis-3 definition remains as high as 51.9% in the intensive care unit (ICU) and 52.1% in hospital [2]. At present, infection control with antibiotic therapy and/or source drainage is the only sepsis-specific therapy available [3]. Given the high morbidity and mortality of septic shock, new therapeutic approaches that focus on mitigating dysregulated host responses in addition to standard therapy are required to improve outcomes [4, 5].
It is well-established that decreased levels of vitamin C and thiamine are prevalent in septic patients [6, 7]. Furthermore, a variety of biological mechanisms have been postulated to account for the potential benefits of vitamin C and thiamine in septic patients. Vitamin C, a potent antioxidant, is an essential cofactor for the biosynthesis of catecholamine and vasopressin and augments vasopressor responsiveness [8,9,10]. It modulates the immune response via several pathways and enhances endothelial function and microcirculatory flow [9,10,11]. Thiamine also plays essential roles in cellular energy production and protects against tissue oxidative damage [10, 12]. Based on these findings, intravenous vitamin C and thiamine administration, either alone or in combination with steroids, has emerged as a potential treatment for septic patients. Several studies have assessed the therapeutic effects of vitamin C and thiamine supplementation in septic patients. Despite some promising results, the effects of these vitamins against sepsis and septic shock remain to be established [7, 13,14,15]. In addition, there is a lack of double-blind, randomized controlled trials (RCT) in the emergency department (ED) to assess the effects of intravenous administration of vitamin C and thiamine during the early phase of septic shock. The ascorbic acid and thiamine effect in septic shock (ATESS) trial aimed to evaluate the effects of early combination therapy with intravenous vitamin C and thiamine on recovery from organ failure, as indicated by changes in Sequential Organ Failure Assessment (SOFA) score during the first 72 h in patients with septic shock.
Methods
Study design
The ATESS trial was a multi-centre, double-blind, RCT in adult patients with septic shock. The study was conducted in four academic EDs in South Korea. The trial protocol was approved by the Institutional Review Boards of the individual participating hospitals and the Ministry of Food and Drug Safety in Korea. For all study participants, written informed consent was obtained from either the patient or the patient’s legal representative. The detailed study protocol was published previously [16]. Patients were enrolled from December 2018 through January 2020, with last patient follow-up in April 2020.
Study population
Adult patients (19–89 years old) who presented to an ED and were diagnosed with septic shock during ED stay were eligible for the study. Septic shock was defined as sepsis with persisting hypotension requiring vasopressors to maintain a mean arterial pressure (MAP) ≥ 65 mmHg and having a serum lactate level > 2 mmol/L despite adequate fluid challenge [1]. Sepsis was diagnosed in patients with suspected infection and organ dysfunction, which was defined as an acute increase in the total SOFA score of 2 or more due to infection. If the baseline SOFA score was unknown, it was assumed to be 0 [1].
Patients who met any of the following criteria were excluded: patients who were transferred from another hospital with vasopressor administration or mechanical ventilator support, patients who had limitations on treatment (e.g., patients with a signed do-not-resuscitate order), patients with an underlying terminal-stage disease; patients taking at least 1 g/day of vitamin C or receiving intravenous thiamine prior to enrolment, patients experiencing cardiac arrest prior to enrolment, patients diagnosed with renal or ureteral stones, patients who met the inclusion criteria more than 24 h after ED arrival, and patients who declined to participate in the trial (directly or by legal proxy). Detailed exclusion criteria are presented elsewhere (eTable 1 in Supplements).
Study randomization and intervention
The patients were randomized 1:1 to either the treatment or the placebo group. The randomization sequence was generated by an independent biostatistician using a permuted block size of four, stratified by site. The patients, attending clinicians, and researchers were blinded to the allocated groups throughout the trial. An identical number of treatment drugs or placebo were pre-packaged for each patient. The sequential randomization code for each site was assigned to the trial pack according to the allocation order. As each patient enrolled in the study, a specific randomization number was assigned to the patient in the order of enrolment, and the treatment drug or placebo in the trial pack with the same number was administered.
In the treatment group, vitamin C (50 mg/kg, maximum single dose 3 g, daily dose 6 g) and thiamine (200 mg) were mixed in a 50-ml 0.9% saline bag, respectively, and intravenously administered to patients over 60 min every 12 h for a total of 48 h. In the placebo group, an identical volume of 0.9% saline from the placebo drug ampoule was administered to patients using the same protocol.
Co-interventions
Initial resuscitation and management other than study drug administration were provided for the patient according to the latest Surviving Sepsis Campaign guidelines [17]. Broad-spectrum antibiotics were intravenously administered as soon as possible after recognition of sepsis and septic shock. Surgical or radiological interventions for the source control were also implemented as soon as possible. At least 30 mL/kg of intravenous crystalloid fluid was administered for patients with sepsis-induced hypoperfusion, but fluid dose was titrated up or down depending on patient condition. Norepinephrine (NE) was administered as the first-choice vasopressor to maintain a MAP of at least 65 mmHg. For patients requiring high-dose NE (at least 0.2 μg/kg/min), vasopressin (up to 0.03 U/min) and hydrocortisone (200 mg/day: 50 mg every 6 h or 200 mg over a 24-h continuous infusion) were additionally administered.
Outcome measures
The primary outcome was ΔSOFA score, which was calculated by subtracting the SOFA score at 72-h from the corresponding value at ED enrolment (ΔSOFA score = initial SOFA score at enrolment–SOFA score after 72 h) [18, 19]. If the patient died within 72 h after being enrolled in the study, the worst score before death was used for the analysis.
Secondary outcomes included mortality (7-day, 28-day, 90-day, in-hospital, ICU), shock reversal, vasopressor-free days, vasopressor dose (at 24, 48, and 72 h from enrolment and maximum dose during 72 h), duration of mechanical ventilation, ventilator-free days, new-onset or worsening acute kidney injury (AKI) after enrolment, new use of renal replacement therapy (RRT), RRT-free days, ICU length of stay (LOS), ICU-free days, hospital LOS, reduction of C-reactive protein (CRP) for 72 h, and reduction of procalcitonin for 72 h. For CRP and procalcitonin, if a patient died within the first 72 h, the last follow-up values were used. Shock reversal was defined as maintaining a MAP of 60 mmHg or more for longer than 24 h after discontinuation of all vasopressors [20]. The time frame to calculate the free day-related variables was set to the first 14 days from enrolment day, and if the patient died before 14 days, this metric was counted as 0 days after death. The vasopressor dose was expressed as a NE equivalent dose [21]. AKI was defined as Kidney Disease: Improving Global Outcomes (KDIGO) stage 2 or higher, which indicated new-onset change [22]. For the evaluation of KDIGO stage, baseline creatinine was derived from the lowest value from 1 year to 24 h prior to the ED arrival. If this value was not available, it was estimated according to a predefined formula (creatinine = 0.74–0.2 (if female) + 0.003 × age) [23]. Patients receiving dialysis for chronic end-stage renal failure were excluded from the analysis. Serum vitamin C and thiamine levels were measured by high-performance liquid chromatography at baseline and 72 h. Vitamin C deficiency was defined as vitamin C level < 11.4 μmol/L, and thiamine deficiency was defined as thiamine level < 66.1 nmol/L.
Statistical analysis
The sample size was calculated based on ΔSOFA score. Based on ΔSOFA scores in a control group from a study by Marik et al. [19], the mean and standard deviation of the ΔSOFA score in the placebo group were expected to be 1.0 and 2.7, respectively, and the mean ΔSOFA score in the treatment group was expected to be at least 2.5 [19]. This effect size of a 1.5-point improvement in 72-h SOFA score was set as a surrogate value to indicate reduction in organ dysfunction. Assuming that the standard deviation of the ΔSOFA score was the same in both groups, the required patient number per group was 52, with a statistical significance of 5% and power of 80%. Assuming a dropout rate of 10%, the total number of patients required for each group was 58 (total N = 116).
Data analyses were conducted for the intention-to treat data set. Missing data were not imputed and the numbers of patients with available data are presented. Data are presented as medians with interquartile ranges (IQRs) for continuous data, and numbers with percentages for categorical data. Groups were compared using the Wilcoxon rank-sum test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. Bonferroni corrections were used for post hoc multiple comparisons. Differences with 95% confidence intervals for continuous outcomes are presented using Hodges–Lehmann median differences. For primary outcome analysis, ΔSOFA scores were compared using the Wilcoxon rank-sum test. Multivariable analyses were not performed, because the baseline characteristics were well-balanced between the treatment and placebo groups. Survival duration and time to shock reversal were analysed using the Kaplan–Meier method and compared by the log-rank test. p values < 0.05 were considered statistically significant. Statistical analyses were performed using STATA version 15.1 (STATA Corporation, College Station, TX, USA).
Results
Patient baseline characteristics
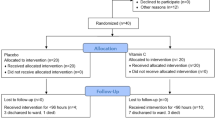
A total of 554 eligible patients were screened for this study. Of these, 438 patients were excluded (Fig. 1). In addition, another five patients in the treatment group were excluded from the analysis after randomization, because three withdrew from the study and two were found to be ineligible for inclusion. Finally, 111 patients were included in the analysis, of which 53 were assigned to the treatment group and 58 were assigned to the placebo group. Of the total cases, 90.1% (100/111) were enrolled at the two institutes. The patient enrolment status for each site is presented in eTable 2 in the Supplements.
The baseline characteristics of the treatment and placebo groups were similar, including age, sex, comorbidities, source of infection, laboratory tests, and severity indexes (Table 1). The most common site of infection was intra-abdominal, followed by the respiratory tract. The median SOFA score at enrolment was 8 (IQR 6–10) in the treatment group and 8 (IQR 6–10) in the placebo group (p = 0.85). The median time to first study drug administration after ED arrival was 8.4 h (IQR 5.7–14.9 h) in the treatment group and 9.9 h (IQR 7.4–15.6 h) in the placebo group (p = 0.21). Overall time from meeting eligibility criteria to first study drug administration and time from randomization to the first study drug were 3.3 h (IQR 1.4–7.5 h) and 1.0 h (IQR 0.5–2.3 h), respectively.
There were no significant differences between the treatment and the placebo groups at enrolment on median vitamin C levels [10.6 μmol/L (IQR 6–20.6) vs. 11.5 μmol/L (IQR 5–24.1), respectively, p = 0.72] or vitamin C deficiency (50.9% vs 47.3%, respectively, p = 0.71) (Fig. 2). However, the median vitamin C level was significantly higher in the treatment group than in the placebo group at 72 h [44 μmol/L (IQR 33.2–72.4) vs. 9 μmol/L (IQR 3.7–16.9), respectively, p < 0.01]. In addition, there were no cases of vitamin C deficiency in the treatment group at 72 h, while vitamin C deficiency persisted in 55.4% of the placebo group. There were no significant differences in thiamine deficiency between the two groups at enrolment (9.4% vs 7.0%, respectively, p = 0.64) or at 72 h (0% vs. 1.8%, respectively, p = 0.35).
Serum levels and deficiency rates of vitamin C and thiamine during the first 72 h from enrolment: a vitamin C levels, b thiamine levels, c vitamin C deficiency rate, d thiamine deficiency rate. The median vitamin C levels in the treatment and placebo groups were 10.6 μmol/L (IQR 6–20.6; n = 53) vs. 11.5 μmol/L (IQR 5–24.1; n = 57) at enrolment (p = 0.72) and 44 μmol/L (IQR 33.2–72.4; n = 49) vs. 9 μmol/L (IQR 3.7–16.9; n = 56) at 72 h (p < 0.01). The median thiamine levels in the treatment group and the placebo group were 133.4 nmol/L (IQR 86–181.5; n = 53) vs. 151.2 nmol/L (IQR 124.7–221.6; n = 57) at enrolment (p = 0.1) and 282.6 nmol/L (IQR 210.1–337; n = 49) vs. 125.6 nmol/L (IQR 94.8–167.6; n = 56) at 72 h (p < 0.01). Vitamin C deficiency was defined as vitamin C level < 11.4 μmol/L, and thiamine deficiency was defined as thiamine level < 66.1 nmol/L. There was a significant difference in vitamin C deficiency between the two groups at 72 h (0% in the treatment group vs. 55.4% in the placebo group; p < 0.01). IQR, interquartile range
Co-interventions
Interventions provided for management of septic shock are presented in Table 2. There was no significant difference in adjunctive steroid administration between the treatment and placebo groups (58.5% vs. 50%, p = 0.37). Furthermore, there were no significant differences between the treatment and placebo groups in the interventions, including times from ED arrival to the first antibiotic and vasopressor administration, vasopressor dose at study enrolment, fluid input before randomization and during the first 72 h after randomization, transfusion during the first 72 h after randomization, and use of mechanical ventilation at study enrolment.
Primary outcomes
There was no significant difference in ΔSOFA scores between the treatment and the placebo groups [3 (IQR − 1 to 5) vs. 3 (0–4), respectively, p = 0.96] (Table 3). Subgroup analysis of patients who received adjunctive steroids showed no significant difference in ΔSOFA score between the treatment and placebo groups [3 (IQR − 1 to 7) vs. 3 (0–4), p = 0.49] (eTable 3 in Supplements). Furthermore, other subgroup analysis showed no significant difference in ΔSOFA score between the treatment and placebo groups according to age (> 75 years vs. ≤ 75 years), sex, malignancy, infection focus (respiratory vs. non-respiratory), serum albumin level (≥ 3 mg/dL vs. < 3 mg/dL), vasopressor requirement (NE equivalent dose < 0.2 μg/kg/min vs. ≥ 0.2 μg/kg/min), time from hypotension to study drug (< 6 h vs. ≥ 6 h), SOFA score (< 10 vs. ≥ 10 points), and deficiencies in vitamin C or thiamine (eTable 3 in Supplements).
Secondary outcomes and adverse events
Predefined secondary outcomes were not significantly different between the treatment and placebo groups (Table 3). Mortality was not significantly different between the treatment and placebo groups at 7 days (9.4% vs. 10.3%, respectively, p = 0.87), 28 days (20.8% vs. 15.5%, respectively, p = 0.47), and 90 days (32.1% vs. 27.6%, respectively, p = 0.61). Kaplan–Meier curves were not significantly different between groups for mortality (p = 0.57) and shock reversal (p = 0.66) according to log-rank tests (Fig. 3).
There were no significant differences between the treatment and placebo groups in terms of shock reversal (83% vs. 84.5%, respectively, p = 0.83), vasopressor-free days [11 (5–12) vs. 11 (10–12), respectively, p = 0.16], or vasopressor dose (at 24 h, 48 h, 72 h and maximal dose for 72 h). No other secondary outcomes showed significant differences between the treatment and placebo groups, including ventilator-free days [11 (2–14) vs. 11 (3–14), p = 0.9], new-onset or worsening AKI [8.1% vs. 4.4%, p = 0.65], ICU-free days [9 (3–11) vs. 9 (0–11), p = 0.42], reduction of CRP for 72 h [7.6% (− 54.4 to 48.8) vs. − 0.7% (− 101.5 to 38.3), p = 0.65) or reduction of procalcitonin [49.2% (− 31.9 to 74.7) vs. 40.3% (− 3.4 to 82.8), p = 0.27].
No adverse events were reported in the treatment group (eTable 4 in Supplements). Two patients (3.5%) in the placebo group reported mild adverse events, including gastrointestinal symptoms.
Discussion
In this study, we found that intravenous administration of vitamin C and thiamine for 48 h during the early phase of septic shock did not significantly improve organ function compared with placebo. Furthermore, there was no statistically significant differences between the groups on secondary outcomes including mortality, shock reversal, vasopressor-free days, ventilator-free days, and ICU-free days.
Vitamin C and thiamine have been studied as potential metabolic resuscitators for critically ill septic patients. However, the treatment effects of these vitamin therapies are controversial. In a phase 1 safety trial for intravenous vitamin C in patients with severe sepsis, Fowler et al. reported significant trends in favour of vitamin C among several surrogate markers, i.e., SOFA score, CRP, and procalcitonin [24]. In the recent CITRIS-ALI trial, high-dose vitamin C infusion did not improve organ function compared with placebo as assessed by a modified SOFA score from baseline to 96 h in patients with sepsis-induced ARDS, although significant mortality reduction was observed as a secondary outcome [15]. Furthermore, various meta-analyses indicated different effects of intravenous vitamin C infusion among a heterogeneous sample of critically ill patients [25,26,27]. In a randomized, double-blind trial in patients (n = 88) with septic shock and elevated serum lactate level, Donnino et al. [28] reported that, in the subgroup with thiamine deficiency, patients who were treated with thiamine had significantly lower lactate level at 24 h and a possible decrease in mortality over time compared to the placebo group. On the other hand, a recent nationwide observational study in Japan did not show any association between thiamine administration and 28-day mortality in patients with septic shock [29].
Several trials using a combination of hydrocortisone, ascorbic acid, and thiamine (HAT) have been conducted since Marik et al. [19] reported profound improvements in mortality and organ function among septic patients treated with HAT. However, recent RCTs evaluating the synergistic effects of HAT in septic patients have produced mixed results. A double-blind RCT by Iglesias et al. indicated that HAT significantly reduced time to resolution of shock compared to the placebo (n = 137; 27 ± 22 vs. 53 ± 38 h, p < 0.001) in patients with sepsis or septic shock [7]. However, change in SOFA score and hospital mortality were not significantly different between groups. A single-blind RCT by Chang et al. [14] investigating combination therapy with HAT vs. placebo in patients with sepsis found no difference in 28-day mortality (n = 80; 27.5% vs. 35%; p = 0.47), although HAT therapy was associated with significant improvement in 72-h ΔSOFA score (p = 0.02). However, this trial was prematurely terminated due to adverse effects of the therapy, leaving the study underpowered. A multi-centre, open-label VITAMINS RCT reported that HAT therapy, compared with hydrocortisone alone, did not improve the duration of time alive and free of vasopressor administration up to day 7 in patients with septic shock [13].
There are several differences between our study and previous studies. First, glucocorticoid was administered to over half of the patients, and 72-h ΔSOFA score did not differ between the two groups regardless of glucocorticoid administration. Second, the interval for vitamin administration in our study was longer (12 h vs. 6 h), while the duration of treatment was shorter (48 h vs. 96 h or more) compared to the previous studies. We found that vitamin C and thiamine levels were normalized in all patients at 72 h in the treatment group, and shock reversal occurred within 72 h in most cases. However, we could not evaluate if shorter intervals and longer durations of vitamin administration had beneficial effects by inducing more repeated vitamin level peaks and extending the therapeutic effects of vitamins. Third, in this study, the time from meeting eligibility criteria to the first study drug administration was relatively short compared to previous studies conducted in the ICU setting [13, 15]. However, it took several hours for patients to meet septic shock eligibility criteria due to the time required for initial resuscitation and repeated lactate measurements. Also, some patients developed septic shock during their ED stay. Because septic shock is a very rapidly progressing inflammatory condition, earlier administration of vitamins, preferably with other bundle-based interventions for sepsis and septic shock, may result in more benefits to patients.
In this study, we measured vitamin C and thiamine levels at baseline and 72 h. At the time of study enrolment, in both groups, about half of the patients had vitamin C deficiency, and about one tenth of the patients had thiamine deficiency. There were no treatment effects associated with combination therapy, although vitamin C and thiamine levels were restored in the treatment group regardless of vitamin deficiencies. In particular, thiamine deficiency in our study population was not prevalent. In addition, even in the placebo group, only 1.8% of patients had thiamine deficiency after 72 h of routine critical care. Therefore, thiamine therapy might not work effectively in this study population.
The ΔSOFA score has been selected as the primary outcome in several clinical trials involving patients with sepsis and septic shock, along with reporting mortality. However, attention should be paid to interpretation of ΔSOFA score in the presence of differences in mortality between treatment and control groups. In the CITRIS-ALI trial, high-dose vitamin C therapy compared with placebo had no effect on the co-primary outcome, the 96-h ΔSOFA score, while it significantly decreased the 96-h and 28-day mortality in the treatment group [15]. Notably, patients who died before 96 h were not included in the primary analysis in that study. Death during the assessment period resulted in missing data for patients with obviously high SOFA score, leading to a survivorship bias which might paradoxically favour the placebo group with higher mortality [30]. In our study, however, the impact of this bias may be minimal, because patients who died within 72 h were rare, and the difference in mortality was not significant between the groups.
Our results should be interpreted in the light of the study’s limitations. First, although this was a multi-centre study, the majority of cases were enrolled at the two institutes. Second, we calculated the required sample size to identify improvements in organ function, but larger samples may be required to estimate the effects of vitamin C and thiamine treatment on mortality. Third, intra-abdominal infection accounted for almost half of the cases of septic shock, and more than half of the patients had either solid cancer or hematologic malignancy. These baseline characteristics might affect our results. Finally, our study drugs included only vitamin C and thiamine, while steroids were only used as part of the co-intervention in patients requiring high-dose vasopressors.
Conclusion
In this multi-centre, double-blind RCT, vitamin C and thiamine administration compared with placebo for 48 h in the early phase of septic shock did not significantly improve organ function, despite improvements in vitamin C and thiamine levels. Our finding does not support routine supplementation of these vitamins in patients with septic shock. Further research is needed to assess the potential benefits of earlier, more frequent, and longer administration of vitamins in combination therapy with corticosteroids.
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315:801–810
Vincent JL, Jones G, David S, Olariu E, Cadwell KK (2019) Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Crit Care 23:196
Vincent J-L (2018) How I treat septic shock. Intensive Care Med 44:2242–2244
De Backer D, Cecconi M, Lipman J, Machado F, Myatra SN, Ostermann M, Perner A, Teboul J-L, Vincent J-L, Walley KR (2019) Challenges in the management of septic shock: a narrative review. Intensive Care Med 45:420–433
Coopersmith CM, De Backer D, Deutschman CS, Ferrer R, Lat I, Machado FR, Martin GS, Martin-Loeches I, Nunnally ME, Antonelli M, Evans LE, Hellman J, Jog S, Kesecioglu J, Levy MM, Rhodes A (2018) Surviving sepsis campaign: research priorities for sepsis and septic shock. Intensive Care Med 44:1400–1426
Donnino MW, Carney E, Cocchi MN, Barbash I, Chase M, Joyce N, Chou PP, Ngo L (2010) Thiamine deficiency in critically ill patients with sepsis. J Crit Care 25:576–581
Iglesias J, Vassallo AV, Patel VV, Sullivan JB, Cavanaugh J, Elbaga Y (2020) Outcomes of metabolic resuscitation using ascorbic acid, thiamine, and glucocorticoids in the early treatment of sepsis: the ORANGES trial. Chest 158:164–173
Carr AC, Shaw GM, Fowler AA, Natarajan R (2015) Ascorbate-dependent vasopressor synthesis: a rationale for vitamin C administration in severe sepsis and septic shock? Crit Care 19:418
Berger MM, Oudemans-van Straaten HM (2015) Vitamin C supplementation in the critically ill patient. Curr Opin Clin Nutr Metab Care 18:193–201
Amrein K, Oudemans-van Straaten HM, Berger MM (2018) Vitamin therapy in critically ill patients: focus on thiamine, vitamin C, and vitamin D. Intensive Care Med 44:1940–1944
May JM, Harrison FE (2013) Role of vitamin C in the function of the vascular endothelium. Antioxid Redox Signal 19:2068–2083
Moskowitz A, Donnino MW (2020) Thiamine (vitamin B1) in septic shock: a targeted therapy. J Thorac Dis 12:S78–S83
Fujii T, Luethi N, Young PJ, Frei DR, Eastwood GM, French CJ, Deane AM, Shehabi Y, Hajjar LA, Oliveira G, Udy AA, Orford N, Edney SJ, Hunt AL, Judd HL, Bitker L, Cioccari L, Naorungroj T, Yanase F, Bates S, McGain F, Hudson EP, Al-Bassam W, Dwivedi DB, Peppin C, McCracken P, Orosz J, Bailey M, Bellomo R, Investigators VT (2020) Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the vitamins randomized clinical trial. JAMA 323:423–431
Chang P, Liao Y, Guan J, Guo Y, Zhao M, Hu J, Zhou J, Wang H, Cen Z, Tang Y, Liu Z (2020) Combined treatment with hydrocortisone, vitamin C, and thiamine for sepsis and septic shock: a randomized controlled trial. Chest 158:174–182
Fowler AA 3rd, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, Fisher B, Thacker LR 2nd, Natarajan R, Brophy DF, Sculthorpe R, Nanchal R, Syed A, Sturgill J, Martin GS, Sevransky J, Kashiouris M, Hamman S, Egan KF, Hastings A, Spencer W, Tench S, Mehkri O, Bindas J, Duggal A, Graf J, Zellner S, Yanny L, McPolin C, Hollrith T, Kramer D, Ojielo C, Damm T, Cassity E, Wieliczko A, Halquist M (2019) Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA 322:1261–1270
Hwang SY, Park JE, Jo IJ, Kim S, Chung SP, Kong T, Shin J, Lee HJ, You KM, Jo YH, Kim D, Suh GJ, Kim T, Kim WY, Kim YJ, Ryoo SM, Choi SH, Shin TG, Korean Shock Society I (2019) Combination therapy of vitamin C and thiamine for septic shock in a multicentre, double-blind, randomized, controlled study (ATESS): study protocol for a randomized controlled trial. Trials 20:420
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP (2017) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43:304–377
Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL (2001) Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 286:1754–1758
Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J (2017) Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest 151:1229–1238
Annane D, Renault A, Brun-Buisson C, Megarbane B, Quenot JP, Siami S, Cariou A, Forceville X, Schwebel C, Martin C, Timsit JF, Misset B, Ali Benali M, Colin G, Souweine B, Asehnoune K, Mercier E, Chimot L, Charpentier C, Francois B, Boulain T, Petitpas F, Constantin JM, Dhonneur G, Baudin F, Combes A, Bohe J, Loriferne JF, Amathieu R, Cook F, Slama M, Leroy O, Capellier G, Dargent A, Hissem T, Maxime V, Bellissant E, Network C-T (2018) Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med 378:809–818
Jentzer JC, Vallabhajosyula S, Khanna AK, Chawla LS, Busse LW, Kashani KB (2018) Management of refractory vasodilatory shock. Chest 154:416–426
Ostermann M, Joannidis M (2016) Acute kidney injury 2016: diagnosis and diagnostic workup. Crit Care 20:299
Zavada J, Hoste E, Cartin-Ceba R, Calzavacca P, Gajic O, Clermont G, Bellomo R, Kellum JA, Investigators AKI (2010) A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transpl 25:3911–3918
Fowler AA 3rd, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, Farthing CA, Larus TL, Martin E, Brophy DF, Gupta S, Medical Respiratory Intensive Care Unit N, Fisher BJ, Natarajan R (2014) Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med 12:32
Hemila H, Chalker E (2019) Vitamin C can shorten the length of stay in the ICU: a meta-analysis. Nutrients. https://doi.org/10.3390/nu11040708
Langlois PL, Manzanares W, Adhikari NKJ, Lamontagne F, Stoppe C, Hill A, Heyland DK (2019) Vitamin C administration to the critically ill: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr 43:335–346
Putzu A, Daems AM, Lopez-Delgado JC, Giordano VF, Landoni G (2019) The effect of vitamin C on clinical outcome in critically ill patients: a systematic review with meta-analysis of randomized controlled trials. Crit Care Med 47:774–783
Donnino MW, Andersen LW, Chase M, Berg KM, Tidswell M, Giberson T, Wolfe R, Moskowitz A, Smithline H, Ngo L, Cocchi MN, Center for Resuscitation Science Research G (2016) Randomized, Double-Blind, Placebo-Controlled Trial of Thiamine as a Metabolic Resuscitator in Septic Shock: A Pilot Study. Crit Care Med 44:360–367
Miyamoto Y, Aso S, Iwagami M, Yasunaga H, Matsui H, Fushimi K, Hamasaki Y, Nangaku M, Doi K (2020) Association between IV thiamine and mortality in patients with septic shock: a nationwide observational study. Crit Care Med. https://doi.org/10.1097/CCM.0000000000004394
de Grooth HJ, Elbers PWG, Vincent JL (2020) Vitamin C for sepsis and acute respiratory failure. JAMA 323:792
Funding
This work was supported by a National Research Foundation of Korea grant funded by the Korean government (No. 2018R1C1B6006821).
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hwang, S.Y., Ryoo, S.M., Park, J.E. et al. Combination therapy of vitamin C and thiamine for septic shock: a multi-centre, double-blinded randomized, controlled study. Intensive Care Med 46, 2015–2025 (2020). https://doi.org/10.1007/s00134-020-06191-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-020-06191-3