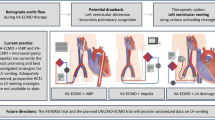
Abstract
During refractory cardiogenic shock and cardiac arrest, veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is used to restore a circulatory output. However, it also impacts significantly arterial oxygenation. Recent guidelines of the Extracorporeal Life Support Organization (ELSO) recommend targeting postoxygenator partial pressure of oxygen (PPOSTO2) around 150 mmHg. In this narrative review, we intend to summarize the rationale and evidence for this PPOSTO2 target recommendation. Because this is the most used configuration, we focus on peripheral VA-ECMO. To date, clinicians do not know how to set the sweep gas oxygen fraction (FSO2). Because of the oxygenator’s performance, arterial hyperoxemia is common during VA-ECMO support. Interpretation of oxygenation is complex in this setting because of the dual circulation phenomenon, depending on both the native cardiac output and the VA-ECMO blood flow. Such dual circulation results in dual oxygenation, with heterogeneous oxygen partial pressure (PO2) along the aorta, and heterogeneous oxygenation between organs, depending on the mixing zone location. Data regarding oxygenation during VA-ECMO are scarce, but several observational studies have reported an association between hyperoxemia and mortality, especially after refractory cardiac arrest. While hyperoxemia should be avoided, there are also more and more studies in non-ECMO patients suggesting the harm of a too restrictive oxygenation strategy. Finally, setting FSO2 to target strict normoxemia is challenging because continuous monitoring of postoxygenator oxygen saturation is not widely available. The threshold of PPOSTO2 around 150 mmHg is supported by limited evidence but aims at respecting a safe margin, avoiding both hypoxemia and severe hyperoxemia.
Similar content being viewed by others
Background
During cardiogenic shock or cardiac arrest refractory to medical treatment, peripheral veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is used to restore adequate oxygen delivery, mainly by increasing systemic blood flow. However, the oxygenator integrated to the VA-ECMO circuit also impacts arterial oxygen saturation of hemoglobin (SaO2) and arterial oxygen partial pressure (PaO2). If the ECMO blood flow management can be guided by lactate and mixed venous oxygen saturation in the pulmonary artery (SvO2) [1], data to guide the sweep gas oxygen fraction (FsO2) management are scarce.
In the recent Extracorporeal Life Support Organization (ELSO) Interim Guidelines for Venoarterial Extracorporeal Membrane Oxygenation in Adult Cardiac Patients, the experts stated that “excessive hypo- and hyperoxemia should be avoided” and that “gas blender should be adjusted to target slight hyperoxemia after the oxygenator (150 mmHg)” [1]. However, no ideal range for oxygenation is provided, and these recommendations open the FsO2 setting to large variations in the VA-ECMO practices. While some data support the harm of severe hyperoxemia [2], recent randomized studies on non-ECMO patients with acute respiratory distress syndrome and sepsis raise concern about the potential risk of a restrictive oxygenation strategy [3,4,5].
In this review, we aim at summarizing the rationale, evidence, and limits of the recent postoxygenator PO2 (PPOSTO2) target recommendation. Because it is the most used configuration, we focus on peripheral VA-ECMO. As so, pathophysiological concepts developed herein are not strictly transposable to central VA-ECMO. Also, while CO2 management during VA-ECMO seems to be another key issue, especially with the risk of hypocapnia [6, 7], it deserves a special focus and is not developed herein.
PO2 during peripheral VA-ECMO support: what are we talking about?
Definitions
During VA-ECMO support, several factors impact patient oxygen delivery: hemoglobin level, native lung function, oxygenator, native cardiac output, and ECMO blood flow.
In this review, we focus on extracorporeal oxygenation which corresponds to both oxygen partial pressure, and oxygen saturation of hemoglobin just after the oxygenator (i.e., PPOSTO2 and SPOSTO2, respectively). Measurement of these parameters needs to sample blood gas on the arterial side of the circuit, after the oxygenator. PPOSTO2 and SPOSTO2 depend on oxygenator gas transfer, which determinants are oxygen saturation of hemoglobin on venous blood before oxygenator (SPREO2), hemoglobin concentration, ECMO blood flow, sweep gas oxygen fraction (FSO2), sweep gas flow, and the oxygenator function. Managing FSO2 needs to incorporate a gas blender on the sweep gas flow circuit (Fig. 1), allowing titration of air and oxygen mixture. Of note, by itself the sweep gas flow little impacts PPOSTO2, whereas it is a major determinant of PaCO2 by influencing the amount of extracorporeal CO2 removal.
Oxygenation parameters during peripheral VA-ECMO support. SaO2: arterial oxygen saturation of hemoglobin; PaO2: arterial partial pressure of oxygen; FIO2: inspired oxygen fraction; FSO2: sweep gas oxygen fraction; SPREO2: preoxygenator oxygen saturation of hemoglobin; SPOSTO2: postoxygenator oxygen saturation of hemoglobin; PPOSTO2: postoxygenator oxygen partial pressure
Extracorporeal oxygenation must be distinguished from the brain and coronary oxygenation, which surrogates are classically the right radial PaO2 and SaO2. These parameters are dependent on several factors: SvO2, hemoglobin concentration, native lung function, inspired oxygen fraction on ventilator (FIO2), positive end expiratory pressure, extracorporeal oxygenation, and ratio between native cardiovascular/lung function and the VA-ECMO support.
An overview of the main oxygenation-related parameters during peripheral VA-ECMO support is provided in Fig. 1.
Dual oxygenation and mixing zone during femoro-femoral VA-ECMO
During femoro-femoral VA-ECMO, while hemodynamic is easily monitored (ECMO blood flow, arterial pressure, etc.), adequate tissue oxygenation monitoring is more challenging. In contrast to the literature on veno-venous ECMO, strong data on oxygenation determinants during VA-ECMO support are lacking [8].
The challenge of oxygenation during femoro-femoral VA-ECMO support is related to the dual oxygenation phenomenon [9], also known as differential hypoxemia, “North–South syndrome,” or “Harlequin syndrome.”
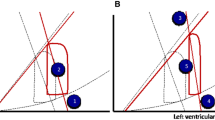
The dual oxygenation phenomenon is linked to the dual circulation phenomenon. During femoro-femoral VA-ECMO configuration, two distinct circulations occur: the native circulation, corresponding to the residual cardiac blood flow, and the extracorporeal circulation. Schematically, in the presence of significant residual cardiac output, the first aortic branches (i.e., the brachio-cephalic trunk and the common left carotid artery) and the upper part of the body (heart and brain) are perfused by the heart and oxygenated by the native lung. The lower part of the body (i.e., gut, liver, kidney, etc.) is perfused by the ECMO flow and oxygenated by the oxygenator. The zone where the two circulations meet is called the mixing zone. Of note, the dual oxygenation phenomenon varies over time. Indeed, the location of the mixing zone, and so the oxygenation level along the aorta, varies according to the degree of VA-ECMO support and the degree of heart impairment [10, 11]. In other words, the higher the ECMO blood flow, the proximal the mixing zone in the aorta. Besides, the lower the native heart ejection, the proximal the mixing zone in the aorta.
During the early phase of resuscitation, VA-ECMO is responsible for near-total hemodynamic support because of cardiogenic shock (high ratio between VA-ECMO blood flow and native cardiac output). In such a situation, the mixing zone is proximal in the aortic arch, and VA-ECMO might be responsible for the oxygenation of the near whole body (Fig. 2a). It should be noted, however, that, at this phase, there is specific concern about possible misdiagnosed coronary hypoxemia (Fig. 2b). Indeed, discrepancies between right radial PaO2 and proximal aorta PaO2 have been described in the setting of peripheral VA-ECMO [12]. Unknown coronary hypoxemia could be particularly deleterious at the myocardial recovery phase.
Clinical pictures illustrating the challenge of oxygenation during peripheral VA-ECMO. The yellow bullet corresponds to the mixing zone location; a When heart function is severely impaired, the mixing zone is in the proximal aorta and the risk is severe hyperoxemia of the whole body; b If there is minimal residual stroke volume and severe lung impairment, the mixing zone is above the coronary arteries but below the brachio-cephalic trunk. Then, the risk is unknown coronary hypoxemia; c When the heart recovers, the mixing zone moves down in the descending aorta. The risk is unknown hyperoxemia because continuous monitoring of PPOSTO2 is not widely available; d When the mixing zone is in the descending aorta, if severe lung impairment is associated, the risk is fulminant differential hypoxemia (Harlequin syndrome) with severe coronary and brain hypoxemia
Later, as the heart recovers, the native cardiac output increases and the VA-ECMO flow can be decreased. The mixing zone moves down in the descending aorta. At this phase, organ oxygenation assessment is more challenging. When oxygenation is monitored at the right radial artery, PaO2 and SaO2 only reflect oxygenation of the upper part of the body. In this situation, physician cannot exclude severe hyperoxemia of the lower part of the body (Fig. 2c). Because PPOSTO2 is not continuously monitored, unknown hypoxemia of the lower part of the body is also theoretically possible in case of oxygenator dysfunction.
Finally, it should be noted that left ventricle unloading with an Impella® also contributes to move down the mixing zone in the aorta.
Relation between extracorporeal oxygenation and systemic oxygenation during femoro-femoral VA-ECMO
While extracorporeal oxygenation firstly impacts oxygenation of the lower part of the body, it might also affect the brain and coronary oxygenation, which clinical surrogates are right radial artery PaO2 and SaO2.
As previously exposed, during the early phase of VA-ECMO support, the mixing zone is proximal in the aortic arch, and systemic oxygenation is mainly ensured by the oxygenator [10]. With an FSO2 commonly set at 100% [13,14,15] for current oxygenator’s characteristics, PPOSTO2 can rise to 500 mmHg at the membrane lung outlet [16]; thus, hyperoxemia is frequently observed on arterial blood gases sampled at the right radial artery [13, 15, 17,18,19,20]. In such setting, VA-ECMO can be responsible for brain and coronary hyperoxemia (Fig. 2a).
When the heart recovers, VA-ECMO might also improve brain and coronary oxygenation, through an increase in the SvO2. It might be clinically relevant in the case of a fulminant differential hypoxemia phenomenon (Fig. 2d). Such a situation appears when the recovering heart ejects severely deoxygenated blood coming from the impaired lungs (for example due to pneumonia). As the blood PaO2 ejected from the left ventricle is very low, the venous oxygen saturation of the upper part of the body measured in the superior vena cava (SSVCO2) is very low. If the venous cannula drains blood from the inferior vena cava (IVC) and not from the superior vena cava (SVC), the low SSVCO2 will result in low SvO2 and finally lead to a lower SaO2 in the aortic root. On the opposite, if the venous cannula tip is moved toward the SVC, deoxygenated blood from the SVC will be drained preferentially by VA-ECMO and oxygenated by the membrane lung. Then, SvO2 will be determined mainly by oxygen saturation of the IVC blood (SIVCO2). As returning from intra-abdominal organs oxygenated by VA-ECMO, it will be moderately deoxygenated, allowing an increased SvO2 and finally increased SaO2 [9, 21,22,23]. In an experimental study on 15 patients supported by VA-ECMO but without Harlequin syndrome, shifting the drainage cannula tip from IVC to SVC increased right radial PaO2 from 127 to 153 mmHg [23]. Then, clinical impact of this moderate oxygenation improvement in case of fulminant differential hypoxemia remains to be determined.
Specificities of femoro-subclavian VA-ECMO
When subclavian (or axillary) artery is preferred for the arterial access, there is no more concern about differential hypoxemia. Indeed, in such a situation, blood oxygenated by the membrane easily reaches the arch vessels, preventing an upper body hypoxemia. Conversion from femoral to subclavian approach is even a therapeutic option in case of severe differential hypoxemia [9].
While of potential interest, it should be noted on the other hand that this configuration may expose brain to hyperoxemia, especially if the right subclavian artery is cannulated, because of its connection with the right common carotid artery. Finally, as with femoro-femoral configuration, there is still a risk of misdiagnosed coronary hypoxemia if the mixing zone is below the brachio-cephalic trunk.
What is recommended for extracorporeal oxygenation management?
Until 2021, ELSO guidelines did not provide any recommendation about FSO2, PPOSTO2 and SPOSTO2 [16]. The recent ELSO Interim Guidelines for Venoarterial Extracorporeal Membrane Oxygenation in Adult Cardiac Patients addressed these points. The experts suggest that “excessive hypo- and hyperoxemia should be avoided.” Despite scarce evidence, they further suggest that “gas blender should be managed to target slight hyperoxemia after the oxygenator (150 mmHg)” [1]. These recommendations do not specify lower and upper limits for PPOSTO2. The experts recommended also monitoring right radial PaO2 to detect differential hypoxemia, but without mentioning PaO2 targets.
Regarding the use of VA-ECMO in resuscitation (ECPR), the recent guidelines do not provide clear recommendation on extracorporeal oxygenation. In the ELSO Interim Guidelines for Extracorporeal Cardiopulmonary Resuscitation in adults, the experts state that “Avoidance of hyperoxia can be achieved through the careful blending of ECMO fresh gas flow with an air and oxygen mix.” They recommend “targeting a patient arterial oxygen saturation of 92–97%” without precision on the monitoring site [24]. Finally, guidelines on postcardiotomy ECMO do not provide any recommendation on extracorporeal oxygenation [25].
What do we know about daily practice?
Reliable data on extracorporeal oxygenation during VA-ECMO support should include FSO2, PPOSTO2 and right radial PaO2. Although some data regarding to FSO2 and PaO2 are available, no study has specifically focused on PPOSTO2.
FSO2 settings
In a retrospective study on 52 VA-ECMO patients, Justus et al. described the evolution of FSO2 during the entire ECMO runs. At baseline, median FSO2 ranged from 72% (interquartile range (IQR) 62–82) to 78% (IQR 70–87). Mean FSO2 was around 80% between day 1 and day 10 and decreased around 60% between day 10 and day 20 of ECMO support [20]. In a retrospective cohort of 240 VA-ECMO patients evaluating the effect of levosimendan, Distelmaier et al. reported a median FSO2 at day 1 of 65% (IQR 60–90) in the levosimendan group and 70% (IQR 60–100) in the control group [26]. In another retrospective study on awake VA-ECMO (n = 57), Ellouze et al. reported a mean FSO2 (± standard deviation) of 66% (± 14) in the extubated group at the day of extubation and of 71% (± 17) in the non-extubated group on the third day of ECMO support [27]. Such description of FSO2 management is rare, and available information regarding FSO2 mainly comes from institutional protocols described in observational studies. Ross et al. reported that they always maintain FSO2 at 100% [13]. In the context of ECPR, Lamhaut et al. set the FSO2 at 50% immediately after ECMO start [28], while Chang et al. set FSO2 at 60% [17] and Halter and Stoll at 100% [14, 15]. Taking together these studies, FSO2 is usually set between 50 and 100% during the early phase of VA-ECMO support.
PPOSTO2 and PaO2
No study specifically provide data on PPOSTO2. However, studies describing general oxygenation in VA-ECMO patients could provide some information. Using a threshold of PaO2 ≥ 300 mmHg, the reported prevalence of severe hyperoxemia in the first 24 h ranged from 12 to 89% [13,14,15, 17,18,19, 29] (Table 1). In the study of Justus et al., the mean right radial PaO2 was higher than 250 mmHg at day 1 and decreased between day 3 and day 10, ranging from 100 to 150 mmHg [20]. In a retrospective study of 79 ECPR patients, the mean right radial PaO2 over the 8 first days was 211 ± 58 mmHg [15]. Based on a study on ECPR, the median value of mean PaO2 in the non-cannulated femoral artery during the first day was 328 mmHg (IQR 228–524) [19].
Why targeting extracorporeal moderate hyperoxemia (PPOSTO2 150 mmHg) during VA-ECMO?
The target of 150 mmHg for PPOSTO2 does not rely on randomized data. However, several observational and preclinical data support this recommendation [14, 17,18,19, 29, 30]. It might correspond to a safety zone, avoiding both hypoxemia and severe hyperoxemia.
To avoid severe hyperoxemia
Hyperoxemia is associated with altered prognosis in VA-ECMO patients
Observational studies, including two pediatric ones, have reported an association between hyperoxemia (usually sampled on right radial artery) and outcomes in VA-ECMO patients [14, 15, 17,18,19, 29,30,31,32,33]. In these studies, severe hyperoxemia that is commonly defined by a PaO2 ≥ 300 mmHg is frequently associated with worst outcomes (Table 1). Despite well-known harmful effect of hyperoxemia, a causative link is still matter to discussion for several reasons. First, hyperoxemia definition was variable, with PaO2 threshold ranging from 101 to 301 mmHg. Second, identification of hyperoxemia was often based on only one arterial blood gas sample in four studies [14, 17, 29, 30]. As so, it represents a small window of oxygen exposure and does not analyze long-term exposure to hyperoxemia. In addition, the site of arterial blood gas sample differs between/within studies. Third, as VA-ECMO was mostly peripherally inserted, high PaO2 might reflect low native cardiac output and high level of circulatory support. In this setting, hyperoxemic patients might be those most severely ill [34]. Fourth, most of these studies analyzed ECPR patients who represent a specifically medical condition in which hyperoxemia seems particularly deleterious. Recently, a retrospective analysis of the ELSO database on 7488 ECPR patients showed that an increase in PaO2 between pre-ECMO and 24 h after ECMO start was associated with in-hospital mortality [7]. Of note, the respective role of hyperoxemia and hypocapnia secondary to the extracorporeal CO2 removal remains matter of debate [6, 7]. Indeed, the rapid decrease in PaCO2 induced by ECMO may also contribute to brain ischemia through cerebral vasoconstriction.
Despite a strong association between early hyperoxemia and death, there are few studies on the mechanism by which hyperoxemia may increase mortality in VA-ECMO patients.
Hyperoxemia affects homeostasis and organ functions
Hyperoxemia induces radical oxygen species (ROS) production even in healthy volunteers exposed to inhaled oxygen [35]. During VA-ECMO, hyperoxemia might act as a booster of ROS production and reperfusion injury [36, 37]. In an experimental animal study, levels of TNF-α and IL-6 significantly increased with a PaO2 greater than 300 mmHg [38]. These findings suggest that hyperoxemia during VA-ECMO enhances systemic inflammation [39]. Severe hyperoxemia also reduced functional capillary density compared to extracorporeal normoxemia [40]. Taking together these phenomenon may contribute to organ dysfunction [41]. Because of shock and VA-ECMO support, ischemia–reperfusion injuries and hyperoxemia alter digestive mucosa barriers, which can be indirectly evaluated by the Intestinal Fatty-Acid Biding Protein (iFABP), a marker of enterocyte damage [42]. High iFABP values are associated with multi-organ failure and mortality [42,43,44]. In an experimental study on pigs supported by VA-ECMO, intestinal mucosa damage and intestinal permeability gradually increased with the duration of ECMO suggesting a role for the duration of hyperoxemia exposition [45, 46]. These results were confirmed by an animal study that demonstrated alteration of gut function in a dose- and time-dependent manner [44] with hyperoxemia. Although there are few clinical data on hyperoxemia during VA-ECMO and gut, it seems that hyperoxemia might enhance gut dysfunction secondary to VA-ECMO. These effects may explain the higher rate of bacterial translocation, and higher value of iFABP when rats are exposed to hyperoxemia [47].
Hyperoxemia has several positive and negative effects on cardiovascular system. Randomized studies in myocardial infarctions have reported conflicting results. While the AVOID trial demonstrated an increase in infarct size, arrhythmia occurrence, and recurrent infarction [48], the DETOX did not [49]. During cardiac surgery with cardiopulmonary bypass, hyperoxemia did not increase cardiovascular complications [50]. A retrospective study in cardiogenic shock after myocardial infarction supported by VA-ECMO did not demonstrate any harm of benefit of hyperoxemia [13].
VA-ECMO is often used for ECPR. In this context, hyperoxemia is potentially harmful. Observational studies have provided conflicting results on the effect of hyperoxemia on neurological outcomes. A randomized study has evaluated the neurological effect of mild hyperoxemia in 120 non-ECMO patients following cardiac arrest. Despite increasing tissue perfusion, hyperoxemia did not increase neuron-specific enolase value, a marker of neurological damage [51]. Equally, a post hoc analysis of the ICU-ROX trial did not demonstrate a decrease in poor neurological outcome at 6 months with conservative oxygen therapy [52].
Hyperoxemia: a question of dose or time exposure, or both?
Despite several animals’ studies demonstrating harmful effect of hyperoxemia, randomized clinical studies during short-term exposure did not demonstrate these effects. Studies performed during cardiopulmonary bypass are of interest because they concern a specific population suffering of cardiovascular disease with controlled ischemia–reperfusion injury, and hyperoxemia. Thus, PaO2 up to 500 mmHg is not associated with worst cardiovascular, renal, and neurological outcomes [50, 53, 54]. For short-term exposure (i.e., during cardiopulmonary bypass), hyperoxemia may not be harmful [50, 54]. Another factor that we should consider may be the time exposure to hyperoxemia. Oxygen therapy is a drug for which studies demonstrated a dose effect and a time exposure effect. Several animals’ studies demonstrated this time exposure effect of hyperoxemia, particularly during ischemia–reperfusion process and systemic inflammation. Hyperoxemia may be a trigger that enhances the host response to injury. These findings were highlighted by a recent meta-analysis. By analyzing more than 5000 ICU patients, Ni et al. demonstrated that conservative oxygen therapy is associated with a shorter mechanical ventilation duration, a decrease in new organ failure during the ICU stays, and a lower risk of renal replacement therapy [55].
To avoid hypoxemia
The ELSO experts recommend avoiding extracorporeal hypoxemia, but they do not define a threshold value. In critically ill patients without ECMO, it is recommended to maintain SaO2 above 92% during mechanical ventilation [56]. Lower limits have even been tolerated in ARDS (SaO2 ≥ 88%, PaO2 ≥ 55 mmHg) [57, 58]. However, several recent randomized studies on oxygenation target have raised concern about possible harm with a PaO2 target lower than 70 mmHg compared to higher levels.
In a post hoc analysis of the ICU-ROX trial focusing on septic patients, there was a trend to higher mortality in the conservative oxygenation arm (pulse oximetry target: 90 to 96%) compared to usual care [4].
As well in the LOCO2 trial (ARDS patients), the mortality at 90 days was higher in the lower oxygenation arm (PaO2 55 to 70 mmHg) [3]. Finally, a secondary analysis of the HOT ICU trial suggested a higher mortality in the lower oxygenation arm (PaO2 60 mmHg) in the subgroup of patients with norepinephrine [5]. In summary, even if hyperoxemia should be avoided, PPOSTO2 should probably not be lower than 70 mmHg.
Because we cannot ensure strict extracorporeal normoxemia
Because of clot formation around the fibers of the membrane, oxygenation performance decreases over time [59]. In a retrospective study on 265 patients supported by veno-venous (VV)-ECMO, 10 patients had membrane lung exchange due to decreasing of gas transfer on oxygenator [60]. Consequently, FSO2 could not reliably predict PPOSTO2 over time, and for a constant FsO2, PPOSTO2 will decrease with time.
It is therefore theoretically necessary to measure continuously PPOSTO2 or SPOSTO2. As VA-ECMO blood flow is not pulsatile, pulse oximetry is unreliable to monitor SPOSTO2. Recently, three devices have been proposed to monitor membrane oxygenation: the LANDING ECMO™ (EUROSET), the System M4™ (SPECTRUM MEDICAL), and the NAUTILUS SMART™ (MEDTRONIC). While of potential interest, these devices are currently not widely available. Furthermore, their reliability has to be tested during prolonged usage. Waiting for such a continuous monitoring system, direct measurement of PPOSTO2 is probably useful at least once a day to rule out severe hyperoxemia and hypoxemia. Attention should also be paid to variation of oxygen transfer determinants (i.e., FSO2, hemoglobin concentration, and ECMO blood flow), which could result in significant change of PPOSTO2, and need to repeat the measure.
Finally, it should be kept in mind that a severe PPOSTO2 drop (resulting in postoxygenator hypoxemia) will be detected by continuous monitoring of near-infrared spectroscopy (NIRS) of the cannulated limb. Indeed, as the cannulated limb oxygenation is totally determined by the oxygenator, a sudden drop of NIRS value indicates reperfusion cannula occlusion, insufficient blood flow, or postoxygenator hypoxemia.
Landscape of the needed and current studies about extracorporeal oxygenation during VA-ECMO support
Needed and current studies about extracorporeal oxygenation during VA-ECMO support are summarized in Table 2.
Conclusion
Defining extracorporeal oxygenation targets for VA-ECMO patients remains challenging, as there is no published randomized trial. Data from observational studies are limited by their design and the definition of hyperoxemia. There is a need to define oxygenation targets for the right radial PaO2 and the PPOSTO2. Pending specific data on ideal oxygenation targets during VA-ECMO support, avoiding both hypoxemia and severe hyperoxemia, seem reasonable.
Availability of data and materials
Not applicable.
Abbreviations
- ELSO:
-
Extracorporeal Life Support Organization
- VA-ECMO:
-
Veno-arterial extracorporeal membrane oxygenation
- ECPR:
-
Extracorporeal cardiopulmonary resuscitation
- SaO2 :
-
Arterial oxygen saturation of hemoglobin
- PaO2 :
-
Arterial oxygen partial pressure
- FIO2 :
-
Inspired oxygen fraction
- SvO2 :
-
Mixed venous oxygen saturation of hemoglobin in the pulmonary artery
- SVC:
-
Superior vena cava
- SSVCO2 :
-
Oxygen saturation of hemoglobin in the superior vena cava
- IVC:
-
Inferior vena cava
- SIVCO2 :
-
Oxygen saturation of hemoglobin in the inferior vena cava
- PPOSTO2 :
-
Postoxygenator oxygen partial pressure
- SPOSTO2 :
-
Postoxygenator oxygen saturation of hemoglobin
- SPREO2 :
-
Preoxygenator oxygen saturation of hemoglobin
- FsO2 :
-
Sweep gas oxygen fraction
- ROS:
-
Radical oxygen species
- iFABP:
-
Intestinal fatty acid-binding protein
References
Lorusso R, Shekar K, MacLaren G, et al. ELSO interim guidelines for venoarterial extracorporeal membrane oxygenation in adult cardiac patients. ASAIO J Am Soc Artif Intern Organs. 2021;67(8):827–44.
Asfar P, Schortgen F, Boisramé-Helms J, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med. 2017;5(3):180–90.
Barrot L, Asfar P, Mauny F, et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020;382(11):999–1008.
Young P, Mackle D, Bellomo R, et al. Conservative oxygen therapy for mechanically ventilated adults with sepsis: a post hoc analysis of data from the intensive care unit randomized trial comparing two approaches to oxygen therapy (ICU-ROX). Intensive Care Med. 2020;46(1):17–26.
Klitgaard TL, Schjørring OL, Lange T, et al. Lower versus higher oxygenation targets in critically ill patients with severe hypoxaemia: secondary Bayesian analysis to explore heterogeneous treatment effects in the Handling Oxygenation Targets in the Intensive Care Unit (HOT-ICU) trial. Br J Anaesth. 2021;S0007–0912(21):00582–91.
Diehl A, Burrell AJC, Udy AA, et al. Association between arterial carbon dioxide tension and clinical outcomes in venoarterial extracorporeal membrane oxygenation. Crit Care Med. 2020;48(7):977–84.
Tonna JE, Selzman CH, Bartos JA, et al. The association of modifiable mechanical ventilation settings, blood gas changes and survival on extracorporeal membrane oxygenation for cardiac arrest. Resuscitation. 2022;S0300–9572(22):00083–91.
Schmidt M, Tachon G, Devilliers C, et al. Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med. 2013;39(5):838–46.
Falk L, Sallisalmi M, Lindholm JA, et al. Differential hypoxemia during venoarterial extracorporeal membrane oxygenation. Perfusion. 2019;34(1):22–9.
Stevens MC, Callaghan FM, Forrest P, Bannon PG, Grieve SM. Flow mixing during peripheral veno-arterial extra corporeal membrane oxygenation—a simulation study. J Biomech. 2017;55:64–70.
Rozencwajg S, Wu EL, Heinsar S, et al. A mock circulation loop to evaluate differential hypoxemia during peripheral venoarterial extracorporeal membrane oxygenation. Perfusion. 2022;2676591211056567.
Lee JG, Kim N, Narm KS, et al. The effect of additional stepwise venous inflow on differential hypoxia of veno-arterial extracorporeal membrane oxygenation. ASAIO J Am Soc Artif Intern Organs. 2020;66(7):803–8.
Ross P, Miller C, Sheldrake J, McGuiness W, Udy A, Burrell A. Hyperoxia in patients with cardiogenic shock after myocardial infarction supported with venoarterial extracorporeal membrane oxygenation. Aust Crit Care Off J Confed Aust Crit Care Nurses 2020.
Halter M, Jouffroy R, Saade A, Philippe P, Carli P, Vivien B. Association between hyperoxemia and mortality in patients treated by eCPR after out-of-hospital cardiac arrest. Am J Emerg Med 2019.
Stoll SE, Paul E, Pilcher D, Udy A, Burrell A. Hyperoxia and mortality in conventional versus extracorporeal cardiopulmonary resuscitation. J Crit Care. 2022;69: 154001.
ELSO. General guidelines for all ECLS cases. 2015.
Chang W-T, Wang C-H, Lai C-H, et al. Optimal arterial blood oxygen tension in the early postresuscitation phase of extracorporeal cardiopulmonary resuscitation: a 15-year retrospective observational study. Crit Care Med. 2019.
Al-Kawaz MN, Canner J, Caturegli G, et al. Duration of hyperoxia and neurologic outcomes in patients undergoing extracorporeal membrane oxygenation. Crit Care Med 2021.
Bonnemain J, Rusca M, Ltaief Z, et al. Hyperoxia during extracorporeal cardiopulmonary resuscitation for refractory cardiac arrest is associated with severe circulatory failure and increased mortality. BMC Cardiovasc Disord. 2021;21(1):542.
Justus A, Burrell A, Anstey C, Cornmell G, Brodie D, Shekar K. The Association of Oxygenation, Carbon Dioxide Removal, and mechanical ventilation practices on survival during venoarterial extracorporeal membrane oxygenation. Front Med. 2021;8: 756280.
Hou X, Yang X, Du Z, et al. Superior vena cava drainage improves upper body oxygenation during veno-arterial extracorporeal membrane oxygenation in sheep. Crit Care Lond Engl. 2015;19:68.
Lindfors M, Frenckner B, Sartipy U, Bjällmark A, Broomé M. Venous cannula positioning in arterial deoxygenation during veno-arterial extracorporeal membrane oxygenation—a simulation study and case report. Artif Organs. 2017;41(1):75–81.
Cai T, Li C, Xu B, et al. Drainage from superior vena cava improves upper body oxygenation in patients on femoral veno-arterial extracorporeal membrane oxygenation. Front Cardiovasc Med. 2021;8: 807663.
Richardson ASC, Tonna JE, Nanjayya V, et al. Extracorporeal cardiopulmonary resuscitation in adults. Interim guideline consensus statement from the extracorporeal life support organization. ASAIO J Am Soc Artif Intern Organs. 2021;67(3):221–8.
Lorusso R, Whitman G, Milojevic M, et al. 2020 EACTS/ELSO/STS/AATS expert consensus on post-cardiotomy extracorporeal life support in adult patients. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2021;59(1):12–53.
Distelmaier K, Roth C, Schrutka L, et al. Beneficial effects of levosimendan on survival in patients undergoing extracorporeal membrane oxygenation after cardiovascular surgery. Br J Anaesth. 2016;117(1):52–8.
Ellouze O, Lamirel J, Perrot J, et al. Extubation of patients undergoing extracorporeal life support. A retrospective study. Perfusion. 2019;34(1):50–7.
Lamhaut L, Hutin A, Puymirat E, et al. A pre-hospital extracorporeal cardio pulmonary resuscitation (ECPR) strategy for treatment of refractory out hospital cardiac arrest: an observational study and propensity analysis. Resuscitation. 2017;117:109–17.
Munshi L, Kiss A, Cypel M, Keshavjee S, Ferguson ND, Fan E. Oxygen thresholds and mortality during extracorporeal life support in adult patients. Crit Care Med. 2017.
Kashiura M, Yasuda H, Kishihara Y, et al. Association between short-term neurological outcomes and extreme hyperoxia in patients with out-of-hospital cardiac arrest who underwent extracorporeal cardiopulmonary resuscitation: a retrospective observational study from a multicenter registry. BMC Cardiovasc Disord. 2022;22(1):163.
Sznycer-Taub NR, Lowery R, Yu S, Owens ST, Hirsch-Romano JC, Owens GE. Hyperoxia is associated with poor outcomes in pediatric cardiac patients supported on venoarterial extracorporeal membrane oxygenation. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2016;17(4):350–8.
Cashen K, Reeder R, Dalton HJ, et al. Hyperoxia and hypocapnia during pediatric extracorporeal membrane oxygenation: associations with complications, mortality, and functional status among survivors. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2018;19(3):245–53.
Kobayashi M, Kashiura M, Yasuda H, Sugiyama K, Hamabe Y, Moriya T. Hyperoxia is not associated with 30-day survival in out-of-hospital cardiac arrest patients who undergo extracorporeal cardiopulmonary resuscitation. Front Med. 2022;9: 867602.
Joyce CJ, Anderson C, Shekar K. Hyperoxia on venoarterial extracorporeal membrane oxygenation: a modifiable risk? Crit Care Med. 2022;50(1):e99-100.
Hafner C, Pramhas S, Schaubmayr W, et al. Brief high oxygen concentration induces oxidative stress in leukocytes and platelets: a randomized cross-over pilot study in healthy male volunteers. Shock Augusta Ga. 2021;56(3):384–95.
Hayes RA, Shekar K, Fraser JF. Is hyperoxaemia helping or hurting patients during extracorporeal membrane oxygenation? Review of a complex problem. Perfusion. 2013;28(3):184–93.
Wolbarsht ML, Fridovich I. Hyperoxia during reperfusion is a factor in reperfusion injury. Free Radic Biol Med. 1989;6(1):61–2.
Fujii Y, Tatsumi E, Nakamura F, Oite T. PaO2 greater than 300 mmHg promotes an inflammatory response during extracorporeal circulation in a rat extracorporeal membrane oxygenation model. J Thorac Dis. 2020;12(3):749–57.
Adrian K, Mellgren K, Skogby M, Friberg LG, Mellgren G, Wadenvik H. Cytokine release during long-term extracorporeal circulation in an experimental model. Artif Organs. 1998;22(10):859–63.
Kamler M, Wendt D, Pizanis N, Milekhin V, Schade U, Jakob H. Deleterious effects of oxygen during extracorporeal circulation for the microcirculation in vivo. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2004;26(3):564–70.
Geppert A, Steiner A, Zorn G, et al. Multiple organ failure in patients with cardiogenic shock is associated with high plasma levels of interleukin-6. Crit Care Med. 2002;30(9):1987–94.
Nguyen M, Tavernier A, Gautier T, et al. Glucagon-like peptide-1 is associated with poor clinical outcome, lipopolysaccharide translocation and inflammation in patients undergoing cardiac surgery with cardiopulmonary bypass. Cytokine. 2020;133: 155182.
Piton G, Belon F, Cypriani B, et al. Enterocyte damage in critically ill patients is associated with shock condition and 28-day mortality. Crit Care Med. 2013;41(9):2169–76.
Li Y, Tao Y, Xu J, et al. Hyperoxia provokes time- and dose-dependent gut injury and endotoxemia and alters gut microbiome and transcriptome in mice. Front Med. 2021;8: 732039.
Guo M, Yao D, Li L, Lu C, Li Y, Li J. Intestinal conditioning after cardiac arrest: the use of normothermic extracorporeal membrane oxygenation in the non-heart-beating animal model. Artif Organs. 2016;40(8):738–45.
Andrei S, Nguyen M, Berthoud V, et al. Evaluation of the oxiris membrane in cardiogenic shock requiring extracorporeal membrane oxygenation support: study protocol for a single center, single-blind, randomized controlled trial. Front Cardiovasc Med. 2021;8: 738496.
Chou H-C, Chen C-M. Neonatal hyperoxia disrupts the intestinal barrier and impairs intestinal function in rats. Exp Mol Pathol. 2017;102(3):415–21.
Stub D, Smith K, Bernard S, et al. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation. 2015;131(24):2143–50.
Hofmann R, James SK, Jernberg T, et al. Oxygen therapy in suspected acute myocardial infarction. N Engl J Med. 2017;377(13):1240–9.
Abou-Arab O, Huette P, Martineau L, et al. Hyperoxia during cardiopulmonary bypass does not decrease cardiovascular complications following cardiac surgery: the CARDIOX randomized clinical trial. Intensive Care Med. 2019;45(10):1413–21.
Jakkula P, Reinikainen M, Hästbacka J, et al. Targeting two different levels of both arterial carbon dioxide and arterial oxygen after cardiac arrest and resuscitation: a randomised pilot trial. Intensive Care Med. 2018;44(12):2112–21.
Young P, Mackle D, Bellomo R, et al. Conservative oxygen therapy for mechanically ventilated adults with suspected hypoxic ischaemic encephalopathy. Intensive Care Med. 2020;46(12):2411–22.
Abou-Arab O, Huette P, Guilbart M, Dupont H, Guinot P-G. Hyperoxia during cardiopulmonary bypass does not increase respiratory or neurological complications: a post hoc analysis of the CARDIOX study. Br J Anaesth. 2020;125(5):e400–1.
McGuinness SP, Parke RL, Drummond K, et al. A Multicenter, randomized, controlled phase IIb trial of avoidance of hyperoxemia during cardiopulmonary bypass. Anesthesiology. 2016;125(3):465–73.
Ni Y-N, Wang T, Liang B-M, Liang Z-A. The effect of conservative oxygen therapy in reducing mortality in critical care patients: a meta-analysis and trial sequential analysis. Front Med. 2021;8: 738418.
Gottlieb J, Capetian P, Hamsen U, et al. German S3 guideline: oxygen therapy in the acute care of adult patients. Respir Int Rev Thorac Dis. 2022;101(2):214–52.
Papazian L, Forel J-M, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–16.
Guérin C, Reignier J, Richard J-C, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–68.
Epis F, Belliato M. Oxygenator performance and artificial-native lung interaction. J Thorac Dis. 2018;10(Suppl 5):S596-605.
Lubnow M, Philipp A, Foltan M, et al. Technical complications during veno-venous extracorporeal membrane oxygenation and their relevance predicting a system-exchange–retrospective analysis of 265 cases. PLoS ONE. 2014;9(12): e112316.
Acknowledgements
None.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
HW, PGG, MS, GB, GP, AP, RL, AK, and GC made literature review and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Winiszewski, H., Guinot, PG., Schmidt, M. et al. Optimizing PO2 during peripheral veno-arterial ECMO: a narrative review. Crit Care 26, 226 (2022). https://doi.org/10.1186/s13054-022-04102-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-04102-0