Abstract
Cardiogenic shock and cardiac arrest contribute pre-dominantly to mortality in acute cardiovascular care. Here, veno-arterial extracorporeal membrane oxygenation (VA-ECMO) has emerged as an established therapeutic option for patients suffering from these life-threatening entities. VA-ECMO provides temporary circulatory support until causative treatments are effective and enables recovery or serves as a bridging strategy to surgical ventricular assist devices, heart transplantation or decision-making. However, in-hospital mortality rate in this treatment population is still around 60%. In the recently published ARREST trial, VA-ECMO treatment lowered mortality rate in patients with ongoing cardiac arrest due to therapy refractory ventricular fibrillation compared to standard advanced cardiac life support in selected patients. Whether VA-ECMO can reduce mortality compared to standard of care in cardiogenic shock has to be evaluated in the ongoing prospective randomized studies EURO-SHOCK (NCT03813134) and ECLS-SHOCK (NCT03637205). As an innate drawback of VA-ECMO treatment, the retrograde aortic flow could lead to an elevation of left ventricular (LV) afterload, increase in LV filling pressure, mitral regurgitation, and elevated left atrial pressure. This may compromise myocardial function and recovery, pulmonary hemodynamics—possibly with concomitant pulmonary congestion and even lung failure—and contribute to poor outcomes in a relevant proportion of treated patients. To overcome these detrimental effects, a multitude of venting strategies are currently engaged for both preventive and emergent unloading. This review aims to provide a comprehensive and structured synopsis of existing venting modalities and their specific hemodynamic characteristics. We discuss in detail the available data on outcome categories and complication rates related to the respective venting option.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) has emerged as an established therapeutic option for patients suffering from severe cardiogenic shock and/or cardiac arrest [1, 2]. Nowadays, the indication for VA-ECMO support spans a variety of etiologies, which is reflected by increasing numbers of VA-ECMO runs reported by the Extracorporeal Life Support Organization (ELSO) registry. In selected patients with ongoing resuscitation due to refractory ventricular fibrillation, VA-ECMO already proofed its effectiveness in improving survival compared to non-extracorporeal supported standard-of-care (Advanced Cardiac Life Support, ACLS) in the recently published randomized controlled ARREST-trial [2] while previous investigations had described a rather limited effect [3]. In contrast, evidence from adequately powered randomized controlled trials (RCT) on its effectiveness in cardiogenic shock is still missing. In this regard, EURO-SHOCK (NCT03813134) [4] and ECLS-SHOCK [5] (NCT03637205), started recruiting patients and the latter recruited more than half of the patients planned.
The original concept of VA-ECMO relies on venous drainage from the right atrium (RA) and retrograde arterial return towards the aortic valve for temporary circulatory support serving as a bridge to myocardial recovery, durable mechanical circulatory support (MCS), transplantation, or refined decision-making based on the patient's overall prognosis [6]. As an innate drawback of VA-ECMO treatment, the retrograde aortic flow could lead to an elevation of left ventricular (LV) afterload, increase in LV filling pressure, mitral regurgitation, and elevated left atrial (LA) pressure [7]. This may compromise myocardial function and recovery, pulmonary hemodynamics – possibly with concomitant pulmonary congestion and even lung failure – and contribute to poor outcomes in—not all, but—some patients [6, 8, 9]. To overcome these detrimental effects, a multitude of venting strategies are currently engaged for both preventive and emergent unloading. VA-ECMO treated patients in the ARREST trial did not undergo unloading indicating that a uniform venting strategy may not be necessary for survival in all patients receiving VA-ECMO after cardiac arrest. In this review, we aim to provide a comprehensive and structured synopsis over existing venting modalities and their specific hemodynamic characteristics. We will discuss in detail the available data on various outcome categories and complication rates related to the respective venting option.
Rationale and systematization of venting
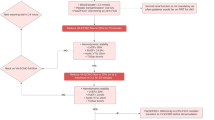
There are fundamental differences in left and right heart adaptation to increased afterload depending on the underlying etiology and chronic preconditions. In general, derivative hemodynamic implications are based on a factitiously high trans-aortic pressure gradient. Assuming, that LV function is preserved, the first coping mechanism is an increase in LV end-diastolic pressure (LVEDP) and consequently elevated calcium sensitivity and contractile power [7]. Albeit both ventricular wall stress and oxygen demand increase, cardiac output, regular aortic valve opening, and arterial pulsatility may be maintained.
In a large proportion of cases, cardiac function is impaired at baseline and the abovementioned system becomes fragile at best. If LV function deteriorates, the demand for increased oxygen need and sufficient endorgan perfusion is not met. Titrating VA-ECMO flow to the lowest acceptable level as well as careful fluid management using diuretics, hemodialysis, or continuous veno-venous hemofiltration (CVVH) may support this state of left heart decompression. Of course, this is not possible in most severe cardiogenic shock patients accompanying completely collapsed LV-function. As one consequence, higher VA-ECMO flow rates are unavoidable, LVEDP rises, and the LV progressively distends. LV volume overload is particularly grave in case of pre-existing aortic valve insufficiency and competent mitral valve. In contrast, in patients suffering from relevant mitral regurgitation, e.g., resulting from chronic dilated cardiomyopathy, the latter can serve as an outlet for elevated LV pressures at the cost of LA and pulmonary congestion. The resulting increase in pulmonary capillary wedge pressure (PCWP) and pulmonary arterial pressure (PAP) facilitates pulmonary congestion and – in a worst-case scenario – causes lung failure. Additionally, if the VA-ECMO sustained aortic mean arterial pressure cannot be overcome by LV systolic pressure, the aortic valve may not open with every beat [10]. Blood stasis and subsequent thrombus formation inside the LV cavity or the aortic root must be feared, potentially leading to fatal thromboembolic complications. Besides, LV distension promotes ventricular arrhythmias and subendocardial ischemia, hinders myocardial recovery and ultimately forestalls VA-ECMO weaning. Furthermore, non-pulsatile flow on ECMO and other MCS devices has been associated with aquired von-Willebrandt syndrome and increased bleeding rates [11]. Continuous clinical, echocardiographic, and radiographic assessment help recognizing early signs of these deleterious effects and might entail considerations for timely decompression (Fig. 1).
Rationale and systematization of venting. LVEDP, LV end-diastolic pressure; LVEDV, LV end-diastolic volume; PCWP, pulmonary capillary wedge pressure; PAP, pulmonary arterial pressure; CVP, central venous pressure; LA, left atrium; LV, left ventricle; PA, pulmonary artery; IABP, intra-aortic balloon pump, VA-ECMO, venoarterial extracorporeal membrane oxygenation
Considering the abovementioned impact of increased afterload and the heterogeneity of the underlying cardiac and/or systemic pathologies, the selection of a tailored venting strategy is a key challenge of successful individualized VA-ECMO support. Addressing the Achilles heel of retrograde aortic flow, different venting options are available and could be promising modifications of VA-ECMO treatment. However, currently there are several approaches that are not systematically applied, and its impact are often insufficiently understood [12]. In the ongoing ECLS-SHOCK trial venting should be considered when there is lack of arterial waveform pulsatility, no aortic valve opening assessed by echocardiography, left-ventricular outflow tract-velocity time interval < 10 cm and left ventricular distension and investigators suggest multiple venting options. In the EURO-SHOCK protocol, indication and mode of left ventricular unloading is rather unspecific and should be instituted as per sites local standard. Here, we propose a holistic classification of decompression strategies based on current clinical practice and available literature (Fig. 1): On one side there are active venting approaches, which directly depend on a pump`s action and imply LV decompression by I) drainage through an additional venous line, which is incorporated via “y”-connection into the VA-ECMO circuit, II) continuous or pulsatile pump devices, which are inserted across the aortic valve and eject LV preload antegrade into the aorta, or III) indirect negative pressure afterload reduction by intra-aortic balloon pumping (IABP). On the contrary, the passive approach in principle utilizes the pressure gradient between LA and RA to reduce LV pre-load and distension. Whereas the latter comprises different percutaneous techniques to disrupt the interatrial septum, active venting strategies have been developed for four anatomical sites, namely the pulmonary artery (PA), LA, LV, and aorta. Finally, each of these four positions can be accessed by either surgical or percutaneous techniques (Fig. 2). Corresponding hypothetical ventricular pressure–volume loops are shown in Supplementary Fig. 1. In the following chapters, we will highlight important features for all venting modalities and summarize currently available studies concerning outcomes as well as major drawbacks of their use.
Venting strategies during venoarterial extracorporeal membrane oxygenation (VA-ECMO). a Active left atrial venting via percutaneously introduced left atrial venting cannula (transseptal approach), which is directly connected to the venous VA-ECMO line. b Active left atrial venting via left atrial venting cannula (transseptal approach), which is directly connected to TandemHeart. c Active left ventricular venting via percutaneously implanted left ventricular pigtail catheter. d Active left ventricular venting using the ECMELLA approach as the combined use of Impella and VA-ECMO support. e The intra-aortic balloon pump (IABP) as an active, indirect LV venting option. f Passive atrial venting percutaneous balloon septostomy
Active pulmonary artery venting
Active drainage from the PA during VA-ECMO support reduces circulating blood volume in the pulmonary vascular system, thus reducing LA volume and LV preload. The PA can be accessed percutaneously or surgically via the internal jugular or femoral vein. Integrating PA venting into a VA-ECMO setup can be performed by adding a PA cannula to a separate RA venous drain (= PaVA-ECMO), by using a PA cannula as singular venous drainage (= PaVA-ECMO), or by a multihole tip (e.g., Medtronic) or double-lumen cannula (e.g., LivaNova) for simultaneous RA and PA drainage. In each case, fluoroscopic and echocardiographic guidance assures correct positioning. Cannulation of the PA can be advantageous if degenerating right heart function or recovering LV function require an adaptation of extracorporeal circulatory support. By inversing PA flow in a PaVA-ECMO configuration, the circuit can be modified quickly at bedside into a right heart assist device or VAPa-ECMO configuration with the very short necessity of stopping ECMO flow. In this type of cannulation, the arterial outflow is divided, with one part towards the aorta and one part towards the PA, enabling a relevant proportion of blood bypassing the compromised RV in an antegrade direction and filling the LV with oxygenated blood [6, 13].
The so far largest retrospective analysis in adult patients by Lorusso et al. reports outcomes of 15 VA-ECMO runs with adjunct PA venting [14] (Table 1). Most patients received PaVA-ECMO for post-cardiotomy shock (60%) and surgical PA cannulation was performed in five patients. All but one patient were successfully weaned from PaVA-ECMO and the overall in-hospital mortality rate was 20%. Of note, the PA cannula was exclusively used for drainage in eight patients, and for dynamic flow management (initial drainage, then perfusion in a VAPa-ECMO configuration) in six patients. Loforte et al. found a comparable successful weaning (87.5%) and in-hospital mortality rate (12.5%) in their patient cohort (n = 8), who received VA-ECMO and PA venting mostly for acute myocardial infarction (AMI) (37.5%) and myocarditis (25%) [15]. The median duration of PaVA-ECMO treatment was comparable between both studies, respectively, 9.0 and 8.5 days. Concerning the hemodynamic effects of PA venting, two case reports demonstrated reduced PCWP (33 mmHg/30 mmHg before and 12 mmHg/10 mmHg after cannulation, respectively), as well as reduced PAP and central venous pressure (CVP) using 14Fr and 15Fr sized cannula, respectively [16, 17]. Additional data from a bovine model showed significantly reduced intracavitary LV pressure with PA venting [18]. Furthermore, Fouilloux et al. and Kimura et al. demonstrated that PA venting may be a safe and effective method for urgent decompression in pediatric patients [19, 20]. In most case reports, PA venting was initiated simultaneously with or shortly after VA-ECMO therapy [15,16,17, 19, 20], but was also successfully used for delayed venting 6 days after VA-ECMO initiation in one case [16]. Overall, procedure-related complications were rarely reported but sufficient data on this are still missing.
In comparison to other unloading strategies, PA venting may be an option in the presence of an LV thrombus, because it requires no direct LA or LV manipulation and, thus, the risk of thrombus mobilization is minimized. In terms of outcome analysis, the limited evidence from case reports and the two retrospective studies showed that PA venting may be a feasible, and effective venting option. However, neither matched retrospective investigations nor RCTs on PA venting are available yet.
Active left atrial venting
Active LA venting enables direct reduction of LA volume. Commonly used techniques for introducing the LA venting cannula are the percutaneous transseptal approach under fluoroscopic and echocardiographic guidance via a femoral vein and the RA, or a direct surgical implantation via the upper-right pulmonary vein, which is preferably used for patients requiring VA-ECMO support after cardiac surgery (Fig. 2) [21]. Standard cannula sizes for percutaneous LA drainage in adult patients range from 19Fr to 28Fr (e.g., Medtronic or CardiacAssist), whereas for pediatric patients smaller sized BioMedicus cannula (Medtronic), the Radiofocus Glidecath (Terumo medical), a pigtail catheter (e.g., Cook), the atrial septal occluder sheath (Amplatzer), or a Mullins sheath (Medtronic) are used. The LA drain is connected to the venous line and flow rates may be adjusted using a cannula clamp. Similar to PA venting, the LA cannula can be added to a separate RA drain, inserted as part of a multistage drain for RA and LA, or even used without RA drainage [22, 23].
Of note, the TandemHeart (LivaNova Plc., London, UK) may represent another option for active LA venting. The TandemHeart is a paracorporeal ventricular assist device with an inflow cannula draining blood from the LA and the outflow cannula pumping blood into the aorta via a femoral access point. Transvenous insertion of the 21Fr LA drain is performed percutaneously via the RA and the interatrial septum. The external pump provides flow rates of up to 5.0 l/min which is returned retrograde into the femoral artery through a 15Fr or 17Fr outflow cannula. By active LA draining, the system reduces LV preload and may therefore be an effective venting option. As an alternative product, the REVAS cannula (Free life medical GmbH, Aachen, Germany) can also be used for active LA relief. This cannula is available in sizes 18/20/22 Fr and can be used with all VA-ECMO systems. Upgrade of the TandemHeart circuit with an in-line oxygenator as well as combination of the TandemHeart LA drainage cannula in conjunction with VA-ECMO are possible but more demanding [24]. To the best our knowledge, however, there are no studies explicitly evaluating the TandemHeart as a venting option, particularly in comparison to other venting options.
In 2021, Kim et al. published the first controlled retrospective trial (n = 124) on outcomes with active percutaneous LA venting compared to patients with an arterial pulse pressure of < 10 mmHg who were treated with isolated VA-ECMO [25] (Table 2). Regarding baseline characteristics in both groups, the authors reported considerable differences: Patients receiving LA venting were younger, less likely to have suffered prior cardiac arrest and more likely to present with acute decompensated heart failure as VA-ECMO indication. Keeping these potential biases in mind, LA venting was associated with a better ECMO weaning rate (61.3% vs. 38.7%, p = 0.012) and lower—albeit not significant—in-hospital mortality (56.5% vs. 69.4%, p = 0.191). Additionally, patients with decompression had a higher median duration of VA-ECMO treatment (237 h vs. 71 h, p < 0.001). In a 1:1 propensity score-matched analysis by Alghanem et al. comparing n = 21 patients undergoing VA-ECMO support with active or passive LA venting to n = 21 VA-ECMO alone controls, in-hospital mortality was unaffected (29% vs. 38%, p = 0.513), whereas both the length of hospitalization and ICU stay were significantly longer with decompression (p = 0.012 and p = 0.008, respectively) [26]. Another retrospective matched analysis by Ok et al. (n = 70) did not show significantly improved survival to discharge or higher weaning rate with decompression (44% vs. 22.2%, p = 0.11 and 37.8% vs. 60%, p = 0.08, respectively), considering substantial differences in baseline variables including age and shock etiology [27]. In two smaller cohorts, each based on seven patients treated with VA-ECMO and percutaneous LA venting, in-hospital mortality rates were 14% and 28%, respectively [28, 29].
A case series by Aiyagari et al. including seven pediatric patients with VA-ECMO support and percutaneous LA venting via transseptal cannulation reported an in-hospital mortality rate of 57%, but echocardiographic improvement of LA dilation after decompression in 71% [30]. Notably, sufficient LA drainage with a large relative sheath size correlated with procedural success (13Fr/m2 vs. 6Fr/m2 indexed to body surface area, p < 0.05). In two larger cohorts, LA pressure decreases by 10 mmHg (mean, p = 0.022) [26] and 7 mmHg after decompression (median, p = 0.002) [31], respectively. Hlavacek et al. observed an LA pressure decrease from 57 mmHg (mean) to 18 mmHg after delayed insertion of a 17Fr LA cannula (n = 1), as well [32]. Other case reports highlighted reduced biventricular filling pressures following active LA venting [24, 33]. As a consequence of these hemodynamic changes, LV function as well as pulmonary congestion and edema may improve [21, 22, 29, 31, 32, 34, 35].
Three larger studies have focused on the timing of LA venting initiation in adult and pediatric cohorts. In a comparative analysis (n = 50) on therapeutic (median interval from VA-ECMO initiation to decompression: 39 h) vs. prophylactic LA venting using surgical and percutaneous techniques, Na et al. found a reduced 30-day mortality rate with prophylactic decompression (34.4% vs. 5.6%, p = 0.036). However, this effect did not reach significance at 90 days (43.8% vs. 22.2%, p = 0.128). On the contrary, Zampi et al. found no difference in survival rates of 137 pediatric patients comparing early (< 18 h interval between VA-ECMO initiation and LA venting) and late (> 18 h) decompression, but longer VA-ECMO treatment duration and ICU length of stay in the late decompression group (p = 0.02 and p = 0.03, respectively) [36]. Hacking et al. published their single-center experience with different LA and LV unloading techniques in pediatric VA-ECMO patients spanning more than 20 years (n = 49) [37]. Elective compared to emergency (median interval from VA-ECMO initiation to decompression: 32 h) venting correlated with reduced VA-ECMO support duration (128 h vs. 236 h, p = 0.013). However, survival to discharge was not affected (p = 0.4).
Although Kim et al. and Ok et al. did not observe significant differences in complication rates between venting and control groups (12.9% vs. 11.3%, p = 0.783 and 29% vs. 8.9%, p = 0.26, respectively), one patient experienced cardiac tamponade after the procedure [25]. Other complications of LA venting included catheter obstruction [28], drain malpositioning [26], cardiac perforation [36], persistent left-to-right shunt after cannula explantation [29, 31, 33], as well as insertion-site bleeding and infection [23].
Prospective RCTs comparing VA-ECMO treatment with or without LA venting have not been published and available retrospective datasets do not allow a clear mortality outcome conclusion. As expected, LA pressure decreases following LA drainage with the appropriate cannulation size and seems to be effective in mitigating at least some of the adverse effects of VA-ECMO-related elevated afterload. Whether the beneficial outcome of early LA unloading exceeds the shorter duration of MCS, and which patient subgroup may particularly gain a survival advantage remains unclear. The data available are also not sufficient to draw a valid conclusion about the safety of the approach. However, due to the invasive nature of transseptal cannula positioning care must be taken with regard to cardiac perforation.
Active left ventricular venting
A great variety of direct LV venting options have been proposed over the past decade. Before the first microaxial pump device was approved for LV venting in 2008, direct LV unloading was performed by percutaneous transaortic catheter insertion or surgical implantation of a venting cannula into the LV (Fig. 2). The fundamental principle of these techniques is the active reduction of LVEDP and LVEDV in conjunction with the venous VA-ECMO drainage, thus preventing progressive LV distension and pulmonary congestion. While transarterial retrograde pigtail catheter implantation is usually performed in the catheterization laboratory or — in special cases—even at bedside [38], surgical cannulation requires a more advanced operating facility and is, therefore, often chosen as a venting strategy for post-cardiotomy patients, who fail to be weaned from cardiopulmonary bypass, or if central VA-ECMO implantation necessitates sternotomy in any case [39, 40]. Access to the LV is obtained by median sternotomy, left thoracotomy [41], right anterior thoracotomy [42], or through a small incision in the diaphragm [43]. The LV cavity is then cannulated through an apical stab incision or the right superior pulmonary vein via LA and mitral valve. Commonly used cannulation sizes range from 20to 32Fr in adult, and 10 to 24Fr in pediatric patients, which allows for higher maximum venting flow rates compared to LA and PA drains, or to 5–8 Fr LV pigtail catheters [42, 44, 45]. Born et al. were able to show in an experimental setup that LV relief is possible with a pigtail catheter. The amount of relief mainly depends on two factors, the negative pressure in the venous line and the hematocrit. With a 7 Fr. pigtail catheter the LV can be relieved with up to 200 ml/min (unpublished). In a remarkable case report, Cheung et al. successfully attempted trans-venous introduction of an 11Fr Mullins transseptal sheath into the LV after blade atrial septostomy, but this did not result in satisfactory decompression [46].
In contrast to active LV drainage by means of the VA-ECMO centrifugal pump, microaxial pump devices not only enable decompression, but also directly contribute to cardiac output by propelling blood from the LV cavity across the aortic valve into the ascending aorta. The Impella microaxial flow pump (Abiomed, Danvers, USA) family for left heart support currently comprises the Impella 2.5 (providing maximum flow rate of 2.5 l/min; 9 Fr, introducer sheath 13 Fr), Impella Cardiac Power (CP and CP Smart Assist) (3.5 l/min and 4.0 l/min; 9 Fr, introducer sheath 14 Fr) and Impella 5.0 and 5.5 Smart Assist (5.0 l/min and 5.5 l/min; 9 Fr, introducer sheath 23 Fr). The first two models are inserted through the femoral or axillary artery and advanced retrograde into the LV under fluoroscopic and echocardiographic guidance. The latter two, the Impella 5.0 and 5.5 require surgical transfemoral, transaxillary, or transubclavian placement, the latter based on a right axillary artery conduit system [47]. The combined use of Impella and VA-ECMO is referred to as the ECMELLA or ECPELLA concept (Fig. 2) [6]. The latter comprises both clinical scenarios: (1) an ongoing Impella therapy is upgraded by VA-ECMO support, e.g., for reinforcement of cardiogenic shock therapy, and (2) Impella is used as a preventive or delayed decompression strategy together with or after VA-ECMO treatment initiation. Similar to Impella, another novel device for active LV mechanical support is the recently developed PulseCath iVAC 2L (PulseCath BV, Arnhem, NL). It creates diastolic antegrade aortic flow of up to 2L/min by a rotating two-way-valve incorporated in a 17Fr trans-aortic catheter, and an extracorporeal membrane pump powered by a standard IABP console [48, 49]. As opposed to the continuous Impella devices [50], pulsatile support by the PulseCath iVAC 2L does not contribute to systolic afterload.
Percutaneous active left ventricular venting options
Left ventricular catheter insertion
Historically, retrograde insertion of a pigtail catheter into the LV was the first percutaneous strategy used for active LV venting (Fig. 2). Since prospective controlled studies are not available, the knowledge on outcomes with this method relies on singular clinical reports and one case series including seven patients with pulmonary edema and severe LV dysfunction published by Hong et al. [38] (Table 3). In their retrospective analysis, the VA-ECMO weaning rate was 58% and overall mortality rate was 42%. Of note, the patients’ median age was less than 40 years, and the majority underwent MCS for AMI. Active decompression with a 5–6 Fr pigtail catheter resulted in decreased LV end-diastolic diameter (LVEDD) (59 mm vs. 50 mm, p = 0.044), a trend towards increased LVEF (18.3% vs. 38.3%, p = 0.094) and an increase in mean arterial pressure (MAP) (70 mmHg vs. 95 mmHg, p = 0.050). Fumagalli et al. observed a noticeable decrease in PCWP (40 mmHg vs. 7 mmHg) and CVP (11 mmHg vs. 3 mmHg) after LV drainage with a 17 Fr pediatric pigtail in a 34-year-old male patient [45]. And even with a smaller 7 Fr pigtail catheter, LVEDV decreased by almost 90 ml after simultaneous VA-ECMO and LV venting initiation compared to baseline without MCS [51]. In another case report by Bloom et al., rapid decline of pulmonary edema within 24 h after percutaneous insertion of a 7 Fr pigtail catheter into LV was observed [44]. Complications related to pigtail catheter insertion were not reported throughout available publications.
The insertion of a pigtail catheter into the LV is an easy and extremely low-cost venting approach which might even be possible to be established at bedside under echocardiographic control. Since this technique is frequently used for diagnostic purposes in catheterization laboratories it might be considered as a low-risk procedure. However, at present there are neither large retrospective data or matched comparisons nor clear evidence showing a mortality benefit, although this method may alleviate LV distension and pulmonary congestion. Furthermore, the maximum venting flow rates are clearly limited by catheter size, which has been demonstrated in an artificial VA-ECMO model [35]. If this limited flow requires stricter anticoagulation is unclear. Definitely more data is needed in the future regarding this easy to establish venting possibility.
ECMELLA
The ECMELLA approach—also called ECPELLA—used as a percutaneous decompression strategy (Fig. 1) has been established by several experienced ECMO centers in recent years and many of which started to publish their outcome data (Table 3). In a large international multicenter 1:1 propensity score-matched analysis of n = 255 patients undergoing ECMELLA compared to n = 255 patients treated with VA-ECMO alone, Schrage et al. found ECMELLA-treatment to be associated with a significantly lower 30-day mortality rate (56.9% vs. 63.5%, p = 0.03) [52]. Early LV unloading shortly before or at VA-ECMO initiation predicted a lower 30-day mortality rate (HR 0.76, p = 0.03) [52]. Another propensity-matched controlled study by Pappalardo et al. previously reported superior in-hospital mortality and VA-ECMO weaning rate in patients with ECMELLA support compared to VA-ECMO alone (48% vs. 74%, p = 0.04 and 48% vs. 28%, p = 0.047, respectively) [53]. Not all centers, however, have seen an overall survival benefit. Akanni et al. found no difference in 30-day mortality and hospital discharge rate with isolated VA-ECMO compared to ECMELLA, regardless of whether VA-ECMO or Impella was the first device instituted (p = 0.913 for mortality) [54]. But owing to the relatively small ECMELLA group comprised of just 29 individuals, the results should be interpreted with caution. In two cohorts without control groups, Tongers et al. and Schrage et al. found that patients undergoing ECMELLA therapy had a 30-day mortality rate of 49% and 64.2%, respectively, which was lower compared to established risk prediction scores [55, 56]. Interestingly, extracorporeal cardiopulmonary resuscitation and a duration of shock onset to first device longer than 13.5 h was associated with inferior 30-day mortality outcome [55, 56].
An elaborated hemodynamic study of 27 patients undergoing ECMELLA treatment by Eliet et al. provided evidence on how an Impella device is contributing to LV decompression [57]. The authors performed an incremental Impella ramp test and compared hemodynamic parameters between the lowest performance level (P1) setting with the individually adjusted optimal Impella performance level determined by the intensive care team. They found not only a significantly decreased LVEDD (49 mm vs. 30 mm, p < 0.0001) and increased MAP (66 mmHg vs. 79 mmHg, p < 0.0001), but also elevated end-tidal CO2 (9 mmHg vs. 19 mmHg, p < 0.0001) and pulmonary arterial velocity time integral (PAVTI), evidencing improved pulmonary vascular compliance (2.3 cm vs. 5 cm, p = 0.001). Aside from these data, other authors reported decreased PCWP [55], decreased PAP [54], reduction in catecholamine requirements and lactate levels [50], as well as resolution of multiorgan failure [58] after Impella addition to VA-ECMO treatment. Regarding additional outcome parameters, Tongers et al. found that ECMELLA patients, who survived to discharge, frequently had acceptable neurologic (CPC I: 73.9%, CPC II: 26.1%) and functional outcome (NYHA I: 17.4%, NYHA II: 47.8%, NYHA III: 34.8%) [56].
Impella devices are contraindicated in presence of LV thrombus, mechanical aortic valve prosthesis, moderate to severe aortic valve disease, and severe peripheral artery disease, which limits its use for critically ill patients. But even for eligible patients, Impella insertion may entail serious risks. Schrage et al. and Pappalardo et al. observed higher rates of major bleeding in the ECMELLA group compared to matched controls (38% vs. 18%, p < 0.01, 38% vs. 29%, p = 0.6, respectively) highlighting the invasiveness of this approach [53]. Tongers et al. found major bleeding in 1% of ECMELLA-treated patients, but only 42% did not show any signs of bleeding [56]. Bleeding requiring intervention occurred in 25% [55], insertion site bleeding in 26% [58], and insertion site ischemia requiring intervention in 22% (p < 0.01) [52] in different centers. Lower limb ischemia distal to the Impella insertion site has been reported as well [56, 58, 59]. Apart from access site and cardiovascular complications, the Impella rotor applies considerable mechanical shear stress to red blood cells, which subjects patients to a higher risk of hemolysis. Across most publications, hemolysis was acknowledged as a drawback of Impella therapy. Schrage et al. found signs of hemolysis in 34% of ECMELLA compared to 22% of VA-ECMO alone patients (p = 0.01) [52], Akanni et al. in 45% compared to 17% (p = 0.002) [54], and Pappalardo et al. in 76% compared to 33% (p = 0.004) [53]. Recent meta-analyses validated these observations [60, 61]. In terms of other general complications, ECMELLA patients more frequently underwent continuous renal replacement therapy [52, 53] and laparotomy for abdominal compartment syndrome [52].
Large retrospective multicenter studies have shown that the ECMELLA concept may translate into survival benefit, despite the indisputable risk of hemolysis, as well as higher bleeding and access site complication rates. In this regard, the 1:1 propensity-matched analysis by Schrage et al. is by now the best available evidence of its effectiveness [52]. To overcome the lack of robust prospective data RCTs are urgently needed [62]. One RCT that already started recruiting patients is the REVERSE trial (NCT03431467). In this trial, 96 patients will be included and randomized to early ECMELLA vs. VA-ECMO alone. Furthermore, the UNLOAD ECMO trial is currently under preparation and aims at including enough patients to be powered to show mortality difference.
VA-ECMO combined with PulseCath iVAC 2L
Hemolysis is a serious adverse event, which can even require withdrawal of Impella support. In the first-in-man case report presented by Tschöpe et al. in 2020, replacement of an Impella CP with the PulseCath iVAC 2L pulsatile device was performed when laboratory assessment displayed persistent signs of hemolysis 6 days after ECMELLA initiation [49] (Table 3). Cardiocirculatory as well as laboratory parameters recovered soon thereafter, and the patient was successfully weaned from PulseCath iVAC 2L after another 5 days.: Whether the iVAC2L will be an alternative to the Impella devices achieving sufficient LV decompression during VA-ECMO support will have to be assessed in future clinical trials.
Surgical active left ventricular venting options
Two older case reports have shown effective LV unloading with a surgically implanted 20Fr cannula [43, 59] (Table 3). The first one presented a 17-year-old patient with myocarditis and progressive pulmonary edema under VA-ECMO therapy who underwent pericardial drain placement shortly before decompression was deemed necessary, the second one a 61-year-old patient who had a transapical drainage cannula placed after going into cardiac arrest during abdominal surgery. No procedure-related complications were noted and both patients survived. However, if the benefit of transapical vent insertion outweighs the risk for a patient who had not undergone cardiac surgery before, remains unanswered. In a retrospective analysis, Takeda et al. compared conventional biventricular assist device implantation to VA-ECMO with simultaneous transapical cannulation for patients with cardiogenic shock and biventricular failure [41]. Although the authors were not primarily focusing on the outcomes of transapical LV cannulation as a venting strategy in VA-ECMO therapy, this study provided insights into its effectiveness and safety. The mortality rate at 30 days in the VA-ECMO with transapical venting group was 13.6% with a weaning rate of 27% and a successful bridge to durable LVAD or heart transplantation rate of 50%. Major bleeding and stroke occurred in 31.8% and 18.2%, respectively.
Further clinical data on surgical LV venting have been published by Schmack et al., who retrospectively evaluated 48 VA-ECMO runs from 2004 to 2014 [39]. Of all 38 patients with central VA-ECMO, 20 patients underwent simultaneous LV drainage with a 24Fr cannula, while the remaining 18 patients and another 10 patients with peripheral VA-ECMO in the control group did not receive any form of LV venting. Only 10% of the patients receiving LV venting had prior cardiac surgery as opposed to 46% in the control group (p < 0.01). Surgical LV decompression was associated with lower mortality during support (25% vs. 57%, p = 0.027) as well as 30-day mortality (45% vs. 75%, p = 0.034), although Kaplan–Meier-estimates of long-term survival did not show significant benefit after 6 and 12 months. Furthermore, the median duration of VA-ECMO support was longer in the LV venting group (7.4 days vs. 5.2 days, p = 0.055). In another smaller cohort including 12 VA-ECMO treated patients with simultaneous decompression the overall mortality rate was 41.7% [63]. The technique is also used in pediatric patients, where in the largest available case series (n = 8) in-hospital mortality rate was 25% and successful weaning rate was 87.5% [64]. In singular cases, LV venting improved LVEF [42, 65], LV distension [64], reopening of the aortic valve [65], and pulmonary edema [64]. Of note, simultaneous rather than delayed LV decompression was performed across most reviewed cohorts. Procedure-related complications were observed in one pediatric patient who died after in-line thrombus development [64] and one adult patient due to deep sternal wound infection [63]. Overall complications observed by Weymann et al. included bleeding requiring intervention in 41.7%, coagulation disorder in 66.7%, renal failure requiring hemodialysis in 50%, and stroke in 8.3% [63]. In most cases, sufficient data on complications are not available.
Surgical techniques allow for large bore LV cannulation and effective LV decompression. Despite surgical trauma and its potential complications there may be signals toward short-term survival benefits based on retrospective data. However, the data on surgical venting options are particularly scarce regarding outcomes and safety and sufficiently powered RCTs are urgently needed but might never be performed.
Active intra-aortic venting
The IABP reduces LV afterload by pulse-synchronous systolic negative pressure generation through deflation of a helium-filled balloon and improves coronary and bypass graft perfusion, when the balloon is inflated in diastole [66, 67]. The device has therefore been considered as an active, indirect LV venting option in patients undergoing VA-ECMO support (Fig. 2). Retrospective analyses and meta-analyses, although not unanimously, have shown IABP to be associated with reduced mortality in VA-ECMO treated patients [60, 61] (Table 4). In a nationwide database from Japan, Aso et al. found a significantly lower 28-day and in-hospital mortality in a 1:1 propensity-matched analysis of patients undergoing VA-ECMO support combined with IABP (n = 533) compared to VA-ECMO alone (n = 533) (48.4% vs. 58.2%, p = 0.001, 55.9% vs. 64.5%, p = 0.004, respectively) [68]. A subgroup analysis of patients without continuous renal replacement therapy found an even more pronounced survival benefit with IABP (28-day mortality rate 42.6% vs. 56.1%, p < 0.001). Last, the VA-ECMO weaning rate was higher in the IABP group (82.6% vs. 73.4%, p < 0.001). In another propensity-matched analysis by Bréchot et al., decompression with an IABP led to a trend towards improved ICU-mortality, but did not reach significance (44.4% vs. 55.5%, p = 0.06) [69]. Additionally, the authors found fewer radiographical signs of pulmonary edema in the IABP group compared to VA-ECMO alone (p < 0.0001). In line with this, Tepper et al. found a reduced, but not significantly lower, 30-day mortality rate in their cohort of 60 patients suffering from post-cardiotomy shock when comparing patients undergoing VA-ECMO treatment with and without IABP (50% vs. 67%, p = 0.06) [70]. The VA-ECMO decannulation rate was 67% in the IABP group and 53% in the VA-ECMO alone group. Lin et al. did not observe a survival benefit 14 days after VA-ECMO initiation (n = 529), but significant differences in baseline characteristics between the VA-ECMO alone and combined treatment group may have strongly influenced this result [71].
Regarding the timing of IABP initiation, Gass et al. evaluated the outcomes of 137 patients, 41% of which received IABP before VA-ECMO initiation compared to controls with delayed IABP insertion [72]. Prior IABP initiation was independently associated with a lower risk of composite outcome of mortality, stroke, or vascular complication requiring intervention (OR 0.353, p = 0.031).
The hemodynamic effects of IABP during VA-ECMO were analyzed in detail by Petroni et al. [73]. In their experimental arrangement various parameters were measured after stopping and re-starting the IABP in 12 consecutive patients after a mean interval from IABP initiation to the hemodynamic test of 4.7 days. In doing so, the authors observed decreased LVEDD (55 mm vs. 47 mm, p = 0.003), mPAP (24 mmHg vs. 19 mmHg, p = 0.02), and PCWP (19 mmHg vs. 15 mmHg, p = 0.01) after IABP re-start. Tepper et al. found CVP to be significantly decreased 48 h after VA-ECMO initiation only in the IABP group (15 mmHg vs. 12 mmHg, p = 0.01) [70]. Interestingly, cerebral blood flow assessed by transcranial Doppler sonography has been shown to improve with IABP counterpulsation only in VA-ECMO patients with preserved pulsatile pressure of > 10 mmHg [74]. Of the patients with persistent aortic valve closure during VA-ECMO, re-opening using an IABP was achieved in eight cases [10].
Complications related to IABP reported by Bréchot et al. include access site bleeding in 14%, access site infection in 1%, minor distal ischemia in 5%, and device dysfunction in 3% [69]. Bonacchi et al. observed leg ischemia in 6.5%, although their study population was not clearly separated into IABP and non-IABP treated patients [75]. Lin et al. have seen significant differences in vascular complication rate requiring fasciotomy in IABP treated patients (2.6% vs. 0%, p = 0.012) [71]. Overall bleeding and stroke rates, as well as vascular complication rate requiring re-perfusion, were mostly indifferent in the IABP and control group [70, 71, 76]. Notably, spinal cord infarction in patients with small aortic size should be considered as a differential diagnosis, if suggestive neurologic deficits occur after IABP initiation [77].
Taken together, the published data on IABP as an active, indirect LV venting option suggest a survival benefit at a relatively low risk of device-related complications and low-cost. Furthermore, IABP can even be implanted at bedside on ICU guided by echocardiography and followed by chest X-ray for positioning. Another advantage of the IABP is the lack of some contraindications which are inherent to active LV-venting devices— most importantly here the LV-thrombus. The latter is of relevance since the most frequent reason for cardiogenic shock is anterior STEMI bearing the greatest risk of LV thrombus formation. These points might be reasons that the IABP is still the most often used venting device outside Germany. Currently, evidence for IABP is even stronger—in terms of the availability of larger matched retrospective trials—compared to the evidence existing for ECMELLA strategy. The latter, however, is much more expensive and associated with a higher complication rate. Like for many other previously described venting strategies, the lack of prospective data calls for future RCTs to evaluate mortality and complication rates as well as to improve implantation timing and therapeutic management of IABP as venting strategy during VA-ECMO support.
Passive atrial venting
Passive atrial venting strategies are based on a volume shift from overloaded LA into RA through an interatrial septum defect. In theory, an iatrogenic left-to-right shunt as an “overflow valve” for the LA aims to reduce LV preload and distension. Interseptal atrial communication may be achieved by percutaneous transvenous septostomy, balloon dilation, or stent implantation (Fig. 2). For transseptal puncture, a Brockenbrough needle (Medtronic) or a transseptal needle (Cook Medical) is used in most cases. Balloon dilation may be performed by serial dilation with increasing balloon sizes or an Inoue balloon (Toray) [78, 79]. In adults, balloons with diameters of 24 mm, 26 mm, or even 30 mm may be selected based on individual patient, whereas for pediatric patients, appropriate sizes range up from 18 mm for infants to 28 mm for older children. The Rashkind atrial septostomy is another catheter-based maneuver using a balloon for shunt creation. After transseptal access to the LA, the deflated balloon is advanced into the LA cavity, then inflated, and retracted into the RA thereby creating a septal defect. O’Byrne et al. have assessed the resulting interatrial shunt dimension in pediatric patients by echocardiographic imaging and found a ratio of defect to maximum balloon diameter of 0.26 [80]. In one case report, Haynes et al. mounted a Palmaz 4010 stent (Johnson&Johnson) onto a 16 mm Balloon (NuMED) which was subsequently implanted into the interatrial septum for urgent left heart decompression [81].
To date, data regarding outcomes are only available from retrospective observational studies without any control groups (Table 5). The in-hospital mortality rate in an adult population with passive transseptal venting observed by Lin et al. (n = 15) was 46.7% [79]. Prasad et al. reported a 30-day mortality rate of 56% in a cohort of nine patients, who underwent septostomy and balloon dilation during VA-ECMO [82]. In a mixed analysis of pediatric and adult patients by Baruteau et al., in-hospital mortality rate was 34.4% [78]. In the largest pediatric cohort of 223 patients published by Desphande et al., the in-hospital mortality rate was 46.19% [83]. Notably, the procedure was performed urgently or even emergently in most cases (80%) and a multivariable analysis showed procedure status (emergent/salvage vs. elective/urgent) to be significantly associated with death less than 7 days post procedure (OR 2.79 (1.2, 6.44), p = 0.017). The large number of emergency procures may explain the difference to the study by O’Byrne et al., who reported—with a median interval of VA-ECMO initiation to decompression of 0 days—a much lower in-hospital mortality rate of 18% [80].
Hemodynamic data from three of the abovementioned studies can serve as a proof on concept for passive atrial venting. Thus, O’Byrne et al. found a significant reduction of LA pressure (− 5 mmHg, p < 0.0001) and LA to RA pressure gradient after decompression (− 7 mmHg, p < 0.0001) [80]. Baruteau et al. and Prasad et al. have seen similar effects after septostomy (-16.4 mmHg, p < 0.0001 and − 11 mmHg, p = 0.001, respectively) [78, 82]. In addition, radiographical signs of pulmonary edema improved in 76.6% and all survivors, respectively and the PaO2/FiO2 ratio improved from baseline to 24 h after decompression (p = 0.002) [78, 82].
Inherently, persisting atrial communication after passive atrial venting is a frequently noticed long-term complication in patients who survived VA-ECMO therapy and did not undergo heart transplantation. Transcatheter or surgical closure may be performed at a later stage, or if durable LVAD implantation requires open heart surgery [78,79,80]. An implanted atrial stent may also be recovered using a trans-catheter or surgical technique [84]. Overall periprocedural complications occurred in 9.4% and VA-ECMO related complications in 25% in the multicenter analysis published by Baruteau et al. [78]. The incidence of bleeding, vascular complications requiring intervention, cardiac tamponade, and device malpositioning/thrombus after septostomy were 3.2%, 0.91%, 5.45%, and 0.46%, respectively, as observed by Desphande et al. [83]. Hence, pericardial tamponade is a relevant complication of this procedure and this should be critically weighted before using this strategy.
Although passive atrial venting may enable effective decompression during VA-ECMO support, mortality and safety outcomes compared to controls have not been investigated, not to mention that there are no RCTs available yet. Obviously, a comparison of the active and passive LA decompression approach would be of particular interest. Due to the frequent use of this technique in children, most data derive from this subgroup and results cannot automatically be transferred to the adult population. The crude data on LA pressure pre and post cannulation or septostomy suggest, that both methods are capable of reducing pressure by ~ 10–15 mmHg. Overall, there are currently no data available to support one of these two options as superior venting strategy in patients undergoing VA-ECMO treatment.
Comparative studies
In a recent retrospective study comparing three different venting strategies by Hasde et al., transapical LV cannulation (n = 16) showed a greater reduction of LA diameter, PCWP, and sPAP than IABP (n = 20) or percutaneous balloon septostomy (n = 17) (p = 0.001) [85] (Table 6). The VA-ECMO indication was acute cardiac decompensation and post-cardiotomy shock in ~ 30% of cases, while only 14.1% underwent VA-ECMO therapy for AMI. The surgically inserted cannulas ranged from 19 to 21Fr and allowed for venting flow rates of 600–1800 ml/min, whereas no further information on balloon diameters or resulting septal defects was provided. Overall weaning (p = 0.783) and in-hospital mortality rates (p = 0.783) did not differ between groups, which may be related to higher rates of procedure-related complications in the surgical venting group, including bleeding, ventricular arrhythmias, cannula thrombosis, and malpositioning.
In a large prospective multicenter cohort of 209 post-cardiotomy shock patients published by Bonacchi et al., all patients without pre-operative IABP insertion were upgraded at the time of VA-ECMO initiation, while 74.2% of the patients additionally received a trans-apical (50.3%) or transpulmonary (49.7%) venting cannula for refractory pulmonary edema or insufficient LV decompression [75]. Intraoperative IABP insertion (OR 0.6, p = 0.038) and trans-apical cannulation (OR 0.6, p = 0.03) were both independent predictors of early mortality.
Piechura et al. retrospectively analyzed Impella CP and IABP for LV decompression in 63 VA-ECMO patients (non-matched) [86]. 30-day mortality rates were 53% in the Impella group and 69% in the IABP group (p = 0.49), but only Impella-treated patients surpassed the predicted mortality calculated by the SAVE-Score (37% vs. 18%). Considering that Impella-treated patients were significantly older (66 years vs. 55 years, p = 0.001) and 25% of the patients in the IABP group required additional LV venting, the majority of patients in each group received LV decompression simultaneously with VA-ECMO initiation. The rate of major complications including hemolysis (p = 0.70), bleeding (p = 0.15), vascular complications (p = 1), ischemic stroke (p = 0.42), and intracardiac thrombus (p = 0.46) were not different between the groups. The authors also evaluated outcomes with respect to the timing of venting initiation. Patients, who received immediate/preventive LV venting, had a similar chance of survival to 30 days compared to patients with reactive LV venting (p = 0.46). However, baseline age was significantly higher in the immediate venting group.
In 2017, Tepper et al. evaluated 45 VA-ECMO runs with concomitant LV venting using Impella (n = 23, Impella 2.5, CP, or 5.0) or surgically implanted LV vent (n = 22, trans-pulmonary, trans-apical, or PA) [87]. The main causes of circulatory failure were AMI (39%) and non-ischemic cardiomyopathy (30%). Diastolic PAP was significantly reduced 48 h after decompression both in the ECMELLA group and VA-ECMO with surgical vent group (23 mmHg vs. 15 mmHg, p = 0.02 and 20 mmHg vs. 15 mmHg, p = 0.01, respectively). Signs of pulmonary edema improved in 65% and 25%, respectively, but 30-day survival rates were not significantly different (43% vs. 32%, p = 0.42).
Conclusion
Currently, two large, randomized trials EURO-SHOCK and ECLS-SHOCK are recruiting patients to answer the question if VA-ECMO therapy in cardiogenic shock improves survival. Both trials will not answer the question whether or not concomitant venting is needed. Hence, adequately powered, prospective RCTs on LV venting strategies will not be available in the upcoming years. However, data gained during the past years have certainly given intensivists a better understanding of the hemodynamics, outcomes, and adverse effects of different venting strategies for patients undergoing VA-ECMO support. Modifications of the basic VA-ECMO circuit and novel devices for LV unloading have added to the complexity of patient management. There is expert consensus that venting should be considered, if aortic flow is non-pulsatile, progressive signs of LA or LV distension, elevated PAP or PCWP, radiographical signs of pulmonary congestion, persistent closure of the aortic valve, or intracavitary blood stasis are detected. Individual patient characteristics, including previous cardiac surgery, vascular preconditions, bleeding risk and presence of LV-thrombus and overall prognosis, should guide the Heart Team’s decision making which decompression method may be the most promising. The classification system of venting techniques, offered in the present review, may be utilized in clinical practice and as a framework for future research. The Heart Team in dedicated high-volume ECMO centers should aim at conducting pioneering RCTs comparing VA-ECMO support with vs. without venting strategies with the currently best investigated strategies active LV-unloading or IABP, to close this massive gap of evidence. Then, remaining questions will still be the timing of venting (immediately or by advent of a distinct condition) and if venting is necessary or beneficial in all patients undergoing VA-ECMO treatment.
Availability of supporting data
Not applicable.
References
Bréchot N, Hajage D, Kimmoun A et al (2020) Venoarterial extracorporeal membrane oxygenation to rescue sepsis-induced cardiogenic shock: a retrospective, multicentre, international cohort study. Lancet 396:545–552. https://doi.org/10.1016/S0140-6736(20)30733-9
Yannopoulos D, Bartos J, Raveendran G et al (2020) Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet 396:1807–1816. https://doi.org/10.1016/S0140-6736(20)32338-2
Napp LC, Sanchez Martinez C, Akin M et al (2020) Use of extracorporeal membrane oxygenation for eCPR in the emergency room in patients with refractory out-of-hospital cardiac arrest. PLoS ONE 15:e0239777. https://doi.org/10.1371/journal.pone.0239777
Banning AS, Adriaenssens T, Berry C et al (2021) Veno-arterial extracorporeal membrane oxygenation (ECMO) in patients with cardiogenic shock: rationale and design of the randomised, multicentre, open-label EURO SHOCK trial. EuroIntervention 16:e1227–e1236. https://doi.org/10.4244/EIJ-D-20-01076
Thiele H, Freund A, Gimenez MR et al (2021) Extracorporeal life support in patients with acute myocardial infarction complicated by cardiogenic shock—design and rationale of the ECLS-SHOCK trial. Am Heart J 234:1–11. https://doi.org/10.1016/j.ahj.2021.01.002
Lüsebrink E, Orban M, Kupka D et al (2020) Prevention and treatment of pulmonary congestion in patients undergoing venoarterial extracorporeal membrane oxygenation for cardiogenic shock. Eur Heart J 41:3753–3761. https://doi.org/10.1093/eurheartj/ehaa547
Burkhoff D, Sayer G, Doshi D et al (2015) Hemodynamics of mechanical circulatory support. J Am Coll Cardiol 66:2663–2674. https://doi.org/10.1016/j.jacc.2015.10.017
Keebler ME, Haddad EV, Choi CW et al (2018) Venoarterial extracorporeal membrane oxygenation in cardiogenic shock. JACC Heart Fail 6:503–516. https://doi.org/10.1016/j.jchf.2017.11.017
Ostadal P, Mlcek M, Kruger A et al (2015) Increasing venoarterial extracorporeal membrane oxygenation flow negatively affects left ventricular performance in a porcine model of cardiogenic shock. J Transl Med 13:266. https://doi.org/10.1186/s12967-015-0634-6
Meani P, Delnoij T, Raffa GM et al (2019) Protracted aortic valve closure during peripheral veno-arterial extracorporeal life support: is intra-aortic balloon pump an effective solution? Perfusion 34:35–41. https://doi.org/10.1177/0267659118787426
Flierl U, Tongers J, Berliner D et al (2017) Acquired von Willebrand syndrome in cardiogenic shock patients on mechanical circulatory microaxial pump support. PLoS ONE 12:e0183193. https://doi.org/10.1371/journal.pone.0183193
Lüsebrink E, Massberg S, Orban M (2021) The multiple options of left atrial and ventricular venting during veno-arterial extra-corporeal membrane oxygenation: practical considerations. Eur Heart J. https://doi.org/10.1093/eurheartj/ehaa1073
Napp LC, Vogel-Claussen J, Schäfer A et al (2017) First-in-man fully percutaneous complete bypass of heart and lung. JACC Cardiovasc Interv 10:e231–e233. https://doi.org/10.1016/j.jcin.2017.07.047
Lorusso R, Raffa GM, Heuts S et al (2020) Pulmonary artery cannulation to enhance extracorporeal membrane oxygenation management in acute cardiac failure. Interact Cardiovasc Thorac Surg 30:215–222. https://doi.org/10.1093/icvts/ivz245
Loforte A, Baiocchi M, Dal Checco E et al (2020) Percutaneous pulmonary artery venting via jugular vein while on peripheral extracorporeal life support. ASAIO J 66:e50–e54. https://doi.org/10.1097/MAT.0000000000000991
de Pommereau A, Radu C, Boukantar M et al (2021) Left ventricle unloading through pulmonary artery in patients with venoarterial extracorporeal membrane oxygenation. ASAIO J 67:e49–e51. https://doi.org/10.1097/MAT.0000000000001179
Avalli L, Loforte Maggioni E, Sangalli F et al (2011) Percutaneous left-heart decompression during extracorporeal membrane oxygenation: an alternative to surgical and transeptal venting in adult patients. ASAIO J 57:38–40. https://doi.org/10.1097/MAT.0b013e3181fe5d0b
von Segesser LK, Kwang K, Tozzi P et al (2008) A simple way to decompress the left ventricle during venoarterial bypass. Thorac Cardiovasc Surg 56:337–341. https://doi.org/10.1055/s-2008-1038664
Fouilloux V, Lebrun L, Macé L et al (2011) Extracorporeal membranous oxygenation and left atrial decompression: a fast and minimally invasive approach. Ann Thorac Surg 91:1996–1997. https://doi.org/10.1016/j.athoracsur.2011.01.005
Kimura M, Kinoshita O, Fujimoto Y et al (2014) Central extracorporeal membrane oxygenation requiring pulmonary arterial venting after near-drowning. Am J Emerg Med 32(197):e1-2. https://doi.org/10.1016/j.ajem.2013.09.031
Kotani Y, Chetan D, Rodrigues W et al (2013) Left atrial decompression during venoarterial extracorporeal membrane oxygenation for left ventricular failure in children: current strategy and clinical outcomes. Artif Organs 37:29–36. https://doi.org/10.1111/j.1525-1594.2012.01534.x
Orozco-Hernandez EJ, Ahmed MI, Von Meering G et al (2020) Femoral venoarterial extracorporeal membrane oxygenation using a novel biatrial cannula for venous drainage and left ventricular venting. J Card Surg 35:3631–3633. https://doi.org/10.1111/jocs.15087
Na SJ, Yang JH, Yang JH et al (2019) Left heart decompression at venoarterial extracorporeal membrane oxygenation initiation in cardiogenic shock: prophylactic versus therapeutic strategy. J Thorac Dis 11:3746–3756. https://doi.org/10.21037/jtd.2019.09.35
Jumean M, Pham DT, Kapur NK (2015) Percutaneous bi-atrial extracorporeal membrane oxygenation for acute circulatory support in advanced heart failure. Catheter Cardiovasc Interv 85:1097–1099. https://doi.org/10.1002/ccd.25791
Kim AR, Park H, Lee SE et al (2021) Outcomes of left ventricular unloading with a transseptal cannula during extracorporeal membrane oxygenation in adults. Artif Organs 45:390–398. https://doi.org/10.1111/aor.13838
Alghanem F, Balasubramanian S, Zampi JD (2019) Impact of left atrial decompression on patient outcomes during pediatric venoarterial extracorporeal membrane oxygenation: a case-control study. Pediatr Cardiol 40:1266–1274. https://doi.org/10.1007/s00246-019-02147-7
Ok YJ, Jung SH, Lee SW et al (2019) Efficacy of left heart decompression during extracorporeal membrane oxygenation: a case-control study. J Thorac Dis 11:865–872. https://doi.org/10.21037/jtd.2019.01.110
Kim S, Kim JS, Shin JS et al (2019) How small is enough for the left heart decompression cannula during extracorporeal membrane oxygenation? Acute Crit Care 34:263–268. https://doi.org/10.4266/acc.2019.00577
Alhussein M, Osten M, Horlick E et al (2017) Percutaneous left atrial decompression in adults with refractory cardiogenic shock supported with veno-arterial extracorporeal membrane oxygenation. J Card Surg 32:396–401. https://doi.org/10.1111/jocs.13146
Aiyagari RM, Rocchini AP, Remenapp RT et al (2006) Decompression of the left atrium during extracorporeal membrane oxygenation using a transseptal cannula incorporated into the circuit. Crit Care Med 34:2603–2606. https://doi.org/10.1097/01.CCM
Eastaugh LJ, Thiagarajan RR, Darst JR et al (2015) Percutaneous left atrial decompression in patients supported with extracorporeal membrane oxygenation for cardiac disease. Pediatr Crit Care Med 16:59–65. https://doi.org/10.1097/PCC.0000000000000276
Hlavacek AM, Atz AM, Bradley SM et al (2005) Left atrial decompression by percutaneous cannula placement while on extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg 130:595–596. https://doi.org/10.1016/j.jtcvs.2004.12.029
Swartz MF, Smith F, Byrum CJ et al (2012) Transseptal catheter decompression of the left ventricle during extracorporeal membrane oxygenation. Pediatr Cardiol 33:185–187. https://doi.org/10.1007/s00246-011-0113-7
Lee SI, Lee SY, Choi CH et al (2017) Left heart decompression in acute complicated myocardial infarction during extracorporeal membrane oxygenation. J Intensive Care Med 32:405–408. https://doi.org/10.1177/0885066617696851
Kim WH, Hong TH, Byun JH et al (2017) Flow rate through pigtail catheter used for left heart decompression in an artificial model of extracorporeal membrane oxygenation circuit. ASAIO J 63:346–350. https://doi.org/10.1097/MAT.0000000000000472
Zampi JD, Alghanem F, Yu S et al (2019) Relationship between time to left atrial decompression and outcomes in patients receiving venoarterial extracorporeal membrane oxygenation support: a multicenter pediatric interventional cardiology early-career society study. Pediatr Crit Care Med 20:728–736. https://doi.org/10.1097/PCC.0000000000001936
Hacking DF, Best D, d’Udekem Y et al (2015) Elective decompression of the left ventricle in pediatric patients may reduce the duration of venoarterial extracorporeal membrane oxygenation. Artif Organs 39:319–326. https://doi.org/10.1111/aor.12390
Hong TH, Byun JH, Lee HM et al (2016) Initial experience of transaortic catheter venting in patients with venoarterial extracorporeal membrane oxygenation for cardiogenic shock. ASAIO J 62:117–122. https://doi.org/10.1097/MAT.0000000000000327
Schmack B, Seppelt P, Weymann A et al (2017) Extracorporeal life support with left ventricular decompression-improved survival in severe cardiogenic shock: results from a retrospective study. PeerJ 5:e3813. https://doi.org/10.7717/peerj.3813
Meani P, Gelsomino S, Natour E et al (2017) Modalities and effects of left ventricle unloading on extracorporeal life support: a review of the current literature. Eur J Heart Fail 19(Suppl 2):84–91. https://doi.org/10.1002/ejhf.850
Takeda K, Garan AR, Ando M et al (2017) Minimally invasive CentriMag ventricular assist device support integrated with extracorporeal membrane oxygenation in cardiogenic shock patients: a comparison with conventional CentriMag biventricular support configuration. Eur J Cardiothorac Surg 52:1055–1061. https://doi.org/10.1093/ejcts/ezx189
Keenan JE, Schechter MA, Bonadonna DK et al (2016) Early experience with a novel cannulation strategy for left ventricular decompression during nonpostcardiotomy venoarterial ECMO. ASAIO J 62:e30–e34. https://doi.org/10.1097/MAT.0000000000000333
Eudailey KW, Yi SY, Mongero LB et al (2015) Trans-diaphragmatic left ventricular venting during peripheral venous-arterial extracorporeal membrane oxygenation. Perfusion 30:701–703. https://doi.org/10.1177/0267659115592468
Bloom JE, Pellegrino V, McGiffin DC et al (2019) Low-flow left ventricle percutaneous venting during peripheral veno-arterial extracorporeal membrane oxygenation for the management of transient left ventricle distension. J Cardio Case Rep. https://doi.org/10.15761/JCCR.1000114
Fumagalli R, Bombino M, Borelli M et al (2004) Percutaneous bridge to heart transplantation by venoarterial ECMO and transaortic left ventricular venting. Int J Artif Organs 27:410–413. https://doi.org/10.1177/039139880402700510
Cheung MM, Goldman AP, Shekerdemian LS et al (2003) Percutaneous left ventricular “vent” insertion for left heart decompression during extracorporeal membrane oxygenation. Pediatr Crit Care Med 4:447–449. https://doi.org/10.1097/01.PCC.0000075325.53339.CA
Boll G, Fischer A, Kapur NK et al (2019) Right axillary artery conduit is a safe and reliable access for implantation of Impella 5.0 microaxial pump. Ann Vasc Surg 54:54–59. https://doi.org/10.1016/j.avsg.2018.10.004
Bastos MB, van Wiechen MP, Van Mieghem NM (2020) PulseCath iVAC2L: next-generation pulsatile mechanical circulatory support. Future Cardiol 16:103–112. https://doi.org/10.2217/fca-2019-0060
Tschöpe C, Alogna A, Faragli A et al (2020) Case report first-in-man method description: left ventricular unloading with iVAC2L during veno-arterial extracorporeal membrane oxygenation: from veno-arterial extracorporeal membrane oxygenation to ECMELLA to EC-iVAC®. Front Cardiovasc Med 7:563448. https://doi.org/10.3389/fcvm.2020.563448
Travis AR, Giridharan GA, Pantalos GM et al (2007) Vascular pulsatility in patients with a pulsatile- or continuous-flow ventricular assist device. J Thorac Cardiovasc Surg 133:517–524. https://doi.org/10.1016/j.jtcvs.2006.09.057
Barbone A, Malvindi PG, Ferrara P et al (2011) Left ventricle unloading by percutaneous pigtail during extracorporeal membrane oxygenation. Interact Cardiovasc Thorac Surg 13:293–295. https://doi.org/10.1510/icvts.2011.269795
Schrage B, Becher PM, Bernhardt A et al (2020) Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international multicenter cohort study. Circulation 142:2095–2106. https://doi.org/10.1161/CIRCULATIONAHA.120.048792
Pappalardo F, Schulte C, Pieri M et al (2017) Concomitant implantation of Impella® on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail 19:404–412. https://doi.org/10.1002/ejhf.668
Akanni OJ, Takeda K, Truby LK et al (2019) EC-VAD: combined use of extracorporeal membrane oxygenation and percutaneous microaxial pump left ventricular assist device. ASAIO J 65:219–226. https://doi.org/10.1097/MAT.0000000000000804
Schrage B, Burkhoff D, Rübsamen N et al (2018) Unloading of the left ventricle during venoarterial extracorporeal membrane oxygenation therapy in cardiogenic shock. JACC Heart Fail 6:1035–1043. https://doi.org/10.1016/j.jchf.2018.09.009
Tongers J, Sieweke JT, Kühn C et al (2020) Early escalation of mechanical circulatory support stabilizes and potentially rescues patients in refractory cardiogenic shock. Circ Heart Fail 13:e005853. https://doi.org/10.1161/CIRCHEARTFAILURE.118.005853
Eliet J, Gaudard P, Zeroual N et al (2018) Effect of Impella during veno-arterial extracorporeal membrane oxygenation on pulmonary artery flow as assessed by end-tidal carbon dioxide. ASAIO J 64:502–507. https://doi.org/10.1097/MAT.0000000000000662
Colombier S, Quessard A, Mastroianni C et al (2019) Benefits of impella and peripheral veno-arterial extra corporeal life support alliance. ASAIO J 65:837–844. https://doi.org/10.1097/MAT.0000000000000922
Guirgis M, Kumar K, Menkis AH, Freed DH (2010) Minimally invasive left-heart decompression during venoarterial extracorporeal membrane oxygenation: an alternative to a percutaneous approach. Interact Cardiovasc Thorac Surg 10:672–674. https://doi.org/10.1510/icvts.2009.228346
Russo JJ, Aleksova N, Pitcher I et al (2019) Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol 73:654–662. https://doi.org/10.1016/j.jacc.2018.10.085
Al-Fares AA, Randhawa VK, Englesakis M et al (2019) Optimal strategy and timing of left ventricular venting during veno-arterial extracorporeal life support for adults in cardiogenic shock: a systematic review and meta-analysis. Circ Heart Fail 12:e006486. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006486
Lüsebrink E, Massberg S, Orban M (2021) The lack of evidence-based therapeutic strategies for left ventricular unloading during venoarterial extracorporeal membrane oxygenation therapy calls for randomized trials. Eur Heart J. https://doi.org/10.1093/eurheartj/ehab120
Weymann A, Schmack B, Sabashnikov A et al (2014) Central extracorporeal life support with left ventricular decompression for the treatment of refractory cardiogenic shock and lung failure. J Cardiothorac Surg 9:60. https://doi.org/10.1186/1749-8090-9-60
Sandrio S, Springer W, Karck M et al (2014) Extracorporeal life support with an integrated left ventricular vent in children with a low cardiac output. Cardiol Young 24:654–660. https://doi.org/10.1017/S1047951113001017
Beyls C, Huette P, Guilbart M et al (2020) An urgent open surgical approach for left ventricle venting during peripheral veno-arterial extracorporeal membrane oxygenation for refractory cardiac arrest: case report. Perfusion 35:82–85. https://doi.org/10.1177/0267659119853949
Sauren LD, Reesink KD, Selder JL et al (2007) The acute effect of intra-aortic balloon counterpulsation during extracorporeal life support: an experimental study. Artif Organs 31:31–38. https://doi.org/10.1111/j.1525-1594.2007.00337.x
Madershahian N, Liakopoulos OJ, Wippermann J et al (2009) The impact of intraaortic balloon counterpulsation on bypass graft flow in patients with peripheral ECMO. J Card Surg 24:265–268. https://doi.org/10.1111/j.1540-8191.2009.00807.x
Aso S, Matsui H, Fushimi K, Yasunaga H (2016) The effect of intraaortic balloon pumping under venoarterial extracorporeal membrane oxygenation on mortality of cardiogenic patients: an analysis using a nationwide inpatient database. Crit Care Med 44:1974–1979. https://doi.org/10.1097/CCM.0000000000001828
Bréchot N, Demondion P, Santi F et al (2018) Intra-aortic balloon pump protects against hydrostatic pulmonary oedema during peripheral venoarterial-extracorporeal membrane oxygenation. Eur Heart J Acute Cardiovasc Care 7:62–69. https://doi.org/10.1177/2048872617711169
Tepper S, Garcia MB, Fischer I et al (2019) Clinical outcomes and reduced pulmonary artery pressure with intra-aortic balloon pump during central extracorporeal life support. ASAIO J 65:173–179. https://doi.org/10.1097/MAT.0000000000000788
Lin LY, Liao CW, Wang CH et al (2016) Effects of additional intra-aortic balloon counter-pulsation therapy to cardiogenic shock patients supported by extra-corporeal membranous oxygenation. Sci Rep 6:23838. https://doi.org/10.1038/srep23838
Gass A, Palaniswamy C, Aronow WS et al (2014) Peripheral venoarterial extracorporeal membrane oxygenation in combination with intra-aortic balloon counterpulsation in patients with cardiovascular compromise. Cardiology 129:137–143. https://doi.org/10.1159/000365138
Petroni T, Harrois A, Amour J et al (2014) Intra-aortic balloon pump effects on macrocirculation and microcirculation in cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation*. Crit Care Med 42:2075–2082. https://doi.org/10.1097/CCM.0000000000000410
Yang F, Jia ZS, Xing JL et al (2014) Effects of intra-aortic balloon pump on cerebral blood flow during peripheral venoarterial extracorporeal membrane oxygenation support. J Transl Med 12:106. https://doi.org/10.1186/1479-5876-12-106
Bonacchi M, Cabrucci F, Bugetti M et al (2020) Outcomes’ predictors in post-cardiac surgery extracorporeal life support. An observational prospective cohort study. Int J Surg 82:56–63. https://doi.org/10.1016/j.ijsu.2020.07.063
Thiele H, Zeymer U, Neumann FJ et al (2012) Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 367:1287–1296. https://doi.org/10.1056/NEJMoa1208410
Samadi B, Nguyen D, Rudham S et al (2016) Spinal cord infarct during concomitant circulatory support with intra-aortic balloon pump and veno-arterial extracorporeal membrane oxygenation. Crit Care Med 44:e101–e105. https://doi.org/10.1097/CCM.0000000000001400
Baruteau AE, Barnetche T, Morin L et al (2018) Percutaneous balloon atrial septostomy on top of venoarterial extracorporeal membrane oxygenation results in safe and effective left heart decompression. Eur Heart J Acute Cardiovasc Care 7:70–79. https://doi.org/10.1177/2048872616675485
Lin YN, Chen YH, Wang HJ et al (2017) Atrial septostomy for left atrial decompression during extracorporeal membrane oxygenation by inoue balloon catheter. Circ J 81:1419–1423. https://doi.org/10.1253/circj.CJ-16-1308
O’Byrne ML, Glatz AC, Rossano JW et al (2015) Middle-term results of trans-catheter creation of atrial communication in patients receiving mechanical circulatory support. Catheter Cardiovasc Interv 85:1189–1195. https://doi.org/10.1002/ccd.25824
Haynes S, Kerber RE, Johnson FL et al (2009) Left heart decompression by atrial stenting during extracorporeal membrane oxygenation. Int J Artif Organs 32:240–242. https://doi.org/10.1177/039139880903200408
Prasad A, Ghodsizad A, Brehm C et al (2018) Refractory pulmonary edema and upper body hypoxemia during veno-arterial extracorporeal membrane oxygenation—a case for atrial septostomy. Artif Organs 42:664–669. https://doi.org/10.1111/aor.13082
Deshpande SR, Kennedy KF, Vincent RN et al (2021) Atrial septostomy in patients supported with venoarterial extracorporeal membrane oxygenation: analysis of the IMPACT registry data. Int J Artif Organs 44:262–268. https://doi.org/10.1177/0391398820953860
Veeram Reddy SR, Guleserian KJ, Nugent AW (2015) Transcatheter removal of atrial septal stent placed to decompress left atrium with VA ECMO. Catheter Cardiovasc Interv 85:1021–1025. https://doi.org/10.1002/ccd.25817
Hasde Aİ, Sarıcaoğlu MC, Dikmen Yaman N et al (2021) Comparison of left ventricular unloading strategies on venoarterial extracorporeal life support. Interact Cardiovasc Thorac Surg 32:467–475. https://doi.org/10.1093/icvts/ivaa284
Piechura LM, Coppolino A, Mody GN et al (2020) Left ventricle unloading strategies in ECMO: a single-center experience. J Card Surg 35:1514–1524. https://doi.org/10.1111/jocs.14644
Tepper S, Masood MF, Baltazar Garcia M et al (2017) Left ventricular unloading by Impella device versus surgical vent during extracorporeal life support. Ann Thorac Surg 104:861–867. https://doi.org/10.1016/j.athoracsur.2016.12.049
Loforte A, Baiocchi M, Gliozzi G et al (2019) Percutaneous pulmonary artery venting via jugular vein while on peripheral extracorporeal membrane oxygenation running: a less invasive approach to provide full biventricular unloading. Ann Cardiothorac Surg 8:163–166. https://doi.org/10.21037/acs.2018.08.06
Fiedler AG, Dalia A, Axtell AL et al (2018) Impella placement guided by echocardiography can be used as a strategy to unload the left ventricle during peripheral venoarterial extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth 32:2585–2591. https://doi.org/10.1053/j.jvca.2018.05.019
Patel SM, Lipinski J, Al-Kindi SG et al (2019) Simultaneous venoarterial extracorporeal membrane oxygenation and percutaneous left ventricular decompression therapy with Impella is associated with improved outcomes in refractory cardiogenic shock. ASAIO J 65:21–28. https://doi.org/10.1097/MAT.0000000000000767
Donker DW, Brodie D, Henriques JPS et al (2019) Left ventricular unloading during veno-arterial ECMO: a review of percutaneous and surgical unloading interventions. Perfusion 34:98–105. https://doi.org/10.1177/0267659118794112
Curran J, Burkhoff D, Kloner RA (2019) Beyond reperfusion: acute ventricular unloading and cardioprotection during myocardial infarction. J Cardiovasc Transl Res 12:95–106. https://doi.org/10.1007/s12265-019-9863-z
Schäfer A, Burkhoff D, Bauersachs J (2019) Haemodynamic simulation and the effect of early left ventricular unloading in pre-shock acute coronary syndrome. ESC Heart Fail 6:457–463. https://doi.org/10.1002/ehf2.12417
Funding
Open Access funding enabled and organized by Projekt DEAL. There was no funding for this study.
Author information
Authors and Affiliations
Contributions
EL and MO designed the study, interpreted data and wrote the manuscript. LB, AK, CM, CS, BS, DJ, TP, DB, SB, SP, JH, SZ, FB, DW, HT, AS, CH and SM interpreted data and critically revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Martin Orban has received speaker honoraria from Abbott Medical, AstraZeneca, Abiomed, Bayer vital, Biotronik, Bristol-Myers Squibb, CytoSorbents, Daiichi Sankyo Deutschland, Edwards Lifesciences Services, Sedana Medical, outside the submitted work. The other authors declare no conflict of interests. Andreas Schäfer has received lecture fees and research support from Abiomed.
Ethical standards
All ethical standards were met in writing and submitting this correspondence.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lüsebrink, E., Binzenhöfer, L., Kellnar, A. et al. Venting during venoarterial extracorporeal membrane oxygenation. Clin Res Cardiol 112, 464–505 (2023). https://doi.org/10.1007/s00392-022-02069-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-022-02069-0