Abstract
Hyperoxia is common practice in the acute management of circulatory shock, and observational studies report that it is present in more than 50 % of mechanically ventilated patients during the first 24 h after intensive care unit (ICU) admission. On the other hand, “oxygen toxicity” due to the increased formation of reactive oxygen species limits its use due to serious deleterious side effects. However, formation of reactive oxygen species to boost bacterial killing is one of the body’s anti-microbial auto-defense mechanisms and, hence, O2 has been referred to as an antibiotic. Consequently, hyperoxia during the peri-operative period has been advocated for surgical patients in order to reduce surgical site infection. However, there is ample evidence that long-term exposure to hyperoxia impaired bacterial phagocytosis and thereby aggravated both bacterial burden and dissemination. Moreover, a recent retrospective study identified the number of days with hyperoxia, defined as a PaO2 > 120 mmHg only, as an independent risk factor of ventilator-associated pneumonia in patients needing mechanical ventilation for more than 48 h. Since so far the optimal oxygenation target is unknown for ICU patients, “conservative” O2 therapy represents the treatment of choice to avoid exposure to both hypoxemia and excess hyperoxemia.
Similar content being viewed by others
Background
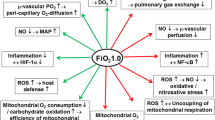
Despite recent evidence that “conservative” oxygenation targets are safe [1, 2], hyperoxia is frequently present in the intensive care unit (ICU) [3, 4]. O2 has friend-and-foe properties because it is vital for ATP production and toxic due to its oxidant effects [5]. On the one hand, “…administration of oxygen should be started immediately to increase O2 delivery…” during the management of circulatory shock in order to counteract the pathognomonic “…imbalance between O2 supply and requirements…” [6]. On the other hand, oxygen toxicity arises from the formation of reactive oxygen species (ROS), in particular during hypoxia/re-oxygenation, e.g., during shock resuscitation. ROS themselves share the janus-headed character of O2: they are vital signaling molecules and their increased release to boost bacterial killing is one of the body’s anti-microbial auto-defense mechanisms [7]. ROS formation is directly related to the PO2 [5] and, hence, O2 was already described as an antibiotic three decades ago [5]. Again, there is contrasting experimental evidence: long-term (48–96 h) exposure to PaO2 values >350–400 mmHg aggravated the pulmonary bacterial burden and the bacteria dissemination [8] due to impaired bacterial phagocytosis and endotoxin-induced cytokine release. Interestingly, ROS scavengers dose-dependently restored the bactericidal capacity.
Hyperoxia during the peri-operative period was reported to reduce post-operative wound infection. Pure O2 ventilation attenuated the anesthesia- and surgery-induced impairment of the phagocytic and microbicidal capacity of alveolar macrophages in healthy patients undergoing prolonged (>6 up to 10 h) elective surgery [9]. The most recent meta-analysis (>8000 patients in 17 randomized control trials (RCTs)) concluded that hyperoxia significantly decreases the risk of surgical site infection during colorectal surgery [10]. Nevertheless, this approach remains highly controversial due to the “moderate evidence” [10] and the deleterious long-term complications reported in patients with cancer [11] and/or cardiovascular disease [12]. Moreover, the question remains unanswered whether hyperoxia affects host defense in mechanically ventilated ICU patients in general: using a multivariate analysis of retrospective data on patients needing mechanical ventilation for >48 h, Six et al. recently identified the number of days with hyperoxia as an independent risk factor of ventilator-associated pneumonia (VAP) [13].
Hyperoxia, lung function, and O2 transport
Hyperoxia impairs pulmonary gas exchange due to inhibition of hypoxic pulmonary vasoconstriction and adsorption atelectasis [5]. Moreover, under healthy conditions blood O2 content only modestly increases upon switching from air to pure O2 breathing due to the near-complete arterial hemoglobin O2 saturation (SaO2) at PaO2 = 90–100 mmHg [5]. Hence, the lower the hemoglobin content, the more pronounced the effect of hyperoxia on blood O2 content, such that hyperoxia may be helpful during hemorrhage [5]. However, any hyperoxia-related rise in blood O2 content may at least in part be counterbalanced by a hyperoxia-induced fall in cardiac output resulting from decreased heart rate and increased systemic vascular resistance, the latter being particularly pronounced in the cerebral and coronary vasculature [5].
The acute hyperoxia-induced impairment of gas exchange must be discriminated from pulmonary O2 toxicity, which presents as pulmonary inflammation and, ultimately, hemorrhagic pulmonary edema [5]. This effect is well-established after long-term hyperoxic exposures and/or when combined with injurious ventilation. The only data in mechanically ventilated patients originate from hyperoxia over 14 h to 30 days and, given the publication year (1972), lung-protective ventilation most likely was not used [5].
Hyperoxia and secondary pulmonary infection
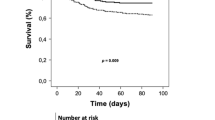
The study by Six et al. [13] clearly suggests an association between hyperoxia and VAP. Of note, hyperoxia was defined as a PaO2 > 120 mmHg, in other words, a PaO2 that is commonly observed in ICU patients [3, 4]. Moreover, the authors showed not only that the numbers of days with hyperoxia, but also “hyperoxemia at ICU admission”, in other words, deliberate, iatrogenic pre-ICU hyperoxia, was an independent risk factor of VAP. Clearly, patients with VAP were older, sicker, and, in particular, more frequently in shock prior to ICU admission. First, shock, as the dysbalance between tissue O2 supply and demand leads to tissue hypoxia, which in turn triggers hyper-inflammation that is aimed to clear pathogens [14]. However, when “too pronounced and/or sustained”, tissue hypoxia may cause anti-inflammation, thereby rendering patients more susceptible to secondary infection [14]. Second, shock is defined as arterial hypotension and/or the need for vasopressor use. Hence, in the study by Six et al., patients with VAP most likely received catecholamines more frequently, for a longer period, and/or at higher doses. Exogenous catecholamines can impair both innate and adaptive immunity and thereby increase bacterial growth and virulence. Despite these potential limits and confounding factors, the study by Six et al. raises an important question with respect to the use of hyperoxia, i.e., whether there is an optimal PaO2 target in the ICU. So far, only retrospective data are available yielding a U-shaped relationship between mortality and arterial PO2, with a nadir at PaO2 values of 110–150 mmHg [3] and 150–200 mmHg [15]. Mortality sharply increased at PaO2 < 65 mmHg and >225 mmHg [3]. However, another study found that even PaO2 > 300 mmHg did not affect outcome, while confirming the harmful impact of PaO2 < 65 mmHg [4]. Consequently, despite a strong signal suggesting an association between hyperoxia and poor hospital outcome, the most recent meta-analyses on hyperoxia [16, 17] are inconclusive due to the high data heterogeneity.
Conclusions
Hyperoxia is common practice during shock management based on experimental evidence that correcting oxygen debt is crucial for survival. On the other hand, oxygen toxicity limits its use, especially during hypoxia/re-oxygenation and/or long-term administration (i.e., >12–24 h). The data of the RCT “Optimal Oxygenation in the Intensive Care Unit (O2-ICU)” (NCT02321072) comparing PaO2 targets of 120 versus 75 mmHg in ICU patients and the detailed results of the preliminary terminated 2 × 2-factorial RCT “Hyperoxia and Hypertonic Saline in Septic Shock (Hyper2S)” (NCT01722422), simultaneously comparing target SaO2 88–95 % versus pure O2 ventilation during the first 24 h and isotonic versus hypertonic saline, are therefore eagerly awaited. The results may allow finding criteria for “…a personalized O2 target…in critically ill patients” [14]. Until then, conservative O2 therapy [1, 2] should be the treatment of choice to avoid both hypoxemia and excess hyperoxia.
Abbreviations
ICU, intensive care unit; RCT, randomized controlled trial; ROS, reactive oxygen species; SaO2, arterial hemoglobin oxygen saturation; VAP, ventilator-associated pneumonia
References
Panwar R, Hardie M, Bellomo R, Barrot L, Eastwood GM, Young PJ, Capellier G, Harrigan PW, Bailey M, CLOSE Study Investigators, ANZICS Clinical Trials Group. Conservative versus liberal oxygenation targets for mechanically ventilated patients. A pilot multicenter randomized controlled trial. Am J Respir Crit Care Med. 2016;193:43–51.
Helmerhorst HJF, Schultz MJ, van der Voort PHJ, Bosman RJ, Juffermans NP, de Wilde RBP, van den Akker-van MArle ME, van Bodegom-Vos L, de Vries M, Eslami S, de Keizer NF, Abu-Hanna A, van Westerloo DJ, de Jonge E. Effectiveness and clinical outcome of a two-step implementation of conservative oxygenation targets in critically ill patients: a before and after trial. Crit Care Med. 2016;44:554–63.
de Jonge E, Peelen L, Keijzers PJ, Joore H, de Lange D, van der Voort PH, Bosman RJ, de Waal RAL, Wesselink R, de Keizer NF. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care. 2008;12:R156.
Eastwood G, Bellomo R, Bailey M, Taori G, Pilcher D, Young P, Beasley R. Arterial oxygen tension and mortality in mechanically ventilated patients. Intensive Care Med. 2012;38:91–8.
Hafner S, Beloncle F, Koch A, Radermacher P, Asfar P. Hyperoxia in intensive care, emergency, and peri-operative medicine: Dr. Jekyll or Mr. Hyde? A 2015 update. Ann Intensive Care. 2015;5:42.
Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369:1726–34.
Magder S. Reactive oxygen species: toxic molecules or spark of life? Crit Care. 2006;10:208.
Patel VS, Sampat V, Espey MG, Sitapara R, Wang H, Yang X, Ashby Jr CR, Thomas DD, Mantell LL. Ascorbic acid attenuates hyperoxia-compromised host defense against pulmonary bacterial infection. Am J Respir Cell Mol Biol. 2016. doi:10.1165/rcmb.2015-0310OC [Epub ahead of print].
Kotani N, Hashimoto H, Sessler DI, Muraoka M, Hashiba E, Kubota T, Matsuki A. Supplemental intraoperative oxygen augments antimicrobial and proinflammatory responses of alveolar macrophages. Anesthesiology. 2000;93:15–25.
Yang W, Liu Y, Zhang Y, Zhao QH, He SF. Effect of intra-operative high inspired oxygen fraction on surgical site infection: a meta-analysis of randomized controlled trials. J Hosp Infect. 2016. doi:10.1016/j.jhin.2016.03.015 [Epub ahead of print].
Meyhoff CS, Jorgensen LN, Wetterslev J, Siersma VD, Rasmussen LS, PROXI Trial Group. Risk of new or recurrent cancer after a high perioperative inspiratory oxygen fraction during abdominal surgery. Br J Anaesth. 2014;113 Suppl 1:i74–81.
Fonnes S, Gögenur I, Søndergaard ES, Siersma VD, Jorgensen LN, Wetterslev J, Meyhoff CS. Perioperative hyperoxia--long-term impact on cardiovascular complications after abdominal surgery, a post hoc analysis of the PROXI trial. Int J Cardiol. 2016;215:238–43.
Six S, Jaffal K, Ledoux G, Jaillette E, Wallet F, Nseir S. Hyperoxemia as a risk for ventilator-associated pneumonia. Crit Care. 2016;20:195.
Kiers HD, Scheffer GJ, van der Hoeven JG, Eltzschig HK, Pickkers P, Kox M. Immunologic consequences of hypoxia during critical iIllness. Anesthesiology. 2016. doi:10.1097/ALN.0000000000001163 [Epub ahead of print].
Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, Abu-Hanna A, de Keizer NF, de Jonge E. Associations of arterial carbon dioxide and arterial oxygen concentrations with hospital mortality after resuscitation from cardiac arrest. Crit Care. 2015;19:348.
Damiani E, Adrario E, Girardis M, Romano R, Pelaia P, Singer M. Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit Care. 2014;18:711.
Helmerhorst HJF, Roos-Blom MJ, van Westerloo DJ, de Jonge E. Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, metaanalysis, and meta-regression of cohort studies. Crit Care Med. 2015;43:1508–19.
Funding
PR was supported by the Deutsche Forschungsgemeinschaft (CRC1149, Project B03) and the German MoD (Vertragsforschungsvorhaben E/U2AD/CF523/DF556).
Authors’ contributions
All authors helped to draft the manuscript and read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
See related research by Six et al. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4917974/
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Nußbaum, B., Radermacher, P., Asfar, P. et al. Does hyperoxia enhance susceptibility to secondary pulmonary infection in the ICU?. Crit Care 20, 239 (2016). https://doi.org/10.1186/s13054-016-1427-x
Published:
DOI: https://doi.org/10.1186/s13054-016-1427-x