Abstract
Introduction
Therapeutic hypothermia (TH) is often used to treat out-of-hospital cardiac arrest (OHCA) patients who also often simultaneously receive insulin for stress-induced hyperglycaemia. However, the impact of TH on systemic metabolism and insulin resistance in critical illness is unknown. This study analyses the impact of TH on metabolism, including the evolution of insulin sensitivity (SI) and its variability, in patients with coma after OHCA.
Methods
This study uses a clinically validated, model-based measure of SI. Insulin sensitivity was identified hourly using retrospective data from 200 post-cardiac arrest patients (8,522 hours) treated with TH, shortly after admission to the intensive care unit (ICU). Blood glucose and body temperature readings were taken every one to two hours. Data were divided into three periods: 1) cool (T <35°C); 2) an idle period of two hours as normothermia was re-established; and 3) warm (T >37°C). A maximum of 24 hours each for the cool and warm periods was considered. The impact of each condition on SI is analysed per cohort and per patient for both level and hour-to-hour variability, between periods and in six-hour blocks.
Results
Cohort and per-patient median SI levels increase consistently by 35% to 70% and 26% to 59% (P <0.001) respectively from cool to warm. Conversely, cohort and per-patient SI variability decreased by 11.1% to 33.6% (P <0.001) for the first 12 hours of treatment. However, SI variability increases between the 18th and 30th hours over the cool to warm transition, before continuing to decrease afterward.
Conclusions
OCHA patients treated with TH have significantly lower and more variable SI during the cool period, compared to the later warm period. As treatment continues, SI level rises, and variability decreases consistently except for a large, significant increase during the cool to warm transition. These results demonstrate increased resistance to insulin during mild induced hypothermia. Our study might have important implications for glycaemic control during targeted temperature management.
Similar content being viewed by others
Introduction
Hyperglycaemia is prevalent in critical care [1]-[4] and increases the risks of further complications and mortality [1],[4],[5]. Glycaemic control has shown benefits in reducing mortality and morbidity [4],[6],[7]. However, many studies have found it difficult to reproduce these results [8]-[10] due in part to metabolic variability [11]. Out-of-hospital cardiac arrest (OHCA) patients often experience hyperglycaemia [12],[13]. These patients belong to one group who can be highly insulin resistant and variable, particularly on the first two days of stay [14], as well as those who may particularly benefit from glycaemic control [4].
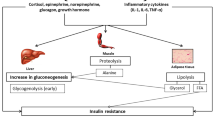
Therapeutic hypothermia (TH) is often used with OHCA patients to protect against brain injury [15],[16], which leads to a lowering of metabolic rate, reduces plasma insulin, induces insulin resistance and alters blood glucose homeostasis [17]. One of the adverse events associated with hypothermic therapy is a decrease in insulin sensitivity and endogenous insulin secretion [18]. However, this decrease may not be observable in a cohort who is already highly insulin resistant and variable [14]. Hence, understanding metabolic evolution and variability would enable safer and more accurate glycaemic control using insulin in this cohort. This study analyses the evolution of a clinically validated model-based measure of insulin sensitivity (SI) in OHCA patients to assess the impact of hypothermia therapy.
Methods
Patients and data
A retrospective analysis of glycaemic control data from 200 OHCA patients (8,522 hours) treated with TH, shortly after admission to intensive care. Data was obtained from intensive care units (ICUs) at Christchurch Hospital, New Zealand, at Erasme Hospital, Belgium, and CHUV-Lausanne Hospital, Switzerland. Patients from Christchurch Hospital (N = 20) were on the specialized relative insulin and nutrition titration (SPRINT) glycaemic control protocol [7], whereas the remaining 180 patients from Erasme (N = 99) and Lausanne (N = 81) hospitals were on local glycaemic control protocols and included in an institutional database (2008 to 2012).
Blood glucose (BG) and temperature readings were taken one to two hourly. Data were divided into three periods: 1) cool (T <35°C); 2) an idle period of two hours as normothermia was restored; and 3) warm (T >37°C). A maximum of 24 contiguous hours and a minimum of 15 hours for each period were considered, ensuring a balance of contiguous data between periods. Overall demographics are shown in Table 1.
Glycaemic targets while treating OHCA patients in the three units were very similar and overlapped. The SPRINT protocol, used in the Christchurch Hospital ICU, targeted 4.0 to 7.0 mmol/L [7]. The protocol used in both the Erasme and Lausanne ICUs differed from SPRINT, but targeted 6.0 to 8.0 mmol/L [17]. Although two different protocols were used in the three units, the targets were very similar and within the relatively tight 4.0 to 8.0 mmol/L range.
Audit of the clinical data from SPRINT was given by the Upper South B Regional Ethics Committee and for the data study by Taccone et al. [17]. No approval was required as it was also a retrospective audit.
Model-based insulin sensitivity
Model-based SI in this study is a patient-specific parameter describing the overall whole-body effect of insulin. SI is identified for each hour, for each patient using a clinically validated glucose-insulin model [19]-[23]. The key model equations are defined:
Where G(t) represents the concentration of blood glucose (mmol/L). I(t) and Q(t) represent the plasma insulin and insulin interstitial concentrations (mU/L) respectively. Model parameters, rates and constants in this model were as fully defined in [21],[24].
Model-based SI is identified hourly from patient data, producing an hourly piece-wise constant profile [25], capturing the whole-body glycaemic response to exogenous insulin and nutrition. The validity and independence of this patient-specific parameter have been validated using data from independent, clinically matched cohorts [19], in comparison to gold-standard insulin sensitivity tests [22] and in clinical glycaemic control [20],[23].
Analyses and metrics
SI level and variability during the cool (T ≤35°C) and warm (T >35°C) periods are analysed on per-cohort and per-patient bases using six-hour blocks of data as per Table 2. SI level is compared between blocks as a cohort median and by per-patient median SI. Similarly, SI variability is calculated as the hour-to-hour percentage change in SI (Δ%SI) and is analysed per cohort for each block.
The use of percentage change, rather than absolute change, normalises the metric so patients with differing SI levels can be compared fairly.
Bagshaw et al. [26] reported an association between both hypoglycaemia and BG variability with mortality during the first 24 hours of ICU stay. Thus, the acute evolution of SI over the first day using six-hour blocks was analysed as SI variability is a key contributor to BG variability. For the cohort analysis, SI and Δ%SI data from all patients was grouped into each appropriate time block. Median values for each time block were calculated for comparison to the previous block, thus capturing overall cohort changes over time in level and hour-to-hour variability.
For the per-patient analysis, the median value of SI and the interquartile range (IQR) of Δ% SI were calculated for each patient, for each time block. The IQR captures the width or degree of variability for a given patient within each six-hour block. Thus, a reduction in the IQR of Δ% SI over time would indicate a reduction in hour-to-hour variability for a given patient.
SI level and variability are non-Gaussian and thus compared using non-parametric statistics and cumulative distribution functions (CDFs). CDFs are particularly useful as they show the entire distribution that is often summarised as a median and IQR. The CDF for a given value of the independent variable (for example SI = x) describes the probability of observing a value less than or equal to x. All distributed data were compared using a Wilcoxon rank-sum test (Mann–Whitney U test), except for SI variability results. SI variability was compared using the Kolmogorov-Smirnov (KS) test as it has greater power to detect differences in the shape of distributions when median values are similar. In all cases, P <0.05 is considered statistically significant.
Results
SIlevel analyses
Figures 1 and 2 present the CDFs of hourly SI level by cohort and median SI per patient, respectively, using six-hour blocks. Table 3 presents the increase in median insulin sensitivity and corresponding P values between successive time blocks.
The results suggest that SI increases for the cohort and per patient are statistically significant for the first 36 hours (P <0.05) in both cases.
Results in Figure 2, Figure 3 and Table 3 are further reflected in Table 4, which shows that SI increases for a large proportion of patients between the six-hour blocks over the first 36 hours of ICU stay. Table 4 also shows that after 48 hours of treatment, only 86% of patients show rise in SI from the first six hours. Thus, while the general trend is obvious for increasing SI, it is not guaranteed for all patients. Equally, these increases decelerate in terms of number of patients with increasing SI over time, going from left to right in the table.
SIvariability analyses
Figures 3 and 4 present the CDFs for changes in SI (%ΔSI) for six-hourly blocks per cohort and 50% range of SI variability per patient, respectively. Table 5 presents the reductions between successive blocks.
Cohort and per-patient variability decreases for the first 24 hours. However, it increases across the cool to warm transition, indicating some potential stress across the cool to warm transition with negative reductions. The decreasing trend returns for all subsequent blocks. The results suggest that %ΔSI decreases per cohort and per patient are statistically significant (P <0.05) for the first 36 hours in both cases.
Discussion
Insulin sensitivity level
The SI level results for both per-cohort and per-patient analysis suggest that OHCA patients undergoing TH treatment have significantly lower SI during the earlier cool period on day 1 than the later warm period on day 2. Both results determine the general trend for overall increasing SI level for critically ill patients over time and are consistent with other ICU studies [14],[27]. Further analysis shows that the increase in SI level during the first 36 hours are large and statistically significant for this cohort. The rapid increases in SI level for the first 36 hours is likely due to significant restart of human physiological systems and metabolic activities for these patients [13]. After 36 hours, the rapid SI increase abates as the patients’ metabolism improves and becomes more stable.
Insulin sensitivity variability
Both per-cohort and per-patient analysis suggest that OHCA patients undergoing TH treatment have high initial variability that decreases over the first 36 hours. However, the cool to warm transition at 24 hours shows an increase in variability likely due to the change of physiological conditions as body temperature increases from cool to warm between 18 and 36 hours. The lower decrease in SI variability after the 36th hour onward suggests that the patients’ metabolic condition has improved and become more stable.
Further analysis and comparison of SI variability between general ICU patients [14] and OHCA patients treated with TH shows that the main difference between them is the SI variability increase during the cool to warm transition period for the latter cohort. These SI variability results do not follow the same trend with other general ICU studies by Pretty et al. [14], and it is a unique finding for this cohort that could significantly impact glycaemic control and safety from hypoglycaemia.
Implications for glycaemic control
Clinically, these results have significant implications for managing glycaemia. Increased SI variability leads to increased variability in BG level for a given insulin intervention [11]. With low and variable insulin sensitivity, glycaemic levels might appear to remain unchanged and difficult to control effectively with exogenous insulin. This situation may result in increased glycaemic variability as well as an increased risk of hyperglycaemia and hypoglycaemia during the first 36 hours of treatment due to greater hour-to-hour SI variability with increased insulin resistance [17]. Thus, since glycaemic variability and hypoglycaemia are independent risk factors for the critically ill, it is important to understand and manage these patient-specific dynamics, especially those unique to a cohort, when implementing glycaemic control. This outcome is particularly important when OHCA patients transition from cool to warm. These results may also generalise to other areas where glycaemic control is applied to hypothermic patients, such as in the operating theatre.
There are several ways that this low and variable insulin sensitivity could be managed during glycaemic control. Reducing exogenous insulin doses, coupled with modulation of the glucose content of nutrition would diminish the impact of sudden changes of insulin sensitivity on glycaemic outcome. Equally, increased BG measurement frequency could improve control and reduce glycaemic variability. Accepting higher glycaemic targets during periods of increased variability would trade off a reduced risk of hypoglycaemia against increased hyperglycaemia. Ultimately, the preferred method for any unit may be influenced by practical considerations, such as clinical workload.
Limitations
The parameters used in the glucose insulin system model are based on general ICU patients with normal body temperature conditions. Thus, the insulin sensitivity values derived during the cool period could be biased by modelling errors or unmodelled effects. However, as noted previously, the validity and independence of this patient-specific parameter has been validated using data from clinically matched cohorts and has been shown to correlate well in gold-standard insulin sensitivity tests.
Insulin sensitivity variability is a key contributor to glycaemic variability. Sechterberger et al. [28] showed an association between high glycaemic variability and mortality is not present in diabetic cohorts. Thus, a subgroup analysis of diabetic OHCA patients in this study would be very interesting. However, only 26 of 200 (13%) OHCA patients in this study had previously diagnosed diabetes (Table 1), which is too few to enable a reliable analysis with these methods. Additionally, in this particular cohort, undiagnosed diabetes or impaired glucose regulation [29] may confound such a subgroup.
Conclusions
This study analyses the metabolic evolution of OHCA patients treated with TH. These analyses characterise the metabolic impact of TH treatment on the level and variability of insulin sensitivity to inform control.
Two main conclusions are drawn as a result for these cohorts.
-
i)
SI level is much lower during TH and consistently increases over time, during both cool and warm periods.
-
ii)
Insulin sensitivity is more variable during the cool period and shows contrasting behaviour during the cool to warm transition period between 18 and 30 hours, which indicates that there are major changes in physiology and metabolic conditions between cool and warm as influenced by human body temperature. Otherwise, it decreases over time.
Finally, this study shows the need for patient-specific glycaemic management to ensure good control and safety during treatment. These results have significant potential clinical impact on the metabolic treatment of these patients, and changes in clinical therapy are required to safely treat patients as they transition from cool to warm.
Key messages
-
OCHA patients treated with TH have significantly lower and highly variable SI during the first 24 hours of the cool period, compared to the later warm period in their ICU stay.
-
There is an overall trend of increasing SI over the first 36 hours, both per-cohort and per-patient results.
-
SI variability decreases consistently over time, except for a large, statistically significant increase during the cool to warm transition at 24 hours.
-
This increase requires special consideration for glycaemic control as it increases risk of hypoglycaemia, BG variability and thus mortality.
Abbreviations
- %ΔSI:
-
hour-to-hour percentage changes in insulin sensitivity
- BG:
-
blood glucose
- CDF:
-
cumulative distribution function
- ICU:
-
intensive care unit
- IQR:
-
interquartile range
- KS:
-
Kolmogorov-Smirnov (test)
- OHCA:
-
out-of-hospital cardiac arrest
- SI:
-
insulin sensitivity metric (model-based)
- SPRINT:
-
specialized relative insulin and nutrition titration
- TH:
-
therapeutic hypothermia
References
Capes SE, Hunt D, Malmberg K, Gerstein HC: Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000, 355: 773-778. 10.1016/S0140-6736(99)08415-9.
McCowen KC, Malhotra A, Bistrian BR: Stress-induced hyperglycemia. Crit Care Clin. 2001, 17: 107-124. 10.1016/S0749-0704(05)70154-8.
Mizock BA: Alterations in fuel metabolism in critical illness: hyperglycaemia. Best Pract Res Clin Endocrinol Metab. 2001, 15: 533-551. 10.1053/beem.2001.0168.
van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R: Intensive insulin therapy in critically ill patients. N Engl J Med. 2001, 345: 1359-1367. 10.1056/NEJMoa011300.
Krinsley JS: Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003, 78: 1471-1478. 10.4065/78.12.1471.
Krinsley JS: Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004, 79: 992-1000. 10.4065/79.8.992.
Chase JG, Shaw G, Le Compte A, Lonergan T, Willacy M, Wong XW, Lin J, Lotz T, Lee D, Hann C: Implementation and evaluation of the SPRINT protocol for tight glycaemic control in critically ill patients: a clinical practice change. Crit Care. 2008, 12: R49-10.1186/cc6868.
Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K: Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008, 358: 125-139. 10.1056/NEJMoa070716.
Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hebert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter R, Robinson BG, Ronco JJ: Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009, 360: 1283-1297. 10.1056/NEJMoa0810625.
Preiser JC, Devos P, Ruiz-Santana S, Melot C, Annane D, Groeneveld J, Iapichino G, Leverve X, Nitenberg G, Singer P, Wernerman J, Joannidis M, Stecher A, Chiolero R: A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009, 35: 1738-1748. 10.1007/s00134-009-1585-2.
Chase JG, Le Compte AJ, Suhaimi F, Shaw GM, Lynn A, Lin J, Pretty CG, Razak N, Parente JD, Hann CE, Preiser JC, Desaive T: Tight glycemic control in critical care-the leading role of insulin sensitivity and patient variability: a review and model-based analysis. Comput Methods Programs Biomed. 2011, 102: 156-171. 10.1016/j.cmpb.2010.11.006.
Taylor NA, Griffiths RF, Cotter JD: Epidemiology of hypothermia: fatalities and hospitalisations in New Zealand. Aust N Z J Med. 1994, 24: 705-710. 10.1111/j.1445-5994.1994.tb01788.x.
Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Bottiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT, Merchant MR, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Vanden Hoek T: Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008, 118: 2452-2483. 10.1161/CIRCULATIONAHA.108.190652.
Pretty CG, Le Compte AJ, Chase JG, Shaw GM, Preiser JC, Penning S, Desaive T: Variability of insulin sensitivity during the first 4 days of critical illness: implications for tight glycemic control. Ann Intensive Care. 2012, 2: 17-10.1186/2110-5820-2-17.
Eisenburger P, Sterz F, Holzer M, Zeiner A, Scheinecker W, Havel C, Losert H: Therapeutic hypothermia after cardiac arrest. Curr Opin Crit Care. 2001, 7: 184-188. 10.1097/00075198-200106000-00007.
Lee R, Asare K: Therapeutic hypothermia for out-of-hospital cardiac arrest. Am J Health Syst Pharm. 2010, 67: 1229-1237. 10.2146/ajhp090626.
Cueni-Villoz N, Devigili A, Delodder F, Cianferoni S, Feihl F, Rossetti AO, Eggimann P, Vincent JL, Taccone FS, Oddo M: Increased blood glucose variability during therapeutic hypothermia and outcome after cardiac arrest. Crit Care Med. 2011, 39: 2225-2231. 10.1097/CCM.0b013e31822572c9.
Hayashi N: Management of pitfalls for the successful clinical use of hypothermia treatment. J Neurotrauma. 2009, 26: 445-453. 10.1089/neu.2008.0648.
Chase JG, Suhaimi F, Penning S, Preiser JC, Le Compte AJ, Lin J, Pretty CG, Shaw GM, Moorhead KT, Desaive T: Validation of a model-based virtual trials method for tight glycemic control in intensive care. Biomed Eng Online. 2010, 9: 84-10.1186/1475-925X-9-84.
Evans A, Shaw GM, Le Compte A, Tan CS, Ward L, Steel J, Pretty CG, Pfeifer L, Penning S, Suhaimi F, Signal M, Desaive T, Chase JG: Pilot proof of concept clinical trials of Stochastic Targeted (STAR) glycemic control. Ann Intensive Care. 2011, 1: 38-10.1186/2110-5820-1-38.
Lin J, Razak NN, Pretty CG, Le Compte A, Docherty P, Parente JD, Shaw GM, Hann CE, Geoffrey Chase J: A physiological Intensive Control Insulin-Nutrition-Glucose (ICING) model validated in critically ill patients. Comput Methods Programs Biomed. 2011, 102: 192-205. 10.1016/j.cmpb.2010.12.008.
McAuley KA, Berkeley JE, Docherty PD, Lotz TF, Te Morenga LA, Shaw GM, Williams SM, Chase JG, Mann JI: The dynamic insulin sensitivity and secretion test–a novel measure of insulin sensitivity. Metabolism. 2011, 60: 1748-1756. 10.1016/j.metabol.2011.05.009.
Fisk LM, Le Compte AJ, Shaw GM, Penning S, Desaive T, Chase JG: STAR development and protocol comparison. IEEE Trans Biomed Eng. 2012, 59: 3357-3364. 10.1109/TBME.2012.2214384.
Pretty CG, Signal M, Fisk L, Penning S, Le Compte A, Shaw GM, Desaive T, Chase JG: Impact of sensor and measurement timing errors on model-based insulin sensitivity. Comput Methods Programs Biomed. 2014, 114: e79-e86. 10.1016/j.cmpb.2013.08.007.
Hann CE, Chase JG, Lin J, Lotz T, Doran CV, Shaw GM: Integral-based parameter identification for long-term dynamic verification of a glucose-insulin system model. Comput Methods Programs Biomed. 2005, 77: 259-270. 10.1016/j.cmpb.2004.10.006.
Bagshaw SM, Egi M, George C, Bellomo R: Early blood glucose control and mortality in critically ill patients in Australia. Crit Care Med. 2009, 37: 463-470. 10.1097/CCM.0b013e318194b097.
Langouche L, Vander Perre S, Wouters PJ, D'Hoore A, Hansen TK, Van den Berghe G: Effect of intensive insulin therapy on insulin sensitivity in the critically ill. J Clin Endocrinol Metab. 2007, 92: 3890-3897. 10.1210/jc.2007-0813.
Sechterberger MK, Bosman RJ, Oudemans-van Straaten HM, Siegelaar SE, Hermanides J, Hoekstra JB, De Vries JH: The effect of diabetes mellitus on the association between measures of glycaemic control and ICU mortality: a retrospective cohort study. Crit Care. 2013, 17: R52-10.1186/cc12572.
Bartnik M, Ryden L, Ferrari R, Malmberg K, Pyorala K, Simoons M, Standl E, Soler-Soler J, Ohrvik J: The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe. The Euro Heart Survey on diabetes and the heart. Eur Heart J. 2004, 25: 1880-1890. 10.1016/j.ehj.2004.07.027.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ASP processed raw patient data from conventional spreadsheet into Matlab format, prepared statistical analysis and summarized results for interpretation. ASP, JGC, CGP, SP, and TD drafted the manuscript, performed analysis and interpretation of the data and critical revision of the manuscript for important intellectual content. GS provided clinical insight and supervised the acquisition of clinical data from Christchurch ICU patients. JCP, FT, JLV and MO provided clinical insight and supervised the acquisition of clinical data from Erasme and Lausanne ICU patients. All authors read and approved the final draft of the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Sah Pri, A., Chase, J.G., Pretty, C.G. et al. Evolution of insulin sensitivity and its variability in out-of-hospital cardiac arrest (OHCA) patients treated with hypothermia. Crit Care 18, 586 (2014). https://doi.org/10.1186/s13054-014-0586-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-014-0586-x