Abstract
Background
This study was designed to examine the effect of resveratrol on mitochondrial biogenesis, oxidative stress (OS), and assisted reproductive technology (ART) outcomes in individuals with polycystic ovary syndrome (PCOS).
Methods
Fifty-six patients with PCOS were randomly assigned to receive 800 mg/day of resveratrol or placebo for 60 days. The primary outcome was OS in follicular fluid (FF). The secondary outcome involved assessing gene and protein expression related to mitochondrial biogenesis, mitochondrial DNA (mtDNA) copy number, and adenosine triphosphate (ATP) content in granulosa cells (GCs). ART outcomes were evaluated at the end of the trial.
Results
Resveratrol significantly reduced the total oxidant status (TOS) and oxidative stress index (OSI) in FF (P = 0.0142 and P = 0.0039, respectively) while increasing the total antioxidant capacity (TAC) (P < 0.0009). Resveratrol consumption also led to significant increases in the expression of critical genes involved in mitochondrial biogenesis, including peroxisome proliferator-activated receptor gamma coactivator (PGC-1α) and mitochondrial transcription factor A (TFAM) (P = 0.0032 and P = 0.0003, respectively). However, the effect on nuclear respiratory factor 1 (Nrf-1) expression was not statistically significant (P = 0.0611). Resveratrol significantly affected sirtuin1 (SIRT1) and PGC-1α protein levels (P < 0.0001 and P = 0.0036, respectively). Resveratrol treatment improved the mtDNA copy number (P < 0.0001) and ATP content in GCs (P = 0.0014). Clinically, the resveratrol group exhibited higher rates of oocyte maturity (P = 0.0012) and high-quality embryos (P = 0.0013) than did the placebo group. There were no significant differences between the groups in terms of chemical or clinical pregnancy rates (P > 0.05).
Conclusions
These findings indicate that resveratrol may be a promising therapeutic agent for patients with PCOS undergoing assisted reproduction.
Trial registration number
http://www.irct.ir; IRCT20221106056417N1; 2023 February 09.
Similar content being viewed by others
Introduction
PCOS is a multifactorial endocrine disorder that affects approximately 8–15% of reproductive-aged women [1]. Recent research has provided new insights into irregular mitochondrial indices in patients with PCOS [2]. The abundance of mitochondria in the granulosa cells (GCs) surrounding a specific oocyte is positively correlated with oocyte maturation, fertilization, and successful embryo development [3]. The mitochondrial biogenesis pathway is a fundamental factor in follicular development [4]. Mitochondrial biogenesis involves the expansion and division of existing mitochondria [5]. It seems that oxidative stress (OS) is caused by insufficient physiological antioxidants to counterbalance excess reactive oxygen species (ROS), which are present in individuals with PCOS [6]. A previous study demonstrated that changes in mitochondrial biogenesis might contribute to hyperandrogenism, while an increase in mitochondrial biogenesis could improve PCOS by reducing ROS in GCs [7]. These findings highlight that mitochondrial dysfunction plays a critical role in the progression and management of PCOS [8]. The literature frequently mentions that the mtDNA copy number is a marker of mitochondrial biogenesis and mitochondrial content [1]. A study demonstrated that impaired mitochondrial function, characterized by decreased mtDNA copy numbers and mutations in mitochondrial genes, might contribute to the progression of PCOS [9]. The transcriptional peroxisome proliferator-activated receptor gamma coactivator (PGC-1α) collaborates with nuclear respiratory factor 1 (Nrf-1), promoting the expression of mitochondrial transcription factor A (TFAM), which directly regulates the replication and transcription of mtDNA. The activation of the TFAM promoter plays a vital role in the transcription of nuclear and mitochondrial genes, serving as a mechanism for mitochondrial biogenesis [10]. Sirtuin 1 (SIRT1) is an NAD+-dependent deacetylase that interacts directly with PGC-1α, resulting in the upregulation of PGC-1α expression [11]. The specific mechanisms linking abnormal mitochondrial markers to the development of PCOS remain unclear. Consistent with this notion, it is imperative to research the molecular processes of PCOS to identify potential therapeutic targets. Recently, there has been a significant focus on antioxidant treatment as a natural and low-risk supplement in medical research for mitigating the impact of PCOS on female fertility [12]. Resveratrol is a natural polyphenolic compound identified as 3-5-4′-trihydroxystilbene that is found in various plant-derived sources, such as grapes, berries, and peanuts [13]. Resveratrol plays a critical role in the regulation of antioxidant enzymes and the inhibition of prooxidant factors [14]. Resveratrol has been used as an activator of SIRT1 to regulate mitochondrial activity in both animals and cell lines [15, 16]. Additionally, numerous clinical trials have suggested that resveratrol supplementation may alleviate symptoms of PCOS [17,18,19]. Furthermore, previous research has revealed that the impact of resveratrol on the mitochondrial biogenesis of GCs may explain the favorable effects of this polyphenol on the female reproductive system, suggesting potential therapeutic implications in clinical practice [20]. This clinical trial aimed to explore the potential of resveratrol as a therapeutic agent for improving mitochondrial biogenesis and assisted reproductive technique (ART) outcomes in patients with PCOS.
Materials and methods
Trial design
This study was a randomized, triple-blind, placebo-controlled trial that evaluated the effects of resveratrol in patients with PCOS. The present study was registered on the Iranian website for registration as a clinical trial (2023 February 09; Registration Code: IRCT20221106056417N1; http://www.irct.ir) before enrolling the first patient. This paper presents some of the outcomes from clinical trials registered with IRCT. Additionally, this study received approval from the Ethics Committee of Tehran University of Medical Sciences, Shariati Hospital, Tehran, Iran (2023 January 07; Ethics Code: IR.TUMS.SHARIATI.REC.1401.021). All participants provided informed consent.
Participants
Inclusion criteria
The Rotterdam criteria define four phenotypes of PCOS: (A) androgen excess, ovulatory dysfunction, and polycystic ovary morphology (PCOM) on ultrasound; (B) androgen excess and ovulatory dysfunction; (C) androgen excess and PCOM; and (D) ovulatory dysfunction and PCOM [21]. The International Evidence-Based Guideline [22] supports these criteria, stating that the diagnosis requires the presence of at least two of the following criteria in adult women: oligo-anovulation, androgen excess (either clinical or biochemical), and ultrasound assessment of ovarian morphology [23]. The inclusion criteria were as follows: (i) 18‒35 years of age; (ii) patients who were diagnosed with PCOS based on the Rotterdam criteria; and (iii) undergoing intracytoplasmic sperm injection (ICSI) treatment at the Department of Omid Fertility Clinic in Tehran, Iran.
Exclusion criteria
The exclusion criteria were as follows: (i) patients with hyperprolactinemia, thyroid disease, congenital adrenal hyperplasia, or Cushing’s syndrome (identified based on the patient’s medical history); (ii) individuals with severe endometriosis (stages 3 and 4 according to the revised AFS-rAFS classification) [24], ovarian tumors, or female infertility factors unrelated to cervical and tubal issues; (iii) those with severe obesity (defined as a body mass index (BMI) > 35); (iv) patients with follicle-stimulating hormone (FSH) levels exceeding 10 mg/mL; (v) individuals with systemic disorders such as metabolic syndrome, diabetes, hyperlipidemia, and cardiovascular disease; (vi) patients who became pregnant, or those who were using any medications affecting reproductive physiology within three months prior to the study; (vii) patients who smoke and/or consume alcohol; (viii) individuals with any autoimmune disease; and (ix) those with severe male factor infertility, particularly nonobstructive azoospermia.
Randomization and blinding
Random allocation was performed through a 1:1 allocation ratio with a balanced block randomization approach, with block sizes of 4 containing letters A and B (representing the resveratrol and placebo groups). The contents were concealed using sequentially numbered sealed opaque envelopes. Patient allocation was conducted by an independent statistician using online platforms. Researchers then enrolled patients, and a gynecologist, who remained blinded throughout the study, diagnosed patients with PCOS based on the Rotterdam criteria before assigning participants to interventions. Researchers, patients, and statisticians were blinded throughout the study and treatment allocation. The appearance of the placebo, the resveratrol capsules, and the packaging were identical to ensure blinding, and the capsules were provided by the same company simultaneously.
Intervention
The effective dose and duration of resveratrol supplementation were derived from previous studies [18, 19, 25]. Based on their findings, a daily dosage of 800 mg/day (2 × 400 mg capsules) of resveratrol (transresveratrol, 99% purified, Mega Resveratrol, UK) was orally administered to patients in the treatment (resveratrol) group, and patients in the placebo group received placebo capsules (maltodextrin and lactose) every day for 60 days before oocyte collection. The shape, scent, and color of the placebo capsules were identical to those of the resveratrol capsules. The intervention and placebo groups were administered two tablets at the total daily dose. Also, it is crucial to control for confounding factors like, physical activity, and food intake. All eligible patients were instructed to maintain their usual lifestyle, including their level of physical activity throughout the study, and to avoid nutritional supplements or foods high in resveratrol, which were listed for the patients. Additionally, patients had to notify the researchers of any changes in their diet. To more closely monitor the effect of resveratrol supplementation, the International Physical Activity Questionnaire-Short Form (IPAQ SF) was used to estimate the level of physical activity at baseline and at the end of the intervention. The questionnaire recorded self-reported activity at four levels: vigorous physical activity (e.g., heavy lifting), moderate physical activity (e.g., carrying light loads), walking, and sitting [26]. To assess participants’ compliance and adherence to the trial protocol, the researcher made telephone calls every 15 days to evaluate capsule consumption and any new symptoms or side effects since the last interview.
Ovarian stimulation procedure
All patients underwent a controlled ovarian stimulation regimen known as the flexible antagonist regime. From the third day of the menstrual cycle, 150–300 IU of rFSH (Gonal-F, Merck Serono SA, Switzerland) was given, with the optimum dosage tailored based on the estradiol concentration and ovarian response. Ovarian monitoring was conducted, and once at least two follicles measuring 14–15 mm in size were observed, the gonadotropin-releasing hormone antagonist cetrorelix acetate cetrotide (0.25 mg/day; Merck Serono SA, Switzerland) was administered. A total of 10,000 IU of human chorionic gonadotropin (hCG) (Ovitrelle, Merck Serono SA, Switzerland) was used as the trigger once at least two follicles reached a diameter of ≥ 18 mm, and cetrotide consumption was discontinued. At 36-hour intervals, each follicle was measured immediately before aspiration, and subsequently, oocyte retrieval was performed under transvaginal ultrasound guidance [27].
Outcomes
The demographic and clinical data of the patients, such as age, BMI, duration of infertility, menstruation, and menstrual cycle duration, were documented. Additionally, the hormonal profiles of patients during the follicular phase (including serum levels of FSH, luteinizing hormone (LH), prolactin (PRL), thyroid stimulating hormone (TSH), testosterone, and anti-Müllerian hormone (AMH)) were evaluated. The primary outcome was the change in OS markers in FF. At the same time, the levels of genes and proteins, as well as the mtDNA copy number and adenosine triphosphate (ATP) content, in GCs and ART outcomes were recorded and subjected to analysis as secondary outcomes.
Sample collection
Following the methods of a previous study [27], individual samples comprising FF and GCs were collected from each patient. The FFs obtained from follicles with a diameter of at least 16 mm were pooled together and immediately subjected to centrifugation at 3000×g for 15 min at room temperature. The resulting supernatant was preserved at − 80 °C until analysis. The sediments were resuspended in 3 mL of phosphate-buffered saline (PBS; Sigma, Germany), gently layered onto 5 mL of Ficoll-Hypaque (Lymphodex, Inno-Train, Germany), and centrifuged at 400×g for 20 min at room temperature to eliminate red blood cells and debris. The GCs were retrieved from the layer at the gradient interface, centrifuged twice at 600×g for 5 min at room temperature with 1 mL of PBS (Sigma, Germany), and stored at − 80 °C until RNA and protein extraction.
Follicular fluid analysis
The levels of OS markers (total antioxidant capacity [TAC] and total oxidant status [TOS]) in the FF samples were evaluated twice for accuracy using readily available NaxiferTM and NatosTM Kits provided by Navand Salamat in Tehran, Iran. Furthermore, the oxidative stress index (OSI), determined by dividing the TOS by the TAC, was assessed in both groups and is expressed in µmol of H2O2/mmol of Fe2+.
Gene expression analysis by qRT‒PCR
Total RNA was extracted from frozen GCs using a column method (RNA Extraction Kit, AnaCell, Iran) following the manufacturer’s instructions. The concentration and purity of the isolated RNA were assessed using a spectrophotometer (Biochrom). Subsequently, complementary DNA (cDNA) synthesis was performed using a cDNA reverse transcription kit (cDNA Synthesis Kit, AnaCell, Iran). Quantitative real-time polymerase chain reaction (qRT–PCR) analysis was performed using SYBR Green RealQ Plus 2× Master Mix Green (Amplicon) on a Real-Time ABI® Step One thermocycler. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for normalization, and the relative expression of each gene was determined using the 2 − ΔΔCt method. For real-time PCR analysis, 24 samples from each group were used. The primer sequences utilized in the study are detailed in Table 1.
Western blot
Total protein was extracted from frozen GCs using RIPA buffer. The protein concentration was determined using a Bradford protein quantification kit (DNAbiotech, Iran) following the manufacturer’s instructions. Equal amounts of protein samples were then separated by SDS‒PAGE and transferred onto an immune-Blot™ polyvinylidene difluoride (PVDF) membrane (Bio-Rad Laboratories, CA, USA; Cat No: 162–017777). Next, the membranes were blocked with 5% bovine serum albumin (BSA) (Sigma Aldrich, MO, USA; Cat No: A-7888) in 0.1% Tween 20 for 1 h. Subsequently, the blots were incubated with specific antibodies against total SIRT1 (Abcam, Cat No: ab189494) and PGC1-α (Abcam, Cat No: ab191838) for 1 h at room temperature. For normalization, β-actin (Abcam, Cat No: ab8227) was used as the internal control. Finally, the membrane was incubated with a goat anti-rabbit IgG H&L (HRP) (Abcam, Cat No: ab6721) secondary antibody. The membrane was subsequently subjected to enhanced chemiluminescence (ECL) for 1–2 min. The densitometry of each band was determined using the gel analyzer version 2010a software (NIH, USA). This process allowed the analysis and comparison of protein expression levels. For western blot analysis, six samples from each group were used.
Measurement of mitochondrial copy number
Total DNA (genomic and mitochondrial) was extracted from GCs using a column method according to the manufacturer’s instructions (DNA Extraction Kit, AnaCell, Iran). mtDNA copy number relative to genomic DNA content was determined based on the ratio of the mtDNA-encoded NADH-dehydrogenase subunit 1 (ND1) gene to β-actin as a nuclear endogenous control region using quantitative polymerase chain reaction (qPCR) assays. mtDNA quantification was performed using the ΔΔCt method, in which all the mtDNA target (ND1) Ct values were normalized to the β-actin values [28, 29]. For the qPCR analysis, 24 samples from each group were used. The primer sequences are outlined in Table 1.
Intracellular ATP level measurement
The ATP concentration in the GCs was measured via an ATP colorimetric assay kit (Adenosine Triphosphate ELISA Kit, Abbexa, Cat. No. abx574124). The absorbance of the samples was measured using an ELISA plate reader set to 450 nm. ATP concentrations in the samples were determined by comparison to a standard curve. Twenty-four samples from each group were used for analysis.
Clinical follow-up
Following oocyte retrieval, the cumulus cells were eliminated through hyaluronidase enzyme (Sigma®, USA) and delicate mechanical pipetting. Then, the oocytes were categorized into three groups based on their nuclear maturity profile immediately after the removal of cumulus cells: (I) MII oocytes, featuring the presence of the first polar body; (II) MI oocytes lacking the first polar body; and (III) germinal vesicle oocytes containing a prominent nucleus within the cytoplasm. The total number of harvested oocytes and the oocyte maturation rate were documented. The oocyte maturation rate was determined using the following formula: rate of maturity oocyte = (number of MII stage oocytes/total oocyte count) ×100% [30].
Injectable MII-stage oocytes were utilized for the ICSI procedure. Sixteen to nineteen hours after the injection, the presence of two pronuclei (2PN) confirmed successful fertilization. The fertilization rate was determined using the following formula: fertilization rate = (2PN/total MII oocyte count) ×100% [31].
Two to three days after ICSI, information on embryo development, including the number of embryos and high-quality embryo rate, was recorded. High-quality embryos were defined as those exhibiting Grade A or B cleavage based on Association for the Study of Reproductive Biology (ASEBIR) standards, which considered factors such as the number of cells (blastomeres), the extent of embryo fragmentation, cell symmetry, and the presence of multinucleation, vacuoles, and pitting relative to low-quality embryos. The high-quality embryo rate was determined as the percentage of high-quality embryos relative to the total number of embryos [32]. Consistent with the standard clinical approach, which aims to mitigate the risk of ovarian hyperstimulation syndrome (OHSS) and enhance overall clinical results, embryos were cryopreserved on days 3 and 5. Subsequently, 2 or 3 embryos were transferred in the following two cycles. Patient follow-up included the computation of chemical and clinical pregnancy rates, which served as the key outcomes of the randomized clinical trial. The chemical pregnancy rate was determined as the ratio of the serum β-hCG level 14 days after embryo transfer to the total number of embryo transfer cycles [33]. Moreover, the clinical pregnancy rate was determined by the presence of a gestational sac and fetal heartbeat observed during an ultrasound scan at approximately 6–7 weeks of gestation divided by the total number of embryo transfer cycles [34].
Statistics
Sample size calculation
The sample size calculation was estimated from a previous study [35], with a significance level of 0.05 and 80% power to detect a 17% increase in the mean FF TAC as the primary outcome in the intervention group compared to the placebo group, while accounting for a potential 10% patient dropout rate during the study, the final sample size was 56 (28 patients in each group).
Statistical methods
The statistical analysis was conducted with SPSS 26.0, and GraphPad Prism 9.0 software was used for data generation. Normally distributed data were tested using the Kolmogorov‒Smirnov test. The differences between the resveratrol and placebo groups were assessed using an Unpaired t test. Nonnormally distributed data were analyzed using the Mann–Whitney test. Chemical and clinical pregnancies were evaluated using Fisher’s exact test. A significance level of P < 0.05 indicated statistical significance for all the statistical assessments. All experimental data are presented as the means and standard deviations (SDs) or as medians (25th–75th percentiles).
Results
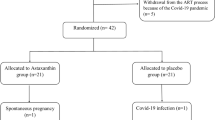
The study was conducted from February 2023 to December 2023. Resveratrol was well tolerated throughout the treatment, with no patients reporting any side effects. Initially, 118 participants were screened, and 62 (52.5%) were subsequently excluded. The remaining 56 (47.4%) participants were randomly allocated to either the 50% resveratrol group (n = 28) or the 50% placebo group (n = 28). During the study, four participants from both the placebo and resveratrol groups withdrew from the trial. In the resveratrol group, two patients withdrew due to pregnancy, and two discontinued the intervention. In the placebo group, one patient withdrew for personal reasons, two patients withdrew due to pregnancy, and one patient discontinued the intervention. Consequently, the study included 24 (50%) participants in each group, as demonstrated by the distribution of participants throughout the trial in the Consolidated Standards of Reporting Trials (CONSORT) diagram depicted in Fig. 1.
Baseline clinical and endocrine characteristics of the study population
No significant differences were observed between the two groups regarding mean age, BMI, duration of infertility, menstruation, menstrual cycle duration, or hormonal profiles on the day of study initiation (P > 0.05 for all; Table 2).
Primary outcome: effects of resveratrol on OS markers
The findings revealed that the TOS and OSI were significantly lower in the treatment group than in the placebo group (P < 0.05; Table 3). Additionally, the TAC in FF, as assessed by the FRAP assay, significantly increased between the two groups (P < 0.05; Table 3).
Secondary outcomes
Effects of resveratrol on mRNA expression levels
We hypothesized that resveratrol might also exert regulatory effects on downstream factors within the PGC-1α pathway. qRT‒PCR measurements indicated that the expression levels of mitochondrial biogenesis-related genes, such as PGC-1α and TFAM, were significantly elevated in the treatment group compared to those in the placebo group (P = 0.0032 and P = 0.0003; Fig. 2A C, respectively). Although there was an increase in Nrf-1 mRNA expression in the treatment group, this increase did not reach statistical significance (P = 0.0611; Fig. 2B). These results provide strong evidence that resveratrol enhances the expression of downstream factors of PGC-1α at the mRNA level by activating the PGC-1α signaling pathway.
The expression of PGC-1α (A), Nrf-1 (B), and TFAM (C) in GCs from the resveratrol and placebo groups. (A, C) The results revealed that the expression of PGC-1α and TFAM was significantly greater in the resveratrol group (P < 0.05). (B) The increase in Nrf-1 levels did not reach statistical significance between the two groups (P > 0.05). The treatment group received a daily dose of 800 mg/day of resveratrol. The data are presented as the means ± SDs. ***P < 0.001, **P < 0.01. (Mann‒Whitney test). PGC-1α, peroxisome proliferator-activated receptor gamma coactivator; Nrf-1, nuclear respiratory factor 1; TFAM, mitochondrial transcription factor A
Effects of resveratrol on the protein expression levels
Resveratrol upregulated the protein expression of SIRT1 and PGC-1α in GCs (Fig. 3). The protein levels of SIRT1 and PGC-1α were significantly greater in the treatment group than in the placebo group (P < 0.0001; P = 0.0036; Fig. 3A and B, respectively).
The protein expression levels of SIRT1 and PGC-1α in GCs from the placebo and resveratrol groups. (A, B) Western blot analysis was performed to assess the protein expression of SIRT1 and PGC-1α, which was subsequently normalized to that of β-actin. The protein expression of SIRT1 and PGC-1α was significantly greater in the treatment group than in the control group (P < 0.05). (C) Blots of SIRT1, PGC-1α, and β-actin. The treatment group received a daily dose of 800 mg/day of resveratrol. The data are presented as the means ± SDs. ****P < 0.0001, **P < 0.01 (Unpaired t test). SIRT1, Sirtuin 1; PGC-1α, Peroxisome proliferator-activated receptor gamma coactivator
Effects of resveratrol on the mtDNA copy number and intracellular ATP content
In contrast to the placebo group, the treatment group exhibited an elevated mtDNA copy number (P < 0.0001; Fig. 4A). To determine the effect of resveratrol treatment on mitochondrial biogenesis and function, we measured the intracellular ATP concentration, which was found to increase in the intervention group (P = 0.0014; Fig. 4B). These findings collectively suggest that resveratrol enhances mitochondrial biogenesis in the GCs of patients with PCOS.
Comparison of mtDNA copy number and intracellular ATP content between the two groups. (A) The relative mtDNA copy numbers were significantly greater in the treatment group than in the control group (P < 0.05). (B) A comparison of the intracellular ATP content in GCs between the resveratrol and placebo groups revealed a significant increase in the resveratrol group (P < 0.05). The treatment group received a daily dose of 800 mg/day of resveratrol. The data are presented as the means ± SDs. ****P < 0.0001, **P < 0.01 (Unpaired t test). ND1, NADH-dehydrogenase subunit; ATP, adenosine triphosphate
Evaluation of ovarian stimulation parameters and ART results
According to the analysis of ovarian stimulation parameters and ART outcomes, resveratrol therapy significantly improved the oocyte maturity rate and percentage of high-quality embryos (P < 0.05). However, there were no significant differences between the two groups in terms of the number of retrieved oocytes, the number of MII oocytes, the total number of embryos, the number of fertilized oocytes, or the fertilization rate (P > 0.05) (Table 4).
Furthermore, there were no significant differences detected between the resveratrol and placebo groups in terms of chemical pregnancy or clinical pregnancy rates (45.83% (11/24) vs. 33.33% (8/24); two-tailed P = 0.5556; 37.50% (9/24) vs. 29.17% (7/24); two-tailed P = 0.7601; Fig. 5).
Comparison of the chemical and clinical pregnancy rates between the resveratrol and placebo groups. In the resveratrol group, the chemical pregnancy rate was 45.83% (11/24), while it was 33.33% (8/24) in the placebo group (Fisher’s test; two-tailed P = 0.5556). Furthermore, the clinical pregnancy rate was 37.50% (9/24) in the resveratrol group and 29.17% (7/24) in the placebo group (Fisher’s test; two-tailed P = 0.7601). Placebo: n = 24, Treatment: n = 24
Discussion
In the context of PCOS, women face hurdles in the progression of oocyte development, resulting in decreased rates of fertilization and challenges in the implantation of embryos [36]. Recent research has highlighted the correlation between increased OS and the progression of PCOS and mitochondrial dysfunction [37]. GCs contribute to the increased accumulation of FF surrounding the oocyte within the developing follicle. Imbalances between antioxidant elements and ROS in FF can potentially have detrimental effects on oocyte quality, fertilization, and embryo development by disrupting the balance within the follicular microenvironment, potentially leading to infertility in patients with PCOS [38]. Considering the OS status of these women, antioxidant therapy may offer potential benefits for individuals with PCOS [6]. In several animal studies, resveratrol treatment was shown to ameliorate oxidative damage by reducing TOS and increasing TAC [14, 39,40,41]. In the present study, a comparison of the OS in FF revealed that the TOS and OSI were significantly lower in the resveratrol group than in the placebo group. TAC serves as an indicator of the abundance of antioxidants actively involved in neutralizing free oxygen radicals in the body. In this study, the levels of TAC were significantly greater in the resveratrol group than in the placebo group, corroborating findings from another clinical trial [25]. The evidence mentioned above indicates potential therapeutic applications of resveratrol within a clinical context. We also focused on exploring the impact of resveratrol treatment on mitochondrial biogenesis in GC patients with PCOS, particularly through activating the SIRT1-PGC-1α signaling pathway. The results of our study can be summarized as follows: (i) The protein levels of SIRT1 and PGC1-α were significantly elevated in response to resveratrol treatment compared to those in the placebo group; (ii) The administration of resveratrol led to an increase in the expression of pivotal genes essential for mitochondrial biogenesis, specifically PGC1-α, Nrf-1, and TFAM mRNA, indicating a positive pharmacological effect; (iii) Interestingly, resveratrol treatment was associated with an increase in mtDNA copy numbers and intracellular ATP content; and (iv) resveratrol ameliorated ART outcomes. Several studies have explored the potential of resveratrol to improve mitochondrial biogenesis in the context of reproduction [42]. The influence of resveratrol on mitochondrial biogenesis in human GCs could explain the positive impacts of this polyphenol on female reproductive system physiology [20]. In bovine models, resveratrol has been shown to stimulate mitochondrial biogenesis and enhance energy metabolism in aged oocytes. Additionally, this treatment has been shown to facilitate bovine oocyte maturation and promote embryonic development [43]. Individuals with PCOS exhibit irregular mitochondrial structure, function, and gene expression within their follicles. These anomalies impact follicular growth, ovulation, and fertilization [37]. Mitochondrial biogenesis in women with PCOS has been explored in limited studies. A study revealed significant mitochondrial suppression in the muscles of women with PCOS. The study indicated a reduction in the expression of the PGC-1α gene, which was linked to decreased expression of OXPHOS genes [44]. In the PCOS cohort, there was a decrease in the gene expression of PGC-1α, accompanied by a high level of methylation in the PGC-1α promoter region. Consequently, methylation of the PGC-1α promoter suppresses the transcription of PGC-1α, leading to a decrease in the rate of mitochondrial biogenesis [45]. A previous study reported that resveratrol can activate PGC-1α and enhance the expression of factors involved in mitochondrial biogenesis [10]. In this study, we noted that the administration of resveratrol contributed to the enhancement of mitochondrial biogenesis by elevating the protein levels of SIRT1 and PGC-1α, as did the upregulation of downstream factors, including Nrf-1 and TFAM, which are associated with increased mitochondrial biogenesis in patients with PCOS. The quantity of mtDNA remains constant, and the preservation of appropriate mtDNA copy numbers is important for sustaining mitochondrial function and cellular growth [46]. Previous research has connected changes in mtDNA copy number with reproductive disorders, including endometrial cancer, premature ovarian aging, and PCOS [47,48,49]. Recent findings indicate that quantitative alterations in mtDNA are linked to the occurrence and progression of PCOS [49]. Most existing investigations have quantified mtDNA levels in the blood of patients with PCOS, and limited research has been carried out in muscle [49,50,51]. One study reported decreased mtDNA copy numbers in women with PCOS relative to those without PCOS [49]. Conversely, another study reported no significant associations between mtDNA copy number and PCOS [50]. A prior animal investigation demonstrated that resveratrol led to an increase in mtDNA copy numbers within oocytes [42]. There is also growing evidence that optimal mtDNA copy numbers and sufficient ATP levels are essential for ensuring proper follicular maturation and blastocyst development [52, 53]. Furthermore, we measured the mtDNA copy number and ATP levels after resveratrol treatment, which were notably elevated in the resveratrol group. In line with our results, resveratrol increased ATP levels in oocytes from aged cows and pigs [42, 54], suggesting its potential for reversing mitochondrial dysfunction and enhancing mitochondrial biogenesis. A recent in vitro study investigated the effects of supplementation with various antioxidants and revealed that it enhanced the developmental competence of oocytes and improved both the quantity and quality of embryos [55]. According to several animal model studies, resveratrol activates SIRT1, thereby improving the biosynthesis of oocyte mitochondria and enhancing the developmental potential of oocytes [56]. More importantly, in vivo, resveratrol administration has been found to influence the maturation of bovine oocytes and embryonic development post in vitro fertilization (IVF) by stimulating progesterone secretion and offering antioxidant properties [43]. Resveratrol supplementation inhibits follicular atresia and increases the reserve of ovarian follicles and the lifespan of ovaries. This supplementation also improves the quantity and quality of oocytes [57, 58]. In addition, in vivo studies have shown the antioxidative properties of resveratrol and its capacity to normalize ovarian morphology [59]. A recent laboratory experiment indicated that the use of resveratrol as a supplement in in vitro maturation (IVM) medium improved oocyte maturation and quality, fertilization rates, and blastocyst formation in both aged mice and humans by improving spindle morphology, chromosome alignment, and mitochondrial function [60]. Several clinical trials have explored the impact of resveratrol on ART outcomes. A previous study reported that resveratrol significantly improved the percentage of high-quality oocytes and embryos [18]. Consistent with our study, resveratrol treatment significantly enhanced the rate of oocyte maturation and the percentage of high-quality embryos. Women with PCOS typically produce a greater number of oocytes during stimulation in an IVF cycle, but the quality of these oocytes is often poor [61]. Previous studies have indicated that the poorer ART outcomes in patients with PCOS than in those without PCOS are primarily due to the lower quality and maturity of oocytes in these patients [6]. This evidence suggests that impaired oocyte maturation and poor oocyte quality in patients with PCOS may result in compromised embryo development [38]. In line with our findings, multiple prospective randomized studies in infertile women undergoing IVF-ET cycles have shown that oral antioxidant therapy increased oocyte maturation, enhanced oocyte quality, and resulted in a greater ratio of high-quality embryos [62,63,64]. Research has also confirmed that the quality of embryos positively affects implantation and pregnancy outcomes [65]. Along with more oocyte maturation and higher embryo quality, we found that resveratrol enhanced fertilization, chemical, and clinical pregnancy rates; however, the difference between the groups was not statistically significant. No significant differences were observed in the clinical data, most likely due to the small sample size. In addition, fertilization and pregnancy outcomes can be significantly affected by the quality and maturity of gametes, uterine receptivity, and competence of the embryologist [66]. In this context, our findings align with those of previous clinical trials [18, 19, 64, 67]. However, some researchers reported contradictory results, highlighting the detrimental effect of resveratrol on the clinical pregnancy rate [68]. In their study, resveratrol was administered during the luteal phase of the treatment cycle, and this adverse effect was likely attributed to its impact on the suppression of decidual senescence. The discrepancy between our trial and another study, which used a low dose of resveratrol (200 mg daily), may be due to the various treatment durations, heterogeneous populations, retrospective design, and different IVF protocols used [67]. More specifically, in our trial, resveratrol was administered before and during ovarian stimulation, and intake was discontinued on the day of oocyte retrieval. The 60-day pretreatment duration was determined by considering the average duration of oogenesis and the stages of follicle development, during which oocytes undergo initial maturation in the ovary [69]. The resveratrol dose used in our study has been demonstrated to be safe and well tolerated in previous research [70, 71]. Additionally, studies have revealed that consuming resveratrol at doses ranging from 700 to 1000 mg/kg body weight/day is not toxic [72,73,74]. Several scientific studies have also reported that a daily dose of up to 1000 mg of resveratrol has antioxidant effects on patients with PCOS and other medical conditions [73, 75,76,77,78,79]. In our study, the effective dose and duration of resveratrol supplementation were derived from a previous study [25]. Oral supplementation with resveratrol has been shown to reduce OS markers (one of the primary goals of this study). Additionally, two clinical trials [18, 19] have investigated the effects of resveratrol at a dosage of 800 mg/day on ART outcomes in patients with PCOS, similar to our study population. However, no studies have examined the impact of resveratrol on mitochondrial biogenesis factors; furthermore, this dose and duration were chosen for this study. The strengths of our study lie in its design, prospective nature, and novel investigation of the effect of resveratrol on mitochondrial biogenesis in the GCs of women with PCOS undergoing assisted reproduction. Additionally, the design of our triple-blind randomized clinical trial with parallel groups (treatment and placebo), along with the inclusion of patients with the same baseline parameters in both groups, remarkably enhances the findings of this study. This study has several limitations and weaknesses. First, the follow-up period was relatively short, and the sample size was small. Additionally, time constraints prevented the evaluation of late pregnancy outcomes, such as live birth. Therefore, future studies with longer follow-ups and larger sample sizes are necessary to analyze pregnancy outcomes comprehensively. Further research with various doses and durations is needed to confirm our findings. In addition, due to limited resources, effectively managing and ensuring that participants adhered to a control diet for 60 days was challenging in this study. It is suggested that future studies utilize both the three-day food recall questionnaire and the Nutritionist IV program (modified for Iranian food composition) to calculate food intake and dietary intake, respectively. In conclusion, our findings indicate that 60 days of supplementation with 800 mg/day resveratrol enhances the expression of specific genes and proteins linked to mitochondrial biogenesis, improving mtDNA copy number and increasing ATP content in GCs. Moreover, our findings suggest that resveratrol may also modulate OS marker levels in the FF of patients, thus protecting against OS damage. Ultimately, resveratrol intervention influences oocyte maturity and embryo quality. These findings reinforce the hypothesis that resveratrol potentially improves mitochondrial biogenesis in GCs and may enhance ART outcomes in patients with PCOS.
Data availability
All data supporting the findings of this study are available within the paper.
Abbreviations
- AMH:
-
Anti-Müllerian Hormone
- ART:
-
Assisted Reproductive Technology
- BMI:
-
Body Mass Index
- cDNA:
-
Complementary DNA
- CONSORT:
-
Consolidated Standards of Reporting Trials
- ECL:
-
Enhanced Chemiluminescence
- FF:
-
Follicular Fluid
- FSH:
-
Follicle-Stimulating Hormone
- GAPDH:
-
Glyceraldehyde-3‐Phosphate Dehydrogenase
- GCs:
-
Granulosa Cells
- hCG:
-
Human Chorionic Gonadotropin
- ICSI:
-
Intracytoplasmic Sperm Injection
- IRCT:
-
Iranian Registry of Clinical Trials
- IVM:
-
In Vitro Maturation
- IVF:
-
In Vitro Fertilization
- LH:
-
Luteinizing Hormone
- mtDNA:
-
Mitochondrial DNA
- ND1:
-
NADH-Dehydrogenase Subunit 1
- Nrf-1:
-
Nuclear Respiratory Factor 1
- OHSS:
-
Ovarian Hyperstimulation Syndrome
- OS/OSI:
-
Oxidative Stress/Oxidative Stress Index
- PBS:
-
Phosphate-Buffered Saline
- PCOM:
-
Polycystic Ovary Morphology
- PCOS:
-
Polycystic Ovary Syndrome
- PGC-1α:
-
Peroxisome Proliferator-Activated Receptor Gamma Coactivator
- PRL:
-
Prolactin
- PVDF:
-
Polyvinylidene Difluoride
- qPCR:
-
Quantitative Polymerase Chain Reaction
- qRT–PCR:
-
Quantitative Real-Time Polymerase Chain Reaction
- SD:
-
Standard deviation
- SIRT1:
-
Sirtuin 1
- TFAM:
-
Mitochondrial Transcription Factor A
- TAC:
-
Total Antioxidant Capacity
- TOS:
-
Total Oxidant Status
- TSH:
-
Thyroid Stimulating Hormone
References
Malamouli M, Levinger I, McAinch AJ, Trewin AJ, Rodgers RJ, Moreno-Asso A. The mitochondrial profile in women with polycystic ovary syndrome: impact of exercise. J Mol Endocrinol. 2022;68(3):R11–23. https://doi.org/10.1530/JME-21-0177.
Mancini A, Bruno C, Vergani E, d’Abate C, Giacchi E, Silvestrini A. Oxidative stress and low-Grade inflammation in polycystic ovary syndrome: controversies and New insights. Int J Mol Sci. 2021;22(4):1667. https://doi.org/10.3390/ijms22041667.
Nishigaki A, Tsubokura H, Tsuzuki-Nakao T, Okada H, Hypoxia. Role of SIRT1 and the protective effect of resveratrol in ovarian function. Reprod Med Biol. 2022;21(1):e12428. https://doi.org/10.1002/rmb2.12428.
Ramadan AG, Abdel-Rehim WM, El-Tahan RA, Elblehi SS, Kamel MA, Shaker SA. Maternal and paternal obesity differentially reprogram the ovarian mitochondrial biogenesis of F1 female rats. Sci Rep. 2023;13(1):15480. https://doi.org/10.1038/s41598-023-42468-5.
Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79(2):208–17. https://doi.org/10.1093/cvr/cvn098.
Gong Y, Luo S, Fan P, Jin S, Zhu H, Deng T, et al. Growth hormone alleviates oxidative stress and improves oocyte quality in Chinese women with polycystic ovary syndrome: a randomized controlled trial. Sci Rep. 2020;10(1):18769. https://doi.org/10.1038/s41598-020-75107-4.
Safaei Z, Bakhshalizadeh S, Nasr-Esfahani MH, Akbari Sene A, Najafzadeh V, Soleimani M, Shirazi R. Vitamin D3 affects mitochondrial biogenesis through mitogen‐activated protein kinase in polycystic ovary syndrome mouse model. J Cell Physiol. 2020;235(9):6113–26. https://doi.org/10.1002/jcp.29540.
Siemers KM, Klein AK, Baack ML. Mitochondrial dysfunction in PCOS: insights into Reproductive Organ Pathophysiology. Int J Mol Sci. 2023;24(17):13123. https://doi.org/10.3390/ijms241713123.
Saeed N, Hamzah IH, Al-Gharrawi SAR. Polycystic ovary syndrome dependency on mtDNA mutation; copy number and its association with insulin resistance. BMC Res Notes. 2019;12(1):455. https://doi.org/10.1186/s13104-019-4453-3.
Zhou J, Yang Z, Shen R, Zhong W, Zheng H, Chen Z, et al. Resveratrol improves mitochondrial Biogenesis function and activates PGC-1alpha pathway in a Preclinical Model of Early Brain Injury following subarachnoid hemorrhage. Front Mol Biosci. 2021;8:620683. https://doi.org/10.3389/fmolb.2021.620683.
Wang SJ, Zhao XH, Chen W, Bo N, Wang XJ, Chi ZF, Wu W. Sirtuin 1 activation enhances the PGC-1alpha/mitochondrial antioxidant system pathway in status epilepticus. Mol Med Rep. 2015;11(1):521–6. https://doi.org/10.3892/mmr.2014.2724.
Alesi S, Ee C, Moran LJ, Rao V, Mousa A. Nutritional supplements and complementary therapies in polycystic ovary syndrome. Adv Nutr. 2022;13(4):1243–66. https://doi.org/10.1093/advances/nmab141.
Dull AM, Moga MA, Dimienescu OG, Sechel G, Burtea V, Anastasiu CV. Therapeutic approaches of Resveratrol on endometriosis via anti-inflammatory and anti-angiogenic pathways. Molecules. 2019;24(4):667. https://doi.org/10.3390/molecules24040667.
Sedlak L, Wojnar W, Zych M, Wygledowska-Promienska D, Mrukwa-Kominek E, Kaczmarczyk-Sedlak I. Effect of Resveratrol, a Dietary-Derived Polyphenol, on the oxidative stress and Polyol Pathway in the Lens of rats with Streptozotocin-Induced diabetes. Nutrients. 2018;10(10):1423. https://doi.org/10.3390/nu10101423.
Davinelli S, Sapere N, Visentin M, Zella D, Scapagnini G. Enhancement of mitochondrial biogenesis with polyphenols: combined effects of resveratrol and equol in human endothelial cells. Immun Aging. 2013;10(1):28. https://doi.org/10.1186/1742-4933-10-28.
Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127(6):1109–22. https://doi.org/10.1016/j.cell.2006.11.013.
Banaszewska B, Wrotynska-Barczynska J, Spaczynski RZ, Pawelczyk L, Duleba AJ. Effects of Resveratrol on Polycystic Ovary Syndrome: a Double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2016;101(11):4322–8. https://doi.org/10.1210/jc.2016-1858.
Bahramrezaie M, Amidi F, Aleyasin A, Saremi A, Aghahoseini M, Brenjian S, et al. Effects of resveratrol on VEGF & HIF1 genes expression in granulosa cells in the angiogenesis pathway and laboratory parameters of polycystic ovary syndrome: a triple-blind randomized clinical trial. J Assist Reprod Genet. 2019;36(8):1701–12. https://doi.org/10.1007/s10815-019-01461-6.
Brenjian S, Moini A, Yamini N, Kashani L, Faridmojtahedi M, Bahramrezaie M, et al. Resveratrol treatment in patients with polycystic ovary syndrome decreased pro-inflammatory and endoplasmic reticulum stress markers. Am J Reprod Immunol. 2020;83(1):e13186. https://doi.org/10.1111/aji.13186.
Ragonese F, Monarca L, De Luca A, Mancinelli L, Mariani M, Corbucci C, et al. Resveratrol depolarizes the membrane potential in human granulosa cells and promotes mitochondrial biogenesis. Fertil Steril. 2021;115(4):1063–73. https://doi.org/10.1016/j.fertnstert.2020.08.016.
Hoeger KM, Dokras A, Piltonen T. Update on PCOS: consequences, challenges, and Guiding Treatment. J Clin Endocrinol Metab. 2021;106(3):e1071–83. https://doi.org/10.1210/clinem/dgaa839.
Teede HJ, Tay CT, Laven JJ, Dokras A, Moran LJ, Piltonen TT, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Eur J Endocrinol. 2023;189(2):G43–64. https://doi.org/10.1093/ejendo/lvad096.
Group REASPCW. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–7. https://doi.org/10.1093/humrep/deh098.
Society TAF. Classification of endometriosis. Fertil Steril. 1979;32(6):633–4. https://doi.org/10.12701/yujm.2020.00444.
Seyyedebrahimi S, Khodabandehloo H, Nasli Esfahani E, Meshkani R. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Acta Diabetol. 2018;55:341–53. https://doi.org/10.1007/s00592-017-1098-3.
Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8:115. https://doi.org/10.1186/1479-5868-8-115.
Qasemi M, Aleyasin A, Mahdian R, Ghanami Gashti N, Shabani Nashtaei M, Ashrafnezhad Z, Amidi F. Cell-free mtDNA level and its biomarker potency for ART outcome are different in follicular fluid of PCOS and non-PCOS women. Mitochondrion. 2021;59:30–6. https://doi.org/10.1016/j.mito.2021.04.003.
Ylikallio E, Page JL, Xu X, Lampinen M, Bepler G, Ide T, et al. Ribonucleotide reductase is not limiting for mitochondrial DNA copy number in mice. Nucleic Acids Res. 2010;38(22):8208–18. https://doi.org/10.1093/nar/gkq735.
Meng F, Jia Z, Zheng J, Ji Y, Wang J, Xiao Y, et al. A deafness-associated mitochondrial DNA mutation caused pleiotropic effects on DNA replication and tRNA metabolism. Nucleic Acids Res. 2022;50(16):9453–69. https://doi.org/10.1093/nar/gkac720.
Ozgur K, Bulut H, Berkkanoglu M, Coetzee K, Ay S. Oocyte maturation-index as measure of oocyte cohort quality; a retrospective analysis of 3135 ICSI cycles. Middle East Fertility Soc J. 2015;20(1):37–42. https://doi.org/10.1016/j.mefs.2014.04.005.
Wang W, Zhang X-H, Wang W-H, Liu Y-L, Zhao L-H, Xue S-L, Yang K-H. The time interval between hCG priming and oocyte retrieval in ART program: a meta-analysis. J Assist Reprod Genet. 2011;28:901–10. https://doi.org/10.1007/s10815-011-9613-x.
The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Human reproduction. 2011;26(6):1270-83. https://doi.org/10.1093/humrep/der037.
Bassiouny YA, Dakhly DMR, Bayoumi YA, Hashish NM. Does the addition of growth hormone to the in vitro fertilization/intracytoplasmic sperm injection antagonist protocol improve outcomes in poor responders? A randomized, controlled trial. Fertil Steril. 2016;105(3):697–702. https://doi.org/10.1016/j.fertnstert.2015.11.026.
Salumets A, Suikkari AM, Makinen S, Karro H, Roos A, Tuuri T. Frozen embryo transfers: implications of clinical and embryological factors on the pregnancy outcome. Hum Reprod. 2006;21(9):2368–74. https://doi.org/10.1093/humrep/del151.
Jabarpour M, Aleyasin A, Nashtaei MS, Lotfi S, Amidi F. Astaxanthin treatment ameliorates ER stress in polycystic ovary syndrome patients: a randomized clinical trial. Sci Rep. 2023;13(1):3376. https://doi.org/10.1038/s41598-023-28956-8.
Dumesic DA, Padmanabhan V, Abbott DH. Polycystic ovary syndrome and oocyte developmental competence. Obstet Gynecol Surv. 2008;63(1):39–48. https://doi.org/10.1097/OGX.0b013e31815e85fc.
Zhang J, Bao Y, Zhou X, Zheng L. Polycystic ovary syndrome and mitochondrial dysfunction. Reprod Biol Endocrinol. 2019;17(1):67. https://doi.org/10.1186/s12958-019-0509-4.
Qiao J, Feng HL. Extra and intraovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update. 2011;17(1):17–33. https://doi.org/10.1093/humupd/dmq032.
Khazaei M, Karimi J, Sheikh N, Goodarzi MT, Saidijam M, Khodadadi I, Moridi H. Effects of Resveratrol on receptor for Advanced Glycation End products (RAGE) expression and oxidative stress in the liver of rats with type 2 diabetes. Phytother Res. 2016;30(1):66–71. https://doi.org/10.1002/ptr.5501.
Asadi S, Moradi MN, Khyripour N, Goodarzi MT, Mahmoodi M. Resveratrol attenuates copper and Zinc Homeostasis and ameliorates oxidative stress in type 2 Diabetic rats. Biol Trace Elem Res. 2017;177(1):132–8. https://doi.org/10.1007/s12011-016-0861-6.
Grujic-Milanovic J, Jacevic V, Miloradovic Z, Milanovic SD, Jovovic D, Ivanov M, et al. Resveratrol improved kidney function and structure in malignantly hypertensive rats by restoration of antioxidant capacity and nitric oxide bioavailability. Biomed Pharmacother. 2022;154:113642. https://doi.org/10.1016/j.biopha.2022.113642.
Sugiyama M, Kawahara-Miki R, Kawana H, Shirasuna K, Kuwayama T, Iwata H. Resveratrol-induced mitochondrial synthesis and autophagy in oocytes derived from early antral follicles of aged cows. J Reprod Dev. 2015;61(4):251–9. https://doi.org/10.1262/jrd.2015-001.
Wang F, Tian X, Zhang L, He C, Ji P, Li Y, et al. Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization. Fertil Steril. 2014;101(2):577–86. https://doi.org/10.1016/j.fertnstert.2013.10.041.
Skov V, Glintborg D, Knudsen S, Jensen T, Kruse TA, Tan Q, et al. Reduced expression of nuclear-encoded genes involved in mitochondrial oxidative metabolism in skeletal muscle of insulin-resistant women with polycystic ovary syndrome. Diabetes. 2007;56(9):2349–55. https://doi.org/10.2337/db07-0275.
Hannon E, Knox O, Sugden K, Burrage J, Wong CCY, Belsky DW, et al. Characterizing genetic and environmental influences on variable DNA methylation using monozygotic and dizygotic twins. PLoS Genet. 2018;14(8):e1007544. https://doi.org/10.1371/journal.pgen.1007544.
Montier LLC, Deng JJ, Bai Y. Number matters: control of mammalian mitochondrial DNA copy number. J Genet Genomics. 2009;36(3):125–31. https://doi.org/10.1016/S1673-8527(08)60099-5.
Sun Y, Zhang L, Ho SS, Wu X, Gu J. Lower mitochondrial DNA copy number in peripheral blood leukocytes increases the risk of endometrial cancer. Mol Carcinog. 2016;55(6):1111–7. https://doi.org/10.1002/mc.22373.
Bonomi M, Somigliana E, Cacciatore C, Busnelli M, Rossetti R, Bonetti S, et al. Blood cell mitochondrial DNA content and premature ovarian aging. PLoS ONE. 2012;7(8):e42423. https://doi.org/10.1371/journal.pone.0042423.
Lee SH, Chung DJ, Lee HS, Kim TJ, Kim MH, Jeong HJ, et al. Mitochondrial DNA copy number in peripheral blood in polycystic ovary syndrome. Metabolism. 2011;60(12):1677–82. https://doi.org/10.1016/j.metabol.2011.04.010.
Rabol R, Svendsen PF, Skovbro M, Boushel R, Schjerling P, Nilas L, et al. Skeletal muscle mitochondrial function in polycystic ovarian syndrome. Eur J Endocrinol. 2011;165(4):631–7. https://doi.org/10.1530/EJE-11-0419.
Eriksen MB, Minet AD, Glintborg D, Gaster M. Intact primary mitochondrial function in myotubes established from women with PCOS. J Clin Endocrinol Metab. 2011;96(8):E1298–302. https://doi.org/10.1210/jc.2011-0278.
Van Blerkom J, Davis PW, Lee J. Fertilization and early embryolgoy: ATP content of human oocytes and developmental potential and outcome after in vitro fertilization and embryo transfer. Hum Reprod. 1995;10(2):415–24. https://doi.org/10.1093/oxfordjournals.humrep.a135954.
Takeuchi T, Neri QV, Katagiri Y, Rosenwaks Z, Palermo GD. Effect of treating induced mitochondrial damage on embryonic development and epigenesis. Biol Reprod. 2005;72(3):584–92. https://doi.org/10.1095/biolreprod.104.032391.
Itami N, Shirasuna K, Kuwayama T, Iwata H. Resveratrol improves the quality of pig oocytes derived from early antral follicles through sirtuin 1 activation. Theriogenology. 2015;83(8):1360–7. https://doi.org/10.1016/j.theriogenology.2015.01.029.
Tripathi SK, Nandi S, Gupta PSP, Mondal S. Antioxidants supplementation improves the quality of in vitro produced ovine embryos with amendments in key development gene expressions. Theriogenology. 2023;201:41–52. https://doi.org/10.1016/j.theriogenology.2022.11.048.
Sato D, Itami N, Tasaki H, Takeo S, Kuwayama T, Iwata H. Relationship between mitochondrial DNA copy number and SIRT1 expression in porcine oocytes. PLoS ONE. 2014;9(4):e94488. https://doi.org/10.1371/journal.pone.0094488.
Liu M, Yin Y, Ye X, Zeng M, Zhao Q, Keefe DL, Liu L. Resveratrol protects against age-associated infertility in mice. Hum Reprod. 2013;28(3):707–17. https://doi.org/10.1093/humrep/des437.
Chen ZG, Luo LL, Xu JJ, Zhuang XL, Kong XX, Fu YC. Effects of plant polyphenols on ovarian follicular reserve in aging rats. Biochem Cell Biol. 2010;88(4):737–45. https://doi.org/10.1139/O10-012.
Ergenoglu M, Yildirim N, Yildirim AG, Yeniel O, Erbas O, Yavasoglu A, et al. Effects of Resveratrol on ovarian morphology, plasma anti-mullerian hormone, IGF-1 levels, and oxidative stress parameters in a rat model of polycystic ovary syndrome. Reprod Sci. 2015;22(8):942–7. https://doi.org/10.1177/1933719115570900.
Liu MJ, Sun AG, Zhao SG, Liu H, Ma SY, Li M, et al. Resveratrol improves in vitro maturation of oocytes in aged mice and humans. Fertil Steril. 2018;109(5):900–7. https://doi.org/10.1016/j.fertnstert.2018.01.020.
Palomba S, De Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update. 2015;21(5):575–92. https://doi.org/10.1093/humupd/dmv029.
Batioglu AS, Sahin U, Gurlek B, Ozturk N, Unsal E. The efficacy of melatonin administration on oocyte quality. Gynecol Endocrinol. 2012;28(2):91–3. https://doi.org/10.3109/09513590.2011.589925.
Unfer V, Raffone E, Rizzo P, Buffo S. Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: a prospective, longitudinal, cohort study. Gynecol Endocrinol. 2011;27(11):857–61. https://doi.org/10.3109/09513590.2011.564687.
Gerli S, Della Morte C, Ceccobelli M, Mariani M, Favilli A, Leonardi L, et al. Biological and clinical effects of a resveratrol-based multivitamin supplement on intracytoplasmic sperm injection cycles: a single-center, randomized controlled trial. J Matern Fetal Neonatal Med. 2022;35(25):7640–8. https://doi.org/10.1080/14767058.2021.1958313.
Agarwal A, Durairajanayagam D, du Plessis SS. Utility of antioxidants during assisted reproductive techniques: an evidence-based review. Reprod Biol Endocrinol. 2014;12:112. https://doi.org/10.1186/1477-7827-12-112.
Group ECPW, Vlaisavljevic V, Apter S, Capalbo A, D’Angelo A, Gianaroli L, et al. The Maribor consensus: report of an expert meeting on the development of performance indicators for clinical practice in ART. Hum Reprod Open. 2021;2021(3):hoab022. https://doi.org/10.1093/hropen/hoab022.
Conforti A, Iorio GG, Di Girolamo R, Rovetto MY, Picarelli S, Cariati F, et al. The impact of resveratrol on the outcome of the in vitro fertilization: an exploratory randomized placebo-controlled trial. J Ovarian Res. 2024;17(1):81. https://doi.org/10.1186/s13048-024-01391-7.
Ochiai A, Kuroda K, Ikemoto Y, Ozaki R, Nakagawa K, Nojiri S, et al. Influence of resveratrol supplementation on IVF–embryo transfer cycle outcomes. Reprod Biomed Online. 2019;39(2):205–10. https://doi.org/10.1016/j.rbmo.2019.03.205.
Erickson GF, Shimasaki S. The physiology of folliculogenesis: the role of novel growth factors. Fertil Steril. 2001;76(5):943–9. https://doi.org/10.1016/s0015-0282(01)02859-x.
Chow HH, Garland LL, Hsu CH, Vining DR, Chew WM, Miller JA, et al. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev Res (Phila). 2010;3(9):1168–75. https://doi.org/10.1158/1940-6207.CAPR-09-0155.
Ramirez-Garza SL, Laveriano-Santos EP, Marhuenda-Munoz M, Storniolo CE, Tresserra-Rimbau A, Vallverdu-Queralt A, Lamuela-Raventos RM. Health effects of Resveratrol: results from human intervention trials. Nutrients. 2018;10(12):1892. https://doi.org/10.3390/nu10121892.
Zhang L-X, Li C-X, Kakar MU, Khan MS, Wu P-F, Amir RM, et al. Resveratrol (RV): a pharmacological review and call for further research. Biomed Pharmacother. 2021;143:112164. https://doi.org/10.1016/j.biopha.2021.112164.
Chow HH, Garland LL, Heckman-Stoddard BM, Hsu CH, Butler VD, Cordova CA, et al. A pilot clinical study of resveratrol in postmenopausal women with high body mass index: effects on systemic sex steroid hormones. J Transl Med. 2014;12:223. https://doi.org/10.1186/s12967-014-0223-0.
Patel KR, Scott E, Brown VA, Gescher AJ, Steward WP, Brown K. Clinical trials of resveratrol. Ann N Y Acad Sci. 2011;1215(1):161–9. https://doi.org/10.1111/j.1749-6632.2010.05853.x.
Khodarahmian M, Amidi F, Moini A, Kashani L, Salahi E, Danaii-Mehrabad S, et al. A randomized exploratory trial to assess the effects of resveratrol on VEGF and TNF-alpha 2 expression in endometriosis women. J Reprod Immunol. 2021;143:103248. https://doi.org/10.1016/j.jri.2020.103248.
Taheri APH, Moradi B, Radmard AR, Sanginabadi M, Qorbani M, Mohajeri-Tehrani MR, et al. Effect of resveratrol administration on ovarian morphology, determined by transvaginal ultrasound for the women with polycystic ovary syndrome (PCOS). Br J Nutr. 2022;128(2):211–6. https://doi.org/10.1017/S0007114521003330.
Asghari S, Rafraf M, Farzin L, Asghari-Jafarabadi M, Ghavami SM, Somi MH. Effects of Pharmacologic Dose of Resveratrol supplementation on Oxidative/Antioxidative status biomarkers in nonalcoholic fatty liver Disease patients: a Randomized, Double-Blind, placebo-controlled trial. Adv Pharm Bull. 2018;8(2):307–17. https://doi.org/10.15171/apb.2018.036.
Mansour A, Samadi M, Sanginabadi M, Gerami H, Karimi S, Hosseini S, et al. Effect of resveratrol on menstrual cyclicity, hyperandrogenism and metabolic profile in women with PCOS. Clin Nutr. 2021;40(6):4106–12. https://doi.org/10.1016/j.clnu.2021.02.004.
Sattarinezhad A, Roozbeh J, Shirazi Yeganeh B, Omrani GR, Shams M. Resveratrol reduces albuminuria in diabetic nephropathy: a randomized double-blind placebo-controlled clinical trial. Diabetes Metab. 2019;45(1):53–9. https://doi.org/10.1016/j.diabet.2018.05.010.
Acknowledgements
We are grateful to all the participants for their invaluable time and commitment, as well as to the staff of the Omid Fertility Clinic, for providing the biological materials needed for this study.
Funding
This work was supported by the Tehran University of Medical Science (Grant Number: 1402-1-371-64482).
Author information
Authors and Affiliations
Contributions
NAA was involved in the data acquisition, drafting, and revision of the work; MAH evaluated the patients and performed the study protocol; MSHN and MK conducted the data analysis and revised the manuscript; MSH contributed to the data analysis and interpretation; MJ and FF contributed to the manuscript’s revision; and TR and FA contributed to the design of the study and conception. All the authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Tehran University of Medical Sciences, Shariati Hospital, Tehran, Iran (2023 January 07; Ethics Code: IR.TUMS.SHARIATI.REC.1401.021). Before participating in the study, all individuals provided informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ardehjani, N.A., Agha-Hosseini, M., Nashtaei, M.S. et al. Resveratrol ameliorates mitochondrial biogenesis and reproductive outcomes in women with polycystic ovary syndrome undergoing assisted reproduction: a randomized, triple-blind, placebo-controlled clinical trial. J Ovarian Res 17, 143 (2024). https://doi.org/10.1186/s13048-024-01470-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13048-024-01470-9